Abstract
Adolescents living in communities with ferromanganese alloy plant activity have been shown to exhibit deficits in olfactory and fine motor function. Household dust may serve as an important manganese (Mn) exposure pathway to children, though dust Mn concentrations have not previously been measured to assess household contamination from ferromanganese alloy plant emissions. Here we determined the association between dust concentrations and surface loadings of Mn and other metals (Al, Cd, Cr, Cu, Fe, Pb, and Zn) in indoor and outdoor household dust from three Italian communities that differ by history of ferromanganese alloy plant activity: Bagnolo Mella, with an active ferromanganese alloy plant (n=178 households); Valcamonica, with historically active plants (n=166); and Garda Lake, with no history of ferromanganese plant activity (n=99). We also evaluated Mn levels in other environmental (soil, airborne particulates) and candidate biomarker (blood, hair, saliva, fingernails) samples from children within the households. Household dust Mn concentrations and surface loadings were significantly different between the three sites, with levels highest in Bagnolo Mella (outdoor median Mn concentration = 4620, range 487 – 183,000 µg/g), intermediate in Valcamonica (median = 876, range 407 – 8240 µg/g), and lowest in Garda Lake (median = 407, range 258 – 7240 µg/g). Outdoor dust Mn concentrations in Bagnolo Mella, but not the other communities, were significantly inversely related with distance from the plant (R2=0.6630, P<0.0001). Moreover, outdoor dust Mn concentrations and loadings were highly predictive of but significantly higher than indoor dust Mn concentrations and loadings by ~2 to ~7-fold (Mn concentrations) and ~7 to ~20-fold (Mn loadings). Finally, both indoor and outdoor dust Mn concentrations and outdoor dust Mn loading values were highly significantly correlated with both soil and air Mn concentrations, and with children’s hair and fingernail Mn concentrations, but weakly or not associated with saliva or blood Mn levels. Given the evidence associating elevated Mn exposure with neurological impairments in children, these data support that dust Mn levels should be reduced in contaminated environments to protect the health of resident children.
Keywords: Manganese, Dust, Ferroalloy, Exposure, Biomarker
Introduction
Manganese (Mn) is an essential nutrient, although Mn elevated exposures have been associated with neurotoxicity in adults and children. (ATSDR, 2012; WHO, 2000; Aschner et al., 2005; Lucchini et al., 2007) Children are considered particularly susceptible to the health impacts of elevated Mn exposure, with studies reporting reduced birth weight, IQ deficits, increased oppositional and attention problems, and fine motor and sensory deficits associated with elevated Mn exposure. (ATSDR, 2012; Bouchard et al., 2011; Wasserman et al., 2006; Bouchard et al., 2007; Lucchini et al., 2012; Zota et al., 2009; Claus Henn et al., 2010; Claus Henn et al., 2012) There are a number of natural and anthropogenic sources of Mn in the environment that may contribute to elevated exposures over the life span, including contaminated groundwater, combustion of the gasoline additive methylcyclopentadienyl manganese tricarbonyl (MMT), the fungicides maneb and mancozeb, and ferromanganese alloy facilities. (ATSDR, 2012; Bouchard et al., 2011; Wasserman et al., 2006; Lucchini et al., 2012; Gunier et al., 2013; Menezes-Filho et al., 2009; Borgese et al., 2013)
The iron and steel industry consumes ~90% of the worldwide Mn produced (Bouaziz et al., 2011), and it represents one of the most regionally significant anthropogenic sources of Mn to the environment - contributing up to 80% of industrial Mn emissions. (ATSDR, 2012; EPA, 2008) Manganese is used as an alloying element in the metal industry, where it is alloyed with silicon and iron forming silicomanganese and ferromanganese. (Pearson et al., 2005) In steel production, addition of Mn to ferromanganese alloys imparts unique physical properties of tensile strength and flexibility compared to ferroalloy steels with lower Mn levels. (ATSDR, 2012; Pearson et al., 2005; Bouaziz et al., 2011) While the Mn content in steel is generally in the range of 0.05 to 12%, standard ferromanganese alloys may contain substantially greater levels of Mn.
Soil naturally contains between 500 and 900 µg Mn/g, and airborne Mn concentrations in areas without anthropogenic sources range from 0.01 – 0.07 µg/m3. (WHO, 2000; Gerber et al., 2002) In areas adjacent to ferro- or silico-Mn industries Mn levels can exceed 0.5 µg/m3, which is well above the world health organization’s (WHO) annual average air guideline value for long-term Mn exposure of 0.15 µg/m3. (WHO, 2000) Coarse Mn particles (defined by WHO as >PM2.5) originating from ferromanganese alloy plant emissions settle within meters to kilometers of the plant, while finer particles (<PM2.5) can be transported larger distances through the air. (WHO, 2000)
The Province of Brescia, Italy contains both historic (Valcamonica valley) and currently active (Bagnolo Mella) ferromanganese alloy industries, and studies have reported associations between environmental Mn exposures and health deficits in children and elderly adults in the impacted communities. (Lucchini et al., 2007; Lucchini et al., 2012; Borgese et al., 2013; Lucchini et al., 2014) In addition, a higher prevalence of Parkinsonian disturbances in aged adults associated with environmental Mn exposure has also been reported. (Lucchini et al., 2007) Environmental Mn associated with airborne particulates and settled dust has been implicated as the exposure source/pathway to children and adults in these areas. Household dust is a well-known exposure pathway for metals, particularly in children, through ingestion and inhalation of particles. (Lanphear et al., 1998, 2002; Lioy et al., 2002; Zota et al., 2011) This exposure pathway is especially well-described for household dust lead (Pb) exposure to children, resulting in elevated blood Pb levels and health problems. (Taylor et al., 2013; Lanphear et al., 1998, 2002; Wilson et al., 2006) Analysis of soil samples in Valcamonica and Garda Lake (control region) demonstrated higher levels of Mn in Valcamonica in the readily extractable fractions, suggesting a higher level of environmental Mn exposure in regions with historic ferromanganese alloy plant activity. (Borgese et al., 2013) In addition, the Mn concentration in air particulates was previously determined to be approximately two to three-times higher in Valcamonica than Garda Lake. (Borgese et al., 2011) An initial screening of household dust samples in Brescia using XRF showed higher concentrations of Mn in samples located nearby municipalities with historic or active ferromanganese alloy plants in comparison to regions without these plants. (Zacco et al., 2009)
In light of the above, indoor and outdoor settled dust from the houses of children in the province of Brescia, Italy were analyzed for Mn and other metals (aluminum, cadmium, chromium, copper, iron, lead, and zinc) to determine whether dust metal levels were significantly elevated in association with local ferromanganese alloy plant activity. We hypothesized that (1) Mn levels in household dust from Bagnolo Mella will be significantly higher than levels in dust from Valcamonica or the Garda Lake reference area; (2) Mn dust concentrations will have an inverse relationship with distance from the active and historic ferromanganese alloy plants in Bagnolo Mella and Valcamonica, respectively; (3) Mn concentrations in indoor household dust will be associated with, but lower than corresponding levels in outdoor house dust; and (4) household dust Mn levels will be associated Mn levels in other environmental media (e.g., soil, airborne Mn) and candidate biomarkers of Mn exposure in resident children (e.g., hair, fingernails, saliva), evidencing that dust may pose an important Mn exposure pathway for adolescents.
Methods
Study Sites
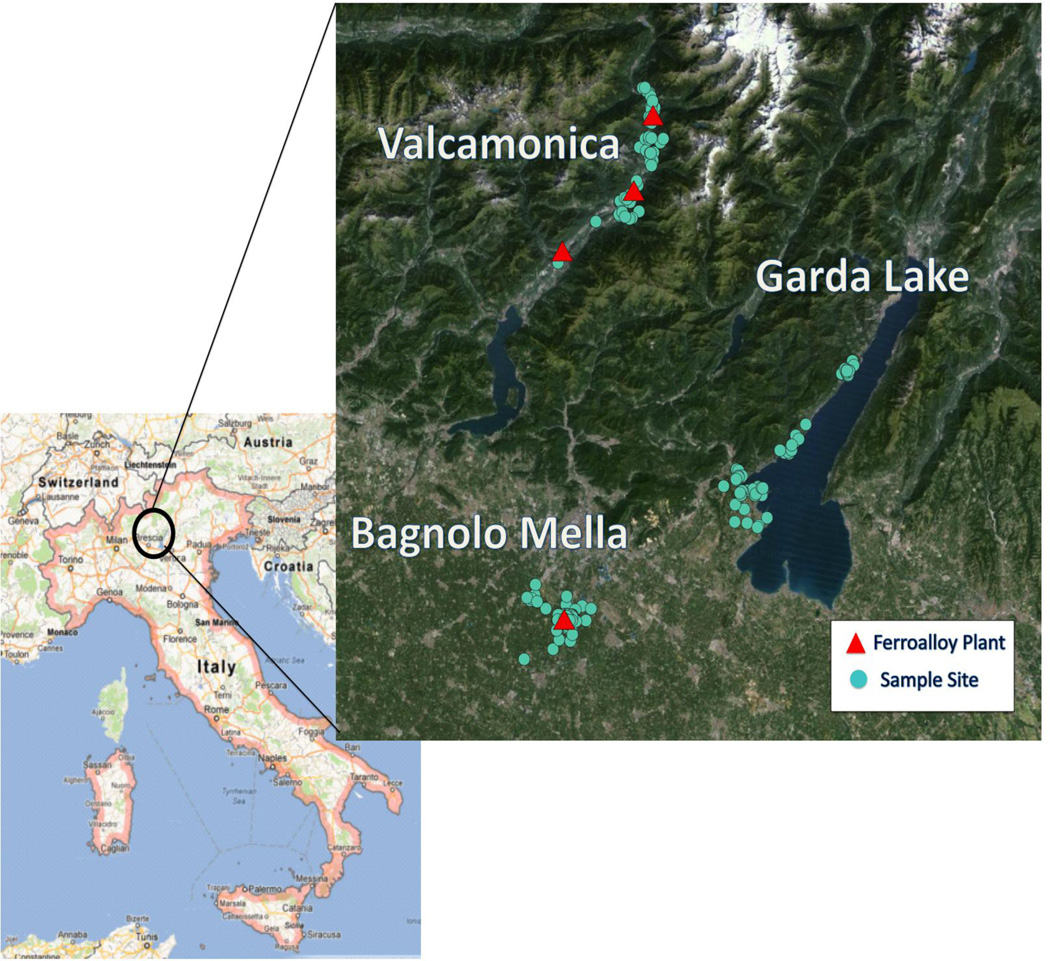
The study focused on households within three areas in the Province of Brescia, Italy: Bagnolo Mella, Valcamonica, and Garda Lake (Figure 1). Bagnolo Mella (population 12,700) is a municipality with an active ferromanganese alloy plant that has produced ferromanganese since 1973, and is situated in a flat plains region. Valcamonica is a valley of the pre-Alps with an average width of approximately 3 km and mountains of about 3000 m on either side. Winds average 5 km/h in the valley, primarily southwest to northeast in the day and northeast to southwest at night. Three ferromanganese alloy plants have operated in the valley in the municipalities of Sellero (population 1500) from 1973 to 1987, Breno (population 5000) from 1921 to 2001, and Darfo (population 13,200) from 1902 to 1995. (Lucchini et al., 2012) Communities within the Garda Lake region of the Province have had no history of ferromanganese alloy plant activity so were used as the reference group.
Figure 1.
Map of study sites in the Province of Brescia, Italy. The red triangles are the locations of each ferroalloy plant. The blue dots are the sampling points. Sellero, Breno and Darfo are located in the valley of Valcamonica.
Sample Collection
Indoor and outdoor dust samples were collected for each household from various horizontal surfaces between December 2010 and October 2013. Dust samples were collected by sweeping a measured area (60 – 35000 cm2, median 1230 cm2) with a plastic brush (cleaned between samplings) into a plastic bag, or using a cyclone vacuum that deposited collected dust into a plastic sample jar. The two methods of sample collection were balanced across the three study sites. The total mass of collected dust ranged from 0.7 to 2673 mg (median 90.8 mg). Indoor and outdoor dust samples were analyzed from 153 households from Bagnolo Mella, 88 households from Valcamonica (Sellero: 50, Breno: 37, Darfo: 1), and 72 households from Garda Lake. The total number of households for each study site for which paired indoor and outdoor dust samples were analyzed were 135 (Bagnolo Mella), 78 (Valcamonica) and 64 (Garda Lake).
Sample Preparation
All laboratory reagents (e.g., HNO3) were trace metal grade. Ultrapure Milli-Q water with resistivity of 18.2 MΩ·cm2 was used for all dilutions. Deionized water (DI) was used to rinse plasticware where stated.
Dust samples were dried in a 60°C oven for at least 2 days, and non-dust debris was manually removed. For dust samples less than 100 mg, the entire sample was transferred to a polypropylene test tube (polytube) to obtain the sample weight. For samples greater than 100 mg, the sample was thoroughly mixed to homogeneity and approximately 100 mg was used. The mass of dust processed for analyses ranged from ~1 – 100 mg. Dust samples were leached in 7.5 N HNO3 at 80°C for 4 hours with vortexing every hour. The resultant leachate was diluted in the polytube with Milli-Q, centrifuged at 3000 × g for 20 minutes, and the supernatant decanted into polyethylene scintillation vials for analyses.
Analysis
Dust metal concentrations were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES; Perkin-Elmer model Optima 4300 DV Series), using emission wavelengths of 257.610 and 260.568 (Mn), 308.215, 394.401, and 396.153 (Al), 214.440, 226.502, and 228.802 (Cd), 267.716 (Cr), 324.752 and 327.393 (Cu), 238.204, 239.562, and 259.939 (Fe), 220.353 (Pb), and 202.548 and 206.200 (Zn). When more than one wavelength was used the average of the concentration from each wavelength was reported. Scandium and yttrium were used as internal standards.
For assessment of analytical accuracy and reproducibility, a certified reference material (CRM BCR-483) was prepared (four replicates per analytical batch of ~50–60 samples) and analyzed in the same manner as the dust samples. Within each analytical batch of samples two household dust samples of adequate mass were selected at random to process and analyze in triplicate. The analytical limits of detection (µg/mL) were 0.0013 (Mn), 0.0254 (Al), 0.0019 (Cd), 0.0023 (Cr), 0.0026 (Cu), 0.0262 (Fe), 0.0367 (Pb), and 0.0065 (Zn). The average RSD’s for samples (N = 20) processed in triplicate were 4.3% (Mn), 3.2% (Al), 6.3% (Cd), 4.6% (Cr), 11.7% (Cu), 4.8% (Fe), 6.0% (Pb), and 6.1% (Zn). The accuracy (percent recovery) of the analysis was 105% (Cd), 123% (Cr), 115% (Cu), 98% (Pb), and 96% (Zn), based on measured versus published indicative values for the certified reference material. The remaining analytes (Mn, Al, Fe) did not have published indicative values, and are compared to previously published values. The analytical and procedural limits of detection, reproducibility, and accuracy based on repeated analysis of the certified reference material are summarized for each metal in Supplementary Table 1.
Other Environmental and Biomarker Measures
This study is part of a larger ongoing study that has recruited a total of ~700 pre-adolescent subjects age 11 – 14 yrs (~200 – 250 subjects/households per study site). Environmental media (household dust, soil, tap water, 24 hour airborne particulates) and candidate biomarker samples (blood, hair, fingernails, saliva) were collected from each subject/household for analyses of metal content, as described in detail elsewhere. (Lucchini et al., 2012; Borgese et al., 2011; Eastman et al., 2013; Smith et al., 2007) Briefly, surface soil metal levels were measured using a portable XRF instrument (Niton), (Lucchini et al., 2012) while 24-hour personal air samples (PM10) were collected using Personal Environmental Monitors connected to a Leland Legacy pump and analyzed by T-XRF. (Borgese et al., 2011) Whole blood samples were collected using a 19-gauge butterfly catheter into Li-heparin Sarstedt Monovette Vacutainers. Passive saliva samples were collected directly into trace metal clean microfuge tubes via a 5 cm plastic straw; prior to collection, subjects rinsed their mouths three-times with ultrapure Milli-Q water, then waited 10 min before dispensing saliva into the tube. Hair samples (2–3 cm section of hair from the occipital lobe, proximal to the scalp) were collected using stainless steel scissors. Fingernail samples were collected using stainless steel nail clippers. In addition, physiological iron status was assessed in the children via measures of blood hemoglobin, total serum iron, and serum ferritin and transferrin levels; for all iron status outcomes, the levels were within the normal clinical range. (Lucchini et al., 2012)
Biological samples were processed and analyzed for Mn as follows (Lucchini et al., 2012; Eastman et al., 2013; Smith et al., 2007): For whole blood, ~0.25 mL of blood was mixed with 0.5 mL 15.7 N quartz distilled nitric acid in a polyethylene tube, and the mixture left overnight at room temperature. Subsequently, 0.25 mL of Ultrex 30% hydrogen peroxide was added, followed by 4 mL of Milli-Q water. The mixture was vortexed and the precipitate allowed to settle overnight. Subsequently, 0.5 mL of supernatant was removed and centrifuged at 13000 × g for 10 minutes for analysis. Saliva samples were vortexed and centrifuged at 1000 × g for 1 minute, and a 0.1 mL aliquot removed and mixed with 0.4 mL 0.8 N quartz distilled nitric acid, and left overnight. Subsequently, the saliva samples were vortexed and centrifuged at 13000 × g for 10 minutes for analyses.
Hair and fingernail samples were cleaned of exogenous metal contamination as described in Eastman et al. (2012). Briefly, samples were placed in 5 mL syringe bodies (hair) or 1.5 mL microfuge tubes (nails) and sonicated (20 min) in 0.5% Triton, rinsed five-times with ultrapure Milli-Q water, sonicated (10 min) in 1 N trace metal grade nitric acid, rinsed with 1 N nitric acid, and rinsed five-times with Milli-Q water. Clean hair and nail samples were dried at 65 °C for 48 hours in a HEPA filtered-air clean room. Subsequently, hair samples were digested in 0.5 mL 15.7 N quartz-distilled nitric acid at 80 °C for 6 h in a Class-100 HEPA filtered-air fume hood. After complete digestion of the hair, samples were diluted with 5 mL Milli-Q water. For analyses, 0.25 mL was transferred to microfuge tube, diluted with 0.25 mL Milli-Q water, and centrifuged at 13000 × g for analysis. Nail samples were digested in 0.1 mL 15.7 N quartz distilled nitric acid at 80 °C for 4 h in a Class-100 HEPA filtered-air hood. After complete digestion, 1.2 mL Milli-Q water was added and samples centrifuged at 13000 × g for 10 min prior to analyses. Rhodium and thallium were added to all samples as internal standards, and samples analyzed by magnetic sector inductively coupled plasma mass spectrometry (Thermo Element XR ICP-MS), as described elsewhere (Smith et al., 2007; Eastman et al., 2013). The analytical detection limit for Mn was 0.0054 ng/mL.
Statistical Analysis
Analysis of variance (ANOVA) or covariance (ANCOVA) were conducted for metal levels to test the hypotheses, using Tukey’s HSD posthoc test for pairwise comparisons when appropriate, as indicated in the Results section. Spearman’s correlation was used to determine the relationship between dust Mn levels and other environmental measures and candidate biomarker data. If data were not normally distributed they were log10 transformed for statistical analyses. In all cases a statistical probability level of P≤0.05 was considered statistically significant. All statistical analysis was carried out using JMP Pro 11 Statistical Discovery (SAS Institute) software.
Results
Dust Mn concentrations and loadings are significantly higher in Bagnolo Mella, the community with an active ferromanganese alloy plant
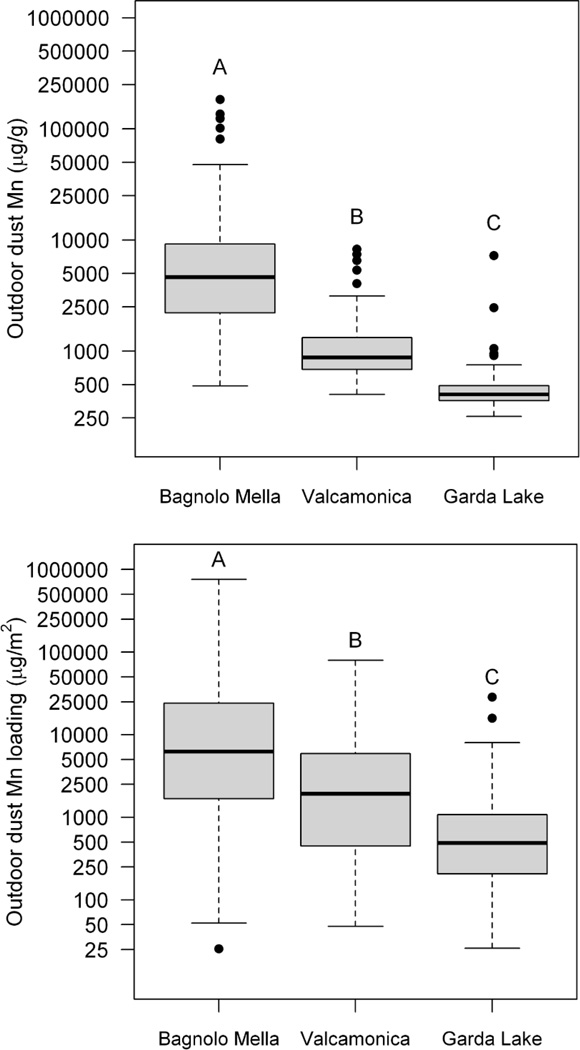
We tested the hypothesis that household dust Mn concentrations (µg/g) are greater in Bagnolo Mella, the community with an active ferromanganese alloy plant compared to communities with historic (Valcamonica) or no history of activity (Garda Lake). Results show that there is a significant effect of ‘study site’ on outdoor dust Mn concentrations (ANOVA on log transformed data F(2, 288) = 179.4, P<0.0001), with concentrations across the three study sites being significantly different (P<0.0001) from one another in the order Bagnolo Mella > Valcamonica > Garda Lake, based on Tukey HSD posthoc analyses. Median outdoor dust Mn concentrations in Bagnolo Mella and Valcamonica were ~11-times and ~2-times higher than levels in the Garda Lake reference community, respectively (Figure 2, Table 1).
Figure 2.
Outdoor household dust Mn concentrations (upper panel, µg/g on log10 scale) and dust Mn loadings (lower panel, (µg Mn/m2 on log10 scale) in the communities with active (Bagnolo Mella, n~142), historic (Valcamonica, n=81) and no history (Garda Lake, n~68) of ferromanganese alloy plant activity. The middle line within each box is the median, the lower and upper margins of the box represent the 25th and 75th percentiles, while the lower and upper whiskers are drawn to the furthest data point within 1.5-times the interquartile range. Boxes with different superscript letters are significantly different from one another, based on Tukey’s post hoc test (p<0.05).
Table 1.
Indoor and outdoor household dust metal concentrations (µg/g) in communities with active (Bagnolo Mella, BM), historic (Valcamonica, VC) and no history (Garda Lake, GL) of ferromanganese alloy plant activity in the Provence of Brescia, Italy.
| Site | Mean | Median | Geo Mean |
Std Dev |
Min | Max | N | ||
|---|---|---|---|---|---|---|---|---|---|
| Mn | BM | Out | 11700A | 4620 | 5040 | 24300 | 487 | 183000 | 142 |
| In | 1240a | 599 | 756 | 1940 | 129 | 18500 | 147 | ||
| VC | Out | 1350B | 876 | 1050 | 1410 | 407 | 8240 | 81 | |
| In | 405b | 308 | 324 | 338 | 59.7 | 2110 | 85 | ||
| GL | Out | 580C | 407 | 459 | 870 | 258 | 7240 | 68 | |
| In | 255c | 185 | 193 | 266 | 20.8 | 2000 | 67 | ||
| Al | BM | Out | 9210A | 9020 | 8750 | 3220 | 2340 | 30600 | 142 |
| In | 4750a | 4260 | 4190 | 2510 | 588 | 16400 | 147 | ||
| VC | Out | 10300B | 10200 | 10000 | 2510 | 5120 | 19400 | 81 | |
| In | 4610ab | 4400 | 4100 | 2220 | 680 | 13900 | 85 | ||
| GL | Out | 6550C | 6720 | 6210 | 2110 | 2320 | 14000 | 68 | |
| In | 4030b | 3510 | 3420 | 2330 | 447 | 11000 | 67 | ||
| Cd | BM | Out | 3.90A | 2.35 | 2.71 | 5.98 | 0.09 | 49.2 | 142 |
| In | 2.00a | 1.47 | 1.20 | 5.02 | 0.09 | 60.8 | 147 | ||
| VC | Out | 2.28B | 1.81 | 1.81 | 2.30 | 0.09 | 16.2 | 81 | |
| In | 2.47a | 1.49 | 1.44 | 5.01 | 0.09 | 39.5 | 85 | ||
| GL | Out | 2.61B | 1.79 | 2.10 | 2.42 | 0.847 | 16.9 | 68 | |
| In | 2.32a | 1.69 | 1.70 | 2.27 | 0.09 | 13.1 | 67 | ||
| Cr | BM | Out | 81.7A | 64.3 | 68.1 | 70.7 | 14.6 | 676 | 142 |
| In | 47.9a | 43.1 | 43.4 | 27.8 | 12.1 | 273 | 147 | ||
| VC | Out | 70.8A | 54.2 | 59.6 | 57.6 | 24.8 | 411 | 81 | |
| In | 47.6a | 42.1 | 43.1 | 29.5 | 19.2 | 264 | 85 | ||
| GL | Out | 143B | 39.6 | 47.3 | 632 | 10.6 | 5170 | 68 | |
| In | 49.0a | 43.6 | 43.8 | 32.6 | 19.0 | 223 | 67 | ||
| Cu | BM | Out | 501AB | 294 | 304 | 1320 | 68.9 | 12200 | 142 |
| In | 359a | 199 | 222 | 747 | 18.9 | 6590 | 147 | ||
| VC | Out | 501B | 197 | 237 | 1480 | 53.2 | 12100 | 81 | |
| In | 327a | 187 | 208 | 577 | 38.3 | 3900 | 85 | ||
| GL | Out | 480A | 332 | 340 | 520 | 58.8 | 3040 | 68 | |
| In | 256a | 200 | 215 | 215 | 43.9 | 1590 | 67 | ||
| Fe | BM | Out | 20300A | 15400 | 17000 | 18700 | 4550 | 162000 | 142 |
| In | 6070a | 4920 | 4980 | 5860 | 1270 | 57800 | 147 | ||
| VC | Out | 21300A | 18600 | 19100 | 15000 | 6870 | 138000 | 81 | |
| In | 5920ab | 5620 | 5020 | 3270 | 728 | 18600 | 85 | ||
| GL | Out | 18300B | 12700 | 14400 | 18700 | 5200 | 139000 | 68 | |
| In | 7750b | 3600 | 3930 | 24400 | 532 | 199000 | 67 | ||
| Pb | BM | Out | 221A | 174 | 184 | 167 | 30.9 | 1380 | 142 |
| In | 113a | 82.3 | 82.1 | 127 | 1.85 | 1110 | 147 | ||
| VC | Out | 195B | 104 | 119 | 400 | 31.6 | 3160 | 81 | |
| In | 101a | 56.9 | 61.7 | 152 | 1.85 | 1030 | 85 | ||
| GL | Out | 342A | 127 | 177 | 650 | 46.5 | 4420 | 68 | |
| In | 101a | 64.6 | 65.4 | 131 | 1.85 | 794 | 67 | ||
| Zn | BM | Out | 1910A | 862 | 991 | 5700 | 116 | 54600 | 142 |
| In | 694a | 524 | 574 | 616 | 66.1 | 5460 | 147 | ||
| VC | Out | 1560B | 583 | 698 | 6020 | 202 | 53900 | 81 | |
| In | 649a | 505 | 516 | 795 | 126 | 7160 | 85 | ||
| GL | Out | 1170B | 684 | 743 | 2260 | 275 | 17800 | 68 | |
| In | 560a | 489 | 487 | 362 | 119 | 2650 | 67 |
A/B/C or a/b/c Capital letter superscripts reflect statistical comparison of outdoor dust samples across sites within each metal; groups with different letters are statistically different from one another based on ANOVA of log10 transformed data. Similarly, lower case letter superscripts reflect statistical comparison of indoor dust samples across sites within each metal; groups with different letters are statistically different from one another.
Similarly, outdoor household dust Mn loadings (µg Mn/m2) differ across site (ANOVA F(2, 283) = 40.58, P<0.0001) in the same pattern, with Bagnolo Mella > Valcamonica > Garda Lake (Figure 2, Supplementary Table 2).These differences in outdoor dust Mn loadings appear largely due to the differences in dust Mn concentrations across sites, as opposed to the mass of dust loadings (i.e., g dust/m2), since outdoor dust mass loadings were not significantly different across site (ANOVA F(2, 282) = 1.98, P=0.14) (Supplementary Table 3). Indoor dust Mn loading is also significantly different across sites (ANOVA F(2, 294) = 18.85, P<0.0001), with Bagnolo Mella > Valcamonica = Garda Lake (Supplementary Table 2), while there is no difference in the mass of indoor dust loadings across sites (ANOVA F(2, 294) = 1.880, P=0.15) (Supplementary Table 3).
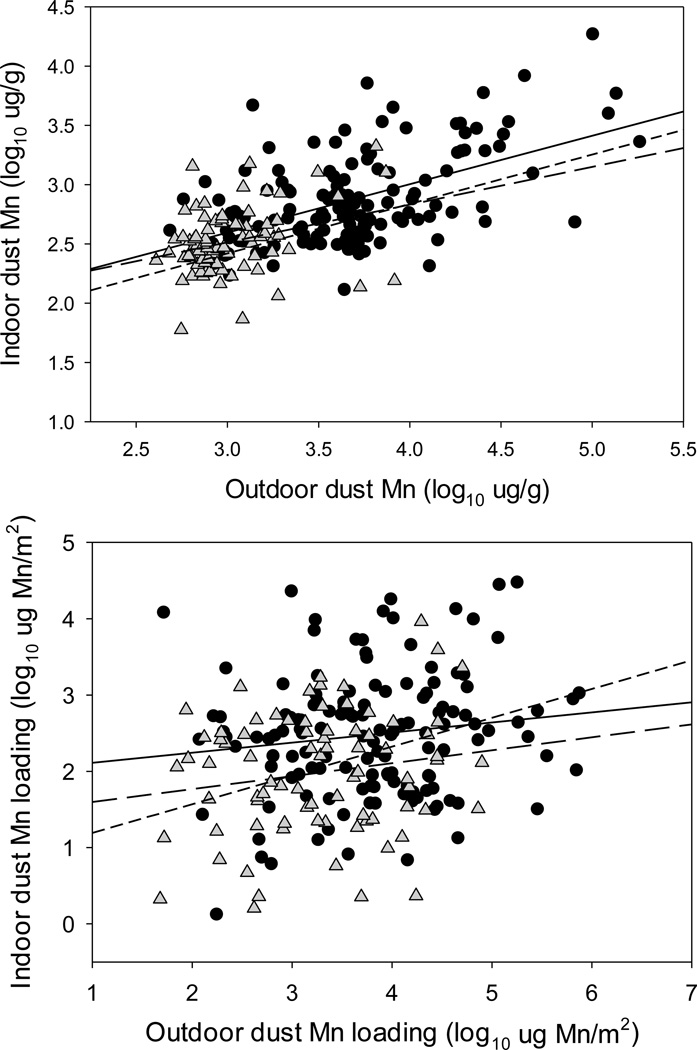
Indoor dust Mn concentrations are associated with but significantly lower than outdoor dust Mn concentrations, with the strength of the linear association being greatest in Bagnolo Mella (R2=0.2848, P<0.0001), and lower in Valcamonica (R2=0.0923, P=0.0065) and Garda Lake (R2=0.0889, P=0.016) (Figure 3). Two-way ANCOVA analyses showed that both outdoor dust Mn concentrations (F(1, 277) = 74.32, P<0.0001) and study site location (F(2, 277) = 4.088, P=0.018) are significant determinants of indoor dust Mn concentrations; the relationship between outdoor and indoor dust Mn concentrations is not significantly different across the three study sites (outdoor dust Mn × site interaction p>0.8) (Figure 3).
Figure 3.
Upper panel: Linear regression of outdoor vs. indoor household dust Mn concentrations across the three study sites: Bagnolo Mella, a community with active ferromanganese plant emissions (filled circles and solid regression line, y = 0.409(x) + 1.371, R2=0.2848, n=137, p<0.0001); Valcamonica, with a past history of past ferromanganese alloy plant activity (grey triangles, long dashed line, y = 0.318(x) + 1.559, R2=0.0923, n=79, P=0.0065), and; Garda Lake, with no history of ferromanganese plant activity (open circles, short dashed line, y = 0.417(x) + 1.170, R2= 0.0889, n=65, P=0.016). Lower panel: Outdoor vs. indoor household dust Mn loadings (log10 µg Mn/m2) across the three study sites: Bagnolo Mella (filled circles and solid regression line, y = 0.132(x) + 1.981, R2=0.0186, n=136, P=0.113); Valcamonica (grey triangles, long dashed line, y = 0.170(x) + 1.429, R2=0.0276, n=79, P=0.143), and Garda Lake (open circles, short dashed line, y = 0.378(x) + 0.813, R2= 0.0992, n=60, P=0.014). Regression lines extended to axis for clarity.
Similarly, indoor dust Mn loading (µg Mn/m2) across all sites is significantly associated with outdoor dust Mn loading levels (R2=0.0872, P<0.0001), though the strength of the relationship varies across site (Bagnolo Mella R2=0.0186, P=0.11; Valcamonica R2=0.0276, P=0.14; Garda Lake R2=0.0992, P=0.014) (Figure 3). Moreover, two-way ANCOVA analyses show that both outdoor dust Mn loading (F(1, 271) = 8.320, P=0.0042) and study site location (F(2, 271) = 7.995, P=0.0004) are significant determinants of indoor dust Mn loading levels; the relationship between outdoor and indoor dust Mn loading is not significantly different across the three study sites (outdoor dust Mn × site interaction p>0.4) (Figure 3).
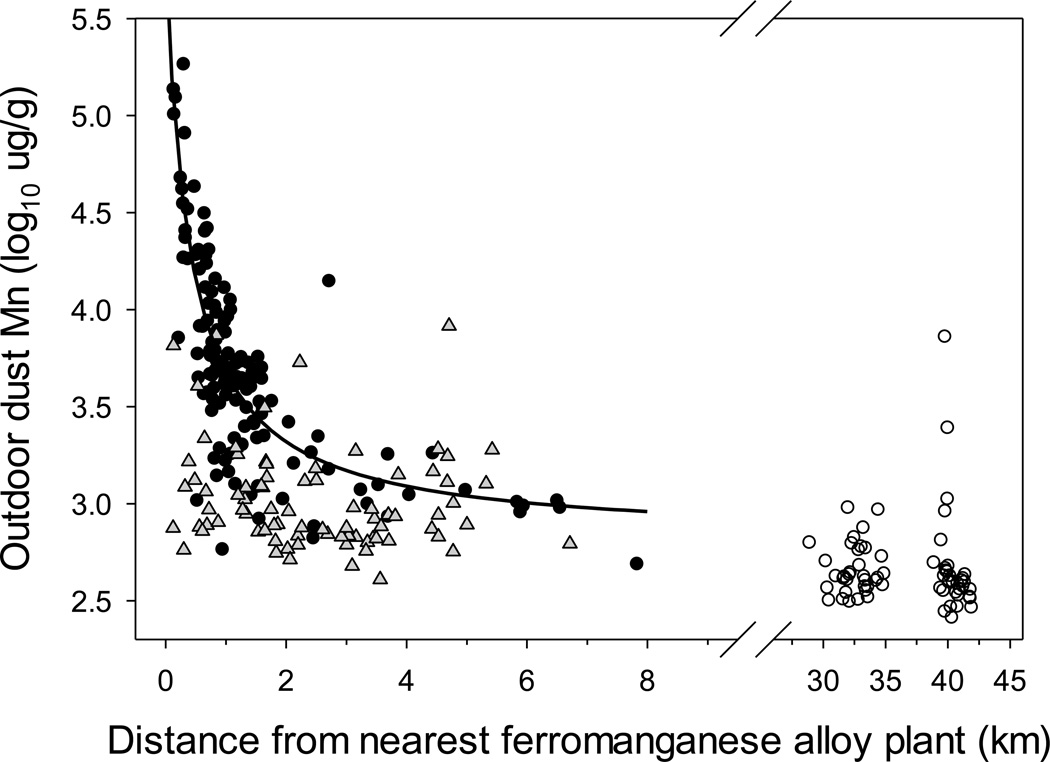
Dust Mn concentrations are inversely related with distance from the active ferromanganese alloy plant in Bagnolo Mella, but not from the historically active plants in Valcamonica
In Bagnolo Mella, site of the active ferromanganese alloy plant, outdoor dust Mn concentrations decrease exponentially with increasing distance from the plant. This relationship is best-fit with modified single exponent three parameter function of the form: Outdoor dust (µg Mn/g) = 2.817 * e(0.4254/(D + 0.5947)); where D=distance in km (R2=0.6630, n=142, p<0.0001) (Figure 4). In contrast, there is no relationship between outdoor dust Mn concentrations and distance from the closest ferromanganese alloy plant in the Valcamonica and Garda Lake sites (Figure 4).
Figure 4.
Outdoor dust Mn concentrations vs. distance (km) from the nearest ferromanganese alloy plant for household dust samples from the three study sites: Bagnolo Mella, a community with active ferromanganese plant emissions (filled circles and solid line best fit regression, [Outdoor dust (µg Mn/g) = 2.817 * e(0.4254/(D + 0.5947)); where D=distance in km; R2=0.6630, n=142, p<0.0001]; Valcamonica, with a past history of past ferromanganese alloy plant activity (grey triangles, n=81), and; Garda Lake, with no history of ferromanganese plant activity (open circles, n=68). Regressions for Valcamonica and Garda Lake (not shown) were non-significant.
Concentrations of Pb, Fe and the other metals in dust across sites follow a different pattern than Mn
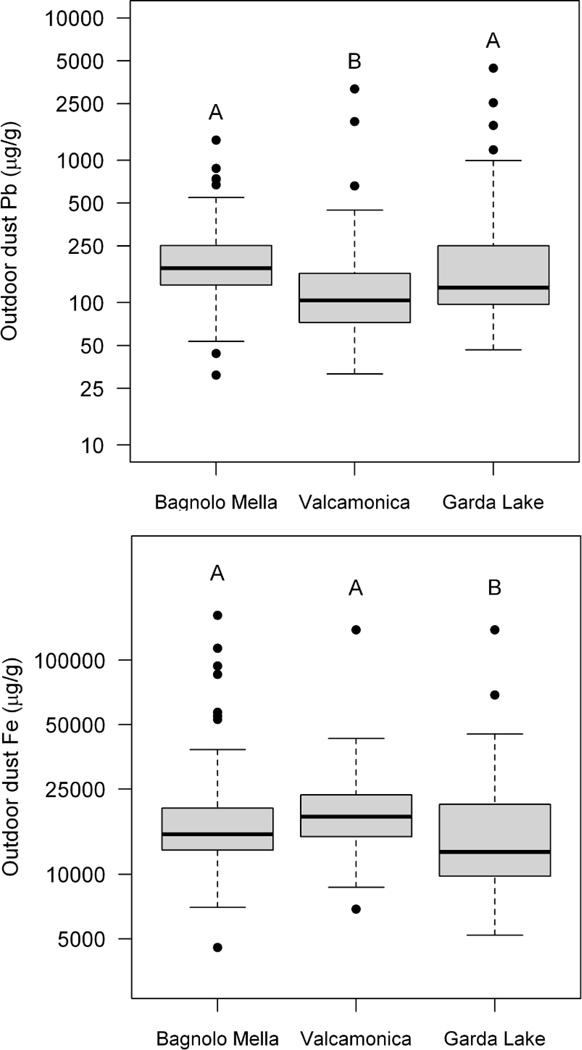
Notably, Mn is the only metal analyzed in dust that shows a site-specific concentration pattern with Bagnolo Mella > Valcamonica > Garda Lake, consistent with the active and historic ferromanganese alloy plant emissions being significant sources of Mn, but not other metals, in settled household dust. This is illustrated with the pattern for dust Pb and iron (Fe) concentrations across study sites. Ferromanganese alloy plants may be a source of Fe emissions, but the extent that emissions contribute to background dust Fe levels may be less than Mn because Fe is typically ~10 – 100-times more abundant in soil/dust than Mn. In contrast, ferromanganese alloy plants are not a significant source of Pb emissions compared to other environmental sources, e.g., historic emissions and resuspension of Pb from leaded gasoline. While there is a significant effect of study site on outdoor dust Pb concentrations (ANOVA F(2, 280) = 9.843, P<0.0001), the pattern across sites is different from Mn, with Garda Lake = Bagnolo Mella > Valcamonica, based on Tukey HSD posthoc analyses (Figure 5). Likewise, there is a significant effect of study site on outdoor dust Fe concentrations (ANOVA F(2, 287) = 6.894, P=0.0012), with the site pattern Valcamonica = Bagnolo Mella > Garda Lake, though differences in median outdoor dust Fe concentrations between study sites are much smaller than with Mn (Figure 5).
Figure 5.
Outdoor household dust Pb (upper panel) and Fe (lower panel) concentrations (µg/g on log10 scale) in the communities with active (Bagnolo Mella, n=142), historic (Valcamonica, n=81) and no history (Garda Lake, n=68) of ferromanganese alloy plant activity. The middle line within each box is the median, the lower and upper margins of the box represent the 25th and 75th percentiles, while the lower and upper whiskers are drawn to the furthest data point within 1.5-times the interquartile range. Boxes with different superscript letters are significantly different from one another, based on Tukey’s post hoc test (p<0.05).
As with Mn, indoor dust Pb concentrations are also associated with but significantly lower than Pb levels in outdoor dust, though the associations were in general statistically weak. For example, linear regression analyses of outdoor vs. indoor household dust Pb concentrations in Bagnolo Mella yielded a best-fit equation of y = 0.183(x) + 1.547, R2=0.0295, n=133, P=0.048. For Valcamonica the best fit equation was y = 0.156(x) + 1.518, R2=0.0241, n=77, P=0.18; and in Garda Lake, it was y = 0.238(x) + 1.325, R2= 0.0974, n=63, P=0.013. ANCOVA analyses with the main factors of ‘study site’ and ‘outdoor dust Pb concentration’ show that both outdoor dust Pb levels (F(1, 269) = 12.34, P=0.0005) and study site location (F(2, 269) = 3.033, P=0.049) are significant determinants of indoor dust Pb levels. The outdoor dust Pb × site interaction is not significant (P>0.8), indicating that the relationship between outdoor and indoor dust Pb concentrations is not significantly different across the three study sites.
The concentrations of aluminum (Al), cadmium (Cd), chromium (Cr), copper (Cu), and zinc (Zn) in outdoor dust were also examined to determine if they exhibited patterns across sites similar to Mn, using one way ANOVA of outdoor dust metal levels by site. There is a significant effect of site for all of these metals (Al F(2, 287) = 46.48, p<0.0001; Cd F(2, 284) =10.83, p<0.0001; Cr F(2, 287) = 12.78, P<0.0001; Cu F(2, 287) = 4.44, P=0.013; Zn F(2, 287) = 5.986, P=0.0028), though in no case does the between-site pattern in outdoor dust metals concentrations match the pattern for Mn (Table 1, Supplementary Figure 1).
Household dust Mn concentrations and loading values are significantly correlated with soil and air Mn levels, as well as biomarkers of environmental Mn exposure in pre-adolescents
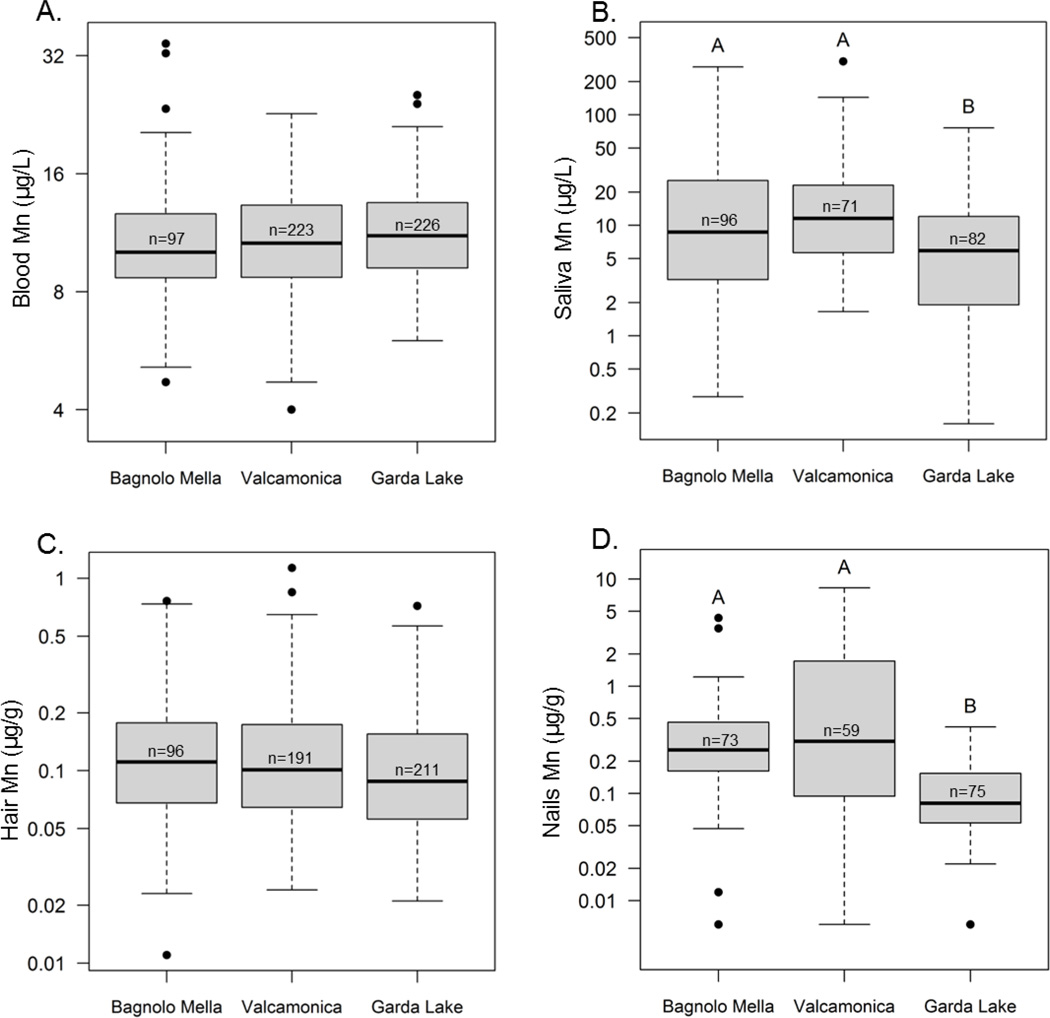
This study is part of a larger ongoing study investigating the impact of ferromanganese plant emissions on neurobehavioral health outcomes in resident pre-adolescents age 11 – 14 yrs. (Lucchini et al., 2012) We assessed Mn levels in whole blood, saliva, hair, and fingernails as candidate biomarkers of environmental Mn exposure. Across all subjects, mean blood Mn levels were 11.3 ng/mL (median 10.8, geometric mean 10.8, SD 3.55, range 4.00 – 34.33, n=546); mean saliva Mn levels were 21.0 ng/mL (median 8.52, geometric mean 8.32, SD 38.6, range 0.160 – 305, n=249); mean hair Mn levels were 0.143 µg/g (median 0.098, geometric mean 0.104, SD 0.139, range 0.011– 1.13, n=501); and mean nail Mn levels were 0.525 µg/g (median 0.181, GM 0.197, SD 1.01, range 0.006 – 8.28, n=207). Both saliva and nail Mn concentrations differed significantly across study site (saliva ANOVA F(2, 246) = 8.36, P=0.0003; nails F(2, 204)=26.8, P<0.0001), with Bagnolo Mella = Valcamonica > Garda Lake (Figure 6). For blood and hair Mn levels, there were no differences across study site (blood ANOVA F(2, 543) = 2.221, P=0.110; hair F(2, 498)=1.89, P=0.152) (Figure 6).
Figure 6.
Manganese concentrations in blood (A), saliva (B), hair (C), and fingernails (D) from pre-adolescent subjects age 11 – 14 years (n = number of subjects per biomarker and study site). The middle line within each box is the median, the lower and upper margins of the box represent the 25th and 75th percentiles, while the lower and upper whiskers are drawn to the furthest data point within 1.5-times the interquartile range. Boxes with different superscript letters are significantly different from one another, based on Tukey’s post hoc test (p<0.05).
To assess whether household dust Mn levels are associated with levels of Mn in the other environmental measures (i.e., surface soil, personal 24 hr airborne PM10 particulates) or candidate biomarkers of environmental Mn exposure (blood, hair, saliva, fingernails), we performed Spearman’s correlations among these various measures. The results show that both indoor and outdoor dust Mn concentrations and outdoor dust Mn loading values were highly significantly correlated with both soil and air Mn concentrations (ρ’s 0.154 – 0.425, all P’s <0.01), while there is no relationship between indoor dust Mn loading values and soil and airborne Mn concentrations (Table 2).
Table 2.
Spearman’s correlation (‘N’) for dust Mn levels vs. environmental and subject exposure biomarker data across all study sites#.
| Indoor Dust [Mn] |
|||||||||
| Outdoor Dust [Mn] |
0.675*** (291) |
Outdoor Dust [Mn] |
|||||||
| Indoor Dust Mn Load |
0.601*** (310) |
0.326*** (289) |
Indoor Dust Mn Load |
||||||
| Outdoor Dust Mn Load |
0.426*** (286) |
0.681*** (296) |
0.287*** (285) |
Outdoor Dust Mn Load |
|||||
| Soil [Mn] | 0.154** (312) |
0.175** (301) |
0.080 (310) |
0.224** (296) |
Soil [Mn] | ||||
| Air [Mn] | 0.291*** (214) |
0.425*** (204) |
0.088 (212) |
0.232** (199) |
0.100* (444) |
Air [Mn] | |||
| Hair [Mn] | 0.280*** (199) |
0.261*** (197) |
0.100 (197) |
0.154* (193) |
0.044 (500) |
0.126* (349) |
Hair [Mn] | ||
| Blood [Mn] |
−0.077 (200) |
−0.160* (197) |
0.002 (198) |
−0.185* (193) |
−0.015 (546) |
−0.155** (376) |
−0.0162 (491) |
Blood [Mn] |
|
| Saliva [Mn] |
0.076 (201) |
0.147* (199) |
−0.095 (199) |
0.083 (195) |
0.135* (249) |
0.204** (202) |
0.013 (239) |
−0.173** (241) |
Saliva [Mn] |
| Nail [Mn] | 0.235** (164) |
0.363*** (161) |
0.145 (162) |
0.284*** (157) |
0.204** (207) |
0.138 (166) |
0.247*** (199) |
−0.154* (201) |
−0.064 (201) |
Dust, soil, and hair Mn concentrations in µg/g, dust Mn loading values in µg/m3 , air Mn concentrations in µg/m3 , blood and saliva Mn concentrations in ng/mL.
Statistical significance of Spearman’s correlations indicated by *0.01 ≤ p ≤ 0.05, **0.01 < p ≤ 0.001, or ***p < 0.001.
Moreover, both indoor and outdoor dust Mn concentrations and outdoor dust Mn loading levels are significantly associated with resident children’s hair and fingernail Mn concentrations, but weakly or not associated with saliva or blood Mn levels (Table 2). Further, these candidate Mn exposure biomarkers show some associations with soil Mn (saliva and fingernail Mn) and airborne Mn (hair, saliva). Notably, blood Mn levels are weakly inversely associated with outdoor dust Mn concentrations, outdoor dust Mn loadings, and 24 hr airborne Mn levels (Table 2).
Discussion
The Province of Brescia, Italy has a prolonged history of ferromanganese alloy plant activity, and of particular importance for environmental health, the ferromanganese alloy plants are located within the confines of small communities where they have produced emissions of Mn-enriched particulates for decades or longer. (Lucchini et al., 2007; Lucchini et al., 2012; Borgese et al., 2013) Here we found that Bagnolo Mella, the community with an active ferroalloy plant since 1973, and Valcamonica, a valley of several communities that each contain historically active ferromanganese alloy plants that ceased a decade or more ago, have significantly higher dust Mn concentrations than the Garda Lake reference community, with Bagnolo Mella containing considerably (~11-fold on average) higher levels (Table 1, Figure 2). Outdoor dust Mn concentrations in Bagnolo Mella are inversely correlated with distance from the ferroalloy plant (Figure 4), and only dust Mn, and not the other metals measured, shows a significant site-based pattern of Bagnolo Mella > Valcamonica > Garda Lake, reflecting the presence of active or historic ferromanganese plant emissions in Bagnolo Mella and Valcamonica. We also found that Mn levels in several candidate exposure biomarkers (hair, fingernails) were significantly associated with outdoor and indoor dust Mn levels. These data evidence a significant impact of ferromanganese alloy plant activity on outdoor and indoor dust Mn levels, and exposure of resident children in these communities.
The background levels of Mn in outdoor dust from the Province of Brescia are conservatively estimated to be ~400–600 µg/g, based on levels from the Garda Lake reference area (median ~400, 75th percentile ~500 µg/g) and the seemingly unimpacted households of Valcamonica (2.5th and 10th percentiles 480 and 590 µg/g, respectively). Only two households from Bagnolo Mella (out of 142) had outdoor dust Mn levels within this background range, with the lowest level (~500 µg/g) from a household furthest (~8 km) from the active ferroalloy plant. In contrast, median outdoor dust Mn levels in Valcamonica and Bagnolo Mella exceeded background levels by ~2 and 10-fold, with levels in the most impacted households in Bagnolo Mella (e.g., 90th percentile, 25,400 µg/g) exceeding background levels by more than 40-fold. The five households in Bagnolo Mella with the highest outdoor dust Mn levels, ranging from 8 – 18% Mn by weight, were all located within several hundred meters of the active plant (Figure 4). This indicates that outdoor household dust from Bagnolo Mella is significantly impacted by the ferromanganese alloy plant, while dust in Valcamonica, where ferromanganese alloy plant operations ceased more than a decade ago, remain significantly impacted, albeit to a lesser extent than Bagnolo Mella.
While outdoor Mn dust concentrations in Bagnolo Mella decrease exponentially with increasing distance from the active ferromanganese alloy plant, even households more than 5 km away have elevated Mn levels in outdoor dust (Figure 4). In contrast, in Valcamonica there is no relationship between outdoor dust Mn levels and distance from the nearest historically active ferromanganese alloy plant (Figure 4). Though the plants in Valcamonica have a longer operational history (i.e., up to ~100 yrs for the Valcamonica plants vs. ~40 yrs for the plant in Bagnolo Mella), the relative impact of current versus historic ferromanganese alloy plant operations is difficult to determine, since the plants are owned by different holding companies and may well have produced ferromanganese alloys with different Mn content. There are also notable differences in the regional landscapes of the Valcamonica (a valley of the pre-Alps with an average width of approximately 3 km and mountains of about 3000 m on either side) and Bagnolo Mella (situated in a flat plains region). These landscape differences are likely important factors influencing the transport and distribution of Mn-enriched particles away from the ferromanganese alloy plants, with widerspread distribution in Bagnolo Mella influenced largely by prevailing winds and other climate conditions, while in Valcamonica the additional factor of a physically constraining valley landscape is likely important.
While background outdoor dust Mn levels in the Province of Brescia, Italy (400 – 600 µg/g) are comparable to or slightly higher than background levels reported in studies from other countries (e.g., Australia, Gunawardana et al., 2012; China, Tong et al., 1998, Zhang and Wang, 2009; Kuwait, Rauret et al., 2000), the dust Mn levels from households impacted by ferromanganese plant activity are substantially higher than dust Mn levels reported elsewhere (Table 3). For example, the outdoor mean (11700 µg/g) and median (4620 µg/g) dust Mn concentrations from Bagnolo Mella are several-fold higher than mean (2260 µg/g) and median (1440 µg/g) street dust Mn concentrations from Witbank, South Africa, (Zibret et al., 2013) site of an active ferromanganese steel plant (Tables 1 and 3), while the highest outdoor dust Mn levels measured in Bagnolo Mella (~80,000 – 180,000 µg/g) were several fold higher than the highest level reported from Witibank (20,000 µg/g). Notably, that study also reported a significant inverse relationship between dust Mn levels and distance from the steel plant, as we observed in Bagnolo Mella. (Zibret et al., 2013) Separately, a study by Zibret et al., (Zibret et al., 2012) reported outdoor dust Mn levels of 2970 µg/g in the vicinity of a ferroalloy plant in Celje, Slovenia, where levels declined by half to 1310 µg/g following installation of emission control filters on the plant. These data show that, in general, active ferromanganese alloy plant operations significantly impact the local dust Mn levels.
Table 3.
Comparison of household and street dust Mn concentrations (µg/g) reported by other studies.
| Location* | Dust sample |
Mean | Median | Std Dev |
Range (min, max) |
N | Notes |
|---|---|---|---|---|---|---|---|
| Salinas, USA1 | House, indoor |
171 | 68 | 2, 414 | 371 2 | Farmworker homes |
|
| Celje, Slovenia2 | House, indoor |
na | na | na | 636, 1330 | 2 | Ferroalloy plant vicinity; post, pre-emission filter installation |
| Street, outdoor |
na | na | na | 1310 , 2970 | 2 | ||
| Bučim, Macedonia3 |
Attic, indoor |
211 | 195 | 70.4 | 113, 454 | 64 | |
| Cape Town, South Africa4 |
School, indoor |
72.5 | 77.5 | 22.6 | 38, 99 | 10 | |
| Johannesburg, South Africa4 |
School, indoor |
404 | 314 | 342 | 17, 959 | 9 | |
| Hong Kong, China5 |
School, indoor |
224 | na | 133 | na | 53 | 53 residential schools |
| School, Outdoor |
532 | na | 291 | na | 53 | ||
| Queensland, Australia6 |
Street, outdoor |
200# | na | 100 | na | 4 | Residential |
| Street, Outdoor |
150# | na | 200 | na | 4 | Industrial, commercial, residential |
|
| Street, outdoor |
90# | na | 10 | na | 4 | Industrial | |
| Street, outdoor |
60# | na | 0 | na | 4 | Commercial | |
| Aqaba, Jordan7 | Street, outdoor |
51 | na | 107 | na | 140 | |
| Hangzhou, China8 |
Street, outdoor |
510 | na | 200 | 179, 935 | 25 | |
| Witbank, South Africa9 |
Street, outdoor |
2260 | 1440 | 2890 | 610, 20000 | 46 | Metal smelter vicinity, traffic |
| Kuwait City, Kuwait10 |
Outdoor | 315 | na | 214 | 36.1, 1280 | 120 | |
Study reference:
Studies have shown that indoor dust may serve as an important reservoir for contaminants originating from industrial emissions (Allott et al., 1994; Lioy et al., 2002; Hwang et al., 1997; Layton and Beamer, 2010). Here, indoor household dust Mn levels also differed significantly across site (Bagnolo Mella > Valcamonica > Garda Lake), with background levels in Garda Lake being comparable to indoor dust levels reported by studies in the Salinas Valley, California, USA (Gunier et al., 2013, 2014), Bučim, Macedonia (Balabanova et al., 2011), and Hong Kong, China (Tong et al., 1998), while levels in Bagnolo Mella were significantly higher than levels reported in those studies (Table 3). Further, indoor household dust Mn concentrations were linearly associated with outdoor dust Mn levels, with a somewhat stronger association in Bagnolo Mella (R2=0.2848, P<0.0001) than Valcamonica or Garda Lake (R2’s ~0.09, P’s = 0.006 and 0.016, respectively) (Figure 3). In all cases, indoor dust Mn levels were significantly lower than outdoor levels, with the median indoor/outdoor dust Mn concentration ratios ranging from 0.15 (Bagnolo Mella) to 0.33 (Valcamonica) to 0.44 (Garda Lake). The differences between indoor and outdoor dust Mn concentrations in Valcamonica and Garda Lake are similar to values of Tong et al. (Tong et al., 1998), who reported mean indoor dust Mn levels (224 µg/g) from schools in Hong Kong that were ~40% of outdoor dust Mn levels (532 µg/g), and Zibret et al. (Zibret et al., 2012) who reported indoor house dust Mn levels that were ~45% of outdoor levels in the vicinity of a ferroalloy plant in Solvenia (Table 3).
The lower Mn concentrations in indoor versus outdoor dust reported here and by others (Table 3) likely reflects that indoor house dust is derived from both outdoor and indoor sources, including particulate matter derived from outdoor air, soil tracked into the house, and organic matter from indoor and outdoor sources (Layton and Beamer, 2010; Thatcher and Layton, 1995; Hunt et al., 2006). Model estimates of the proportion of indoor dust Mn derived from active airborne emissions of Mn versus resuspension and track-in of previously deposited particulate matter from surface soils has not been conducted to our knowledge. However, studies with Pb in house dust have suggested that airborne Pb was likely the dominant source of Pb to indoor dust when leaded gasoline was used (i.e., high airborne emissions), but that as airborne Pb levels declined following the phase out of leaded gasoline, soil resuspension and track-in became the primary sources of Pb in house dust (Layton and Beamer, 2010). Moreover, recent studies of indoor house dust Mn in farmworker communities in Salinas, California, where the Mn-containing fungicides maneb and mancozeb have been used, reported that floor dust Mn concentrations (mean 171 µg/g, range 2 – 414 µg/g) were associated with the presence of a farmworker in the home and the amount of agricultural Mn fungicides applied within 3 km of the residence during the month prior to dust sample collection, presumably reflecting the increased track-in of Mn-contaminated dusts from agricultural fields into the home (Gunier et al., 2014). Notably, indoor floor dust Mn levels in that study were considerably lower than the indoor dust Mn concentrations from Bagnolo Mella, Valcamonica, and even the Garda Lake reference area in the present study (Tables 1 and 3). Given this, it is likely that in Bagnolo Mella both active airborne emissions and surface soil resuspension and track-in of dust are the significant contributors to indoor dust Mn, whereas in Valcamonica and Garda Lake indoor dust Mn is likely derived predominantly from surface soil resuspension and track-in.
Surface dust contaminant loading has been shown to be a significant predictor of exposure risk to household residents (Guinier et al., 2013; Hwang et al., 1997; Wilson et al., 2006; Lanphear et al., 1998; 2006). Here, indoor and outdoor dust Mn loadings (i.e., µg Mn/m2) also differed significantly across site (Figure 2, Supplementary Table 1). The differences in dust Mn loadings across sites can largely be accounted for by differences in dust Mn concentrations (vs. dust mass loadings), since indoor or outdoor surface dust loadings were not different across site (see Results section, Supplementary Table 3); indoor dust mass loadings in the present study (median 0.339, mean 1.15 g/m2) are comparable to or lower than levels reported elsewhere (e.g., ~0.28 g/m2 reported in Layton and Beamer, 2009, for households in six Midwestern states in the USA; median 4 g/m2 floor area reported for households in the agricultural Salinas Valley, California, USA, Gunier et al., 2014). Finally, unlike dust Mn concentrations indoor dust Mn loading was not associated with outdoor dust Mn loading in Bagnolo Mella or Valcamonica (Figure 4), likely reflecting differences across residences in the extent of outdoor dust infiltration into the homes, as well as differences in indoor cleaning practices. (Layton and Beamer, 2009)
This study is part of a larger ongoing investigation of the impacts of ferromanganese plant operations on Mn exposure and neurobehavioral health outcomes in resident pre-adolescent children (Lucchini et al., 2012) and elderly. (Lucchini et al., 2014) While prior studies have shown that Valcamonica possesses elevated outdoor settled dust and surface soil Mn levels associated with the historically active ferromanganese alloy plants (Zacco et al., 2009; Borgese et al., 2011; Lucchini et al., 2012), the associations between environmental measures of Mn contamination (e.g., soil, airborne particles, and surface dust) with biomarker measures of Mn exposure in children from communities with current versus historic ferromanganese plant operations was not known. Here, we found that across sites both indoor and outdoor dust Mn concentrations and outdoor (but not indoor) dust Mn loading values were highly significantly correlated with both soil and airborne Mn concentrations (Spearman’s ρ’s range from 0.154 – 0.425, Table 2), consistent with studies showing that surface soil and airborne particulate matter are important contributors to household dust. (Layton and Beamer, 2009; Al-Awadhi and AlShuabi, 2013) As noted above, contaminants deposited onto surficial soils may be redistributed via aeolian resuspension and serve as a major source to household dust via airborne infiltration. (Nicholson, 1988) Direct track-in of soil contaminants on footwear is also important, depending upon a number of factors such as foot traffic and particle adherence to and deposition from footwear, and the chemical content of the particles, the latter of which is controlled in part by the source(s) of soil contamination. (Hunt et al., 2006; Layton and beamer, 2009)
In general, human particulate exposure risk is influenced by a number of biotic and abiotic factors, and for many inorganic contaminants indirect ingestion often serves as a principle childhood exposure route. (Lanphear et al., 1998; 2002; Hwang et al., 1997; Wilson et al., 2006; Lioy et al., 2002) While there remains uncertainty over the extent that biomarker tissues reflect environmental Mn exposures (Smith et al., 2007), emerging evidence suggests that Mn levels in hair, nails, and shed deciduous teeth are associated both with exposure level and health deficits in children and adults. (Bouchard et al., 2007; Menezes-Filho et al., 2011; Guinier et al., 2013; Eastman et al., 2013; Laohaudomchok et al., 2011; Lucchini et al., 2012)
Here, we found that saliva and fingernail Mn concentrations differ significantly across study site, with higher levels in children from communities with current (Bagnolo Mella) and historically active (Valcamonica) ferromanganese alloy plants, compared to children from the Garda Lake reference area (Figure 6). Hair Mn levels follow a similar but non-significant trend across study site (P=0.15), while blood Mn levels show an opposite but non-significant trend (P=0.11) (Figure 6). Notably, both indoor and outdoor dust Mn concentrations and outdoor dust Mn loading levels are significantly associated with resident children’s hair and fingernail Mn concentrations, but in general weakly or not associated with saliva or blood Mn levels (Table 2). Consistent with this, several of these candidate Mn exposure biomarkers show some associations with soil Mn (saliva and fingernail Mn) and airborne Mn (hair, saliva). Somewhat counterintuitively, however, blood Mn levels are weakly inversely associated with outdoor dust Mn concentrations, outdoor dust Mn loadings, and 24 hr airborne Mn levels (Table 2). The basis for this inverse association is not clear, though in general blood Mn has been shown to be a poor exposure biomarker in environmental settings, or in some occupational studies to be positively associated with exposure (Smith et al., 2007; Lucchini et al., 2012; Mora et al., 2015; Gunier et al., 2015).
It is also noteworthy that hair and nail Mn levels were significantly positively associated with each other, while blood Mn was negatively associated with both saliva and nail Mn levels (Table 2). The bio/toxicokinetics of Mn in these biomarker tissues are different, with blood and saliva reflecting exposure durations of days to a week or so, whereas hair and fingernails are presumed to reflect exposures and circulating Mn levels integrated over the 2 – 4 month period of hair/nail growth (Smith et al., 2007; Eastman et al., 2012). Also, the hair Mn levels reported here are in general lower than levels reported in other studies (Eastman et al., 2012), possibly because the methods used here to clean the hair and fingernails and reduce exogenous metal contamination have been shown to be more effective than cleaning methods typically used in other studies (see Eastman et al., 2012 for comparison and thorough discussion of this point). Finally, given that blood Mn is homeostatically regulated in young adults/adults, and the potential interactions between Mn regulation and age and physiological iron status, further study is needed to better understand the inverse associations between blood Mn and saliva/nails and household dust.
In conclusion, the results here show that historic and active ferromanganese plant operations significantly impact both outdoor and indoor household dust Mn levels. The significant correlations between hair/fingernail and dust Mn concentrations supports the conclusion that resuspension of dust contaminated with Mn from ferromanganese alloy plant emissions is an important Mn exposure pathway to local residents, particularly children and the elderly (Menezes-Filho et al., 2011; Lucchini et al., 2012; Lucchini et al., 2014). While the health impacts of these associations requires further analyses to elucidate, studies have shown associations with elevated environmental Mn (e.g., soil, water) and/or Mn levels in a candidate biomarker tissue and poorer motor, behavioral, and cognitive performance in children and elderly (Claus Henn et al., 2010; Menezes-Filho et al., 2011; Bouchard et al., 2011; Lucchini et al., 2012; Lucchini et al., 2014). These findings are consistent with the well-known importance of dust Pb as both a reservoir of environmental Pb contamination and a source of Pb exposure to children. (Lanphear et al., 1998, 2002; Wilson et al., 2006) Given the emerging evidence associating elevated Mn exposure with growth and neurological impairments in children (Zota et al, 2009, Claus-Henn et al., 2010, 2012; Wasserman et al., 2006; Lucchini et al., 2012, Bouchard et al., 2007; Menezes-Filho et al., 2011), these data support that dust Mn levels should be reduced in contaminated environments to protect the health of resident children, akin to recommendations in place for abating and reducing household dust Pb levels.
Supplementary Material
Highlights.
Dust Mn was significantly elevated in communities with a ferromanganese plant.
Indoor dust Mn was significantly associated with Mn in outdoor dust.
Dust Mn was correlated with soil and air Mn, and children’s hair and fingernail Mn.
Dust Mn from ferromanganese plants poses an important exposure pathway for children
Acknowledgements
We thank Rob Franks for analytical assistance, and Neil Zimmermann, Elza Bontempi, and Laura Borgese for their valuable contributions to broader aspects of the study. This research was supported by the National Institute of Environmental Health Sciences (NIEHS) grant number RO1ES019222, and by a gift from the Centro Servizi Multisettoriale Tecnologico, Bescia, Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Manganese. Atlanta: U.S. Department of Health and Human Services; 2012. [PubMed] [Google Scholar]
- Al-Awadhi JM, AlShuabi AA. Dust fallout in Kuwait city: Deposition and characterization. Sci. Total. Environ. 2013;461–462:139–148. doi: 10.1016/j.scitotenv.2013.03.052. [DOI] [PubMed] [Google Scholar]
- Al-Khashman OA. The Investigation of metal concentrations in street dust samples in Aqaba city, Jordan. Environ. Geochem. Hlth. 2007;29:197–207. doi: 10.1007/s10653-006-9065-x. [DOI] [PubMed] [Google Scholar]
- Allott RW, Kelly M, Hewitt CN. A model of environmental behaviour of contaminated dust and its application to determining dust fluxes and residence times. Atmos. Environ. 1994;28:679–687. [Google Scholar]
- Aschner M, Erikson K, Dorman D. Manganese Dosimetry: Species Differences and Implications for Neurotoxicity. Cr. Rev. Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Balabanova B, Stafilov T, Sajn R, Baceva K. Distribution of chemical elements in attic dust as reflection of their geogenic and anthropogenic sources in the vicinity of the copper mine and flotation plant. Arch. Environ. Con. Tox. 2011;61:173–184. doi: 10.1007/s00244-010-9603-5. [DOI] [PubMed] [Google Scholar]
- Borgese L, Zacco A, Pal S, Bontempi E, Lucchini R, Zimmerman N, Depero LE. A new non-destructive method for chemical analysis of particulate matter filters: The case of manganese air pollution in Vallecamonica (Italy) Talanta. 2011;84:192–198. doi: 10.1016/j.talanta.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese L, Federici S, Zacco A, Gianoncelli A, Rizzo L, Smith DR, Donna F, Lucchini R, Depero LE, Bontempi E. Metal fractionation in soils and assessment of environmental contamination in Vallecamonica, Italy. Environ. Sci. Pollut. R. 2013;20:5067–5075. doi: 10.1007/s11356-013-1473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz O, Allain S, Scott CP, Cugy P, Barbier D. High manganese austenitic twinning induced plasticity steels: A review of the microstructure properties relationships. Curr. Opin. Solid St. M. 2011;15(4):141–168. [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ. Health Persp. 2007;115(1):122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual Impairment in School-Age Children Exposed to Manganese from Drinking Water. Environ. Health Persp. 2011;119(1):138. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21(4):433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnass L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernandez-Afila M, Amarasiriwardena C, Hu H, Bellinger DC, Wright RO, Tellez-Rojo MM. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Persp. 2012;120(1):126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environ. Sci. Technol. 2013;47:1629–1637. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GB, Leonard A, Hantson P. Carcinogenicity, mutagenicity and tetratogenicity of manganese compounds. Cr. Rev. Oncol-Hem. 2002;42(1):25–34. doi: 10.1016/s1040-8428(01)00178-0. [DOI] [PubMed] [Google Scholar]
- Gunawardana C, Goonetilleke A, Egodawatta P, Dawes L, Kokot S. Source characterisation of road dust based on chemical and mineralogical composition. Chemosphere. 2012;87(2):163–170. doi: 10.1016/j.chemosphere.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, Vedar M, Arora M, Eskenazi B. Determinants of Manganese in Prenatal Dentin of Shed Teeth from CHAMACOS Children Living in an Agricultural Community. Environ. Sci. Tech. 2013;47:11249–11257. doi: 10.1021/es4018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Jerrett M, Smith DR, Jursa T, Yousefi P, Camacho J, Hubbard A, Eskenazi B, Bradman A. Determinants of manganese levels in house dust samples from the CHAMACOS cohort. Sci. Total Environ. 2014 doi: 10.1016/j.scitotenv.2014.08.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Mora AM, Smith DR, Arora M, Austin C, Eskenazi B, Bradman A. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Env. Sci. Technol. 2015 doi: 10.1021/es503866a. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health effects support document for manganese. 2008 Available from: http://www.epa.gov/safewater/ccl/pdfs/reg_determine1/support_cc1_magnese_healtheffects.pdf.
- Hunt A, Johnson DL, Griffith DA. Mass transfer of soil indoors by track-in on footwear. Sci. Total. Environ. 2006;370:360–371. doi: 10.1016/j.scitotenv.2006.07.013. [PubMed: 16962161] [DOI] [PubMed] [Google Scholar]
- Hwang YH, Bornschein RL, Grote J, Menrath W, Roda S. Environmental arsenic exposure of children around a former copper smelter site. Environ. Res. 1997;72:72–81. doi: 10.1006/enrs.1996.3691. [PubMed: 9012374] [DOI] [PubMed] [Google Scholar]
- Kubova J, Stresko V, Bujdos M, Matus P. Fractionation of various elements in CRMs and in polluted soils. Anal. Bioanal. Chem. 2004;379(1):108–114. doi: 10.1007/s00216-004-2505-5. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Matte TD, Rogers J, Clickner RP, Dietz B, Bornschein RL, Succop P, Mahaffey KR, Dixon S, Galke W, Rabinowitz M, Farfel M, Rohde C, Schwartz J, Ashley P, Jacobs DE. The contribution of lead-contaminated house dust and residential soil to children's blood-lead levels. A pooled analysis of 12 epidemiologic studies. Environ. Res. 1998;79(1):51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Ho M, Howard CR, Eberly S, Knauf K. Environmental lead exposure during early childhood. J. Pediatr. 2002;140(1):40–47. doi: 10.1067/mpd.2002.120513. [DOI] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, Weisskopf MG. Toenail, blood, and urine as biomarkers of manganese exposure. J. Occup. Environ. Med. 2011;53(5):506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton D, Beamer PI. [2009 November 1];Migration of Contaminated Soil and Airborne Particulates to Indoor Dust Environ Sci Technol. 2009 43(21):8199–8205. doi: 10.1021/es9003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ, Freeman NCG, Millette JR. Dust: a metric for use in residential and building exposure assessment and source characterization. Environ. Health Persp. 2002;110:969–983. doi: 10.1289/ehp.02110969. [PubMed: 12361921] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am. J. Ind. Med. 2007;50(11):788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, Salmistraro M, Bontempi E, Zimmerman NJ, Smith DR. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. NeuroToxicology. 2012;33(4):687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Benedetti C, Fedrighi C, Peli M, Donna F, Bontempi E, Borgese L, Micheletti S, Ferri R, Marchetti S, Smith DR. Neurofunctional dopaminergic impairment in elderly after lifetime exposure to manganese. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.05.006. PubMed PMID: 24881811. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Paes CR, Pontes AM, Moreira JC, Sarcinelli PN, Mergler D. High levels of hair manganese in children living in the vicinity of a ferro-manganese alloy production plant. NeuroToxicology. 2009;30:1207–1213. doi: 10.1016/j.neuro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, et al. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res. 2011;111(1):156–63. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, van Wendel de Joode B, Mergler D, Córdoba L, Cano C, Quesada R, Smith DR, Menezes-Filho JA, Eskenazi B. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Env. Res. 2015;136:47–56. doi: 10.1016/j.envres.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KW. A review of particle resuspension. Atmos. Environ. 1988;22:2639–2651. [Google Scholar]
- Pearson GF, Greenway GM. Recent Developments in Manganese Speciation. Trend. Anal. Chem. 2005;24(9):803–809. [Google Scholar]
- Rauret G, Lopez-Sanchez JF, Sahuquillo A, Barahona E, Lachica M, Ure A, Muntau H, Quevauviller P. Indicative Values for Extractable Contents (mass fractions) of Cd, Cr, Cu, Ni, Pb, and Zn in Sewage Sludge Amended Soil (CRM 483) Following the Modified BCR-sequential Extraction (three-step) procedure. Luxemberg: European Commission; 2000. [Google Scholar]
- Rollin H, Mathee A, Levin J, Theodorou P, Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ. Res. 2005;97:93–99. doi: 10.1016/j.envres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Smith DR, Gwiazda R, Roels H, Park R, Bowler R, Apostoli P, Lucchini R. Biomarkers of Mn exposure in humans. Am. J. Ind. Med. 2007;50(11):801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Sutherland RA, Tack FMG. Determination of Al, Cu, Fe, Mn, Pb and Zn in certified reference materials using the optimized BCR sequential extraction procedure. Anal. Chim. Acta. 2002;454:249–257. [Google Scholar]
- Taylor M, Camenzuli D, Kristensen L, Forbes M, et al. Environmental lead exposure risks associated with children’s outdoor playgrounds. Environ. Pollut. 2013;178:447. doi: 10.1016/j.envpol.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Thatcher TL, Layton DW. Deposition, resuspension, and penetration of particles within a residence. Atmos. Environ. 1995;29:1487–1497. [Google Scholar]
- Tong STY, Lamb KC. Are nursery schools and kindergartens safe for our kids? The Hong Kong study. Sci. Total. Environ. 1998;216:217–225. doi: 10.1016/s0048-9697(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Persp. 2006;114(1):124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The WHO Regional Office for Europe. Air Quality Guidelines for Europe. 2nd ed. Copenhagen: WHO Regional Publications; 2000. [Google Scholar]
- Wilson J, Dixon S, Galke W, McLaine P. An investigation of dust lead sampling locations and children’s blood lead levels. J. Exposure. Sci. Environ. Epidemiol. 2006;17:2–12. doi: 10.1038/sj.jes.7500514. [DOI] [PubMed] [Google Scholar]
- Zacco A, Resola S, Lucchini R, Albini E, Zimmerman N, Guazzetti S, Bontempi E. Analysis of settled dust with X-ray Fluorescence for exposure assessment of metals in the province of Brescia, Italy. J. Environ. Monitor. 2009;11:1579–1585. doi: 10.1039/b906430c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang H. Concentrations and chemical forms of potentially toxic metals in road-deposited sediments from different zones of Hangzhou, China. J. Environ. Sci. 2009;21:625–631. doi: 10.1016/s1001-0742(08)62317-7. [DOI] [PubMed] [Google Scholar]
- Zibret G. Impact of Dust Filter Installation in Ironworks and Construction on Brownfield Area on the Toxic Metal Concentration in Street and House Dust (Celje, Slovenia) Ambio. 2012;41:292–301. doi: 10.1007/s13280-011-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibret G, Tonder D, Zibret L. Metal content in street dust as a reflection of atmospheric dust emissions from coal power plants, metal smelters, and traffic. Environ. Sci. Pollut. R. 2013;20(7):4455–4468. doi: 10.1007/s11356-012-1398-7. [DOI] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20(3):367–373. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Schalder LA, Ettinger AS, Wright RO, Shine JP, Spengler JD. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Env. Epid. 2011;21:495–505. doi: 10.1038/jes.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.