Abstract
Clinical studies suggest cardiovascular and renal benefits of ingesting small amounts of ethanol. Effects of ethanol, role of alcohol dehydrogenase (ADH) or of 20-hydroxyeicosatetraenoic acid (20-HETE) in podocytes of the glomerular filtration barrier have not been reported. We found that mouse podocytes at baseline generate 20-HETE and express ADH but not CYP2e1. Ethanol at high concentrations altered the actin cytoskeleton, induced CYP2e1, increased superoxide production and inhibited ADH gene expression. Ethanol at low concentrations upregulated the expression of ADH and CYP4a12a. 20-HETE, an arachidonic acid metabolite generated by CYP4a12a, blocked the ethanol-induced cytoskeletal derangement and superoxide generation. Ethanol at high concentration or ADH inhibitor increased glomerular albumin permeability in vitro. 20-HETE and its metabolite produced by ADH activity, 20-carboxy-arachidonic acid, protected the glomerular permeability barrier against an ADH inhibitor, puromycin or FSGS permeability factor. We conclude that ADH activity is required for glomerular function, 20-HETE is a physiological substrate of ADH in podocytes and that podocytes are useful biosensors to understand glomeruloprotective effects of ethanol.
Keywords: Ethanol, Podocytes, Glomerular Filtration Barrier, Chronic Kidney Disease, Oxidative stress, Alcohol dehydrogenase, Proteinuria, 20-Hydroxyeicosatetraenoic Acid, 20-Carboxy-Arachidonic Acid
1. Introduction
The effect of ethanol on human behavior and metabolism with its psychosocial and economic implications has been widely studied. Negative effects of excessive ethanol consumption include injury to brain and liver tissues as well as exacerbation of hypertension and cardiovascular disease [1]. In contrast, ingestion of moderate amounts of ethanol in the form of alcoholic beverages has been associated with improved cardiovascular survival and other health benefits [2, 3]. A recent review suggests that small amounts of ethanol may lower the risk of kidney disease and may have clinically beneficial effect on renal function and the progression of chronic kidney disease [4]. Potential renoprotection by small amounts of ethanol indicate its role in glomerular function. However, the mechanism of dose-dependent switch in its effect from toxic to beneficial is not clear.
Ethanol at high concentrations injures cells by direct toxicity, through its metabolites and oxidative stress. Ethanol is mainly metabolized by two pathways namely, alcohol dehydrogenase (ADH) and cytochrome P4502E1 (human CYP2E1, mouse CYP2e1). ADH oxidizes ethanol to aldehyde in an NAD+-dependent reaction followed by transport of aldehyde to mitochondria where it is converted into acetate by aldehyde dehydrogenase. In another reaction CYP2E1, the main enzyme of the microsomal ethanol oxidation system, converts ethanol into acetaldehyde with production of free radicals leading to oxidative stress in a NADPH-dependent reaction. Thus, the metabolic fate of ethanol depends on its concentration in the target cells.
Effects of ethanol ingestion on the kidney and specifically on podocytes (glomerular visceral epithelial cells) responsible for maintaining the filtration barrier have not been reported. Podocytes are terminally differentiated cells that control the glomerular macromolecular permeability barrier function and determine the passage of proteins into urine. They are characterized by a unique actin cytoskeleton, interdigitating foot processes and specialized intercellular slit-junctions [5]. Derangement of the actin cytoskeleton, foot process fusion and loss of slit-junctions are associated with glomerular dysfunction, and precede proteinuria [6].
Proteinuria is associated with chronic kidney disease (CKD) and is an independent indicator of cardiovascular disease [7]. Patients with systemic diseases including hypertension and diabetes mellitus often develop CKD progressing to end-stage renal disease (ESRD). Thus, changes in podocyte structure and the resulting alteration in glomerular filtration barrier function are early events in the progressive loss of glomerular function, onset of proteinuria and chronic disease. Due to its wide-ranging effects, ethanol may positively and negatively affect several metabolites and signaling pathways involved in regulating glomerular barrier function.
Arachidonic acid is converted to 20-hydroxyeicosatetraeneoic acid (20-HETE) by cytochrome P450 (CYP450) 4A omega hydroxylases. We have found that 20-HETE is required for maintaining the glomerular permeability barrier function. 20-HETE also protects the glomerular filtration barrier from a circulating factor in the plasma from patients with recurrent focal segmental glomerulosclerosis (FSGS permeability factor), a progressive glomerular disease that leads to ESRD [8]. Similarly, 20-HETE protects against glomerular injury caused by puromycin aminonucleoside-induced nephrosis in a model of minimal change disease [9]. Since podocytes provide most of the resistance to passage of plasma proteins through the glomerular filtration barrier, the glomeruloprotective effect of 20-HETE may well originate from podocytes. The omega-OH group in 20-HETE is the molecular site for ADH catalytic activity. Indeed, it has been shown that 20-HETE is oxidized by ADH to form 20-carboxy (COOH)-arachidonic acid (AA) [10].
We studied selected aspects of the effects of low and high concentrations of ethanol (0.02–0.4 mmol/mL) on immortalized mouse podocytes and isolated rat glomeruli. Results summarized here show that high concentrations of ethanol induce CYP2e1, cause oxidative stress and alter the actin cytoskeleton, downregulate ADH protein and CYP4a12a gene in mouse podocytes. In contrast, low concentrations of ethanol upregulate ADH and CYP4a12a expression, and do not derange the actin cytoskeleton. Inhibition of ADH resulted in increased glomerular albumin permeability similar to that caused by PAN or FSGS permeability factor. 20-HETE or its metabolite 20-COOH-AA blocked the increase in glomerular permeability. Our results suggest the possibility that 20-HETE is a physiological substrate of ADH activity in glomerular podocytes and that very small amounts of ethanol protect the glomerular filtration barrier, an effect that is reversed by higher amounts of ethanol.
2. Methods
2.1. Animals and Institutional Approvals
These studies were carried out using protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the Kansas University Medical Center, Kansas City, KS, USA and by the IACUC, Safety Subcommittee and the Research and Development Committee at the VA Medical Center, Kansas City, MO, USA. Animals were obtained from national vendors and maintained at the Animal Resource Facility under control temperature, humidity and a light/dark cycle of 12/12 hr with unrestricted access to standard diet and water. ARF is approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
2.2. Cell culture
Immortalized mouse podocytes with thermosensitive tsA58 mutant T-antigen were grown on collagen in 75 cm2 flasks, plastic petri dishes, glass coverslips or multiwell plates. Medium consisted of RPMI 1640 (Mediatech, Manassas, VA) with L-glutamine supplemented with 10% fetal bovine serum, interferon-γ (IFN-γ), 100 units/mL penicillin and 0.1 mg/mL streptomycin with 5% CO2 and 95% humidity in the atmosphere. Cells grown to 80% confluence in medium containing mouse interferon-γ at 33°C were divided and grown at 37°C in the same medium without IFN-γ to differentiate and used on day 14 [11].
2.3. Treatment of cells with ethanol
Differentiated podocytes were treated with 1, 2, 5, 10, and 20 μL/mL (v/v) ethanol (approximately 0.02–0.4 mmol/mL, 20–400 mM) in the culture medium and incubated for 1–24 hr at 37°C. Time-matched control cells were treated with equivalent volumes of the medium. Molecular biology grade ethanol (Sigma-Aldrich) was used. ADH inhibitor fomepizole (4-methylpyrazole, Sigma-Aldrich, St Louis, MO), 20-hydroxyeicosatetraenoic acid (20-HETE, Cayman Chemical, Ann Arbor, MI) or 20-carboxy-arachidonic acid (20-COOH-AA, Cayman Chemical) were used in some experiments.
2.4. Visualization of ethanol-induced changes in the actin cytoskeleton using confocal microscopy
Podocytes grown on coverslips were incubated with ethanol for 8 hr, fixed in 4% paraformaldehyde, permeabilized and incubated with Alexa Fluor 568 Phalloidin (1:200) for 30 minutes in the dark and washed with PBS. The coverslips mounted were viewed using a Carl Zeiss LSM 510 confocal microscope (63x) with laser at 543 nm. Laser intensity, gain settings, scaling, individual section depth, and pinhole settings were kept constant.
2.5. Determination of ethanol-induced apoptotic changes using flow cytometry
Podocytes were analyzed using flow cytometry to determine apoptotic changes at 6 and 24 hr after incubation with ethanol (0.2 or 0.4 mmol/mL). For each experimental group, 1–5 × 105 cells were collected by centrifugation and resuspended in 500 μL of 1X Annexin V Binding Buffer. Annexin V-FITC 5 μL and propodium iodide 5 μL (Life Technologies) were added and each group incubated for 10 minutes at room temperature, and injected into a BD LSR II flow cytometer at KU Medical Center, Kansas City, KS and data were analyzed using FlowJo software.
2.6. Gene expression studies using Quantitative RT-PCR (RT-qPCR)
Podocytes incubated with ethanol (0.2 or 0.4 mmol/mL) were analyzed for CYP2e1 at 4 hr in parallel with determination of cellular superoxide at 4 hr when superoxide production due to 0.2 and 0.4 mmol/mL ethanol was noticeable. Podocytes were incubated with ethanol (0.02–0.4 mmol/mL) for 1, 8 and 24 hr to study CYP4a12a gene expression at early to late time points in these experiments.
TRI Reagent (Molecular Research Center, Cincinnati, OH) and RNase-free DNase (Qiagen, Valencia, CA) were used to isolate total RNA and to remove DNA, respectively. First-strand cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Total RNA (1 μg, 20 μL) was reverse transcribed followed by RT-qPCR using 100 ng of total RNA in each reaction. The iCycler iQ Real-Time PCR Detection System and iQ SYBR Green Supermix (Bio-Rad) were used for quantitative RT-PCR analysis. The relative gene expression was expressed using cycle threshold (Ct) values of the gene of interest normalized with GAPDH in the same sample. The sequences of primers used in the real–time PCR analyses of various genes are:
| CYP2e1 | Forward:
GCTGCCCATCATTATCCCTTAC Reverse: TCTTACCCACTGAGCCATCT |
| GAPDH | Forward:
GCCTTCCGTGTTCCTACCC Reverse: TGCCTGCTTCACCACCTTC |
2.7. 20-HETE quantitation using Liquid Chromatography-Mass Spectrometry (LC-MS) analysis
Differentiated podocytes were used for determination of 20-HETE levels using established protocols at the Mass Spectrometry Facility, Department of Pharmacology, Medical College of Wisconsin, Milwaukee, WI [12, 13]. Liquid chromatography-electrospray ionization-mass spectrometry (Agilent 1100 LC-MSD, SL model) was used for chromatography on C18 column (Kromasil 100, 250×2.0 mm, 5μm, Phenomenex, Torrance, CA) using a gradient of water (solvent A) and acetonitrile (solvent B) with 0.005% acetic acid as the mobile phase (200 μl/min). Mass spectrometry detection was carried out in negative mode and analytes were quantitated comparing the ratios of peak areas (compounds/internal standards) to the standard curves and expressed as pg/mg protein.
2.8. Western Blot analysis for protein expression and phosphorylation
Podocytes were incubated with ethanol (0.02–0.4 mmol/mL) for one hr to determine changes at the earliest time point in these experiments. Change in total ADH protein levels and phosphorylation of Akt and/or ERK1/2 were determined.
ADH protein expression Akt and ERK1/2 phosphorylation were analyzed in cell lysates by SDS-PAGE using TGX gels (BioRad) followed by electro-transfer to PVDF membranes and detection using specific antibodies in TBST containing5% dry milk as the blocking agent. β-actin was used as loading control. Primary antibodies included anti-ADH antibody (1:200 dilution) from Abcam, (Cambridge, MA). Anti-phosphoERK (1:2000 dilution), anti-total ERK (1:5000 dilution), anti-phosphoAkt (1:2000), anti-total Akt (1:1000) were obtained from Cell Signaling Technology, Danvers, MA. Anti-β actin (1:10,000 dilution) and peroxidase conjugated secondary antibodies (1:5000) were used (Sigma-Aldrich, St. Louis, MO). ECL (GE Healthcare, Piscataway, NJ) was used for visualizing and images were recorded using Kodak Gel Logic 2200 imaging system (Carestream Health Inc, New Haven, CT).
2.9. Fluorescence microscopy to assay superoxide generation
Podocytes on glass coverslips were incubated with ethanol (EtOH) 0.2 mmol/mL and 0.4 mmol/mL for 1 or 4 hr at 37°C. After washing with HEPES buffer cells were incubated with dihydroethidium (HE, 15μM) for 15 min, mounted on microscope slides with 5% n-propylgallate 9:1 glycerol:PBS and observed using a Leica microscope (excitation-550 nm and emission-650 nm). Fifteen to twenty cells were observed in each group in 2 separate experiments and fluorescence intensities were quantified using the microscope software. Results are presented as change in fluorescence (fold increase) compared to vehicle-treated control group expressed as 1.
2.10. In vitro glomerular albumin permeability (Palb) assay
Details of the method have been described [14]. Briefly, fresh preparations of glomeruli isolated from Sprague Dawley rats (200–250 g) were suspended in modified Ringer’s solution containing 5% BSA. Glomeruli were incubated with control or test agent (final volume 1 mL) for 15 minutes at 37°C. Five glomeruli from one rat were observed individually using videomicroscopy. First set of images was obtained with 5% BSA in the bath medium. A second image of each glomerulus was obtained after changing the bath medium to 1% BSA to generate an oncotic gradient. Images were analyzed for change in glomerular volume following change of medium by morphometry. The ratio of ΔV of experimental to ΔV of control glomeruli in response to identical oncotic gradients was used to calculate Reflection coefficient (σalb) = ΔVexperimental/ΔVcontrol. Convectional permeability to albumin (Palb): (1−σalb) describes the movement of albumin consequent to water flow. When σalb is zero, albumin moves at the same rate as water and Palb is 1.0. When σalb is 1.0, albumin cannot cross the membrane with water and Palb is zero.
2.11. Statistical analyses
Descriptive statistics such as means and proportions were used. Mean values were compared using Student’s t-test and P<0.05 was accepted as significant.
3. Results
3.1. Ethanol altered the actin cytoskeleton of cultured podocytes
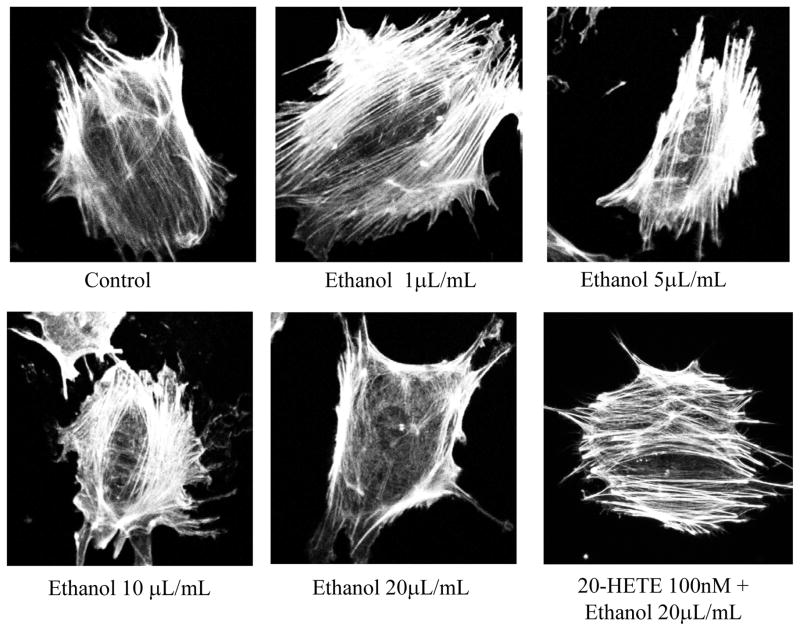
Control and cells treated with lower concentrations of ethanol (0.02 or 0.1 mmol/mL) showed linear and parallel arrangement of thick actin filament bundles. The actin cytoskeleton underwent changes at higher concentrations of ethanol (0.2 or 0.4 mmol/mL). The linear and parallel pattern was lost, and the thin bundles of short actin filaments acquired mesh-like random orientation. Pre-treatment with 100 nM 20-HETE prevented the formation of actin bundles (Fig. 1).
FIGURE 1. Ethanol at high concentrations causes derangement of the actin cytoskeleton in podocytes.
Differentiated podocytes were incubated with ethanol (0.02–0.4 mmol/mL) for 8 hr. Cells were stained for actin filaments using phalloidin and observed using confocal microscope. Representative images (63x objective) show that lower amounts of ethanol 0.04–0.1 mmol/mL did not alter the actin filament arrangement. A parallel arrangement of thick bundles running across the cell was maintained. Higher concentration of ethanol (0.2–0.4 mmol/mL) caused visible change in the thickness, length and arrangement of actin filaments. These changes were visible at 0.02 mmol/mL ethanol and a cortical ring like structure was observed at 0.4 mmol/mL ethanol. Pre-treatment of cells with 100 nm 2-HETE for 15 minutes before incubation with 0.4 mmol/mL ethanol prevented the cytoskeletal derangement.
3.2. Flow cytometry analysis of ethanol-treated cells
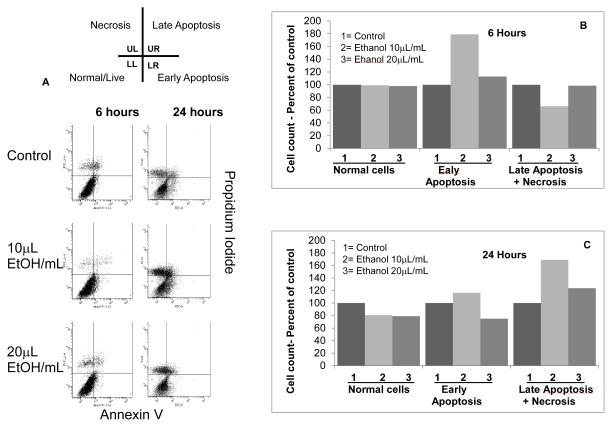
We followed the microscopy observations by flow cytometry to determine apoptotic changes caused by ethanol. Figure 2 shows the effect of ethanol 0.2 and 0.4 mmol/mL at 6 and 24 hr. At 6 hr, normal cells constituted approximately 80% of the total in control and ethanol-treated (0.2 and 0.4 mmol/mL) groups. However, the abundance of normal cells decreased by 20% at 24 hr in both ethanol-treated groups compared to the control group.
FIGURE 2. Ethanol causes dose- and time-dependent apoptotic changes in podocytes.
Control and ethanol-treated (0.2 or 0.4 mmol/mL) podocytes were stained using annexin V-FITC (x axis) and propidium iodide (y axis) at 6 and 24 hr and analyzed using flow cytometry. Data are presented as four quadrant distribution dot plots - Upper Left (UL), Upper Right (UR), Lower Left (LL) and Lower Right (LR). Combined data for late apoptosis and necrosis are presented (Late Apoptosis+Necrosis). Bar graphs summarize these observations at 6 and 24 hr that suggest increase in the number of apoptotic cells by 0.2 mmol/mL ethanol.
Normal cells (LL): At 6 hr, normal cells were comparable in all groups (70–80% of total cell count). At 24 hr, Ethanol-treated groups showed 20% decrease compared to the control.
Early Apoptosis (LR): Early apoptotic cells count was 79% (6h) and 16% (24h) higher than the time matched control in the 0.2 mmol/mL ethanol group. Cells showing early apoptosis in the ethanol-treated group (0.4 mmol/mL) were 13% (6h) higher and 25% (24h) lower than the control.
Late Apoptosis+Necrosis (UL+UR): Ethanol 0.2 mmol/mL caused a decline in the number of these cells at 6 hr but a 29% increase over control by 24 hr. The 0.4 mmol/mL ethanol-treated group showed 23% (24h) higher number of cells with late apoptosis+necrosis compared to the control group. Values represent average of two separate experiments.
Early apoptotic cells in the 0.2 mmol ethanol/mL group were 79% and 16% higher than the control group at 6 and 24 hr, respectively. Cells showing early apoptosis in the 0.4 mmol ethanol group were 13% higher and 25% lower than the control group at 6 and 24 hr, respectively.
The abundance of cells undergoing Late apoptosis+necrosis increased by 69% and 23% in the ethanol-treated groups (0.2 and 0.4 mmol/mL), respectively, by 24 hr.
Although we did not determine cytotoxicity of different concentrations of ethanol in parallel experiments, these data suggest that ethanol 0.4 mmol/mL caused cell death and therefore we did not observe a gradual increase in apoptotic cell. Thus, ethanol at 0.1–0.2 mmol/mL concentrations would be optimum concentrations to study the chronicity of cellular changes in future experiments.
3.3. Normal podocytes synthesize 20-HETE
We have previously shown that 20-HETE preserves and protects the glomerular filtration barrier [9]. LC-MS results demonstrate that 20-HETE is present in normal untreated podocytes. Briefly, the concentration of 20-HETE was 530.9±50.4 pg/mg protein compared to 19-HETE at 236.2±5.3 pg/mg protein (mean±SD, n=4 biological replicates each). Thus, 20-HETE is formed in podocytes under basal physiological conditions.
3.4. Ethanol at high concentrations increases superoxide generation in podocytes and induces the expression of superoxide producing cytochrome P450 2e1 in podocytes
High concentrations of ethanol significantly increased superoxide production in podocytes and thereby oxidative stress as shown using dihydroethidium (HE) (Figure 3A, 3B and 3C). CYP2e1 plays an important role in ethanol metabolism, particularly in settings of high concentrations and/or chronic exposure to alcohol. Ethanol metabolism by CYP2e1 causes production of superoxide. The presence of CYP2e1 in podocytes has not been previously described.
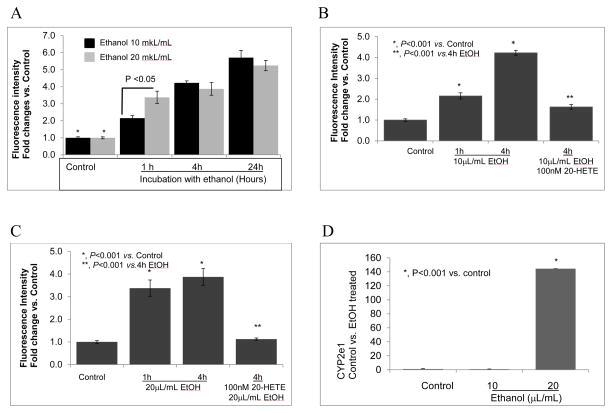
FIGURE 3. Ethanol increases superoxide generation in podocytes.
A. Immortalized podocytes were incubated with ethanol 0.2 or 0.4 mmol/mL. Dihydroethidium (15 μM) was used to detect superoxide. Significant increase in fluorescence due to ethanol (0.2 or 0.4 mmol/mL) was detectable at 1, 4 and 24 hr compared to control. Fluorescence caused by 0.4 mmol/mL was significantly greater than that by 0.2 mmol/mL ethanol only at 1 hr (P<0.05, n=20 cells in two separate experiments). Ethanol at 0.2 and 0.4 mmol/mL caused significant increase in superoxide production at 1, 4 and 24 hr compared to the their respective control groups (*, P<0.05)
B. Pre-treatment of cells with 100 nM 20-HETE blocked the effect of 0.2 mmol/mL ethanol on superoxide production at 4 hr (P<0.001 vs. ethanol treated group).
C. Pre-treatment of cells with 100 nM 20-HETE blocked the effect of 0.4 mmol/mL ethanol on superoxide production at 4 hr (P<0.001 vs. ethanol treated group).
D. Total RNA was prepared from untreated control and ethanol-treated podocytes (0.2 or 0.4 mmol/mL, 4 hr) and analyzed using RT-qPCR. CYP2e1 gene expression was significantly increased in podocytes treated with 0.4 mmol/mL (P<0.001 vs control). (n=4/group, each value represents average of 3 determinations).
Ethanol at 0.2 or 0.4 mmol/mL caused significant increase in superoxide production at 1, 4 and 24 hr (P<0.05, Figure 3A). At 1 hr, superoxide production caused by 0.2 mmol/mL ethanol was lower (P<0.05) than that caused by 0.4 mmol/mL, comparable superoxide production was observed at 4 and 24 hr (Figure 3A). Pre-treatment of cells with 100 nM 20-HETE for 15 minutes blocked the effect of ethanol 0.2 or 0.4 mmol/mL (Figure 3B, 3C). To determine whether ethanol at high concentrations induces expression of CYP2e1 in parallel with increased superoxide production, podocytes were incubated with ethanol 0.2 and 0.4 mmol/mL for 4 hr, and expression of CYP2e1 was examined using RT-qPCR. As shown in Figure 3D, ethanol (0.4 mmol/mL) markedly increased the expression of CYP2e1 at 4 hr (P<0.001 vs. control).
3.5. High concentration of ethanol down regulates CYP4a12a gene expression at 24 hr
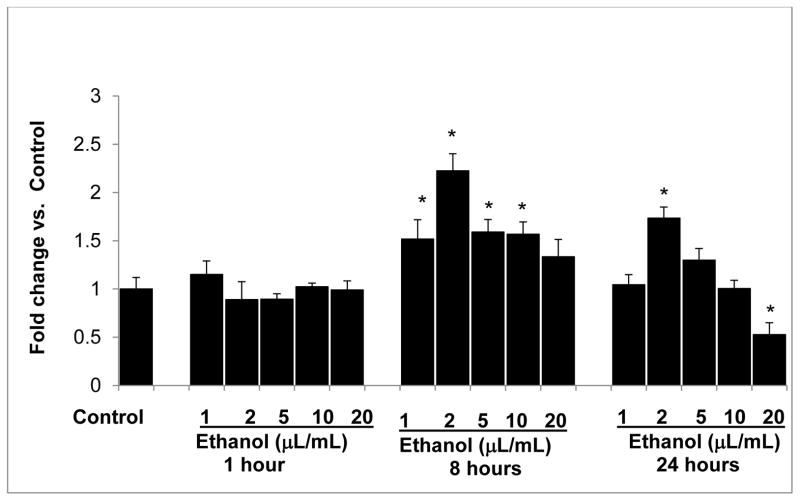
Since 20-HETE protects the glomerular barrier function, we determined the effect of ethanol on CYP4a12a omega hydroxylase gene expression. Podocytes were incubated with ethanol at various concentrations (0.02, 0.04, 0.1, 0.2 and 0.4 mmol/mL). Results are shown in Figure 4. Ethanol at 0.02, 0.04, 0.1, 0.2 mmol/mL caused significant increase in the expression of CYP4a12a at 8 hr (P<0.05 compared to control). However, by 24 hr, ethanol 0.4 mmol/mL caused decreased expression of CYP4a12a (P<0.05 compared to control). Thus, any beneficial effect of ethanol may only be at low concentrations since higher amounts down regulated CYP4a12a gene expression by 24 hr. This finding also suggest that CYP4a12a is expressed in podocytes which corresponds with the presence of 20-HETE detected in untreated podocytes.
FIGURE 4. Ethanol at high concentrations downregulates CYP4a12a gene expression.
Differentiated podocytes were treated with 0.02–0.4 mmol/mL ethanol for 1, 8 and 24 hr. Numbers 1–5 indicate treatment groups in the bar graph. Thus, 1= 0.02; 2=0.04; 3=0.1; 4=0.2 and 5=0.4 mmol ethanol/mL. Total RNA was analyzed for CYP4a12a expression as described. GAPDH was used as the housekeeping gene control. Data calculated as ΔΔCt were normalized with GAPDH gene expression and average fold-change values of 4 replicate experiments are presented. Ethanol at 0.02, 0.04, 0.1 and 0.2 mmol/mL caused significant increase in CYP4a12a at 8 hr. Ethanol at 0.4 mmol/mL caused a significant decrease in CYP4a12a gene expression. (*, P<0.05 vs. control n=4 replicate experiments each analyzed in triplicates)
3.6. Podocytes express ADH and ethanol causes a dose-dependent effect on total ADH protein
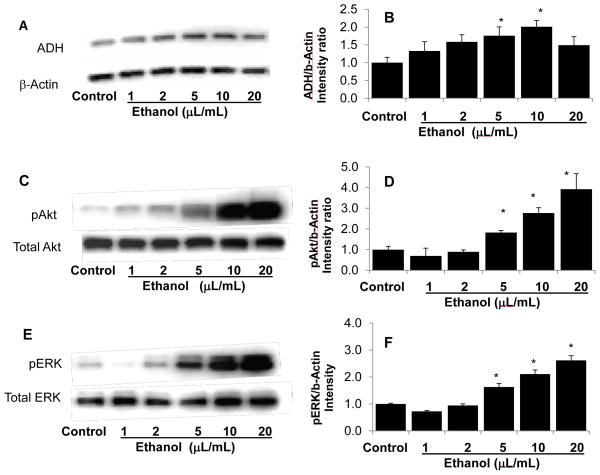
We next determined the effect of ethanol on the alternative pathway for alcohol metabolism that does not result in superoxide production. Ethanol (0.04–0.1 mmol/mL for 1 hr) caused a dose-dependent increase in ADH protein in cultured podocytes (P<0.05 vs. control, Figure 5A and 5B) followed by a decrease at the highest concentration of ethanol tested (0.4 mmol/mL). These data suggest a rapid effect of ethanol on ADH protein in podocytes.
FIGURE 5. Ethanol at high concentrations down regulates ADH protein expression and up regulates Akt and ERK1/2 phosphorylation.
Podocytes were incubated with ethanol (0.02–0.4 mmol/mL) for 1 hr at 37°C. Representative Western Blots and bar graphs of image intensity ratios (protein/β-actin ratios) are shown (mean±SEM).
A and B. Ethanol-induced expression of total ADH in a dose-dependent manner that was noticeable at 0.04 mmol/mL and significantly increased at 0.1 and 0.2 mmol/mL ethanol (*, P<0.05, n=3) but not at 0.4 mmol/mL ethanol.
C and D. Ethanol dose-dependently upregulated phosphorylation of Akt between 0.1 and 0.4 mmol/mL concentrations (P<0.05, n=5). Levels of total Akt protein expression remained unchanged.
E and F. Ethanol dose-dependently upregulated phosphorylation of ERK 1/2 between 0.1 and 0.4 mmol/mL concentrations (P<0.05, n=5). Total ERK 1/2 protein expression did change.
We selected changes in Akt and ERK phosphorylation as representative molecules of pathways that ethanol is known to modulate. Akt is a key enzyme in cell survival and regulates a number of interconnected signaling molecules. Ethanol at 0.02 and 0.04 mmol/mL showed a trend towards decreasing pAkt that did not reach statistical significance (Figure 5D). In contrast, higher concentrations of ethanol (0.1–0.4 mmol/mL) significantly increased pAkt in cultured podocytes (P<0.05 compared to control, Figure 5C, 5D).
Ethanol is known to alter several signaling pathways and its effect on MAPK pathways may show cell-specificity. Intensity ratios for pERK1/2 (protein/β-actin) increased significantly at ethanol concentrations 0.1, 0.2 and 0.4 mmol/mL (P<0.05) (Figure 5E, 5F).
3.7. Ethanol causes dual changes in glomerular albumin permeability (Palb) in vitro
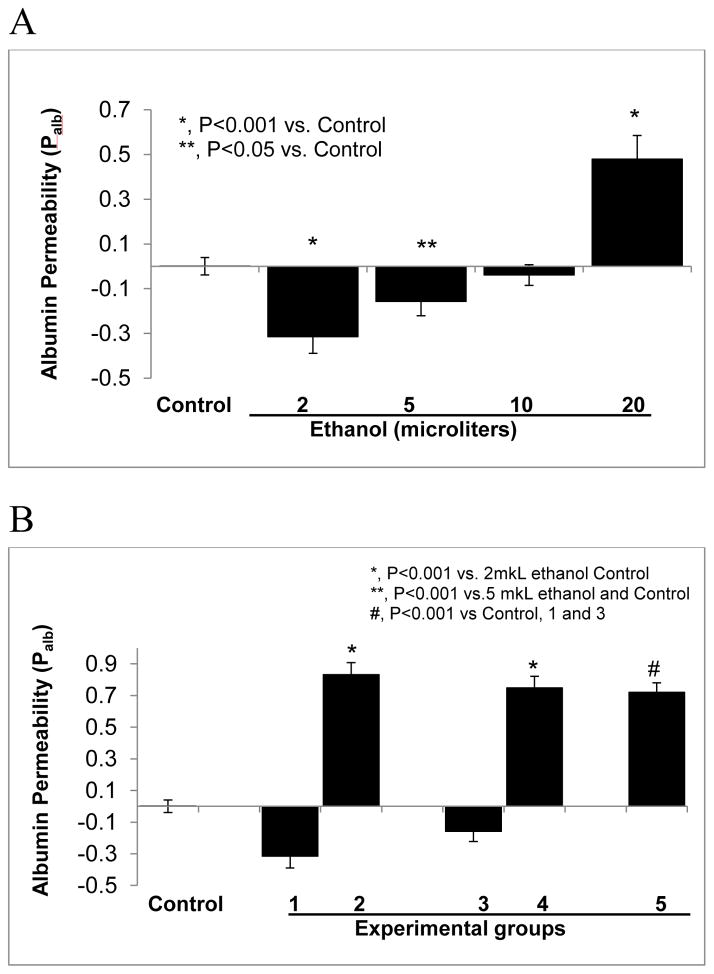
The effect of ethanol at low or high concentrations on glomerular filtration barrier is not known. We determined the effect of ethanol on Palb using isolated glomeruli. Glomeruli were incubated with ethanol (0.04–0.4 mmol/mL) in the incubation medium. Control glomeruli were incubated in the medium without ethanol. Glomeruli treated with ethanol 0.4 and 0.1 mmol/mL showed Palb values lower than the control (P<0.001 and P<0.05, respectively, Figure 6A). Ethanol at 0.4 mmol/mL significantly increased Palb (P<0.001), while 0.2 mmol/mL did not alter Palb significantly. In some experiments we determined the effect of ADH inhibitor fomepizole on Palb in glomeruli treated with ethanol 0.04 or 0.1 mmol/mL. Results (Figure 6B) show that pre-treatment with fomepizole blocked the effect of 0.04 or 0.1 mmol ethanol and caused an increase in Palb (P<0.001 vs. ethanol 0.04 or 0.1 mmol).
FIGURE 6.
Isolated rat glomeruli were incubated with (A) ethanol 0.02–0.4 mmol or with (B) 0.4 mmol/mL ethanol after pre-treatment with fomepizole (10 μM) for 15 minutes at 37°C. Palb was determined using an in vitro video-microscopy assay.
A. Ethanol at 0.04 and 0.1 mmol/mL resulted in Palb lower than the control group (*, P<0.001 and **, P<0.05 vs. control, respectively). Palb after incubation with ethanol 0.2 mmol/mL was not different from control. However, Palb after incubation with ethanol at 0.4 mmol/mL increased significantly (*, P<0.001 vs. control, n= 15 glomeruli, 3 rats, 5 glomeruli from 1 rat in each group).
B. Isolated glomeruli were incubated with ethanol alone, with fomepizole followed by ethanol or with fomepizole alone. Experimental groups are indicated by numbers 1–5. (1) Ethanol 0.04 mmol/mL, (2) 15 minute pre-incubation with 10μM fomepizole followed by addition of 0.04 mmol/mL ethanol and incubation for 15 minutes, (3) ethanol 0.1 mmol/mL, 15 minutes, (4) pre-incubation for 15 minutes with 10μM fomepizole followed by addition of 0.1 mmol/mL ethanol and incubation for 15 minutes (5) 10μM fomepizole for 15 minutes. Control glomeruli incubated in the medium for 15 minutes. Pre-treatment of glomeruli with fomepizole (10μM) blocked the effect of ethanol 0.04 or 0.1 mmol/mL (*, P<0.001 vs. ethanol alone groups) and increased Palb that was comparable to fomepizole alone (#, P<0.001 vs. control, n= 15 glomeruli, 3 rats, 5 glomeruli from each rat).
3.8. ADH inhibitor fomepizole increases Palb and 20-HETE blocks this effect
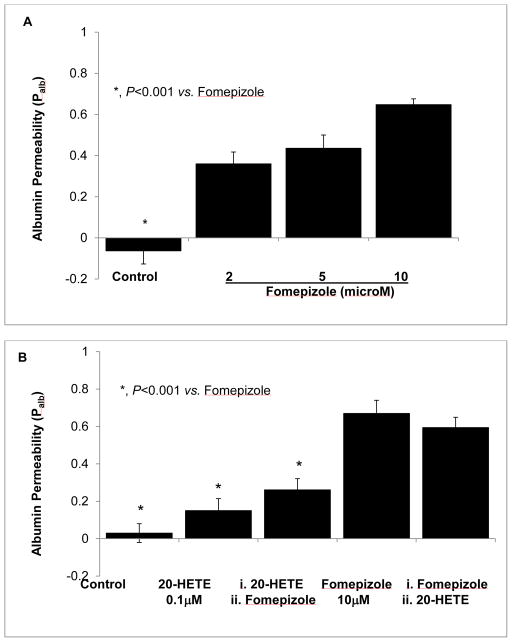
Detection of ADH in podocytes and its response to ethanol suggests a possible role of this enzyme activity in glomerular barrier function. We studied the effect of ADH inhibition with fomepizole on Palb in vitro. Fomepizole (2–10 μM) increased Palb in a dose-dependent manner (P<0.001 compared to control, Figure 7A). Pre-treatment of glomeruli with 20-HETE (0.1 μM) for 15 minutes blocked the increase in Palb caused by fomepizole. However, adding 20-HETE to glomerular suspension 5 minute after fomepizole did not block the increase in Palb (Figure 7B). These results suggest that ADH and 20-HETE are required for glomerular protein permeability barrier function.
FIGURE 7. 20-HETE blocks fomepizole-induced increase in glomerular albumin permeability (Palb).
Isolated rat glomeruli were incubated with (A) fomepizole (2–10 μM) or with (B) 20-HETE (0.1 μM) for 15 minutes at 37°C. Palb was determined using an in vitro video-microscopy assay.
A. Fomepizole increased glomerular Palb at 2 μM (P<0.001) and showed incremental effect with dose. n= 15 glomeruli, 3 rats (5 glomeruli from 1 rat) in each group, *, P<0.001 vs. fomepizole 2, 5 or 10 μM.
B. Pre-treatment with 20-HETE (0.1 μM) for 15 minutes before adding fomepizole blocked the increase in Palb caused by fomepizole. However, adding 20-HETE 15 minute after fomepizole did not block the increase in Palb. n= 15 glomeruli, 3 rats (5 glomeruli from 1 rat) in each group, *, P<0.001 vs. fomepizole alone.
3.9. 20-HETE and its metabolite 20-COOHarachidonic acid block the effect of FSGS factor or PAN on glomerular albumin permeability
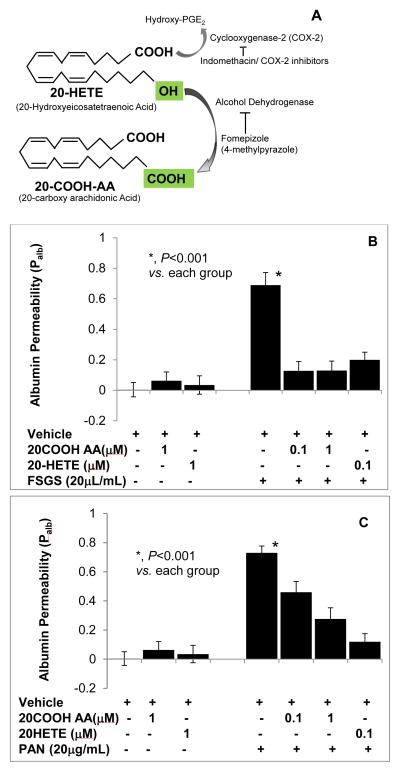
ADH metabolizes 20-HETE to 20-carboxy (COOH)-arachidonic acid (AA) by converting the C20 omega-hydroxyl group to carboxyl group (Figure 8A). C20-COOH-AA is an intracellular metabolite of 20-HETE that activates PPAR-α and PPAR-γ [10].
FIGURE 8. 20-HETE and its metabolite 20-COOH-arachidonic acid block the effect of FSGS factor or PAN on glomerular albumin permeability.
Isolated rat glomeruli were incubated with 20-HETE (0.1 μM), 20-COOH-AA (0.1, 1 μM) followed by FSGS serum (20μL/mL) or by PAN (20 μg/mL) and incubated for 15 minutes. Change in Palb was determined using an in vitro assay. 20-HETE or 20-COOH-AA did not alter glomerular permeability.
A. Schematic shows that 20-HETE is metabolized by ADH to form 20-COOH-AA that can be blocked by ADH inhibitor fomepizole.
B. FSGS increased albumin permeability (P<0.001) and its effect was blocked by 20-HETE (0.1 μM) or 20-COOH-AA (0.1 μM).
C. PAN increased albumin permeability (P<0.001). 20-HETE blocked the increase in permeability at 0.1 μM and 20-COOH-AA at 1 μM. n= 15 glomeruli, 3 rats (5 glomeruli from 1 rat) in each group, *, P<0.001.
Figure 8B shows the results of treating isolated glomeruli with 20-HETE (0.1 μM), 20-COOH-AA (0.1, 1 μM) followed by FSGS serum (20 μL/mL) or by PAN (20 μg/mL). Change in Palb was determined using an in vitro assay. 20-HETE or 20-COOH-AA did not alter glomerular permeability. FSGS increased albumin permeability (P<0.001) and its effect was blocked by 20-HETE (0.1 μM) or 20-COOH-AA (0.1 μM) (Figure 8B). Incubation of glomeruli with PAN also increased albumin permeability (P<0.001). 20-HETE (0.1 μM) or 20-COOH-AA (1 μM) blocked the increase in permeability caused by PAN (Figure 8C). Thus, both 20-HETE and its ADH metabolite protect the glomerular barrier function from injury. ADH may be involved in converting 20-HETE to 20-COOH-AA to maintain glomerular barrier function under physiological conditions.
4. Discussion
This report contains several novel observations regarding the effect of ethanol on podocyte structure and function. Ethanol showed dual effects on podocytes. At low concentrations, ethanol upregulated the expression of ADH and CYP4A isoforms. Others have shown that ADH can metabolize 20-HETE to 20-COOH-AA. We show here that untreated podocytes generate 20-HETE that may be a substrate for ADH under basal conditions in the absence of ethanol. ADH is known to metabolize 20-HETE to 20-COOH-AA and we show that 20-COOH-AA protects glomerular barrier function similar to the protective effect of 20-HETE. At higher concentrations, ethanol induced the expression of CYP2e1, increased superoxide production and altered cytoskeleton and glomerular function. We hypothesize that these events overcome protection afforded by lower concentrations of ethanol. These results are consistent with the clinical observation that moderate intake of alcoholic beverages may have health benefits. Our findings raise the intriguing possibility that low to moderate alcohol intake increases the synthesis of the salutary eicosanoids 20-HETE and 20-COOH-AA in the glomerulus and that these metabolites contribute to the health benefits of moderate ethanol use.
The effect of alcohol consumption on kidney function in health and disease is not clear. Alcohol can damage the kidney directly [19–21] or through hypertension that is induced or exacerbated by alcohol consumption [21]. Consumption of more than moderate amounts of alcohol is associated with increased risk of renal failure [21, 22]. Alcohol consumption is an independent risk factor for renal failure following liver transplantation [23]. Chronic alcohol abuse in humans has been linked to tubular dysfunction and necrosis, IgA nephropathy as well as renal structural and functional abnormalities in the fetal alcohol syndrome [20]. Prenatal exposure to alcohol reduces nephron number and increases glomerular volume [24].
In contrast, moderate alcohol consumption may reduce the risk for cardiovascular disease and diabetes [25, 26] and thus protect against kidney disease. In addition to a secondary protective effect, i.e. lower risk of cardiovascular disease and diabetes and therefore less kidney disease, moderate alcohol consumption may have other effects. Moderate alcohol consumption is associated with decreased risk of myocardial infarction irrespective of the type of beverage, i.e. beverages other than red wine also appear to confer benefit [27]. Thus, there is growing recognition that moderate alcohol consumption may have a beneficial effect on the development and progression of CKD [4].
CKD associated with diabetes and hypertension is characterized by a gradual loss of glomerular function and increase in urinary protein excretion. These pathophysiological changes directly relate to the early changes in glomerular cells. Podocytes constitute the main cellular barrier to regulate the passage of plasma macromolecules into glomerular filtrate. Derangement of podocyte cytoskeleton and foot process fusion are important clinical indicators of glomerular dysfunction that precede the onset of proteinuria [28, 29]. Podocytes are the principal site of glomerular damage in familial and idiopathic forms of focal segmental glomerulosclerosis (FSGS) and in puromycin aminonucleoside (PAN) induced nephrosis model of minimal change disease. Circulating factor(s) in idiopathic FSGS target podocytes to increase glomerular albumin permeability prior to the onset of albuminuria [8, 30]. We use cell culture and glomerular models to study the pathophysiology of the glomerulus.
Effects of ethanol on the structure and function of glomerular cells are not known. Ethanol alters the expression of a large number of genes involved in several cellular processes including stress response, protein modification, gene regulation, cell signaling and ethanol metabolism itself [31]. Effects of ethanol in humans are determined by a number of factors including genetic background, gender, body fat, age, quantity, and the rate and frequency of alcohol consumption [32]. The legal limit of blood alcohol in the USA is 0.08%. We used ethanol at concentrations covering a wide range that would correspond to moderate drinking to high amounts of ethanol consumed during binge drinking that may be fatal. Concentrations used were 0.1% to 2% (v/v) or 0.086–1.72% (w/v) equivalent to approximately ethanol 0.02–0.4 mmol/mL (20–400 mM) medium. Also, published studies indicate that response to identical concentration of ethanol varies with the cell type [33].
We found that the concentration of ethanol determines its metabolic route in podocytes. CYP2E1 takes on greater importance in ethanol oxidation in settings of high concentrations of ethanol. CYP2E1 generates superoxide [34, 35] and oxidizes drugs [36]. CYP2E1 expression is induced in diabetes, starvation and obesity [37]. Normal glomeruli do not express CYP2E1 [32] but proximal tubule cells express CYP2E1 which is upregulated by ethanol [38]. Preliminary work showed that the gene expression of CYP2e1 in mouse kidney cortex is only about 12.5% of that in the liver. CYP2e1 expression in glomerular cells has not been described. We found that untreated mouse podocytes do not express CYP2e1 while a high concentration of ethanol (0.4 mmol/mL) induced CYP2e1 expression (Figure 3D).
As anticipated, induction of CYP2e1 gene expression was associated with increased superoxide production in podocytes treated with ethanol (0.4 mmol/mL, Figure 3D) indicating oxidative stress. Oxidative stress is a major mechanism of ethanol toxicity in humans [38]. Ethanol induces oxidative stress in neurons [39] and hepatocytes [40] and alters the oxidation state and antioxidant defense system in the kidney [41]. These effects may depend on the dose [42] and chronicity of exposure [43–46], and on the nature of the beverage [47].
A dose-dependent derangement of the actin cytoskeleton at ethanol concentrations 0.1–0.4 mmol/mL (Figures 1 and 3) suggests that oxidative stress is associated with the disruption of cytoskeletal integrity. However, cytoskeletal changes in podocytes [48, 49, 50], neurons [51], hepatic cells [52] and pancreatic acini [53] vary and appear to be cell-type dependent [54]. Oxidative stress-induced structural changes involve altered intracellular signaling [55]. Ethanol influences multiple signaling pathways and alters the expression of a number of genes. We selected ERK and Akt phosphorylation as examples of the potential link between increased superoxide production, signaling events and cellular changes observed. Ethanol alters MAPK signaling directly or through its metabolites and oxidative stress. Changes in MAPK and Akt signaling with derangement of the actin cytoskeleton in podocyte and mesangial cells have been linked to superoxide production/oxidative stress [56, 57]. Transient upregulation of p38 MAPK phosphorylation with actin cytoskeleton reorganization in podocytes was observed in oxidative stress-mediated nephropathy induced by adriamycin or puromycin [58]. We found that ethanol upregulated both ERK1/2 and Akt phosphorylation (Figures 5C–5F). Increased Akt phosphorylation inactivates GSK-3β and prevents mitochondrial permeability transition pore (mPTP) opening [59]. While Akt/NRF2-dependent mechanism mediates the cardioprotection provided by moderate amounts of ethanol [60], detrimental role of Akt associated with oxidative stress in specific pathological conditions (e.g., myocardial infarction or severe dyslipidemia) is also known [61]. Thus, high amounts of ethanol induce oxidative stress and may alter podocyte structure through changes in MAPK and Akt signaling pathways.
The finding that ethanol at low concentrations upregulates ADH expression that is attenuated at high concentrations (Figures 5A, 5B) suggests that cellular response to metabolize ethanol through the ADH reaction reaches a peak at low to moderate concentrations of ethanol while CYP2e1 is not induced at these concentrations. We speculate that the potential beneficial effects of ethanol are likely only in the narrow range of concentrations where ethanol is metabolized by the ADH reaction without induction and action of CYP2e1.
The dual effect of ethanol on ADH protein expression suggests that ethanol regulates its own metabolism in podocytes. Also, ADH expression at basal conditions indicated the possibility of additional substrates for ADH isoenzymes. ADH class IV is known to oxidize retinol and omega-hydroxy fatty acids [15-18]. ADH activity converts omega hydroxyl group of 20-HETE into its dicarboxylic acid form 20-COOH-AA in coronary endothelial, renal epithelial, endothelial and smooth muscle cells [16]. Notably, 20-COOH-AA is more stable than 20-HETE and functions as a ligand of peroxisome proliferator-activated receptors-α and γ (PPAR-α, PPAR-γ), nuclear receptor proteins that function as transcription factors for mediating the effects of eicosanoids including 20-HETE. Additionally, 20-COOH-AA causes vasorelaxation in endothelin-constricted microvessels whereas 20-HETE shows no effect [10, 16].
LC-MS analysis showed that 20-HETE is present in untreated podocytes but the role of this abundant eicosanoid in the glomerular filtration barrier is not known. It is possible that ADH converts 20-HETE into 20-COOH-AA in podocytes under physiological conditions. We found a significant increase in CYP4a12a gene expression by 8 hr of incubation with lower concentrations of ethanol (0.02–0.1 mmol/mL) followed by a significant decrease by 24 hr in the 0.4 mmol/mL group (Figure 4). While detailed analyses of enzyme expression and activity remain to be determined, we have shown that 20-HETE preserves and protects the glomerular filtration barrier function indicated by its blocking effect on PAN-induced increase in Palb [9]. Presently, a significant increase in Palb (Figure 6A) at high concentration of ethanol (0.4mmol/mL) suggests direct glomerular injury that parallels changes observed in cultured podocytes. Inhibition of ADH in isolated glomeruli resulted in increased Palb (Figure 7A) suggesting a role for ADH in glomerular function under physiological conditions. We also found that 20-HETE and 20-COOH-AA each blocked the effect of the ADH inhibitor fomepizole (Figure 7B). Thus, comparable protective effect of 20-HETE and 20-COOH-AA suggests that 20-COOH-AA may be the functional mediator of 20-HETE in maintaining the glomerular filtration barrier.
In summary, our results show that ethanol at high concentration causes podocyte actin cytoskeleton derangement, increased production of superoxide, upregulation of pAkt and pERK1/2, downregulation of CYP4a12a and ADH and increased glomerular albumin permeability. In contrast, ethanol at low concentrations upregulates ADH protein and CYP4a12a omega hydroxylase gene expression. Active ADH appears to be required to maintain the glomerular permeability barrier. The ADH metabolite of 20-HETE, 20-COOH-AA, may be the intracellular mediator of ADH-catalyzed preservation and protection of the glomerular barrier function. We hypothesize that benefit or harm of alcohol consumption in humans may include changes in podocyte structure and function particularly by altering eicosanoid metabolism.
HIGHLIGHTS.
Ethanol consumption at low quantities appears to have beneficial effect on chronic kidney disease, proteinuria and cardiovascular function. However, the effect of ethanol on the kidney or glomerular podocytes has not been reported.
Podocytes express alcohol dehydrogenase (ADH) that metabolizes alcohol at the hydroxyl group. Podocytes produce 20-HETE by omega hydroxylation of arachidonic acid (AA) by cytochrome P450 hydroxylase (CYP450 hydroxylase) and do not express superoxide forming CYP450 2e1.
Ethanol at high concentrations inhibits ADH and CYP450 omega hydroxylase, induces CYP450 2e1 and increases superoxide production in podocytes. Ethanol at high concentrations increases glomerular albumin permeability. ADH inhibitor fomepizole increases glomerular protein permeability, an effect blocked by 20-HETE or its metabolite 20-COOH-AA generated by ADH activity. Thus, ADH and CYP450 hydroxylase activities are required for maintaining podocyte integrity and glomerular barrier function.
Ethanol at relevant low concentrations maintains podocyte actin cytoskeleton and, upregulates CYP450 omega hydroxylase and ADH. Small quantities of alcohol appear to protect glomerular protein filtration function.
Acknowledgments
We thank Dr. Peter Mundel, Dr. Jochen Reiser and Dr. Jeffrey Kopp for providing immortalized mouse podocytes. We thank Ms. Shuhua Wang for laboratory assistance. We thank Ms. Marilyn Isbell, Dr. Kasem Nithipatikom and Dr. William B. Campbell, Shared MSMS Facility, Pharmacology Department, Medical College of Wisconsin, Milwaukee, WI. We thank the Dr. Dale Abrahamson and Dr. Michael Werle, Confocal Imaging Facility, Department of Cell Biology and Anatomy, KUMC, Kansas City, KS. Parts of the results were presents at the Winter Eicosanoid Conference, Baltimore 2012 and 2014.
FINANCIAL SUPPORT
This work was supported in part by grants from NIH DK 1RO1 DK064969 (McCarthy), the VA BX001037 (Savin) and funds from the Midwest Biomedical Research Foundation (Savin, Sharma).
Footnotes
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare regarding the contents of the paper.
ETHICS STATEMENT
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. Rats used in these studies were obtained from reputed vendors and maintained at the Animal Resource Facility, Kansas City VA Medical Center, Kansas City, MO, USA. All studies involving animal subjects were conducted following protocols approved by the Institutional Animal Care and Use Committee (IACUC), KC VA Medical Center, Kansas City, MO.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–9. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–14. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 3.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffner E, Ritz E. Alcohol and kidney damage: a Janus-faced relationship. Kidney Int. 2012;81:816–8. doi: 10.1038/ki.2012.14. [DOI] [PubMed] [Google Scholar]
- 5.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiser J, Sever S. Podocyte biology and pathogenesis of kidney disease. Annu Rev Med. 2013;64:357–66. doi: 10.1146/annurev-med-050311-163340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2:581–90. doi: 10.2215/CJN.03190906. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–21. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy ET, Sharma R, Sharma M. Protective effect of 20-hydroxyeicosatetraenoic acid (20-HETE) on glomerular protein permeability barrier. Kidney Int. 2005;67:152–6. doi: 10.1111/j.1523-1755.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- 10.Fang X, Dillon JS, Hu S, Harmon SD, Yao J, Anjaiah S, Falck JR, Spector AA. 20-carboxy-arachidonic acid is a dual activator of peroxisome proliferator-activated receptors alpha and gamma. Prostaglandins Other Lipid Mediat. 2007;82:175–84. doi: 10.1016/j.prostaglandins.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava S, McCarthy ET, Sharma R, Kats A, Carlton CG, Alon US, Cudmore PA, El-Meanawy, Sharma M. Fluid flow shear stress upregulates prostanoid receptor EP2 but not EP4 in murine podocytes. Prostaglandins and other Lipid Mediat. 2013;104–105:49–57. doi: 10.1016/j.prostaglandins.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Nithipatikom K, Grall AJ, Holmes BB, Harder DR, Falck JR, Campbell WB. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem. 2001;298:327–36. doi: 10.1006/abio.2001.5395. [DOI] [PubMed] [Google Scholar]
- 13.Nithipatikom K, Laabs ND, Isbell MA, Campbell WB. Liquid chromatographic-mass spectrometric determination of cyclooxygenase metabolites of arachidonic acid in cultured cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;785:135–45. doi: 10.1016/s1570-0232(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, McCarthy ET, Reddy DS, Patel PK, Savin VJ, Medhora M, et al. 8,9-Epoxyeicosatrienoic acid protects the glomerular filtration barrier from the Focal Segmental Glomerulosclerosis permeability factor. Prostaglandins and Other Lipid Mediat. 2009;89:43–51. doi: 10.1016/j.prostaglandins.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allali-Hassani A, Peralba JM, Martras S, Farrés J, Parés X. Retinoids, omega-hydroxyfatty acidsand cytotoxic aldehydes as physiological substrates, and H2-receptor antagonists as pharmacological inhibitors, of human class IV alcohol dehydrogenase. FEBS Lett. 1998;426:362–6. doi: 10.1016/s0014-5793(98)00374-3. [DOI] [PubMed] [Google Scholar]
- 16.Collins XH, Harmon SD, Kaduce TL, Berst KB, Fang X, Moore SA, Raju TV, Falck JR, Weintraub NL, Duester G, Plapp BV, Spector AA. Omega-oxidation of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral microvascular smooth muscle and endothelium by alcohol dehydrogenase 4. J Biol Chem. 2005;280:33157–64. doi: 10.1074/jbc.M504055200. [DOI] [PubMed] [Google Scholar]
- 17.Mardh G, Dingley AL, Auld DS, Vallee BL. Human class II (IT) alcohol dehydrogenase has a redox-specific function in norepinephrine metabolism (alcohol dehydrogenase substrate specificity/aldehyde reduction) Proc Natl Acad Sci USA. 1986;83:8908–12. doi: 10.1073/pnas.83.23.8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivusalo M, Baumann M, Uotila L. Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett. 1989;257:1059. doi: 10.1016/0014-5793(89)81797-1. [DOI] [PubMed] [Google Scholar]
- 19.Van Thiel DH, Gavaler JS, Little JM, Lester R. Alcohol: its effect on the kidney. Metabolism. 1977;26:857–66. doi: 10.1016/0026-0495(77)90004-x. [DOI] [PubMed] [Google Scholar]
- 20.Cecchin E, De Marchi S. Alcohol misuse and renal damage. Addict Biol. 1996;1:7–17. doi: 10.1080/1355621961000124656. [DOI] [PubMed] [Google Scholar]
- 21.Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr Opin Nephrol Hypertens. 2001;10:385–90. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Perneger TV, Whelton PK, Puddey IB, Klag MJ. Risk of end-stage renal disease associated with alcohol consumption. Am J Epidemiol. 1999;150:1275–81. doi: 10.1093/oxfordjournals.aje.a009958. [DOI] [PubMed] [Google Scholar]
- 23.Gayowski T, Singh N, Keyes L, Wannstedt CF, Wagener MM, Vargas H, et al. Late-onset renal failure after liver transplantation: role of posttransplant alcohol use. Transplantation. 2000;69:383–8. doi: 10.1097/00007890-200002150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Gray SP, Denton KM, Cullen-McEwen L, Bertram JF, Moritz KM. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Nephrol. 2010;21:1891–902. doi: 10.1681/ASN.2010040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajani UA, Hennekens CH, Spelsberg A, Manson JE. Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch Intern Med. 2000;160:1025–30. doi: 10.1001/archinte.160.7.1025. [DOI] [PubMed] [Google Scholar]
- 26.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev. 1993;15:328–51. doi: 10.1093/oxfordjournals.epirev.a036124. [DOI] [PubMed] [Google Scholar]
- 27.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Jr, Stampfer MJ, Willett WC. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 28.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–8. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 29.Jefferson JA, Alpers CE, Shankland SJ. Podocyte biology for the bedside. Am J Kidney Dis. 2011;58:835–45. doi: 10.1053/j.ajkd.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savin VJ, McCarthy ET, Sharma M. Permeability factors in nephrotic syndrome and focal segmental glomerulosclerosis. Kidney Res Clin Pract. 2012;31:205–213. doi: 10.1016/j.krcp.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uddin RK, Singh SM. Ethanol-responsive genes: identification of transcription factors and their role in metabolomics. Pharmacogenomics J. 2007;7:38–47. doi: 10.1038/sj.tpj.6500394. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan JB, Jr, Hauptman M, Bronstein AC. Lack of observable intoxication in humans with high plasma alcohol concentrations. J Forensic Sci. 1987;32:1660–5. [PubMed] [Google Scholar]
- 33.Castañeda F, Kinne RK. Cytotoxicity of millimolar concentrations of ethanol on HepG2 human tumor cell line compared to normal rat hepatocytes in vitro. J Cancer Res Clin Oncol. 2000 Sep;126(9):503–10. doi: 10.1007/s004320000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Baliga M, Baliga R. Effect of cytochrome P450 2E1 inhibitors on cisplatin-induced cytotoxicity to renal proximal tubular epithelial cells. Anticancer Res. 2002;22:863–8. [PubMed] [Google Scholar]
- 35.Liu H, Baliga M, Bigler SA, Baliga R. Role of cytochrome P450 2B1 in puromycin aminonucleoside-induced cytotoxicity to glomerular epithelial cells. Nephron Exp Nephrol. 2003;94:e17–24. doi: 10.1159/000070815. [DOI] [PubMed] [Google Scholar]
- 36.Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168–79. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- 37.Ronis MJ, Huang J, Longo V, Tindberg N, Ingelman-Sundberg M, Badger TM. Expression and distribution of cytochrome P450 enzymes in male rat kidney: effects of ethanol, acetone and dietary conditions. Biochem Pharmacol. 1998;55:123–9. doi: 10.1016/s0006-2952(97)00381-x. [DOI] [PubMed] [Google Scholar]
- 38.Zima T, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, et al. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci. 2001;8:59–70. doi: 10.1007/BF02255972. [DOI] [PubMed] [Google Scholar]
- 39.Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–50. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–38. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinu D, Nechifor MT, Movileanu L. Ethanol-induced alterations of the antioxidant defense system in rat kidney. J Biochem Mol Toxicol. 2005;19:386–95. doi: 10.1002/jbt.20101. [DOI] [PubMed] [Google Scholar]
- 42.Scott RB, Reddy KS, Husain K, Schlorff EC, Rybak LP, Somani SM. Dose response of ethanol on antioxidant defense system of liver, lung, and kidney in rat. Pathophysiology. 2000;7:25–32. doi: 10.1016/s0928-4680(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo R, Rivera G, Orellana M, Araya J, Bosco C. Rat kidney antioxidant response to long-term exposure to flavonol rich red wine. Life Sci. 2002;71:2881–95. doi: 10.1016/s0024-3205(02)02140-9. [DOI] [PubMed] [Google Scholar]
- 44.Das SK, Mukherjee S, Vasudevan DM. Effects of long-term ethanol consumption on adhesion molecules in liver. Indian J Exp Biol. 2010;48:394–401. [PubMed] [Google Scholar]
- 45.Shanmugam KR, Ramakrishna CH, Mallikarjuna K, Reddy KS. Protective effect of ginger against alcohol-induced renal damage and antioxidant enzymes in male albino rats. Indian J Exp Biol. 2010;48:143–9. [PubMed] [Google Scholar]
- 46.Das SK, Varadhan S, Gupta G, Mukherjee S, Dhanya L, Rao DN, Vasudevan DM. Time-dependent effects of ethanol on blood oxidative stress parameters and cytokines. Indian J Biochem Biophys. 2009;46:116–21. [PubMed] [Google Scholar]
- 47.Lassaletta AD, Chu LM, Elmadhun NY, Burgess TA, Feng J, Robich MP, et al. Cardioprotective effects of red wine and vodka in a model of endothelial dysfunction. J Surg Res. 2012;178:586–92. doi: 10.1016/j.jss.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vega-Warner V, Ransom RF, Vincent AM, Brosius FC, Smoyer WE. Induction of antioxidant enzymes in murine podocytes precedes injury by puromycin aminonucleoside. Kidney Int. 2004;66:1881–9. doi: 10.1111/j.1523-1755.2004.00962.x. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Mariscal L, Quiros M, Diaz-Coranguez M. ZO proteins and redox-dependent processes. Antioxid Redox Signal. 2011;15:1235–53. doi: 10.1089/ars.2011.3913. [DOI] [PubMed] [Google Scholar]
- 50.Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal. 2011;23:317–23. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero AM, Esteban-Pretel G, Marin MP, Ponsoda X, Ballestin R, Canales JJ, et al. Chronic ethanol exposure alters the levels, assembly, and cellular organization of the actin cytoskeleton and microtubules in hippocampal neurons in primary culture. Toxicol Sci. 2010;118:602–12. doi: 10.1093/toxsci/kfq260. [DOI] [PubMed] [Google Scholar]
- 52.Shepard BD, Tuma PL. Alcohol-induced alterations of the hepatocyte cytoskeleton. World J Gastroenterol. 2010;16:1358–65. doi: 10.3748/wjg.v16.i11.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwata T, Nozu F, Imawari M. Ethanol impairs the assembly and disassembly of actin cytoskeleton and cell adhesion via the RhoA signaling pathway, catenin p120 and E-cadherin in CCK-stimulated pancreatic acini. Biochem Biophys Res Commun. 2011;405:558–63. doi: 10.1016/j.bbrc.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 54.Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–64. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 55.El-Mas M, Abdel-Rahman A. Enhanced oxidative stress/DAPK3/Akt/ERK signaling accounts for estrogen exacerbation of cardiac dysfunction caused by ethanol in male rats. FASEB J. 2014;28(1):S652.2. [Google Scholar]
- 56.Yan Q, Gao K, Chi Y, Li K, Zhu Y, Wan Y, et al. NADPH oxidase-mediated upregulation of connexin43 contributes to podocyte injury. Free Radic Biol Med. 2012;53:1286–97. doi: 10.1016/j.freeradbiomed.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 57.O’Connell S, Tuite N, Slattery C, Ryan MP, McMorrow T. Cyclosporine A--induced oxidative stress in human renal mesangial cells: a role for ERK 1/2 MAPK signaling. Toxicol Sci. 2012;126:101–13. doi: 10.1093/toxsci/kfr330. [DOI] [PubMed] [Google Scholar]
- 58.Koshikawa M, Mukoyama M, Mori K, Suganami T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, Sugawara A, Nakao K. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol. 2005;16:2690–701. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 59.Zhou K, Zhang L, Xi J, Tian W, Xu Z. Ethanol prevents oxidant-induced mitochondrial permeability transition pore opening in cardiac cells. Alcohol Alcohol. 2009;44:20–4. doi: 10.1093/alcalc/agn098. [DOI] [PubMed] [Google Scholar]
- 60.Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, Al-Rubaiee M, et al. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res. 2013;37:1253–60. doi: 10.1111/acer.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kerr BA, Ma L, West XZ, Ding L, Malinin NL, Weber ME, et al. Interference with Akt signaling protects against myocardial infarction and death by limiting the consequences of oxidative stress. Sci Signal. 2013;6(287):ra67. doi: 10.1126/scisignal.2003948. [DOI] [PMC free article] [PubMed] [Google Scholar]