Abstract
Purpose/Objective(s)
To calculate planning target volume (PTV) margins for chest wall and regional nodal targets using daily orthogonal kV imaging, and to study residual setup error after kV alignment using volumetric cone-beam computed tomography (CBCT).
Methods and Materials
Twenty-one post-mastectomy patients were treated with IMRT with 7mm PTV margins. Population-based PTV margins were calculated from translational shifts following daily kV positioning and/or weekly CBCT data for each of 8 patients, whose surgical clips were used as surrogates for target volumes. Errors from kV and CBCT data were mathematically combined to generate PTV margins for 3 simulated alignment workflows: 1) skin marks alone, 2) weekly kV imaging, 3) daily kV imaging.
Results
kV data from 613 treatment fractions indicated a 7mm uniform margin would account for 95% of daily shifts if patients were positioned using only skin marks. Total setup errors incorporating both kV and CBCT data were larger than those from kV alone, yielding PTV expansions of 7mm anterior-posterior (AP), 9mm left-right (LR), and 9mm superior-inferior (SI). Required PTV margins following weekly kV imaging were similar in magnitude as alignment to skin marks, but rotational adjustments of patients were required in 32%±17% of treatments. These rotations would have remained uncorrected without the use of daily kV imaging. Despite the use of daily kV imaging, CBCT data taken at the treatment position indicates that an anisotropic PTV margin of 6mm AP, 4mm LR, 8mm SI must be retained to account for residual errors.
Conclusions
CBCT provides additional information on three-dimensional reproducibility of treatment setup for chest wall targets. 3D data indicate that a uniform 7mm PTV margin is insufficient in the absence of daily IGRT. Inter-fraction movement is greater than suggested by two-dimensional imaging, thus a margin of at least 4–8mm must be retained despite the use of daily IGRT.
Introduction
Post-mastectomy radiation therapy has been shown to improve disease-free and overall survival in breast cancer patients (1–3). Emerging data regarding the application of inverse-planned intensity modulated radiotherapy (IMRT) for treatment of breast and chest wall targets, including the regional nodal chain, demonstrates dosimetric advantages in certain clinical scenarios. For example, IMRT (4) and volumetric modulated arc therapy (5) can significantly reduce high doses (i.e., > 30Gy) to the heart and ipsilateral lung when the internal mammary chain is targeted for treatment. IMRT treatments have a steep decline in dose outside the target volume, thus requiring stringent controls to ensure planning target volume (PTV) coverage is maintained and dose limits to organs-at-risk are not exceeded. A challenge to delivering effective treatment is daily setup reproducibility, which contributes to definition of the appropriate treatment target margins.
Image guidance is often used to detect large setup errors and improve the accuracy of radiotherapy delivery (6). These techniques could also be used to calculate more appropriate target volume margins for decreasing dosimetric impact on nearby organs. While image guidance has been used to quantify setup reproducibility of the lumpectomy cavity for accelerated partial breast irradiation (APBI) (7–9), few studies have quantified daily setup uncertainties for the entire breast and regional nodal targets. The majority have focused on the effects of respiratory motion in patients with an intact breast (10, 11). While planning studies utilized a 7mm margin for IMRT to breast targets (4, 12), no population study of setup reproducibility for post-mastectomy chest wall irradiation has been performed to our knowledge.
Our goals are: 1) to calculate an appropriate PTV margin for chest wall and nodal targets using orthogonal kV image data, and 2) to study residual setup error after daily kV alignment using volumetric cone-beam computed tomography (CBCT) data. This study utilized daily online kV imaging to investigate setup reproducibility in an initial series of patients treated with inverse-planned IMRT to the chest wall and regional lymph nodes. Post-mastectomy chest wall targets are expected to be minimally affected by soft tissue deformation, thus both bony anatomical landmarks and surgical clips were used as target surrogates for adjustment of patient position. In contrast to orthogonal x-ray imaging, which only provides a two-dimensional (2D) representation of the treated volume, we also track three-dimensional (3D) deviation of targets via volumetric CBCT imaging. The residual errors quantified by CBCT acquired at the treatment position were used to characterize the overall three-dimensional setup reproducibility. We report on setup uncertainties and appropriate PTV margins for post-mastectomy chest wall IMRT in a variety of patient alignment workflows depending upon the frequency of image guidance.
Methods and Materials
From October 2009 through May 2013, 21 post-mastectomy patients were treated with inverse-planned intensity-modulated radiation therapy (IMRT) to deliver a median dose of 50.4Gy in 28 fractions. 16 patients also received a median boost dose of 10Gy in 5 fractions delivered with 3D conformal radiotherapy (3DCRT) or IMRT. Clinical indications for IMRT included treatment of recurrent chest wall disease, treatment of bilateral internal mammary nodes (IMN) following detection of PET-positive or pathologically confirmed disease, inflammatory breast cancer, or improved sparing of critical organs for left-sided chest wall irradiation based on comparison to 3DCRT. The targeted chest wall, IMN, supraclavicular, and level I-III axillary nodes comprised the clinical target volume (CTV) and were contoured according to the RTOG Breast Cancer Atlas. To account for setup uncertainty and respiratory motion, the planning target volume (PTV) was generated by expanding the CTV uniformly by 7mm to within 5mm of the skin while avoiding the interior of the esophagus. In general, IMRT plans were generated using at least 9 beams separated by 20–30° spanning 185°, in addition to 1–2 non-coplanar beam orientations (4). Treatment planning goals included covering the PTV with at least 95% of the prescription dose while minimizing 20Gy, 30Gy, and mean doses to the heart and lungs when compared to conventional 3D plans, which were generated for each patient. Dose calculations were performed in Pinnacle v8.0–9.0 (Philips Systems, Andover, MA) using the convolution/superposition algorithm to correct for tissue heterogeneities. Patients were treated with 6 MV photons on a Varian Trilogy linear accelerator (Varian Medical Systems, Palo Alto, CA) with an OBI console.
All patients were immobilized with custom upper and lower alpha-cradles. For 4 patients, these devices were additionally secured to a C-Qual breast board (CIVCO Medical Solutions, Orange City, IA). Respiratory motion was assessed in 15 patients by acquiring both free-breathing and end-exhalation gated computed tomography (CT) scans using RPM (Varian Systems) at the time of simulation on a Brilliance BigBore scanner (Philips Healthcare, Andover, MA). Comparison of end-exhalation to free-breathing CT scans (FBCT) indicated minimal displacement (< 3mm) of the chest wall targets due to breathing. Thus, FBCT was used for all patients.
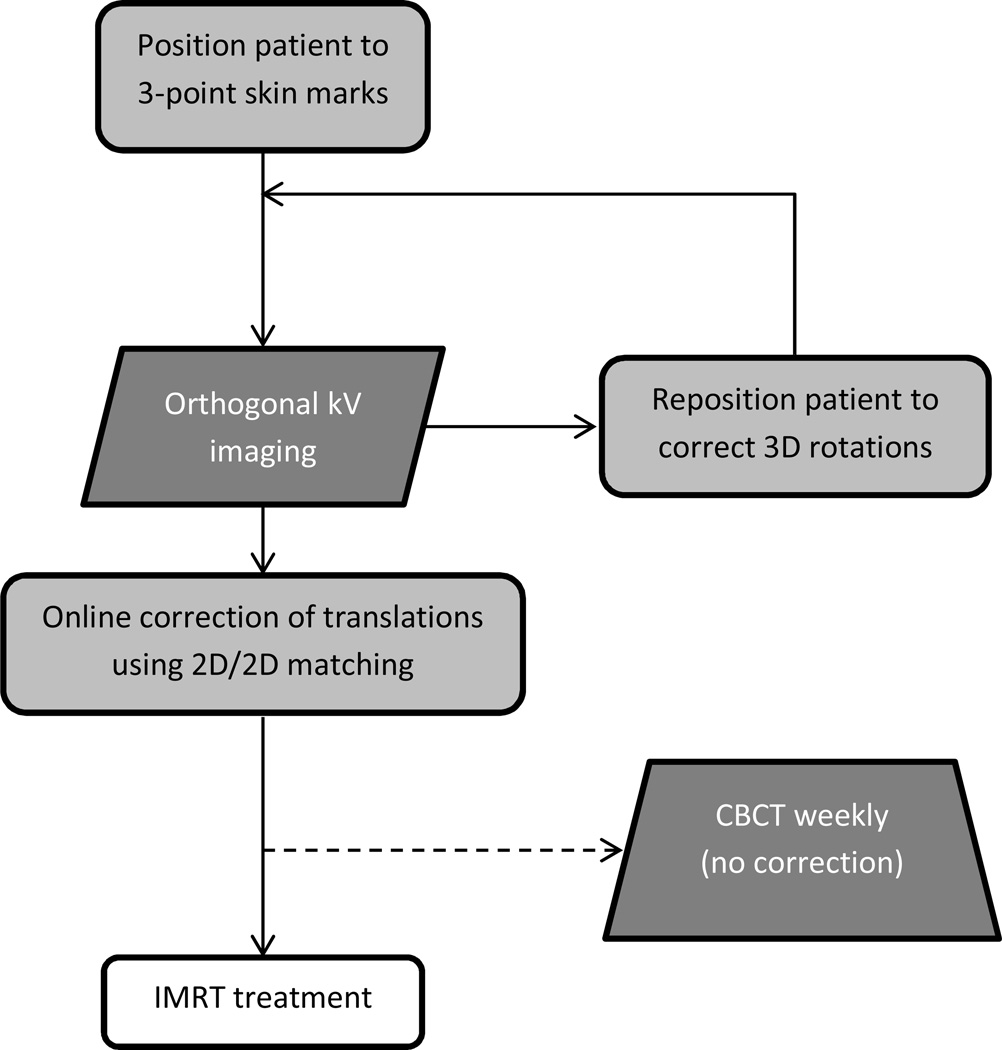
Figure 1 shows the treatment workflow. For daily treatments, patients were initially aligned to skin marks then filmed. If the first set of orthogonal kV images indicated rotations were present (estimated to be > 2° from surface imaging data taken at our institution for an independent breast cancer patient cohort (13)), patients were repositioned manually and another orthogonal kV pair was acquired. 2D/2D matching of bony anatomy and clips (as shown in Figure 2a), when present, was used for online correction of translations. CBCT was acquired weekly for 8 patients in the treatment position but not used for alignment. All patients provided written consent according to institutional review board guidelines (15837A).
Figure 1.
Treatment alignment workflow utilized for 21 post-mastectomy patients treated with IMRT. CBCT was acquired weekly for a subset of 8 patients, for whom OBI panels did not encounter any potential collisions.
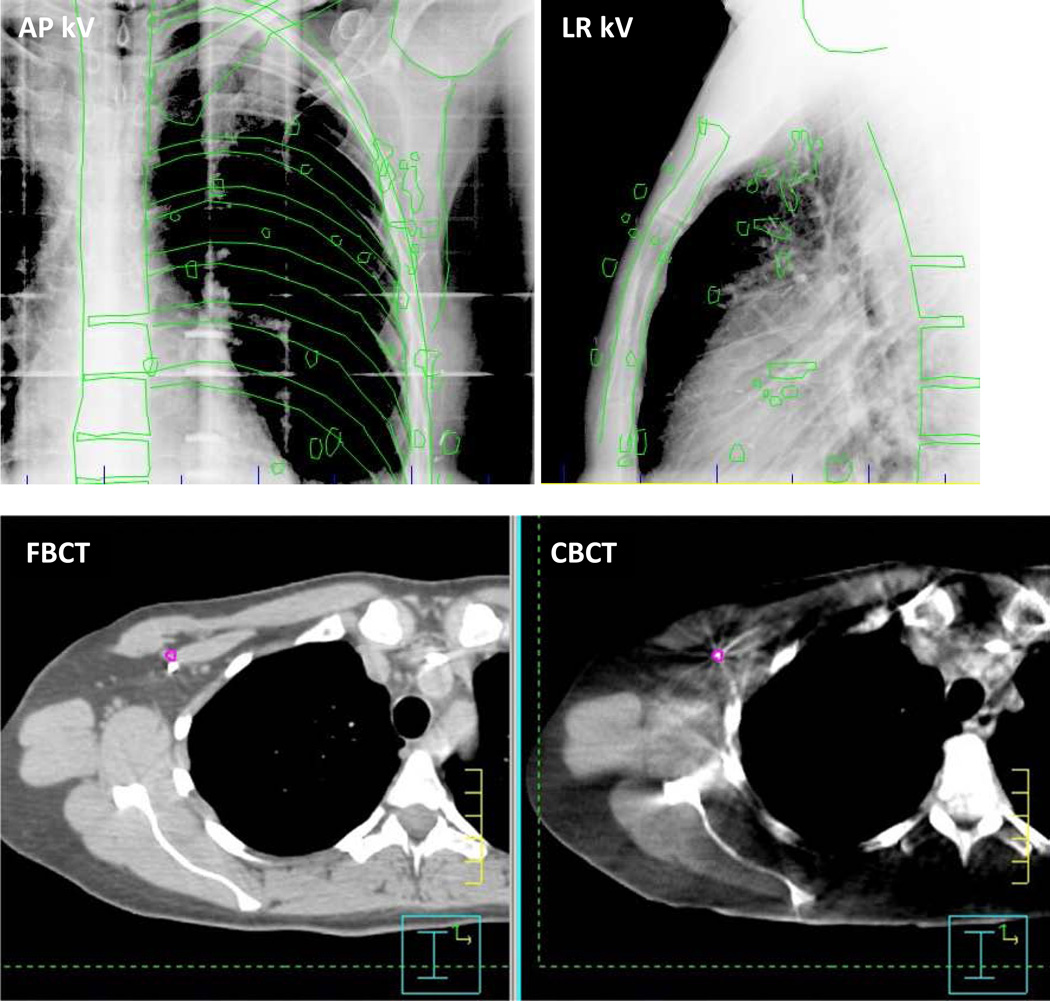
Figure 2.
(a) Orthogonal kilovolt x-ray images obtained prior to treatment, in the AP (left) and lateral (right) dimensions. The ideal positions of bony anatomic markers and surgical clips are projected onto the images in green. (b) Paired axial slices from FBCT (left) and CBCT (right). A surgical clip is outlined in color on CBCT and its corresponding 3D location is shown on the planning CT. Averaged over all 20 clips, the mean 3D displacement between clip centers on planning CT and CBCT was 3.2 mm for this treatment fraction.
Translational shifts in three axes (AP: anterior-posterior, LR: left-right, and SI: superior-inferior) following kV positioning were analyzed for 613 treatment fractions. The total setup error, defined as the sum of setup error and target motion, was calculated from the mean of the average shifts in each axis. PTV margins for each dimension were calculated using the van Herk recipe (14): 2.5(Σ) + 0.7(σ), where Σ and σ are standard deviations for systematic and random errors, respectively. These parameters ensure the delivery of at least 95% of the prescribed dose to the CTV for 90% of patients whose patient treatment positions are not corrected via image guidance. Although only shifts subsequent to correction for rotations were included, according to van Herk’s methodology (14), the proportion of fractions requiring rotational correction was tracked.
To analyze residual setup error following image-guided radiotherapy (IGRT), CBCT images were aligned offline to the FBCT. Manual rigid registration was implemented in Pinnacle with priority given to accurate registration of the sternum and ribs to achieve a position that would provide the most accurate dose deliverability. Surgical clips were then manually contoured and matched to their counterparts in the FBCT. Surgical clips that were visually indistinguishable from one another due to physical proximity and/or overlap were consolidated and contoured as one. The coordinates for the center of mass of each surgical clip volume were collected in three axes and used as an independent translational shift for that patient in the PTV margin analysis. A total of 34 CBCT scans were analyzed with a median of 5 per patient. PTV margins were calculated to simulate three alignment workflows: 1) skin marks alone, 2) weekly kV imaging, and 3) daily kV imaging. Most studies simulate the use of skin marks by analyzing either 2D or 3D images (7–9). In contrast, we compare daily setup errors calculated from 2D images to those calculated by combining 2D and 3D data. Mean setup errors were added linearly while standard deviations were added in quadrature (15) prior to calculating systematic and random errors. The rationale for combining 2D and 3D errors stems from previous studies illustrating that 2D data can underestimate bony anatomy setup errors when compared to CBCT (16). Thus, if online kV guidance correctly aligns the patient, each clip would be expected to match in the CBCT taken following kV imaging. If residual errors are detected by CBCT, this would indicate that the kV correction was not ideal. To simulate weekly kV imaging, setup errors for every fifth fraction were eliminated from the analysis. Finally, margins calculated from CBCT alone indicate the accuracy of daily kV imaging since they highlight residual errors that remain following daily kV imaging.
Margin calculations yield a single number in each axis, so it is not possible to compare margins directly between those generated from 2D data versus 2D/3D data. Instead, the individual data used to generate the PTV margin was compared. This method is justified because PTV margins are calculated by a linear combination of systematic and random errors. Systematic errors, which are calculated from the standard deviation of the group means, were compared using the F-test (p<0.05) which analyzes standard deviations. Random errors were compared using a two-tailed paired t-test (p<0.05).
Results
Patient Characteristics
Patient characteristics are shown in Table 1. Of 21 patients, 17 had a median of 21 surgical clips. In 5 of the 17 patients, a median of 8 clips was localized to the axilla only whereas the remaining 12 patients had clips throughout the chest wall and axilla.
Table 1.
Patient and treatment characteristics for kV analysis (n=21) and for the CBCT subset analysis (n=8)
| Age (years) | ||
| Median (range) | 46.0 (23–70) | 48.5 (23–70) |
| Body Mass Index (kg/m2) | ||
| Median (range) | 27.9 (18.7–40.8) | 23.7 (18.7–25.4) |
| Stage | ||
| IIB | 2 | 2 |
| IIIA | 5 | 3 |
| IIIB | 3 | 2 |
| IIIC | 8 | 1 |
| IV | 3 | 0 |
| Left | 11 | 5 |
| Right | 10 | 3 |
| Dose | ||
| Median chest wall dose (range) | 50.4 Gy (48.0–66.0 Gy) | 50.4 Gy (48.0–50.4 Gy) |
| Median boost dose (range) | 10.0 Gy (10.0–16.0 Gy) | 10.0 Gy (10.0–16.0 Gy) |
| Neoadjuvant Chemotherapy | ||
| Yes | 16 | 7 |
| No | 5 | 1 |
| Cardiotoxic Chemotherapy | ||
| Herceptin use | 3 | 2 |
| Adriamycin use | 15 | 6 |
| Previous Contralateral Breast RT | 4 | 2 |
| IMN Disease | 10 | 3 |
Daily inter-fractional setup error (kV analysis)
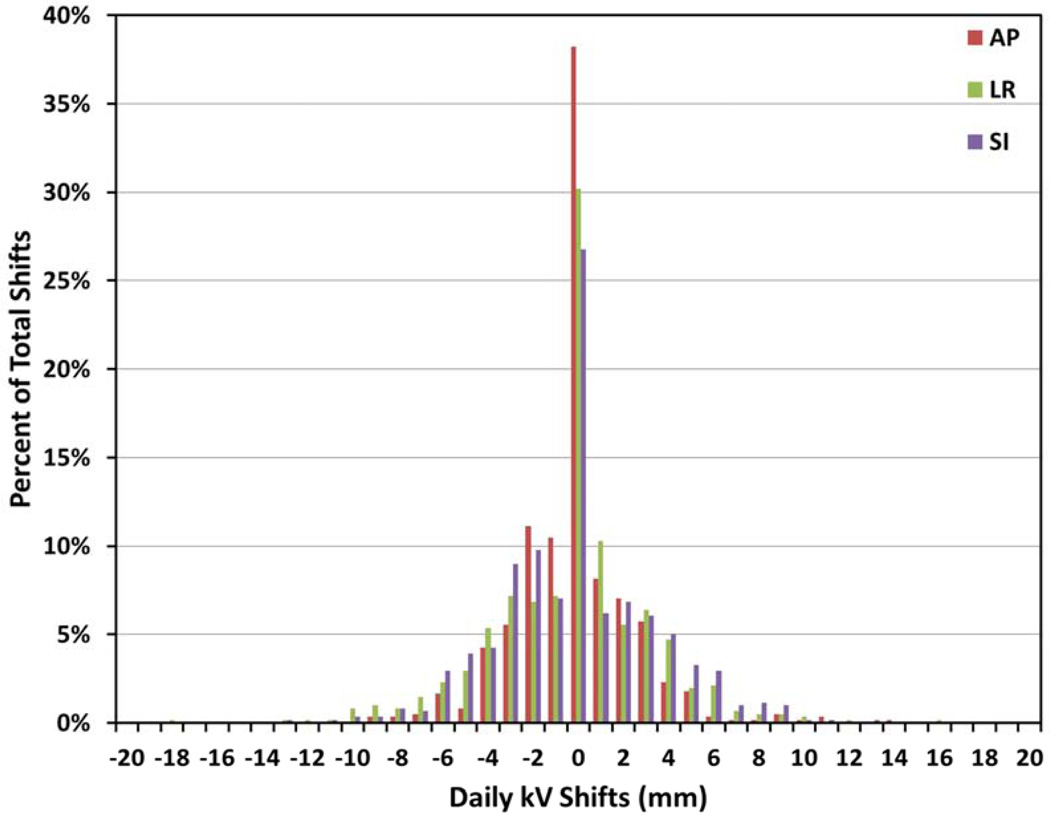
A total of 613 orthogonal kV image pairs, as shown in Figure 2a, were acquired. Figure 3 shows a histogram distribution of all resulting translational shifts.
Figure 3.
Percentage of shifts of a certain magnitude and direction in anterior-posterior (AP), left-right (LR), and superior-inferior (SI) axes for 613 treatment fractions from 21 patients.
Table 2 displays the means of absolute shifts and total setup errors, and standard deviations of systematic and random errors computed from 2D kV images. By definition, the calculation of the total setup error combined the positive and negative shifts, resulting in smaller total errors than absolute shifts. According to van Herk’s methodology, the values indicate that a 7mm uniform margin would account for more than 95% of daily shifts without the use of IGRT during treatment setup (i.e., setup to skin marks alone). In fact, the data in Table 2 indicate that the AP margin could be reduced to 4mm.
Table 2.
Mean of absolute shifts, setup errors, and PTV margins calculated from 613 kV image pairs for AP, LR, and SI axes in units of millimeter. These PTV margins apply to patients positioned daily to skin marks alone.
| AP | LR | SI | |
|---|---|---|---|
| Absolute shifts (± SD) | 1.7 (±2.3) | 2.4 (±2.6) | 2.5 (±2.4) |
| % shifts within 5 mm | 95.3 | 88.6 | 88.1 |
| % shifts within 7 mm | 97.9 | 95.1 | 95.6 |
| % shifts within 10 mm | 99.4 | 99.0 | 99.4 |
| Total setup error | −0.1 | −0.4 | 0.0 |
| SD of systematic error | 0.7 | 1.9 | 1.6 |
| SD of random error | 2.8 | 3.1 | 3.2 |
| PTV expansion (mm) | 3.7 | 6.9 | 6.2 |
When patients without surgical clips (n = 4) were excluded from the analysis, the calculated PTV margins remained comparable to those of Table 2 to within 0.5mm. For the 12 patients with clips in the chest wall/axilla, the PTV margins were likewise comparable to within 1mm. This population-averaged data indicates that utilizing an anisotropic margin (4mm in AP and 7mm in LR/SI) may be considered for all patients regardless of the location of these clips. This anisotropic margin may potentially be applied to patients without surgical clips once a larger patient cohort is analyzed.
Rotational adjustments of patients were required in 32% ± 17% of treatment sessions. Fractions requiring rotation increased towards the second half of the treatment course, and could potentially be linked to observed patient pain and discomfort from radiation therapy. These rotational errors would not have been detected if patients were aligned to skin marks alone.
Residual setup error (CBCT analysis)
A total of 964 surgical clip markers were manually contoured from 34 CBCT scans taken during 3–5 treatments (median = 5) from 8 patients; patients had a median of 28 surgical clips (range: 18–37). The total setup errors differed from those calculated from orthogonal kV images, which can only identify an average matched position (i.e., individual clips are not necessarily all matched). The 3D data (see Table 3) indicate that despite daily use of kV for positioning, residual errors in clip localization warrant continued use of an anisotropic margin (5.9mm AP, 3.9mm LR, 7.9mm SI).
Table 3.
PTV margins in units of millimeter for three simulated IGRT workflows calculated from either 613 kV image pairs (2D data) and/or 34 CBCT datasets with 964 total clips (3D data) for AP, LR, and SI axes.
| Uses 2D Data |
Uses 3D Data |
AP | LR | SI | |
|---|---|---|---|---|---|
| Skin marks only | Yes | No | 3.7 | 6.9 | 6.2 |
| Skin marks only | Yes | Yes | 7.0 | 8.3 | 8.1 |
| kV weekly | Yes | No | 3.8 | 7.0 | 6.5 |
| kV daily | No | Yes | 5.9 | 3.9 | 7.9 |
Figure 3b depicts an axial slice from registered CT and CBCT scans for a single treatment fraction. Despite kV-guided positioning prior to treatment, residual error in clip localization persisted. There was no measurable trend (Pearson’s correlation coefficient r < 0.5) between the magnitude of clip shift and treatment fraction number, indicating minimal clip migration during the course of treatment.
Total setup error (kV/CBCT analysis
The systematic and random errors from CBCT measurements were combined in quadrature with kV x-ray data from the same 8 patients during the same treatment course to yield total errors from both 2D and 3D imaging data. The combined random errors for all three axes were significantly different from those derived from kV x-ray data alone (p<0.05). The combined systematic errors were statistically different only for the AP dimension. Table 3 compares the derived PTV margins for the simulated alignment workflows and lists the data sets (2D and/or 3D) used in the calculations. Combining 2D and 3D data resulted in larger PTV margins (by 3.3mm AP, 1.4mm LR, 1.9mm SI) than those calculated from 2D data alone. If patients were positioned to skin marks alone, a margin of 7–9mm would be required to account for 3D setup errors. The data also indicate use of weekly or daily kV imaging to correct patient positioning would result in similar PTV margins within 0.1–0.3mm. If the 3D data alone are used for analysis, the PTV margins remain large (4–8mm). These 3D data demonstrate that the largest errors exist in the SI direction, requiring a PTV margin of 8mm, even after positioning with daily kV imaging.
Discussion
Studies of APBI, delivered with 3DCRT, have demonstrated daily variability in setup to skin marks ranging between 7–12mm (8, 17, 18). Similar results have been reported for treatment of intact breast cancer patients (16, 19). Two APBI studies used kV and CBCT imaging in a workflow similar to ours (8, 9). kV was first used for online alignment of the surgical cavity then CBCT was used to quantify residual errors allowing margin calculations for daily kV imaging. In order to simulate a workflow that forgoes daily IGRT, we combined kV and CBCT data to determine the offset of each surgical clip from the alignment to skin marks resulting in margins of 7–9mm. While chest wall targets are generally expected to be less mobile, there is a relative lack of published guidelines quantifying setup errors. Jain et al. studied intact breast treatments utilizing CBCT data to quantify 3D residual errors following alignment to daily MV imaging (19). Using van Herk’s equation to sum their reported systematic and random errors yields margins of 9mm AP, 17mm LR, and 8mm SI. In our population, margins calculated from 3D data following daily kV alignment are smaller in 2 axes (6mm AP, 4mm LR, and 8mm SI), which could be attributed to less soft tissue deformation of chest wall targets. However, other contributing factors include the higher contrast of kV imaging and differences in patient immobilization. By studying individual clip alignment we can estimate 3D errors for chest wall and regional nodal targets independently. To our knowledge, no study has explicitly reported on required margins for inverse-planned IMRT including regional nodal targets for a variety of IGRT workflows.
Our data demonstrate that while weekly and daily kV imaging would require similar PTV margins, patient rotations would remain uncorrected in approximately one-third of treatment fractions by forgoing daily kV imaging. Because van Herk’s methodology only utilizes translational shifts, the calculated PTV margins are considered to be the minimum threshold necessary to ensure accurate dose delivery (14). Thus, IGRT would still be necessary for each treatment fraction in order to detect these rotational errors. In a large target such as the chest wall, small rotational inaccuracies could result in translational inaccuracies (20), as shown in Figure 3b, on the order of the PTV margin. For larger patients, daily imaging may be particularly important to reduce positioning errors, as there was a strong correlation (Pearson’s correlation coefficient r = 0.71) between the magnitude of daily shifts and body mass index in the 12 patients with clips in the chest wall and axilla.
kV data yielded smaller overall error than the combined kV/CBCT data, likely a result of the different intrinsic properties of the imaging modalities. The NKI group previously compared two-dimensional electronic portal image devices (EPID) and CBCT-driven patient positioning and concluded that CBCT quantifies setup error more accurately, particularly in the SI axis (16). Our results are consistent with these findings in that combining 3D data with 2D reports larger errors and contributes to larger PTV margins, particularly in the SI direction. Due to the fact that systematic errors were not significantly different for all 3 axes for 2D/3D data compared to 2D data alone, we could rule out the possibility of patient movement or bony landmark mis-alignment between acquisition of kV and CBCT images. Furthermore, CBCT data provide increased sensitivity for small out-of-plane rotations (see Figure 3b) and deformation errors near the humeral head, which are factored into the overall calculations when individual clips are tracked. When the CBCT analysis was repeated for the subset of clips (n=427) in the supraclavicular and axillary nodal targets, the calculated PTV margins were 7.1mm AP, 4.3mm LR, 8.9mm SI. Although the systematic and random errors were not significantly different from those used to calculate margins for clips in the chest wall using CBCT data, the resulting PTV margins were consistently larger by 0.4–1.2mm in all axes. These data imply that arm positioning may more difficult than alignment of the chest wall, which has been reported by others (17). This distinction between nodal target and chest wall positioning would not be possible without 3D data, which allows for translational shifts to be calculated on the basis of individual clips rather than the entire image.
By combining systematic and random errors calculated from kV and CBCT data, we captured information about the true target 3D position. The shifts in each axis were added linearly and random errors added in quadrature based on the lack of covariance between the two data sets (15). The combined total setup error yielded PTV expansions of 7mm in the AP axis, 9mm in the LR axis, and 9mm in the SI axis. These margins would adequately cover the target volume and account for setup errors in the absence of IGRT. Currently, a uniform 7mm expansion of the CTV is used for treatment planning of chest wall targets (4). Even with daily kV imaging, a uniform 7mm margin would be insufficient in the SI direction, which requires an 8mm margin.
Increases in PTV margins based on the van Herk formula using this data would ensure greater coverage of chest wall targets, but would also increase radiation to nearby organs-at-risk. These results indicate a need for improvement in setup technique and technology, as the inter-fraction deviations in positioning have an impact on total IMRT dose to the chest wall target. This potential clinical consequence suggests a need for daily IGRT. While it may never be possible to set up a patient with absolute reproducibility, improvements in immobilization devices or online three-dimensional IGRT equipment such as surface imaging could help reduce these setup errors (13, 17).
There are some limitations to our findings. We did not characterize intra-fraction fluctuations and their effect on setup errors, as these were previously shown to have lesser effects on dose distributions than inter-fraction changes (21). There are a few simplifications in the van Herk formula used to derive suggested PTV margins. While it takes into account random and systematic errors, there are other clinical factors which may also affect treatment reproducibility that are not factored into the calculation. In addition, CBCT imaging has limited resolution in the SI axis due to a 3mm slice thickness. This may result in underestimation of residual error (16, 22) and inaccurate PTV margin recommendations. The inherent properties of CBCT technology also make resulting images more susceptible to breathing artifact during image acquisition. However, CBCT is currently the only three-dimensional imaging modality available at our treatment machines. Another limitation is the small sample size of this study. This, combined with the low-BMI range of our patients due to physical restrictions rotating the CBCT imaging panels around the patient (8), results in limited extrapolation of this 3D data to all post-mastectomy breast cancer patients.
In conclusion, CBCT analysis provided additional information on the effect of three-dimensional reproducibility of treatment setup for chest wall targets. In the absence of daily image-guided treatment setup, an anisotropic PTV expansion of 7–9mm can be used. Because the inter-fraction movement is significantly greater than suggested by two-dimensional kV x-ray imaging, a PTV margin of 4–8mm must be retained for accurate target localization even following daily kV imaging.
Summary.
Daily IGRT data (n=613) were analyzed to quantify setup uncertainty in 21 post-mastectomy breast cancer patients treated with inverse-planned IMRT to the chest wall and regional nodes including the internal mammary chain. While 2D kV images indicate that a uniform PTV margin of 7 mm is sufficient, CBCT demonstrated persistent rotations and deformations. Thus, a margin of 4–8 mm must be retained despite daily kV imaging. This is the largest population-based study in post-mastectomy patients.
Acknowledgments
Support for institutional review board approval was provided by University of Chicago Comprehensive Cancer Center support grant P30 CA014599. This work was supported in part by the National Institutes of Health (NIH) Grant No. 2T35AG029795-06. The authors thank Dimple Modgil, Ph.D., for assistance in data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary versions of this study were presented at the 54th Annual Meeting of the American Society of Radiation Oncology in Boston, MA, October 28 – 31, 2012.
Conflict of Interest: None
References
- 1.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N. Engl. J. Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with highrisk breast cancer receiving adjuvant chemotherapy, 20-year results of the British Columbia randomized trial. J. Natl. Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 4.Beckham WA, Popescu CC, Patenaude VV, et al. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int. J. Radiat. Oncol. Biol. Phys. 2007;69:918–924. doi: 10.1016/j.ijrobp.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 5.Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 6.Bujold A, Craig T, Jaffray D, et al. Image-guided radiotherapy: has it influenced patient outcomes? Semin. Radiat. Oncol. 2012;22:50–61. doi: 10.1016/j.semradonc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Hasan Y, Kim L, Martinez A, et al. Image guidance in external beam accelerated partial breast irradiation: comparison of surrogates for the lumpectomy cavity. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:619–625. doi: 10.1016/j.ijrobp.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 8.Kim LH, Wong J, Yan D. On-line localization of the lumpectomy cavity using surgical clips. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:1305–1309. doi: 10.1016/j.ijrobp.2007.07.2365. [DOI] [PubMed] [Google Scholar]
- 9.Fatunase T, Wang Z, Yoo S, et al. Assessment of the residual error in soft tissue setup in patients undergoing partial breast irradiation: results of a prospective study using cone-beam computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:1025–1034. doi: 10.1016/j.ijrobp.2007.07.2344. [DOI] [PubMed] [Google Scholar]
- 10.Frazier RC, Vicini FA, Sharpe MB, et al. Impact of breathing motion on whole breast radiotherapy: a dosimetric analysis using active breathing control. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:1041–1047. doi: 10.1016/j.ijrobp.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Roeske JC, Chmura SJ, et al. Calculation and prediction of the effect of respiratory motion on whole breast radiation therapy dose distributions. Med. Dosim. Off. J. Am. Assoc. Med. Dosim. 2009;34:126–132. doi: 10.1016/j.meddos.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Goddu SM, Yaddanapudi S, Pechenaya OL, et al. Dosimetric consequences of uncorrected setup errors in helical Tomotherapy treatments of breast-cancer patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009;93:64–70. doi: 10.1016/j.radonc.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Padilla L, Kang H, Washington M, et al. Assessment of interfractional variation of the breast surface following conventional patient positioning for whole-breast radiotherapy. J. Appl. Clin. Med. Phys. 2014;15 doi: 10.1120/jacmp.v15i5.4921. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Herk M, Remeijer P, Rasch C, et al. The probability of correct target dosage: dosepopulation histograms for deriving treatment margins in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 15.Stroom J, Gilhuijs K, Vieira S, et al. Combined recipe for clinical target volume and planning target volume margins. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:708–714. doi: 10.1016/j.ijrobp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Topolnjak R, Sonke J-J, Nijkamp J, et al. Breast patient setup error assessment: comparison of electronic portal image devices and cone-beam computed tomography matching results. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:1235–1243. doi: 10.1016/j.ijrobp.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Gierga DP, Riboldi M, Turcotte JC, et al. Comparison of target registration errors for multiple image-guided techniques in accelerated partial breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:1239–1246. doi: 10.1016/j.ijrobp.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Hasan Y, Kim L, Wloch J, et al. Comparison of planned versus actual dose delivered for external beam accelerated partial breast irradiation using cone-beam CT and deformable registration. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:1473–1476. doi: 10.1016/j.ijrobp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Jain P, Marchant T, Green M, et al. Inter-fraction motion and dosimetric consequences during breast intensity-modulated radiotherapy (IMRT) Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009;90:93–98. doi: 10.1016/j.radonc.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Ezzell LC, Hansen EK, Quivey JM, et al. Detection of treatment setup errors between two CT scans for patients with head and neck cancer. Med. Phys. 2007;34:3233–3242. doi: 10.1118/1.2751074. [DOI] [PubMed] [Google Scholar]
- 21.Yue NJ, Goyal S, Zhou J, et al. Intrafractional target motions and uncertainties of treatment setup reference systems in accelerated partial breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:1549–1556. doi: 10.1016/j.ijrobp.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Woodford C, Yartsev S, Van Dyk J. Optimization of megavoltage CT scan registration settings for brain cancer treatments on tomotherapy. Phys. Med. Biol. 2007;52:N185–N193. doi: 10.1088/0031-9155/52/8/N04. [DOI] [PubMed] [Google Scholar]