Abstract
Recent preclinical evidence suggests that leptin may modulate the stress response and may increase nociception. In this study, we examined for the first time the extent to which cigarette smoking is associated with leptin levels during an extended rest period and in response to noxious stimuli. Repeated blood samples were collected during a laboratory session from smokers and nonsmokers and assayed for leptin. Pain experiences, as well as neuroendocrine and cardiovascular measures, were collected across cold pressor and thermal heat pain tests. Both analysis of variance and correlations confirmed that smokers demonstrated dysregulations in leptin responsivity and association with pain relative to nonsmokers. The flat pattern of leptin release and the weak associations of this hormone with pain in smokers suggest a long-term effect of tobacco dependence on this regulatory hormone. In light of leptin’s influence on reward pathways, further investigation of leptin’s involvement in nicotine dependence is warranted.
Keywords: leptin, pain, smoking, mood
Introduction
Leptin, a protein product of the ob gene produced primarily by adipocytes (Campfield, Smith, Guisez, Devos, & Burn, 1995), regulates hypothalamic centers involved in body weight, energy homeostasis, and gene expression of corticotrophin-releasing hormone and pro-opiomelanocortin (Cheung, Clifton, & Steiner, 1997; Enriori, Evans, Sinnayah, & Cowley, 2006; Mantzoros, 1999). Evidence also suggests an involvement of appetite hormones in the regulation of drug reward (Bruijnzeel, 2012; Dickson et al., 2011; Funahashi et al., 2000; Harris, Wimmer, & Aston-Jones, 2005; Hollander, Lu, Cameron, Kamenecka, & Kenny, 2008; Li et al., 2000; Opland, Leinninger, & Myers, 2010).
Basic research suggests a role for leptin in regulating nociception. Specifically, research on nociception has demonstrated leptin’s involvement in the development of allodynia (high sensitivity to pain) and exacerbation of neuropathic pain (Maeda et al., 2009). Increased pain sensitivity has also been demonstrated in response to peripheral administration of leptin using multiple animal models (Kutlu et al., 2003; Tian et al., 2011). In addition, leptin modulates systems involved in the hypothalamic-pituitary-adrenocortical (HPA) stress response leading to reduced adrenocortical output, presumably by acting at the level of the hypothalamus (Ahima et al., 1996; Heiman et al., 1997). Consistent with this, research has shown that reduced cortisol levels are associated with increased pain sensitivity (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Godfrey et al., 2014), although little is known about this in humans.
The extent to which leptin’s associations with pain are affected by chronic smoking has not been investigated. Existing leptin research with smokers has addressed the effects of smoking or abstinence on changes in this hormone with some studies finding differences (Koc, Bulucu, Karadurmus, & Sahin, 2009; Reseland et al., 2005; Perkins & Fonte, 2002) and others finding no effect of short-term smoking abstinence on the hormone (Klein, Corwin, & Ceballos, 2004). However there has been no systematic investigation of the differences between smokers and nonsmokers in baseline and repeated measures of this hormone as this relates to pain modulation. In light of recent findings showing disrupted endogenous pain modulation in smokers (Nakajima & al’Absi, in press), manifested by increased pain and absence of stress-induced analgesia, it is important to examine the hormonal association with pain in this population. To that end, comparing smokers with nonsmokers allows for an examination of the impact of chronic smoking behavior on this hormone. The goal of this study was to examine the extent to which circulating leptin levels are associated with nicotine dependence during resting baseline and following exposure to noxious stimuli. We measured leptin during rest, before and after completing two pain-induction procedures, and during an extended recovery period. We predicted that smoking would be associated with a disrupted pattern of leptin production across time, and that leptin levels would be associated with increased pain perception.
Methods
Participants
Participants were recruited from the community by posters and newspaper advertisements to participate in a larger study (al'Absi, Hatsukami, & Davis, 2005). Smokers were included if they had smoked at least 10 cigarettes per day for the past two years and were not interested in cessation at the time of the study. Nonsmokers were included if they had never smoked over the last five years or if they had smoked fewer than 100 cigarettes over their lifetime, but none over the previous year. All participants had to meet the following criteria: 1) no regular use of prescribed or over-the-counter medications except contraceptives; 2) no current or prior treatment for hypertension, renal or hepatic disease, no current or history of chronic diseases (e.g., cardiovascular, respiratory, endocrine, and neurological disorders, thyroid, respiratory disorders); 3) no current or history of major psychiatric disorders (e.g., depression, schizophrenia, alcohol and drug abuse); 4) no current opiate dependence, recent daily opiate use, or use of any narcotic medication within 3 days prior to the study; 5) non-pregnancy; and 6) weight within ± 30% of Metropolitan Life Insurance norms. Smokers were asked to maintain their normal smoking patterns and were to smoke one cigarette of their preferred brand 30 minutes prior to each laboratory session to minimize withdrawal effects. They did not smoke during the laboratory session (approximately 4 hours). Data used in this study were collected in a larger project that was conducted to examine endogenous opioid blockade and pain sensitivity (al'Absi, Wittmers, Hatsukami, & Westra, 2008). We include here data from the placebo day (described below). Participants received a monetary incentive for participation (approximately $20 US per hour), and they signed a consent form approved by the Institutional Review Board of the University of Minnesota
Apparatus and Measures
Pain Measures
The cold pressor test (CPT) apparatus consisted of a one-gallon container that was filled with ice-water slurry (temperature range: 0–1 °C). Participants were asked to rate their pain at 15-second intervals throughout the 90-second hand immersion in ice-water slurry and the 90-second recovery period. The rating was based on a visual, numerical rating scale with a range from 0 (not at all painful) to 100 (extremely painful). Average ratings were calculated during and after the task, respectively. Subjective pain experience was also measured using the short form of the McGill Pain Questionnaire (MPQ; Melzack, 1987) after CPT.
For thermal pain, a computer-controlled 2 cm2 Peltier contact thermode affixed in place with a Velcro strap was used to deliver thermal pain stimuli to the skin of the right volar forearm. Temperature was monitored by a contactor-contained thermistor (Medoc TSA 2001, Minneapolis, MN). The thermode was returned to the adapting temperature (32°C) between trials by active cooling at a rate of 10° C/sec. Thermal pain threshold and tolerance were assessed using an ascending method of limits with a staircase ramp of 1°C /sec. Participants were instructed to press a button when the thermal stimulus first felt painful (i.e. pain threshold). For the tolerance assessment, subjects were instructed to press the button when the pain became intolerable. The assessment was repeated four times and the average of the last three trials was calculated to determine thermal pain threshold and tolerance. Each stimulus was presented four times in random order, and responses were averaged for each temperature. Participants rated their estimate of pain at each of the 5 levels between 45 – 49°C using a visual analogue scale of 0 to 100. Participants also completed the MPQ after the completion of the thermal pain test.
Hormonal & Cardiovascular Measure
Blood samples were collected during baseline rest (first), after one hour rest (second sample), 30 minutes after exposure to the pain induction procedures (third sample), and after a 60-minute recovery period (fourth sample). Blood was collected using a 20-gauge intravenous Teflon catheter inserted in a left forearm vein. The catheter was fitted with a rubber infusion plug through which samples were drawn. Sterile saline was used to flush the system. Each sample was collected in an 8 ml EDTA Vacutainer tube. At the end of the session, samples were centrifuged and stored at −70°C. Plasma leptin was assayed using a direct sandwich ELISA (Linco, Missouri). Inter- and intra-assay coefficients of variance for these assays were below 8%. Adrenocorticotropic hormone (ACTH) was assayed using RIA kits with a lower sensitivity of 1 pg/mL. Plasma cortisol was assayed using EIA (DSL, Sinsheim, Germany) with a lower sensitivity of 0.1 µg/dL. Inter- and intra-assay coefficients of variance for these assays were below 10%. Systolic blood pressure (SBP), diastolic blood pressure (DBP, and heart rate (HR) were collected across the laboratory session prior to blood sampling using a Danamap oscillometric monitor system (Critikon, Tampa, FL). Demographic information was collected from the entire sample. Smoking history and nicotine dependence levels (Fagerström Test of Nicotine Dependence; FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) were assessed in smokers.
Procedures
All testing sessions started at approximately 12:00 PM to control for circadian rhythm. Women were tested during the follicular phase of their menstrual cycle (2–13 days after last menses) to avoid hormonal fluctuation. Prior to each session, participants were asked to abstain from alcohol or analgesic medication for 24 hours and narcotic medication for 3 days. They were instructed to have a light lunch at least one hour prior to the session and were provided specific suggestions for items to consume. Those who reported hunger at the beginning of the session were provided two oatmeal granola bars.
At the beginning of the session an IV catheter was inserted, a blood pressure cuff was placed on the opposite arm, and participants were asked to sit quietly for 30 minutes prior to ingesting a placebo double blind capsule that included placebo or naltrexone (data reported here included the placebo condition only). This was followed by a 60-minute rest period; after which the two pain induction procedures were administered in a counterbalanced order, separated by a 20-minute rest period. After the second pain test (CPT or thermal pain), participants rested for 60 minutes.
Dependent Variables and Data Analyses
The primary dependent variables were leptin, ACTH, plasma cortisol, salivary cortisol, and pain measures. Smoking status was categorized as smoker or nonsmoker as described above. Bivariate correlation analysis indicated that BMI did not correlate with any of the appetite measures and was therefore not controlled for in the analyses. A series of one-way analysis of variance (ANOVA) were used to test differences as a function of smoking status on all demographic variables (e.g., age, body mass index (BMI), education) and smoking or other substance use history variables (caffeine, smoking rate, duration at that rate, age first smoked and nicotine dependence score). We conducted a 2 (Smoking Status) × 4 (Sample; 2 before and 2 after the pain assessment procedures) repeated measures of analysis of covariance (ANCOVA) with sex as a covariate to examine the extent to which leptin, ACTH, plasma and salivary and cortisol changed acutely during the lab session. Sex was included as a covariate due to limited numbers of females in the study (9 nonsmokers; 6 smokers). Cardiovascular measures were analyzed by a series of 2 (Smoking status) × 3 (Sample: baseline, rest, post pain recovery) repeated measures ANCOVA (gender as the covariate). Greenhouse-Geisser correction of degrees of freedom was used in the event to violations of sphericity (Jennings, 1987). Separate one-way ANOVAs were conducted in each smoking group to examine significant interactions and simple comparisons with Bonferroni correction were used to examine significant time effects. Differences between smokers and nonsmokers in mean pain ratings during CPT, after CPT, thermal heat pain threshold and tolerance, and MPQ scores after each pain task were analyzed by one-way ANCOVAs. A 2 (Smoking status) × 5 (temperature level) mixed ANCOVA was conducted using pain report to temperature at each degree (45 – 49° C) as the within subjects factor and smoking status as the between subjects factor. Gender was again a covariate. Preliminary analyses found that the order of drug was not associated with hormonal and pain measures. This variable was not included in the subsequent analysis to save degrees of freedom. Finally, a series of bivariate correlation analysis was also conducted to examine association of appetite hormones with pain measures. P values less than 0.05 were considered statistically significant.
Results
Participant Characteristics
A total of 43 participants (23 smokers and 20 nonsmokers) had plasma samples available to be assayed for leptin. Due to missing data, variations exist in degrees of freedom for the reported variables, and only 39 participants (21 smokers) had the total set of blood samples for the entire session. Participants’ characteristics are included in Table 1. Smokers and nonsmokers did not differ in age, BMI, or years of education (Fs < 1.3). Smokers reported consuming more daily caffeinated drinks than nonsmokers (F (1, 41) = 9.19, p < 0.01). Leptin levels from all 4 time points were not associated with demographic variables (i.e., age, length of education, and body mass index; correlation range = .20 to .24, ps > .10) or smoking variables (i.e., age when first smoked, cigarettes per day, years of smoking, and FTND scores; correlation range = −.07 to .16, ps > .10).
Table 1.
Sample Characteristics.
| Nonsmokers (n=20) | Smokers (n=23) | |
|---|---|---|
| Age (yrs) | 21.7 (1.6) | 24.0 (1.5) |
| BMI (kg/m2) | 24.7 (0.7) | 24.9 (0.7) |
| Education (yrs) | 14.7 (0.5) | 14.7 (0.4) |
| Caffeine use (serving)* | 1.0 (0.4) | 2.7 (0.4) |
| Smoking rate (cigs/day) | n/a | 17.2 (1.3) |
| Duration at this rate (yrs) | n/a | 6.0 (1.2) |
| Age first smoked (yrs) | n/a | 16.5 (0.5) |
| FTND | n/a | 4.2 (0.4) |
Entries show mean (standard error of the mean); BMI, body mass index; FTND, Fagerström Test of Nicotine Dependence.
p < 0.01.
Leptin Levels
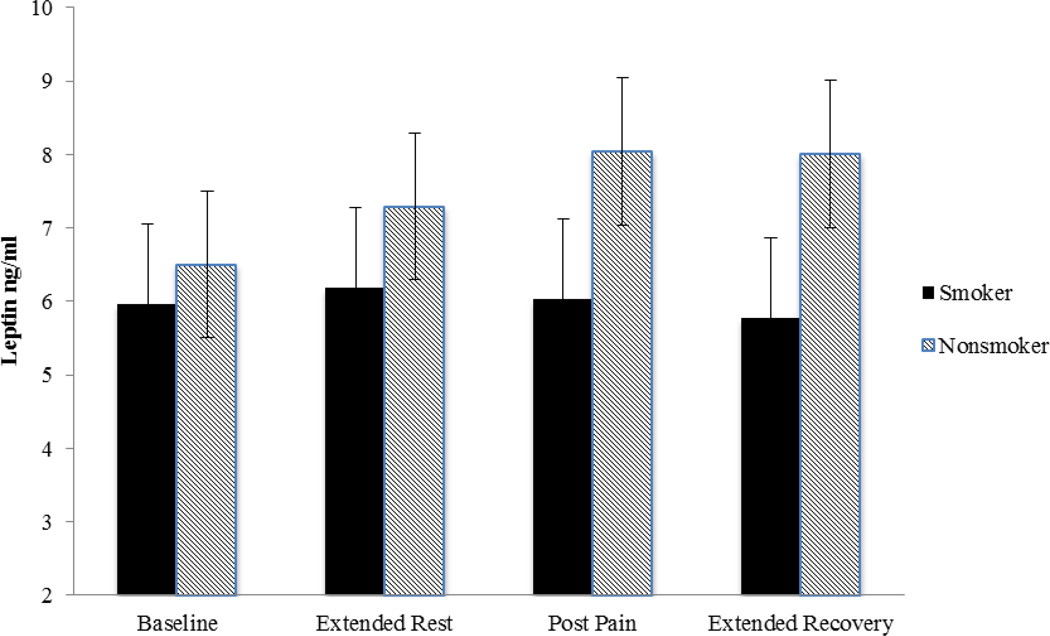
Significant changes in circulating leptin levels were noted over time, as indicated by a significant time main effect (F(1.58, 53.81) = 3.55, p < 0.05). This was, however, qualified by a significant group by time interaction (F(1.58, 53.81) = 7.98, p < 0.01). Follow-up analysis using one-way ANOVAs conducted in each smoking group indicated that there was a significant increase over time in nonsmokers but not in smokers (see Figure 1). Planned contrasts showed that there was a significant increase during two pre-pain periods (time 1 versus time 2; F(1, 15) = 14.82, p < .01) and it remained elevated after the pain assessments (time 1 versus time 4: F(1,15) = 11.67, p < .01; time 1 versus time 3: F(1,15) = 10.51, p < .01) in nonsmokers. In contrast, the smokers did not show any significant time effects (p > .40). As shown in Figure 1, the smokers and nonsmokers diverge over time. At their greatest difference (sample 4, post thermal pain) the smokers were marginally lower than the nonsmokers (F(1, 36) = 3.76, p = .06).
Figure 1.
Mean (SEM) of circulating leptin levels obtained during baseline after extended rest, post pain induction, and after extended recovery period in smokers and nonsmokers.
Other physiological and endocrine measures
ACTH did not differ by smoking groups or across time (Fs < 2.5, p > .10; see Table 2). A significant time effect in plasma cortisol (F (2.15, 81.68) = 7.24, p < .005) indicated greater levels in the initial compared to the second (F(1, 38) = 14.68, p < .001), third (F(1, 38) = 9.50, p < .005), and the final sample (F(1, 38) = 5.17, p < .05). Neither group nor group by time interaction was significant (Fs < 2.22, p > .11). There was a significant time effect in salivary cortisol (F (2.03, 79.08) = 6.11, p < .005), indicating higher levels in the first sample relative to the second (F(1, 39) = 12.73, p < .005), third (F(1, 39) = 8.00, p < .01), and the last sample (F(1, 39) = 9.13, p < .005). Smokers also had greater salivary cortisol levels than nonsmokers (F(1, 39) = 13.18, p < .005). The interaction of smoking group by time was not significant (F = .14, p = .84). Neither SBP nor DBP showed any significant time, group or time by group interaction effects (All Fs < 2.5, ps > .09). In contrast, HR showed a significant time by group interaction (F(1.58, 63.41) = 5.12, p < .05) with greater levels during the initial baseline than during the subsequent periods in smokers (F(1.56, 34.21) = 14.57, p < .001; baseline vs. rest (F(1, 22) = 15.38, p < .005); baseline vs. recovery (F(1, 22) = 20.12, p < .001). This pattern was not found in nonsmokers (F < 1.8).
Table 2.
Physiological measures.
| Nonsmokers (n=20) | Smokers (n=23) | ||
|---|---|---|---|
| ACTH (pg/mL) | |||
| Pre-pain baseline | 13.8 (1.6) | 14.1 (1.4) | |
| Pre-pain rest | 12.0 (1.5) | 13.1 (1.3) | |
| Post-pain rest 1 (30 m after) | 13.5 (1.6) | 11.9 (1.4) | |
| Post-pain rest 2 (60 m after) | 13.1 (1.6) | 13.1 (1.4) | |
| Plasma cortisol (µg/dL)a | |||
| Pre-pain baseline | 10.7 (1.2) | 8.2 (1.1) | |
| Pre-pain rest | 6.6 (0.8) | 6.1 (0.7) | |
| Post-pain rest 1 (30 m after) | 6.7 (0.7) | 6.6 (0.6) | |
| Post-pain rest 2 (60 m after) | 6.4 (0.8) | 7.4 (0.7) | |
| Salivary cortisol (nmol/L)ab | |||
| Pre-pain baseline | 7.0 (1.0) | 10.4 (0.9) | |
| Pre-pain rest | 4.2 (0.7) | 6.9 (0.7) | |
| Post-pain rest 1 (30 m after) | 4.1 (0.7) | 7.1 (0.6) | |
| Post-pain rest 2 (60 m after) | 3.5 (0.8) | 6.7 (0.7) | |
| Systolic BP (mmHg) | |||
| Pre-pain baseline | 112.8 (2.7) | 115.7 (2.5) | |
| Pre-pain rest | 110.8 (2.5) | 113.6 (2.4) | |
| Post-pain rest | 113.2 (2.4) | 115.6 (2.3) | |
| Diastolic BP (mmHg) | |||
| Pre-pain baseline | 61.2 (1.9) | 63.2 (1.7) | |
| Pre-pain rest | 61.5 (1.7) | 62.7 (1.6) | |
| Post-pain rest | 64.2 (1.9) | 64.8 (1.8) | |
| Heart rate (bpm)c | |||
| Pre-pain baseline | 63.3 (1.8) | 67.5 (1.7) | |
| Pre-pain rest | 61.4 (1.9) | 64.7 (1.7) | |
| Post-pain rest | 62.2 (1.9) | 62.2 (1.8) | |
Entries show mean (standard error of the mean). Note: ACTH = Adrenocorticotropic hormone; BP = blood pressure; bpm = beats per minute.
Time effect was significant.
Group effect was significant.
Group × time interaction was significant.
Pain measures
Smokers reported greater MPQ Total scores after CPT than nonsmokers (F(1, 40) = 6.14, p < .05). MPQ scores after thermal pain did not differ by smoking status (F < 1). No smoking group differences were observed in VAS pain ratings during (F(1, 40) = 0.1, p > .79) and after CPT (F(1, 40) = 3.60, p < .07). There was no difference between smokers and nonsmokers in heat pain threshold (F < 2) or pain tolerance (F < 1.1). There was, however, the expected increase in self-reported pain to rising heat over time (F(2.24, 87.41) = 52.52, p < .001) that did not differ by smoking group (F < 1).
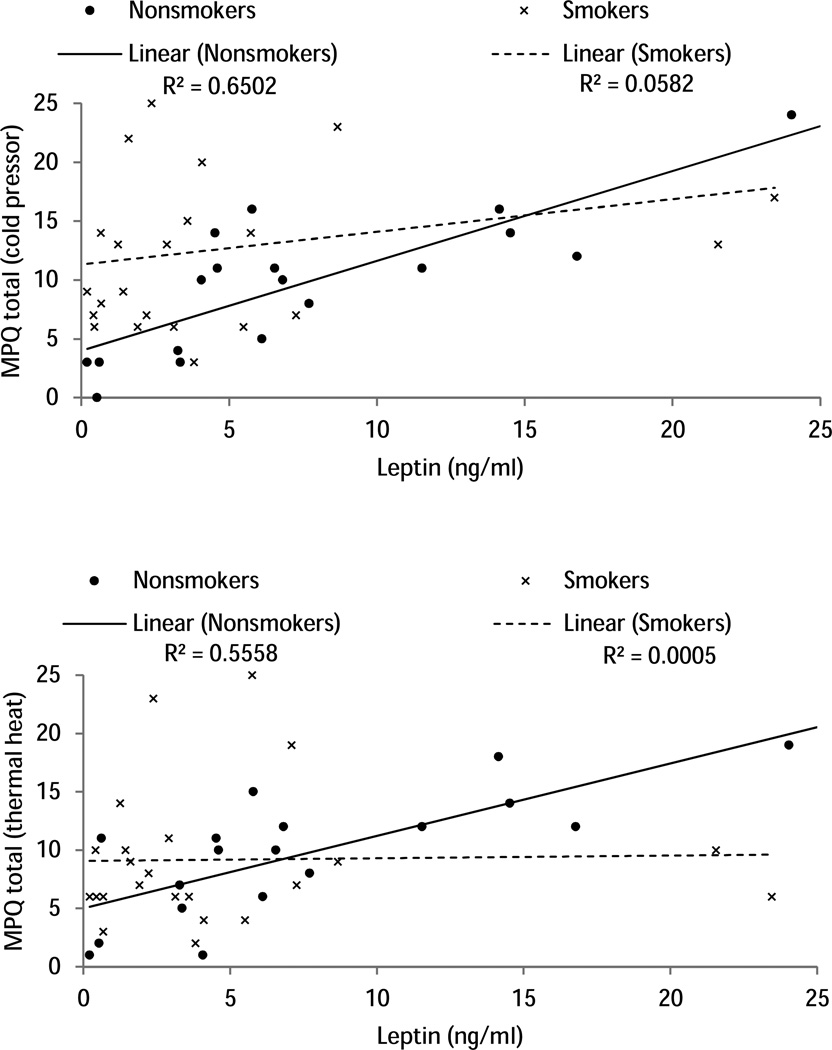
The correlation analysis showed that higher leptin levels throughout the study were positively associated with greater MPQ Total scores after CPT (correlation range = .39 to .43, ps<.05) and after thermal heat pain (rs >.32, ps < .05). When the correlational analysis was conducted in each group separately, these positive correlations were highly significant in nonsmokers (correlation range after CPT = .74 to .81, ps < .001; after thermal heat pain = .73 to .75, ps < .001), but not significant in smokers (correlation range after CPT = .20 to .24, ps > .10; after thermal heat pain = −.02 − .02, ps > .10; see Figure 2 for an example).
Figure 2.
Scatter plot depicting the relationship between leptin levels (ng/mL) during baseline and MPQ total scores after cold pressor pain and after thermal pain in smokers (x symbols) and nonsmokers (closed circles).
Discussion
Two primary findings were obtained in this study. First, smokers showed minimal changes in leptin concentrations throughout the laboratory session. This is in contrast to nonsmokers who had a robust leptin response to the laboratory manipulations of pain. The fact that the rise in self-reported pain across the laboratory session that did not differ by smoking status confirms that the pain induction was successful for both groups. Thus, the smoker’s failure to show a leptin effect cannot be presumed to be a failure of the experimental manipulation. Instead, other explanations must account for these findings.
Previous studies have shown an association between reduced leptin level and smoking, although this has not been consistent (Eliasson & Smith, 1999; Koc et al., 2009; Perkins & Fonte, 2002; Reseland et al., 2005). We show not only that the smokers failed to demonstrate the pain-related change in leptin evident in nonsmokers, but that the smokers also showed a trend towards lower leptin. Although marginal, this trend supports the previous findings cited above indicating that active smokers have lower leptin than non-smokers (Koc, Bulucu, Karadurmus, & Sahin, 2009; Reseland et al., 2005). It is possible that the blunted changes in leptin concentrations throughout the session in the current study reflected a long-term effect of tobacco on leptin release that may contribute to maintenance of tobacco use. This effect may be mediated by leptin’s effects on the dopaminergic pathway. Indeed, a role for leptin in the modulation of reward pathways has been demonstrated in animal models (Kalra & Kalra, 2004; Palmiter, 2007). Leptin has been shown to inhibit dopamine signalling in the nucleus accumbens (Laviolette & van der Kooy, 2004) and it can reduce reward benefits of drug or electrical stimulation (Fulton et al., 2006; Fulton, Woodside, & Shizgal, 2000). If this can be applied to humans, then the implication is that lower leptin in smokers would facilitate enhanced reward of drug exposure. This is consistent with reports of a strong negative correlation between leptin and the number of cigarettes smoked per day and evidence of lower leptin in smokers (Koc, Bulucu, Karadurmus, & Sahin, 2009; Reseland et al., 2005). It has been suggested that this animal literature supports the possibility of a leptin-related reward deficit among tobacco dependent individuals (Laviolette & van der Kooy, 2004). It is also possible that the regulation effects of leptin are weakened among smokers due to chronic stimulatory effects of nicotine on dopaminergic transmission, contributing to dysregulation of dopaminergic activity. In turn nicotine may be used as a way to compensate for this dysregulation by increasing the reinforcing effects of smoking. Previous observations indicating a positive association between leptin levels and craving for cigarettes (al'Absi et al., 2011; von der Goltz et al., 2010) are consistent with this possibility.
Secondly, in addition to replicating previous findings of increased pain reports in smokers (al'Absi, Nakajima, & Grabowski, 2013; Nakajima & al'Absi, in press), it was also interesting to find positive associations between pain reports and leptin levels across the session in nonsmokers but not in smokers. The strength of these correlations with pain measures suggests common underlying pathways that link leptin release and endogenous regulation of pain perception. There is evidence indicating that leptin may be involved in regulating nociception. As reviewed in the introduction, leptin appears to be critical in the development of allodynia and it may contribute to the development of neuropathic pain (Maeda et al., 2009). Some have suggested that a decoupling of leptin and neuropeptide Y signaling may account for the higher pain report in smokers with fibromyalgia (Bokarewa, Erlandsson, Bjersing, Dehlin, & Mannerkorpi, 2014). Again the lack of human studies requires that existing animal literature guide the interpretation of these results. Experiments using rats and mice have demonstrated increased pain sensitivity in response to peripheral administration of leptin (Kutlu et al., 2003; Tian et al., 2011). There is also evidence to suggest that leptin can produce nociceptive effects (Guneli, Gumustekin, & Ates, 2010; Maeda et al., 2009; Tian et al., 2011). It should be noted that, in the current study, the association between leptin and pain was not significant in smokers possibly reflecting disruptive links between physiological mechanisms and motivational processes in smokers similar to those observed among other drug using populations (al'Absi, Nakajima, & Grabowski, 2013; Lovallo, 2007).
There are potentially important clinical implications of these observations. In addition to the well-known association between smoking and weight regulation (O'Hara et al., 1998; Koopmann et al., 2011; Williamson et al., 1991), pain complaints are heightened in both smokers (Parkerson, Zvolensky, & Asmundson, 2013) and obese individuals (Arranz, Rafecas, & Alegre, 2014; Thomazeau et al., 2014). Leptin dysregulation and possibly leptin-resistence has been proposed as an important contributing factor for obesity (Sainz, Barrenetxe, Moreno-Aliaga, & Martinez, 2015) and leptin appears to interact with nicotine to further compound negative health effects. For example, leptin is associated with blood pressure and metabolic syndrome in male smokers, but only those who are obese (Kim, Won, Ko & Roh, 2014). Adiposity also appears to mediate the association between leptin and depressive affect (Morris et al., 2012). This, and observed disparities in the prescription of smoking cessation medicines based on obesity (Yu, Rajan, Essien, Yang, & Abughosh, 2014), has led some physicians to advocating for different treatment approaches with the co-morbid obese and chronic pain smoker (Aronoff, 2009). Appetite-regulating neuropeptides, like leptin, have been suggested as a possible mediator of tobacco and other drug craving (Aguiar-Nemer, Toffolo, da Silva, Laranjeira, & Silva-Fonseca, 2013; Li et al., 2000). The mechanisms responsible for the flat fluctuations of appetite hormones across pain manipulation demonstrated in this study are not clear and the implications of this leptin and pain relationship for smoking cessation should be investigated in the context of examining effects of stress, either laboratory induced or that associated with the stress of abstinence, on craving, relapse, appetite and weight.
The results of this study open several questions related to the complex interplay between stress, smoking, leptin and pain. In addition to the uniqueness of these findings, the strengths of this study include the use of a control group of nonsmokers and the repeated measures design. The pain induction was also carefully controlled and assessed. It should be noted, however, that this study is limited by the small sample of twenty-three smokers, and therefore reported results should be considered preliminary. Nevertheless, in light of the novelty and consistency of the reported results with preclinical studies, these results provide directions for future research.
In summary, we demonstrate here that leptin in nonsmokers, but not smokers, is responsive to laboratory induced pain. Further, we demonstrate that nonsmokers are the only group to show a strong correlation between leptin and reported pain regardless of the mode of pain induction. We hypothesize that chronic exposure to tobacco may have disrupted this leptin and pain association, though further research is needed to clarify this. Finally, replication of this study is needed to clarify the unexpected finding of no change in leptin for smokers but increased levels of leptin in response to the pain manipulation in the nonsmokers. These results augment the growing literature on appetite hormones and smoking and they support leptin as a new, and potentially important, factor in the negative effects of chronic smoking.
Highlights.
Despite normal pain sensitivity, smokers fail to show a change in leptin following experimental pain induction.
Among nonsmokers only, leptin is positively correlated with perceived pain.
The leptin-pain association has implications for defining the nature of endogenous pain dysregulation in smokers
Acknowledgements
This research was supported in part by grants to the first author from the National Institute of Health (R01DA016351 and R01DA027232).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar-Nemer AS, Toffolo MC, da Silva CJ, Laranjeira R, Silva-Fonseca VA. Leptin influence in craving and relapse of alcoholics and smokers. Journal of Clinical & Medical Research. 2013;5(3):164–167. doi: 10.4021/jocmr1159w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181(1):107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hooker S, Fujiwara K, Kiefer F, von der Goltz C, Cragin T, Wittmers LE. Circulating leptin levels are associated with increased craving to smoke in abstinent smokers. Pharmacology Biochemistry Behavior. 2011;97(3):509–513. doi: 10.1016/j.pbb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al'Absi M, Nakajima M, Grabowski J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biological Psychology. 2013;93(1):1–8. doi: 10.1016/j.biopsycho.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosomatic Medicine. 2008;70(8):928–935. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff G. Chronic pain, smoking, and obesity: a pain physician's perspective on patient selection. Pain Med. 2009;10(6):962–965. doi: 10.1111/j.1526-4637.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- Arranz LI, Rafecas M, Alegre C. Effects of obesity on function and quality of life in chronic pain conditions. Current Rheumatology Reports. 2014;16(1):390. doi: 10.1007/s11926-013-0390-7. [DOI] [PubMed] [Google Scholar]
- Bokarewa MI, Erlandsson MC, Bjersing J, Dehlin M, Mannerkorpi K. Smoking is associated with reduced leptin and neuropeptide Y levels and higher pain experience in patients with fibromyalgia. Mediators of Inflammation. 2014;2014:627041. doi: 10.1155/2014/627041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neuroscience & Biobehavioral Reviews. 2012;36(5):1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Molecular and Cellular Endocrinology. 2011;340(1):80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Eliasson B, Smith U. Leptin levels in smokers and long-term users of nicotine gum. European Journal of Clinical Investigation. 1999;29(2):145–152. doi: 10.1046/j.1365-2362.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring) 2006;14(Suppl 5):254s–258s. doi: 10.1038/oby.2006.319. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287(5450):125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Funahashi H, Hori T, Shimoda Y, Mizushima H, Ryushi T, Katoh S, Shioda S. Morphological evidence for neural interactions between leptin and orexin in the hypothalamus. Regulatory Peptides. 2000;92(1–3):31–35. doi: 10.1016/s0167-0115(00)00146-4. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Strachan E, Dansie E, Crofford LJ, Buchwald D, Goldberg J, Afari N. Salivary cortisol and cold pain sensitivity in female twins. Annals of Behavioral Medicine. 2014;47(2):180–188. doi: 10.1007/s12160-013-9532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneli E, Gumustekin M, Ates M. Possible involvement of ghrelin on pain threshold in obesity. Medical Hypotheses. 2010;74(3):452–454. doi: 10.1016/j.mehy.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proceedings of the National Academy of Sciences U S A. 2008;105(49):19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J. Editorial policy on analyses of variance with repeated measures. Psychophysiology. 1987;24:474–475. [Google Scholar]
- Kalra SP, Kalra PS. Overlapping and interactive pathways regulating appetite and craving. Journal of Addictive Diseases. 2004;23(3):5–21. doi: 10.1300/J069v23n03_02. [DOI] [PubMed] [Google Scholar]
- Kim KW, Won YL, Ko KS, Roh JW. Smoking Habits and Neuropeptides: Adiponectin, Brain-derived Neurotrophic Factor, and Leptin Levels. Toxicological Research. 2014;30(2):91–97. doi: 10.5487/TR.2014.30.2.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ, Ceballos RM. Leptin, hunger, and body weight: Influence of gender, tobacco smoking, and smoking abstinence. Addictive Behaviors. 2004;29(5):921–927. doi: 10.1016/j.addbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Koc B, Bulucu F, Karadurmus N, Sahin M. Lower leptin levels in young non-obese male smokers than non-smokers. Upsala Journal of Medical Sciences. 2009;114(3):165–169. doi: 10.1080/03009730902761631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann A, Dinter C, Grosshans M, von der Goltz C, Hentschel R, Dahmen N, Kiefer F. Psychological and hormonal features of smokers at risk to gain weight after smoking cessation--results of a multicenter study. Hormones and Behavior. 2011;60(1):58–64. doi: 10.1016/j.yhbeh.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Kutlu S, Canpolat S, Sandal S, Ozcan M, Sarsilmaz M, Kelestimur H. Effects of central and peripheral administration of leptin on pain threshold in rats and mice. Neuroendocrinology Letters. 2003;24(3–4):193–196. [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Reviews Neuroscience. 2004;5(1):55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Research. 2000;867(1–2):157–164. doi: 10.1016/s0006-8993(00)02283-6. [DOI] [PubMed] [Google Scholar]
- Lovallo W. Individual differences in response to stress and risk for addiction. In: al'Absi M, editor. Stress and addiction: Biological and psychological mechanisms. London: Elsevier; 2007. pp. 265–284. [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ikuta T, Ozaki M, Kishioka S. Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulation. Proceedings of the National Academy of Sciences U S A. 2009;106(31):13076–13081. doi: 10.1073/pnas.0903524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS. Leptin and the hypothalamus: neuroendocrine regulation of food intake. Molecular Psychiatry. 1999;4(1):8–12. 16–17. doi: 10.1038/sj.mp.4000497. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Morris AA, Ahmed Y, Stoyanova N, Hooper WC, De Staerke C, Gibbons G, Vaccarino V. The association between depression and leptin is mediated by adiposity. Psychosomatic Medicine. 2012;74(5):483–488. doi: 10.1097/PSY.0b013e31824f5de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, al'Absi M. Nicotine withdrawal and stress-induced changes in pain sensitivity: A cross-sectional investigation between abstinent smokers and nonsmokers. Psychophysiology. doi: 10.1111/psyp.12241. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. American Journal of Epidemiology. 1998;148(9):821–830. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- Opland DM, Leinninger GM, Myers MG., Jr Modulation of the mesolimbic dopamine system by leptin. Brain Research. 2010;1350:65–70. doi: 10.1016/j.brainres.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends in Neuroscience. 2007;30(8):375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Parkerson HA, Zvolensky MJ, Asmundson GJ. Understanding the relationship between smoking and pain. Expert Review of Neurotherapeutics. 2013;13(12):1407–1414. doi: 10.1586/14737175.2013.859524. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C. Effects of smoking status and smoking cessation on leptin levels. Nicotine & Tobacco Research. 2002;4(4):459–466. doi: 10.1080/1462220021000018434. [DOI] [PubMed] [Google Scholar]
- Reseland JE, Mundal HH, Hollung K, Haugen F, Zahid N, Anderssen SA, Drevon CA. Cigarette smoking may reduce plasma leptin concentration via catecholamines. Prostaglandins, Leukotrienes and Essentential Fatty Acids. 2005;73(1):43–49. doi: 10.1016/j.plefa.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Thomazeau J, Perin J, Nizard R, Bouhassira D, Collin E, Nguyen E, Lloret-Linares C. Pain management and pain characteristics in obese and normal weight patients before joint replacement. Journal of Evaluation in Clinical Practice. 2014 doi: 10.1111/jep.12176. [DOI] [PubMed] [Google Scholar]
- Tian Y, Wang S, Ma Y, Lim G, Kim H, Mao J. Leptin enhances NMDA-induced spinal excitation in rats: A functional link between adipocytokine and neuropathic pain. Pain. 2011;152(6):1263–1271. doi: 10.1016/j.pain.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Rockenbach C, Grosshans M, Kiefer F. Orexin and leptin are associated with nicotine craving: a link between smoking, appetite and reward. Psychoneuroendocrinology. 2010;35(4):570–577. doi: 10.1016/j.psyneuen.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. New England Journal of Medicine. 1991;324(11):739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- Yu Y, Rajan SS, Essien EJ, Yang M, Abughosh S. The Relationship between Obesity and Prescription of Smoking Cessation Medications. Population Health Management. 2014 doi: 10.1089/pop.2013.0059. [DOI] [PubMed] [Google Scholar]