Abstract
Depressed people perform poorly on cognitive tasks, however under certain conditions they show intact cognitive performance with physiological reactivity consistent with needing to recruit additional cognitive control. We hypothesize that this apparent compensation is driven by the presence of affective processes (e.g., state anxiety) which in turn are moderated by the depressed individual’s motivational state. Clarifying these processes may help researchers identify targets for treatment that if addressed may improve depressed patients’ cognitive functioning. To test this hypothesis, 36 participants with unipolar depression and 36 never-depressed controls completed a problem-solving task modified to elicit anxiety. Participants completed measures of motivation, anxiety, sadness, and rumination, while pupillary responses were continuously measured during problem-solving as an index of cognitive control. Anxiety increased throughout the task for all participants, while both sadness and rumination were decreased during the task. In addition, anxiety more strongly affected planning accuracy in depressed participants relative to controls, regardless of participants’ levels of motivation. In contrast, differential effects of anxiety on pupillary responses were observed as a function of depressed participants’ levels of motivation. Consistent with behavioral results, less-motivated and anxious depressed participants demonstrated smaller pupillary responses, whereas more highly-motivated and anxious depressed participants demonstrated larger pupillary responses than controls. Strong effects of sadness and rumination on cognitive control in depression were not observed. Thus, we conclude that anxiety inhibits the recruitment of cognitive control in depression and that a depressed individual’s motivational state determines, in part, whether they are able to compensate by recruiting additional cognitive control.
Keywords: Motivation, anxiety, rumination, cognitive control, problem-solving, depression
Introduction
Major depressive disorder (MDD) is associated with reduced performance at work, with an estimated cost to US businesses of $31 billion a year (Stewart, Ricci, Chee, Hahn, & Morganstein, 2003). Limited evidence suggests that cognitive deficits in depression are responsible for this decrease in work performance (Godard, Grondin, Baruch, & Lafleur, 2011). In contrast, under certain conditions, depressed individuals display intact cognitive performance at the apparent expense of recruiting additional cognitive control (e.g., van Tol et al., 2011; Wagner et al., 2006). In this context, cognitive control refers to the mechanisms of attentional selection, goal maintenance, and inhibition that facilitate adaptive goal-directed behavior (Chiew & Braver, 2013). Currently, we do not have a clear understanding of the processes that hinder or facilitate cognitive performance in depression (Rose, Simonotto, & Ebmeier, 2006; Walter, Wolf, Spitzer, & Vasic, 2007). In particular, previous investigations have not examined the impact of negative emotional states occurring during difficult tasks or the impact of motivation to persist in goal-directed behavior on cognitive control in depression (Berggren & Derakshan, 2013; Jones, Siegle, Muelly, Haggerty, & Ghinassi, 2010). If negative emotional states and motivation are found to be relevant, it may be possible to target these processes with brief interventions to facilitate performance—for example, listening to music to reduce anxiety (Iwanaga, Kobayashi, & Kawasaki, 2005) or setting task goals to increase motivation (Scheurich et al., 2008).
Cognitive control is predominately supported by activation within the prefrontal cortex (Harvey et al., 2005; van Tol, et al., 2011; Wagner, et al., 2006). Relative to healthy controls, depressed individuals demonstrate both hypoactivation (e.g., Elliott, Baker, Rogers, O’Leary, & et al., 1997) and hyperactivation (e.g., Harvey, et al., 2005; van Tol, et al., 2011; Walter, et al., 2007) in the dorsolateral prefrontal cortex (DLPFC) in different experimental contexts. These abnormal patterns of cognitive activity have been hypothesized to stem from the fact that depressed individuals have difficulty inhibiting negative emotional states (Harvey, et al., 2005). Depressed individuals are thought to compensate for their emotional state by engaging greater cognitive control to maintain the same level of behavioral performance as healthy individuals (Harvey, et al., 2005; Rose, et al., 2006; Walter, et al., 2007). Under conditions of resource exhaustion, decreased recruitment of cognitive control and cognitive deficits are then posited to occur (Harvey, et al., 2005).
Confirmatory evidence for this hypothesis comes from studies using cognitive tasks that can generate stress and emotional arousal particularly when the task’s difficulty closely matches the individual’s ability (Gianaros et al., 2008). Specifically, during the Stroop task, depressed individuals show hyperactivity in the rostral anterior cingulate (rACC) combined with hyperactivation in the DLPFC and intact behavioral performance (Wagner, et al., 2006), as well as hypoactivation in the DLPFC and performance deficits (Holmes & Pizzagalli, 2008). In this context, increased rACC activity is inferred to reflect increased affective interference stemming from increased amygdala activity (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Gianaros, et al., 2008) and possibly increased rumination (Yoshimura et al., 2013; Zhu et al., 2012). Here, rumination is defined as the tendency to focus on one’s symptoms of depression and its causes and consequences (Nolen-Hoeksema & Morrow, 1991). However, none of the reviewed studies assessed participants’ ongoing emotional state or their engagement in rumination during the cognitive task. Hence, it is not possible to determine whether the observed rACC activity reflects increased anxiety, sadness, or rumination during a task. Moreover, it remains unclear what processes facilitate the apparent compensatory recruitment of cognitive control.
Induced negative emotional states, in the form of stress and anxiety (Clarke & Johnstone, 2013; Qin, Hermans, van Marle, Luo, & Fernández, 2009), as well as sadness (Deckersbach et al., 2008) occurring during cognitive tasks are associated with hypoactivation and hyperactivation in the DLPFC. In addition, behavioral evidence suggests that induced state rumination is also associated with deficits in cognitive control (Watkins & Brown, 2002; Whitmer & Gotlib, 2012). Thus, some evidence supports the hypothesis that negative emotional states occurring during a cognitive task is associated with abnormal cognitive control and performance deficits in MDD. Furthermore, evidence suggests that taking an individual’s motivational state into consideration may help explain whether or not compensatory recruitment of cognitive control is observed (c.f., Berggren & Derakshan, 2013). In particular, in healthy populations, motivation improves task performance by enhancing recruitment of cognitive control (Chiew & Braver, 2013, 2014; Kouneiher, Charron, & Koechlin, 2009; Pochon et al., 2002) and by suppressing activation in brain regions associated with affective processing (Pochon, et al., 2002). Motivation may also influence the recruitment of cognitive control in depression given evidence indicating that increasing depressed individuals’ motivation to engage in a cognitive task improves behavioral performance (Scheurich, et al., 2008). Thus, compensatory cognitive control and intact performance are likely to be observed when depressed individuals are experiencing negative emotion and are motivated to accomplish a demanding cognitive task.
The present study aimed to clarify this possibility by examining whether motivation moderates the effects of negative emotional states on cognitive control (as indexed by pupillary responses) while unipolar depressed individuals and never-depressed controls engaged in a modified version of the Tower of London (TOL; Shallice, 1982; Unterrainer et al., 2004). The TOL is a visuospatial problem-solving task that requires cognitive control and activates the DLPFC (Unterrainer & Owen, 2006). We modified the TOL to induce predominately anxiety and possibly sadness as well as rumination by (a) notifying participants prior to the task that their performance was a reflection of their intelligence; (b) requiring participants to solve challenging problems with a sense of urgency by including a countdown timer; and (c) requiring that participants hold information in working memory as they plan the entire solution to the problem.
During problem-solving, pupillary responses were continuously recorded as an index of cognitive and emotional load (Granholm & Steinhauer, 2004). Pupillary responses were used because the pupil dilates in response to cognitive load (Beatty, 1982; Granholm & Steinhauer, 2004; Hess & Polt, 1964; Kahneman & Beatty, 1966), remains dilated throughout expenditure of cognitive load (Granholm, Asarnow, Sarkin, & Dykes, 1996), and responds proportionately to conditions that challenge cognitive efficiency, such that increased anxiety yields higher pupillary reactivity on cognitive tasks (Wilson, Smith, Chattington, Ford, & Marple-Horvat, 2006), as does increased rumination (Siegle, Steinhauer, Carter, Ramel, & Thase, 2003). Moreover, pupil dilation is associated with activation in relevant cortical regions supporting cognitive control (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005; Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003) and has been shown to be sensitive to the effects of motivational manipulations on cognitive control (Chiew & Braver, 2013, 2014). In addition to cognitive load, pupil dilation has also been associated with anxiety-related resource consumption (e.g., anticipation; Bitsios, Szabadi, & Bradshaw, 2004; Gray & Williams, 1969; Murray, 1971; Simpson & Molloy, 1971) and arousal (Bakes, Bradshaw, & Szabadi, 1990; Lader, 1983). We also obtained self-reports of motivation, anxiety, sadness, and rumination at different points throughout the task to examine their effects on cognitive control.
We predicted that the differential impact of anxiety, sadness, and rumination, on pupil dilation and task performance in unipolar depressed individuals and never-depressed controls would be moderated by participants’ levels of motivation. Specifically, we hypothesized that motivated depressed individuals also experiencing elevated levels of anxiety, sadness, and/or rumination would demonstrate greater pupil dilation and equivalent task performance relative to never-depressed controls. In contrast, we hypothesized that unmotivated depressed individuals also experiencing elevated levels of anxiety, sadness, and/or rumination would demonstrate decreased pupil dilation and decreased task performance relative to never-depressed controls.
Method
Participants
Thirty six individuals diagnosed as having a current major depressive episode (age range: 18–55 years: mean age: 30.9 yrs., SD=10.5 yrs.; 18 females; 78% Caucasian, 11% African American, 3% Asian, 3% Hispanic/Latino, 5% Caucasian/Native American) via a structured clinical interview (SCID-I; First, Spitzer, Gibbon, & Williams, 1996) were compared with an age-matched group of 36 never-depressed controls with no current or past psychiatric diagnoses based on the SCID-I (First, et al., 1996), and with no known first-degree relatives with psychiatric diagnoses (age range, 18–52 years; Mage=31.1 yrs.; SD=10.6 yrs.; 20 females, 92% Caucasian, 5% African American, 3% Asian). Twelve depressed participants (33%) had a comorbid anxiety diagnosis. The age difference between groups was not significant, t(70)=0.08, p=.938, nor was the distribution of gender χ2(1)=0.22, p=.637 (due to small cell sizes statistical differences in the distribution of ethnicity could not be obtained). All participants reported no significant eye problems, interfering health problems, psychoactive drug or alcohol abuse within the past 6 months, or a history of psychosis or manic episodes. Two depressed patients had started a trial of antidepressant medication (Zoloft, or Lexapro) within the past 12 days; all other participants were unmedicated. All participants were required to pass a cognitive screen (Full Scale Intelligence Quotient Equivalent; FSIQE > 80; Nelson & Willison, 1991). There were no differences between depressed participants (M=108.5, SD=6.95) and controls (M=109.8, SD=7.21) in FSIQE, t(69)=0.76, p=.449.
Apparatus
Forty participants (20 depressed and 20 controls) completed a computer-based version of the TOL, which was presented on a monitor 65 cm from the participant. Pupillary data were collected at 60 Hz (every 16.7 msec) in a moderately lit room (0.56-fc illuminance) using a table-mounted ISCAN, Inc., RK464 video-based infrared pupilometer. Lighting was held constant across participants. Video digitization and pupil diameter calculation were completed by the equipment. The remaining 32 participants (16 depressed and 16 controls) completed the computer-based TOL while undergoing a functional magnetic resonance imaging (fMRI) assessment. These participants were part of a later cohort that was recruited for an fMRI study. As participants lay in the moderately lit 3T Siemens Trio scanner, stimuli were presented on a back-projection screen approximately 127 cm from a mirror that was placed approximately 12 cm above their eye (varied slightly by head size). Pupil dilation was acquired using an ASL Model 504 eye-tracker. This device consisted of a video camera and an infrared light source positioned outside the magnet’s bore. The pupil was automatically tracked through a mirror anchored to the headcoil. Pupil size was recorded at 60 Hz (every 16.7 ms) along with signals marking the beginning of trials, the end of fixation, and stimulus onset time. Because of the camera’s considerable distance from the participant’s eye (over 10 feet), pupil resolution was poorer than for pupil dilation collected using the table mounted pupilometer.
Procedure
Participants were recruited through flyers and electronic postings, as well as from an existing database of previous participants who had agreed to be contacted for future research studies. During an initial assessment, participants were screened using a SCID-I at the Mood Disorders Treatment Research Program (MDTRP) or were screened via telephone to determine if they met preliminary eligibility requirements. Eligible participants were invited to participate in a study of problem-solving in depression. All healthy control subjects received a complete SCID-I interview during preliminary assessment. Depressed participants recruited from the MDTRP completed the depression module of the SCID-I if they had received their initial SCID assessment at the MDTRP more than 2 weeks prior (M=2.8 wks; SD=1.7 wks; range: 1.7 – 5.1). All other depressed participants received a complete SCID-I interview. Participants then completed a cognitive screen and were trained on the TOL task (~ 10 minutes) and completed the task in the lab or fMRI scanner environment. Finally, participants completed several questionnaires via computer. This study was approved by the University of Pittsburgh Institutional Review Board. All participants provided informed consent prior to the SCID-I interview and were compensated for their participation.
Task
Participants completed three blocks of TOL problems (Unterrainer, et al., 2004), whose optimal solution required 4, 5, or 6 moves. Participants were presented with a 1.67s fixation cue, followed by a TOL problem in which they were instructed to “plan the fewest number of moves that will be necessary to make the ‘Current State’ picture match the ‘Goal State’ picture” (Fig. 1) while following three rules: (1) only one disc may be moved at a time; (2) a disc cannot be moved when another disc is lying on top of it; and (3) three discs may be placed on the tallest peg, two discs on the middle peg, and one disc on the shortest peg. Participants had 35.07s to plan their solution and select their answer using the mouse. Next, participants were given 20.04s to show the plan they had developed—even if the plan didn’t solve the problem—using a mouse. Subsequently, participants were given performance feedback for 5.01s, followed by a 11.69s fixation. Planning time (time needed to plan their response) and accuracy (percentage of problems solved correctly) were recorded. Task timing was adapted from a previous study (Unterrainer, et al., 2004) and pilot testing was done to ensure sufficient time for participants to complete planning, show their plan, and read the presented feedback. Duration of the initial fixation cue and the post-feedback fixation were selected to allow the hemodynamic response to fully resolve prior to the onset of the next trial. At the start of the task and after each block, participants were asked to rate their mood, engagement in rumination, and motivation on brief Likert-like scales described below.
Fig. 1.
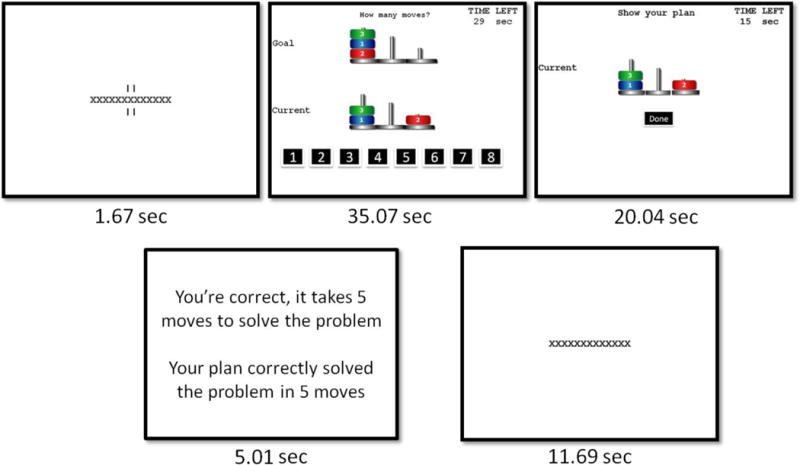
Modified computer version of the Tower of London.
Measures
The North American Adult Reading Test (NAART)
The NAART (Nelson & Willison, 1991) was administered to ensure that participants had the cognitive and linguistic ability to complete the proposed task. The NAART is a quick cognitive screening measure that asks individuals to pronounce a list of 50 words. The test has been shown to have high correlations with WAIS–R verbal intelligence quotient (VIQ) scores (r=0.83) and is appropriate for use with depressed individuals because it is not affected by poor concentration or motivation.
Motivation, emotion, and rumination scales
At baseline, participants rated how motivated they were to do well at the task, how sad they were feeling, and how anxious they were feeling in the current moment. In addition, they rated how focused they were on their current feelings and personal problems; these two items were combined to form a measure of state rumination (Moberly & Watkins, 2008). After each block, they completed analogous ratings in reference to the last block of problems. All questions were rated on a scale from 1 (not at all) to 6 (a great deal). The motivation question was designed to capture participants’ motivation to engage in behavior directed towards task completion. Our composite measure of rumination, although broad and potentially capturing other self-referential processes such as worry, was chosen due to: its established relationship to rumination in daily contexts (Moberly & Watkins, 2008), research demonstrating that ruminative thoughts typically temporal transition from rumination to worry (McLaughlin, Borkovec, & Sibrava, 2007), and its brevity.
Data Selection, Cleaning, and Reduction
Data were cleaned using our laboratory’s standard procedures (Siegle, Ichikawa, & Steinhauer, 2008), including interpolation through blinks, removal of trials with over 50% blinks, and smoothing. Absolute dilation could not be calculated for data collected in the fMRI scanner, therefore an index representing the proportion of maximal dilation was used as a proxy for diameter for all participants. Hence, pupil values were range-corrected to standardize according to the 95% maximally dilated pupil diameter and the 95% maximally constricted pupil diameter [(current pupil diameter – minimum pupil diameter)/(maximum pupil diameter – minimum pupil diameter)] (Siegle, Steinhauer, Stenger, et al., 2003) and baseline corrected by subtracting out pupil dilation in the 1.67 s prior to planning onset. The data was then downsampled to 1-Hz yielding 37 time points during the planning phase of TOL trial. Time was downsampled to reduce the number of parameters (37 vs. 2,220) to facilitate the simultaneous examination of main and interaction effects among continuous covariates, clinical status, and task difficulty on pupillary responses over time.
Analysis Strategy
Motivational, emotional, and rumination data
Behavioral data were analyzed using a marginal mixed effects analysis that modeled the covariance structure for time as autoregressive. We examined the main effects of clinical status (unipolar depressed vs. never-depressed controls) and time (baseline, block 1, block 2, block 3), and clinical status by time interactions on each of the following dependent variables: motivation, anxiety, sadness, and rumination.
Behavioral performance data
We conducted mixed effects analyses to determine whether the differential impact of anxiety, sadness, or rumination on behavioral performance (planning accuracy and planning time) in depressed individuals relative to never-depressed controls was moderated by motivation after controlling for task difficulty. Six mixed effects analyses were conducted. We modeled the covariance structure for task difficulty as autoregressive (AR1). After controlling for the effects of task difficulty (4, 5, and 6 move problems), we evaluated the hypothesized motivation-by-clinical status-by-inhibitory process interaction after including the relevant main effects and two-way interactions. Significant three-way interactions were probed at low levels of motivation (1 SD below the mean=Z-score=−1) and high levels of motivation (1SD above the mean=Z-score=1) using published methods (Aiken & West, 1991). The Johnson-Neyman procedure was used to determine whether the MDD group differed from never-depressed controls at specific points along the simple slopes, for example at low levels of motivation (Z-score=−1) and at high levels of anxiety (Z-score=1). Three multivariate outliers on anxiety were observed in the depressed group and were removed from analyses incorporating this variable.
Pupillary data
Mixed effects analyses were conducted to determine whether the differential impact of anxiety, sadness, and rumination on pupillary responses in depressed individuals relative to never-depressed controls was moderated by motivation. Three mixed effects analyses were conducted. We modeled the covariance structure for task difficulty as unstructured and the covariance structure for time as autoregressive (AR1). After controlling for the effects of task difficulty (4, 5, and 6 move problems), time (0 to 36 sec), and a difficulty-by-time interaction,1 we evaluated the hypothesized motivation-by-clinical status-by-inhibitory process interaction after including the relevant main effects and two-way interactions. Significant three-way interactions were probed using methods described above. Three multivariate outliers on anxiety were observed in the depressed group and were removed from analyses.
Reporting
In all cases, results were considered significant for p<.05, and were reported as non-significant for p>.1. Results in between p<.05 and p<.1 were probed for clinical significance.
Results
Behavioral Data
Motivational, Emotional, and Ruminative States
Motivation
There was a main effect of clinical status, F(1,70)=8.45, p=.005, d=0.69, which indicated that the MDD group was less motivated than the control group to perform well on the task (Fig. 2A). The main effect of time was not significant, F(2,213)=0.95, p=.416, η2=.11, indicating that there was no change in motivation across task blocks (SS Table 1).
Fig. 2.
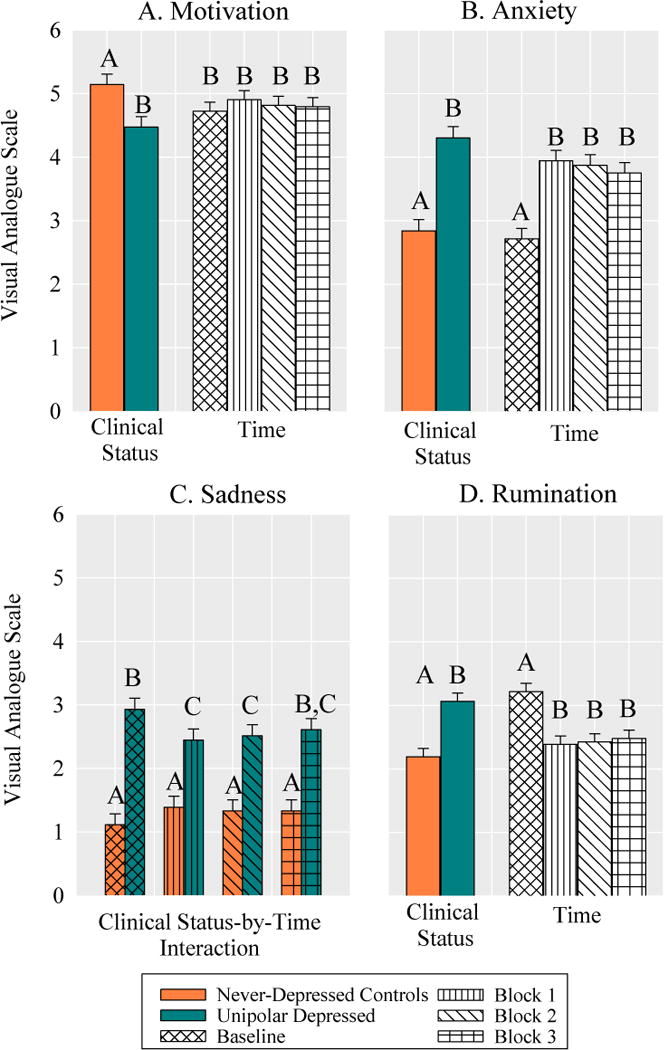
Between group differences and changes in motivation, anxiety, sadness, and rumination at baseline and across task blocks. Bars with differing letters within an effect are significantly different from one another.
Table 1.
Mixed effects analyses evaluating whether motivation moderated the effects of anxiety, sadness, and rumination on planning accuracy and planning time differently for the MDD group relative to never-depressed controls.
| DF | Moderator Anxiety | DF | Moderator Sadness | DF | Moderator Rumination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Num | Den | F | p | η2 | Num | Den | F | p | η2 | Num | Den | F | p | η2 | |
| Planning Accuracy | |||||||||||||||
| Difficulty | 2 | 136 | 52.4 | <0.001 | 0.44 | 2 | 142 | 51.81 | <0.001 | 0.42 | 2 | 142 | 50.5 | <0.001 | 0.42 |
| Clinical Status | 1 | 61 | 4.41 | 0.040 | 0.07 | 1 | 64 | 0.37 | 0.543 | 0.01 | 1 | 64 | 0.52 | 0.473 | 0.01 |
| Motivation | 1 | 61 | 1.27 | 0.265 | 0.02 | 1 | 64 | 0.59 | 0.446 | 0.01 | 1 | 64 | 1.26 | 0.265 | 0.02 |
| Moderator | 1 | 61 | 16.9 | 0.001 | 0.22 | 1 | 64 | 0.70 | 0.406 | 0.01 | 1 | 64 | 0.08 | 0.781 | 0.00 |
| Motivation × Moderator | 1 | 61 | 0.96 | 0.331 | 0.02 | 1 | 64 | 2.39 | 0.127 | 0.04 | 1 | 64 | 2.11 | 0.151 | 0.03 |
| Motivation × C.S. | 1 | 61 | 1.33 | 0.253 | 0.02 | 1 | 64 | 3.60 | 0.062 | 0.05 | 1 | 64 | 0.00 | 0.951 | 0.00 |
| Moderator × C.S. | 1 | 61 | 5.96 | 0.018 | 0.09 | 1 | 64 | 0.29 | 0.589 | 0.00 | 1 | 64 | 0.00 | 0.987 | 0.00 |
| Motivation × M. × C.S | 1 | 61 | 0.20 | 0.653 | 0.00 | 1 | 64 | 3.46 | 0.067 | 0.05 | 1 | 64 | 0.33 | 0.566 | 0.01 |
|
| |||||||||||||||
| Planning Time | |||||||||||||||
| Difficulty | 2 | 136 | 72.8 | <0.001 | 0.50 | 2 | 142 | 74.64 | <.001 | 0.52 | 2 | 142 | 77.40 | <0.001 | 0.52 |
| Clinical Status | 1 | 61 | 0.09 | 0.769 | 0.00 | 1 | 64 | 0.01 | 0.934 | 0.00 | 1 | 64 | 1.04 | 0.311 | 0.02 |
| Motivation | 1 | 61 | 0.06 | 0.804 | 0.00 | 1 | 64 | 0.01 | 0.907 | 0.00 | 1 | 64 | 0.03 | 0.873 | 0.00 |
| Moderator | 1 | 61 | 1.96 | 0.170 | 0.03 | 1 | 64 | 2.06 | 0.157 | 0.03 | 1 | 64 | 3.24 | 0.076 | 0.05 |
| Motivation × Moderator | 1 | 61 | 0.38 | 0.541 | 0.01 | 1 | 64 | 0.15 | 0.704 | 0.00 | 1 | 64 | 0.00 | 0.980 | 0.00 |
| Motivation × C.S. | 1 | 61 | 1.43 | 0.237 | 0.02 | 1 | 64 | 2.55 | 0.115 | 0.04 | 1 | 64 | 4.83 | 0.032 | 0.07 |
| Moderator × C.S. | 1 | 61 | 0.10 | 0.750 | 0.00 | 1 | 64 | 1.07 | 0.304 | 0.02 | 1 | 64 | 1.55 | 0.218 | 0.02 |
| Motivation × M. × C.S. | 1 | 61 | 0.01 | 0.908 | 0.00 | 1 | 64 | 0.01 | 0.914 | 0.00 | 1 | 64 | 0.55 | 0.459 | 0.01 |
Note. C.S.=Clinical Status, M.=Moderator=either anxiety, sadness, or rumination.
Anxiety
There was a main effect of clinical status F(1,70)=34.61, p<.001, d=1.39 on anxiety, which indicated that the MDD group reported higher levels of anxiety than the control group (Fig. 2B). There was also a main effect of time, F(3,213)=24.73, p<.001, η2=.26, which indicated that on average participants demonstrated an increase in anxiety from baseline that remained stable across task blocks (SS Table 1).
Sadness
The main effects of clinical status, F(1,70)=48.30, p<.001, η2=.41, and time, F(3,210)=0.36, p=.782, η2=.01 on sadness were qualified by a clinical status-by-time interaction, F(3,210)=4.30, p=.006, η2=.06. Simple contrasts indicated that the MDD group reported higher levels of sadness than the control group across all time points (Baseline: t(210)=7.42, p<.001, d=−7.75; Block 1: t(210)=4.30, p<.001, d=−1.01; Block 2: t(210)=4.81, p<.001, d=−1.13; Block 3: t(210)=5.21, p<.001, d=−1.23, Fig. 2C). The MDD group demonstrated a significant decrease in sadness from baseline to block 1 and block 2, which rebounded by block 3 (SS Table1). The level of sadness did not change for the control group across task blocks (SS Table1). Thus, the task increased levels of anxiety during problem-solving for all participants and decreased levels of sadness for MDD group, although not to the level observed in the control group.
Rumination
There was a significant main effect of clinical status, F(1,70)=22.32, p<.001, d=1.11, on rumination, which indicated that the MDD group endorsed a greater tendency to engage in rumination than the control group across all time points (Fig. 2D). There was also a main effect of time F(3,213)=14.33, p<.001, η2=.17, which indicated that on average participants demonstrated a decrease in rumination from baseline that remained stable across task blocks (SS Table 1).
Behavioral Performance
Did motivation determine whether or not the MDD group demonstrated poorer planning accuracy relative to never-depressed controls in the presence of anxiety?
Motivation did not moderate the association between clinical status and anxiety, p>.100 (see Table 1). However, there was a significant anxiety-by-clinical status interaction. Probing this interaction indicated that anxiety was more strongly negatively correlated with planning accuracy in the MDD group, β=−0.67, SE=0.17, p=.001, than in the control group, β=−0.17, SE=0.11, p=.118 (Fig. 3A), regardless of participants motivational state. These results suggest that acute anxiety is potentially more impairing for depressed individuals than controls, although at high levels of anxiety the MDD group did not statistically differ in planning accuracy relative to the control group, t(61)=0.44, p=0.661, d=0.10 (Fig. 3B).
Fig. 3.
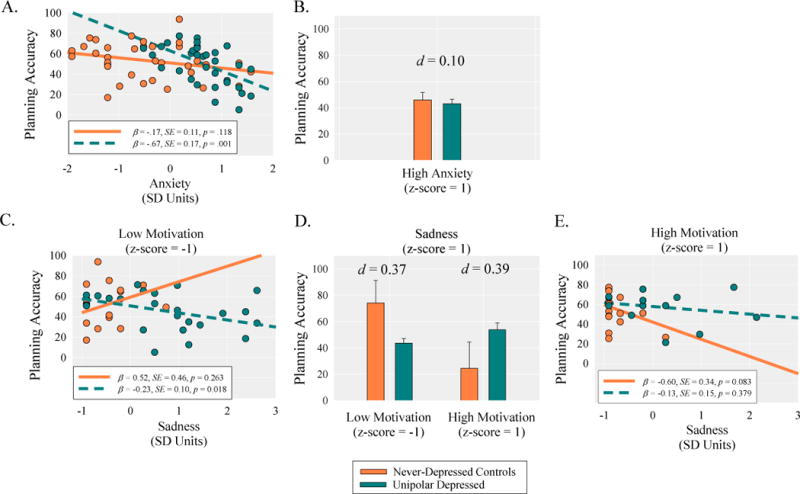
The top and bottom panels shows the decomposition of the anxiety-by-clinical status and motivation-by-sadness-by-clinical status interactions on planning accuracy. The panels show (A) the simple slopes for the effect of anxiety on planning accuracy as a function of clinical status (never-depressed controls vs. unipolar depressed); (B) the differences in planning accuracy at high levels of anxiety as a function of clinical status; (C) the simple slopes for the effect of sadness on planning accuracy as a function of clinical status at low levels of motivation; (D) the differences in planning accuracy at high levels of sadness as a function of clinical status and motivation; (E) the simple slopes for the effect of sadness on planning accuracy as a function of clinical status at high levels of motivation.
Did motivation determine whether or not the MDD group demonstrated poorer planning accuracy relative to never-depressed controls in the presence of sadness?
Main effects and two-way interaction effects were qualified by a marginal motivation-by-sadness-by-clinical status interaction (see Table 1). At low levels of motivation, sadness was significantly negatively correlated with planning accuracy within the MDD group, β=−0.23, SE=0.10, p=.018, but not the control group, β=0.52, SE=0.46, p=.263 (Fig. 3C). These results suggest that at low levels of motivation, acute sadness is potentially more impairing for depressed individuals than controls, although at low levels of motivation and high levels of sadness, the MDD group did not significantly differ from the control group in their planning accuracy, t(64)=1.51, p=.135, d=0.37 (Fig. 3D). In contrast, at high levels of motivation, sadness was not significantly correlated with planning accuracy within the MDD group, β=−0.13, SE=0.15, p=.379, but was marginally negatively correlated with planning accuracy in the control group, β=−0.60, SE=0.34, p=.083 (Fig. 3E). These results suggest that at high levels of motivation, sadness is marginally more impairing in the control group than in the MDD group, although at high levels of motivation and high levels of sadness, the MDD group did not differ in their planning accuracy relative to the control group, t(64)=1.65, p=.104, d=0.39 (Fig. 3D).
Did motivation determine whether or not the MDD group demonstrated poorer planning accuracy relative to never-depressed controls in the presence of rumination?
As shown in Table 1, there were no significant main effects or two-way interactions involving rumination, and these effects were not qualified by a motivation-by-rumination-by-clinical status interaction. These results indicate that rumination does not interact with motivation to predict planning accuracy.
Did motivation determine whether or not the MDD group demonstrated longer planning times relative to never-depressed controls in the presence of inhibitory processes?
As shown in Table 1, there were no significant motivation-by-clinical status-by-inhibitory processes (i.e., anxiety, sadness, rumination) interactions, ps >.100. These results indicate that inhibitory processes do not interact with motivation to predict planning time.
Peripheral Physiological Data
Instrumentation Check
Supplemental analyses to verify the comparability of both pupilometers indicated that the pattern of results for task activity was nearly identical across the ISCAN RK464 pupilometer and the ASL Model 504 eye-tracker. This suggests that the cognitive processes recorded between the two pupil monitoring systems and in and out of the fMRI scanning environment can be considered to be comparable (Fig. 4). Decreased resolution led to the more jagged appearance of waveforms acquired in the scanner, along with greater standard errors. Both outside and inside the scanner, pupil dilation increased parametrically with task difficulty in both amplitude and duration during planning stages of the TOL. Analyses did indicate that participants assessed using the ISCAN RK464 pupilometer demonstrated greater dilation during 4 and 5 move problems relative to participants scanned using the ASL Model 504 eye-tracker. Supplementary analyses to verify that pupil dilation is indexing a measure of cognitive control relevant to behavioral performance indicated that both planning accuracy (SA Fig. 1A) and planning Time (SA Fig. 1B) were associated with pupil dilation during the task.
Fig. 4.
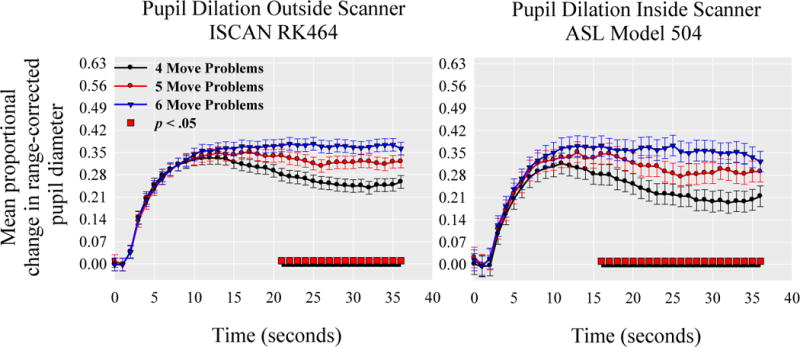
Pupil dilation in response to increasing levels of task load during the Tower of London separated by the type of pupilometer. The red bar under each pupil dilation waveform reflects where the omnibus test of differences in task-load at each time-point is statistically significant at p< 0.05. Black underlined segments indicate regions that are statistical significant after controlling for multiple comparisons.
Did motivation determine whether or not the MDD group demonstrated compensatory cognitive control relative to never-depressed controls in the presence of anxiety?
As shown in Table 2, main effects and two-way interaction effects were qualified by a significant motivation-by-anxiety-by-clinical status interaction. At low levels of motivation, anxiety was significantly negatively correlated with pupil dilation within the MDD group, β=−0.77, SE=0.19, p<.001, but was non-significantly correlated with pupil dilation in the controls, β=0.20, SE=0.13, p=.112 (Fig. 5A). These results indicate that at low levels of motivation, anxiety had a stronger inhibitory effect on the magnitude of pupil dilation in the MDD group relative to the control group. Consistent with this interpretation, at low levels of motivation and high levels of anxiety, the MDD group demonstrated significantly less pupil dilation relative to the control group, t(61)=3.19, p=.002, d=0.75 (Fig. 5B). In contrast, at high levels of motivation, anxiety was not significantly correlated with pupil dilation within the MDD group, β=−0.22, SE=0.21, p=.294, or the control group, β=−0.14, SE=0.08, p=.074 (Fig. 5C). These results indicate that anxiety had minimal effects on pupil dilation in more highly-motivated participants. Furthermore, at high levels of motivation and high levels of anxiety, the MDD group demonstrated significantly greater pupil dilation relative to the control group, t(61)=2.26, p=.027, d=0.53 (Fig. 5B). Overall these findings support the conclusion that motivation facilitates the compensatory recruitment of cognitive control in depression.
Table 2.
Mixed effects analyses evaluating whether motivation moderated the effects of anxiety, sadness, and rumination on pupillary responses differently for the MDD group relative to never-depressed controls.
| DF | Moderator Anxiety | Moderator Sadness | Moderator Rumination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Num | Den | F | p | η2 | F | p | η2 | F | P | η2 | |
| Time | 36 | 2448 | 55.16 | <.001 | .45 | 54.44 | <.001 | .44 | 54.15 | <.001 | .44 |
| Difficulty | 2 | 136 | 7.96 | .001 | .10 | 7.33 | <.001 | .10 | 7.05 | .001 | .09 |
| Difficulty × Time | 72 | 4896 | 1.44 | .010 | .02 | 1.42 | .011 | .02 | 1.42 | .012 | .02 |
| Clinical Status | 1 | 61 | 6.91 | .011 | .10 | 2.66 | .108 | .04 | 0.00 | .986 | .00 |
| Motivation | 1 | 61 | 0.35 | .559 | .01 | 0.00 | .953 | .00 | 6.71 | .012 | .10 |
| Moderator | 1 | 61 | 12.65 | .001 | .17 | 1.55 | .218 | .02 | 0.01 | .939 | .00 |
| Motivation × Moderator | 1 | 61 | 0.31 | .581 | .01 | 5.64 | .021 | .08 | 0.08 | .776 | .00 |
| Motivation × C.S. | 1 | 61 | 0.97 | .329 | .02 | 14.49 | <.001 | .19 | 5.18 | .026 | .08 |
| Moderator × C.S. | 1 | 61 | 16.3 | <.001 | .21 | 0.02 | .887 | .00 | 0.10 | .751 | .00 |
| Motivation × Moderator × C.S. | 1 | 61 | 5.91 | .018 | .09 | 3.90 | .053 | .06 | 0.62 | .433 | .01 |
Note. C.S.=Clinical Status: 1=unipolar depressed, 0=never-depressed controls=0. Moderator=either anxiety, sadness, or rumination.
Fig. 5.
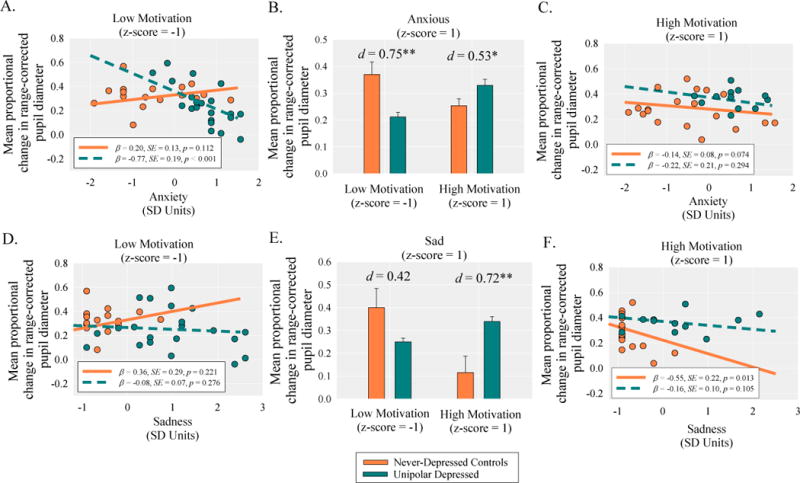
The top and bottom panels shows the decomposition of the significant motivation-by-emotion-by-clinical status interactions on pupil dilation. The panels show (A) the simple slopes for the effect of anxiety on pupil dilation as a function of clinical status (never-depressed controls vs. unipolar depressed) at low levels of motivation; (B) the differences in pupil dilation at high levels of anxiety as a function of clinical status and motivation; (C) the simple slopes for the effect of anxiety on pupil dilation as a function of clinical status at high levels of motivation; (D) the simple slopes for the effect of sadness on pupil dilation as a function of clinical status at low levels of motivation; (E) the differences in pupil dilation at high levels of sadness as a function of clinical status and motivation; (F) the simple slopes for the effect of sadness on pupil dilation as a function of clinical status at high levels of motivation.
Did motivation determine whether or not the MDD group demonstrated compensatory cognitive control relative to never-depressed controls due to the presence of sadness?
As shown in Table 2, main effects and two-way interaction effects were qualified by a marginal motivation-by-sadness-by-clinical status interaction. At low levels of motivation, sadness was not significantly correlated with pupil dilation within the MDD group, β=−0.08, SE=0.07, p=.276, or the control group, β=0.36, SE=0.29, p=.221 (Fig. 5D). These results indicate that in less-motivated individuals, sadness had no significant effects on pupil dilation. At low levels of motivation and high levels of sadness, the MDD group did not significantly differ from the control group, in their pupil dilation t(61)=1.75, p=.085, d=0.42 (Fig. 5E). At high levels of motivation, sadness was not significantly correlated with pupil dilation within the MDD group, β=−0.16, SE=0.10, p=.105, but was significantly negatively correlated with pupil dilation in the control group, β=−0.55, SE=0.22, p=.013 (Fig. 5F). These results indicate that at high levels of motivation, sadness had a greater inhibitory impact on cognitive control in the control group relative to the MDD group. Consistent with this interpretation, at high levels of motivation and high levels of sadness, the MDD group demonstrated significantly greater pupil dilation relative to the control group, t(61)=2.97, p=.004, d=0.72 (Fig. 5E).
Did motivation determine whether or not the MDD group demonstrated compensatory cognitive control relative to never-depressed controls due to the presence of rumination?
As shown in Table 2, there were no significant main effects or two-way interactions involving rumination, and these effects were not qualified by a motivation-by-rumination-by-clinical status interaction p>.100. These results indicate that rumination did not influence cognitive control as indexed by pupil dilation.
Discussion
This study aimed to clarify whether negative emotional states occurring during a cognitive task inhibits the recruitment of cognitive control in depressed individuals and to determine whether the influence of negative emotional processing on cognitive control depends on the individuals’ motivational state (Berggren & Derakshan, 2013; Jones, et al., 2010). The results strongly supported the conclusion that anxiety exerts an inhibitory effect on the recruitment of cognitive control in depression, and that motivation can facilitate the compensatory recruitment of cognitive control. We did not find a strong influence of sadness and there was no influence of rumination on the recruitment of cognitive control in depression.
Behavioral results demonstrated that, across levels of motivation, anxiety impaired planning accuracy more in the MDD group than the control group. The analyses of pupillary responses closely mirrored the behavioral data. Less-motivated and anxious depressed individuals demonstrated decreased pupil dilation relative to similarly less-motivated and anxious never-depressed controls. In contrast, more highly-motivated depressed individuals demonstrated greater pupil dilation at high levels of anxiety relative to similarly anxious more highly-motivated never-depressed controls. These results are consistent with past research and clarifies that it is task-induced anxiety that inhibits the recruitment of cognitive control in depressed individuals (Wagner, et al., 2006). They also extend previous research (Harvey, et al., 2005; Matsuo et al., 2007; van Tol, et al., 2011; Walter, et al., 2007) by demonstrating that motivation is a critical factor in determining whether depressed individuals recruit compensatory cognitive control.
A similar pattern of results was observed for sadness, however these results failed to reach statistical significance, and as such should be interpreted with caution. The behavioral results demonstrated that sadness was more strongly associated with poorer planning accuracy in the less-motivated depressed participants, whereas the opposite was true at high levels of motivation. At high levels of motivation, sadness was more strongly associated with poor planning accuracy in the never-depressed controls relative to depressed individuals. Somewhat consistently, the experience of sadness was not associated with pupil dilation in the depressed group regardless of their level of motivation, while sadness was associated with decreased pupil dilation in highly motivated never-depressed controls. This unexpected pattern of findings was driven by a small group of four highly-motivated never-depressed controls who experienced increased sadness over the course of the task, potentially due to their poor performance. In contrast, the high levels of sadness reported by depressed participants did not occur in response to the task and was likely a preexisting feature of their depression. Thus, the greater pupil dilation observed in sad, highly-motivated, depressed individuals relative to sad, highly-motivated never-depressed controls actually reflects a failure of a small group of healthy controls to recruit cognitive control.
We did not observe an association between rumination and behavioral performance or pupil dilation in either the depressed participants or never-depressed controls. In light of past research indicating that induced rumination can impact cognitive performance (Watkins & Brown, 2002; Whitmer & Gotlib, 2012), our results suggest that naturally-occurring rumination was not strong enough to interfere with the recruitment of cognitive control.
Given the non-significant findings described above and the observation that the task significantly suppressed rumination for all participants and largely suppressed sadness in the depressed participants, we conclude that these processes were not proximal drivers of cognitive control. Instead, we believe these factors are independent signatures of depression that are likely suppressed with active engagement in a current cognitive task (Walter, et al., 2007). As such, these findings are consistent with research indicating that engagement in a cognitive task can suppress activation in brain regions associated with self-referential (Harvey, et al., 2005) and affective processing (Kanske, Heissler, Schönfelder, Bongers, & Wessa, 2011; McRae et al., 2010; Van Dillen, Heslenfeld, & Koole, 2009).
Multiple limitations warrant mentioning; given our use of a unidimensional physiological measure of cognitive and emotional load, combined with a subjective measure of emotion processing, it is not truly possible to determine the precise nature of emotional and motivational contributions to cognitive inefficiency in this depressed sample. It is possible that the observed differences in pupil dilation between depressed individuals and never-depressed controls reflect the additive influence of an unmeasured emotional state (Bradley, Miccoli, Escrig, & Lang, 2008; Jones et al., 2011). Our results suggest that this is an unlikely explanation. Task engagement actually led to decreased engagement in rumination for all participants, and it even appeared to actively suppress levels of sadness in depressed participants. In addition, our self-report measures of task-induced anxiety and sadness were associated with decreased pupil dilation rather than increased pupil dilation. Thus, we believe that increased pupil dilation in anxious highly-motivated depressed participants relative to anxious highly-motivated controls reflects the additive contribution of motivation to cognitive control (Chiew & Braver, 2013, 2014). Ideally, the effect of emotion on processing would be measured on the same time-scale as the use of cognitive resources (Siegle, Steinhauer, Friedman, Thompson, & Thase, 2011; Siegle, Steinhauer, Stenger, et al., 2003). In addition, our sample was predominately female, and as such, findings may not adequately generalize to depressed males. However, given that females are more likely to be depressed than males (Cyranowski, Frank, Young, & Shear, 2000), our findings do generalize to the larger population of depressed individuals. Lastly, many of the self report measures were sampled at different parts of the range in each group, which could have affected our ability to detect certain patterns of findings. In particular, while the experience of anxiety was relatively equal between the depressed and anxious groups, there was a smaller group of motivated anxious depressed participants. Thus, failure to find an effect of anxiety on pupil dilation in this group may have been driven the absence of depressed subjects demonstrating this combined pattern of responding.
In summary, our results supported the conclusion that anxiety exerts an inhibitory effect on the recruitment of cognitive control in depression, and that motivation can facilitate the compensatory recruitment of cognitive control. Interventions aimed at improving depressed individuals’ cognitive function should be aimed at increasing their ability to regulate their anxiety during demanding cognitive tasks and improving their motivational state. It appears that addressing these two issues may increase cognitive control and improve depressed individuals’ ability to execute goal-directed behavior.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health [MH086811 to N.J.), [MH074807, MH082998 to G.S.). The Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. We thank Ashley F. McFarland, Olivia L. Conner, and Jillian Rodgers for their role in data collection as well as the staff of the Mood Disorders Treatment and Research Program at Western Psychiatric Institute and Clinic.
Footnotes
There were no significant interactions with time or task difficulty other than the significant task difficulty × time interaction. Thus, we controlled for these effects when testing the study’s main hypotheses that did not involve time
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Bakes A, Bradshaw CM, Szabadi E. Attenuation of the pupillary light reflex in anxious patients. British Journal of Clinical Pharmacology. 1990;30(3):377–381. doi: 10.1111/j.1365-2125.1990.tb03787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91(2):276–292. [PubMed] [Google Scholar]
- Berggren N, Derakshan N. Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biological Psychology. 2013;92(3):440–446. doi: 10.1016/j.biopsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Szabadi E, Bradshaw C. The fear-inhibited light reflex: importance of the anticipation of an aversive event. International Journal of Psychophysiology. 2004;52(1):87–95. doi: 10.1016/j.ijpsycho.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. [Original Research] Frontiers in psychology. 2013;4 doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Dissociable influences of reward motivation and positive emotion on cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2014:1–21. doi: 10.3758/s13415-014-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Johnstone T. Prefrontal inhibition of threat processing reduces working memory interference. [Article] Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear M. Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Nierenberg AA, Buhlmann U, Ostacher M, Dougherty D, Loh R, Sachs GS. The influence of sadness on working memory in bipolar disorder: an fMRI investigation. [Meeting Abstract] Bipolar Disorders. 2008;10:928–942. doi: 10.1111/j.1399-5618.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker S, Rogers R, O’Leary D, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: A study using positron emission tomography. Psychological Medicine. 1997;27(4):931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. http://dx.doi.org/10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition. Vol. 20. New York: Biometrics Research Department New York State Psychiatric Institute; 1996. [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. The Journal of Neuroscience. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard J, Grondin S, Baruch P, Lafleur MF. Psychosocial and neurocognitive profiles in depressed patients with major depressive disorder and bipolar disorder. Psychiatry Research. 2011;190(2):244–252. doi: 10.1016/j.psychres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33(4):457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Granholm E, Steinhauer SR. Pupillometric measures of cognitive and emotional processes. International Journal of Psychophysiology. 2004;52(1):1–6. doi: 10.1016/j.ijpsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gray KC, Williams DE. Anticipation and stuttering: A pupillographic study. Journal of Speech, Language and Hearing Research. 1969;12(4):833. doi: 10.1044/jshr.1204.833. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, LeBastard G, Lehéricy S, Dubois B. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. NeuroImage. 2005;26(3):860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil Size in Relation to Mental Activity during Simple Problem-Solving. Science. 1964;143(3611):1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives General Psychiatry. 2008;65(2):179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M, Kobayashi A, Kawasaki C. Heart rate variability with repetitive exposure to music. Biological Psychology. 2005;70(1):61–66. doi: 10.1016/j.biopsycho.2004.11.015. http://dx.doi.org/10.1016/j.biopsycho.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Jones NP, Siegle GJ, Muelly ER, Haggerty A, Ghinassi F. Poor performance on cognitive tasks in depression: Doing too much or not enough? Cognitive, Affective and Behavioral Neuroscience. 2010;10(1):129–140. doi: 10.3758/CABN.10.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NP, Siegle GJ, Proud L, Silk JS, Hardy D, Keljo DJ, Szigethy E. Impact of inflammatory bowel disease and high-dose steroid exposure on pupillary responses to negative information in pediatric depression. Psychosomatic Medicine. 2011;73(2):151–157. doi: 10.1097/PSY.0b013e318207ffea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Beatty J. Pupil Diameter and Load on Memory. Science. 1966;154(3756):1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to Regulate Emotion? Neural Networks for Reappraisal and Distraction. Cerebral Cortex. 2011;21(6):1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Lader M. The psychophysiology of anxiety. L’Encephale. 1983;9(4 Suppl 2):205B–210B. [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P, Soares JC. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry. 2007;12(2):158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Borkovec TD, Sibrava NJ. The effects of worry and rumination on affect states and cognitive activity. Behavior Therapy. 2007;38(1):23–38. doi: 10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly NJ, Watkins E. Ruminative self-focus and negative affect: an experience sampling study. Journal of Abnormal Psychology. 2008;117(2):314–323. doi: 10.1037/0021-843X.117.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DC. Talk, silence and anxiety. Psychological Bulletin. 1971;75(4):244. doi: 10.1037/h0030801. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison JR. National Adult Reading Test (NART): test manual. Second. Nfer-Nelson Publishing Co; Berkshire, England: 1991. [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99(8):5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute Psychological Stress Reduces Working Memory-Related Activity in the Dorsolateral Prefrontal Cortex. Biological Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. http://dx.doi.org/10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. NeuroImage. 2006;29(1):203–215. doi: 10.1016/j.neuroimage.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Scheurich A, Fellgiebel A, Schermuly I, Bauer S, Wolfges R, Muller MJ. Experimental evidence for a motivational origin of cognitive impairment in major depression. Psychological Medicine. 2008;38(2):237–246. doi: 10.1017/S0033291707002206. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ichikawa N, Steinhauer S. Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008;45(5):679–687. doi: 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitve Therapy and Research. 2003;27(3):365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biol Psychiatry. 2011;69(8):726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Simpson H, Molloy F. Effects of audience anxiety on pupil size. Psychophysiology. 1971;8(4):491–496. doi: 10.1111/j.1469-8986.1971.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289(23):3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Owen AM. Planning and problem solving: From neuropsychology to functional neuroimaging. Journal of Physiology-Paris. 2006;99(4–6):308–317. doi: 10.1016/j.jphysparis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Rahm B, Kaller CP, Ruff CC, Spreer J, Krause BJ, Halsband U. When Planning Fails: Individual Differences and Error-related Brain Activity in Problem Solving. Cerebral Cortex. 2004;14(12):1390–1397. doi: 10.1093/cercor/bhh100. [DOI] [PubMed] [Google Scholar]
- Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage. 2009;45(4):1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJA, Demenescu LR, Nielen MMA, Aleman A, Renken R, Veltman DJ. Functional MRI correlates of visuospatial planning in out-patient depression and anxiety. Acta Psychiatrica Scandinavica. 2011;124(4):273–284. doi: 10.1111/j.1600-0447.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel HJ, Schlösser RGM. Cortical Inefficiency in Patients with Unipolar Depression: An Event-Related fMRI Study with the Stroop Task. Biological Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. http://dx.doi.org/10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: An event-related, parametric, performance-controlled fMRI study. Journal of Affective Disorders. 2007;101(1–3):175–185. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Watkins E, Brown RG. Rumination and executive function in depression: an experimental study. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72(3):400–402. doi: 10.1136/jnnp.72.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer AJ, Gotlib IH. Switching and backward inhibition in major depressive disorder: The role of rumination. Journal of Abnormal Psychology. 2012;121(3):570. doi: 10.1037/a0027474. [DOI] [PubMed] [Google Scholar]
- Wilson M, Smith NC, Chattington M, Ford M, Marple-Horvat DE. The role of effort in moderating the anxiety – performance relationship: Testing the prediction of processing efficiency theory in simulated rally driving. Journal of Sports Sciences. 2006;24(11):1223–1233. doi: 10.1080/02640410500497667. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, Yamawaki S. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S. Evidence of a Dissociation Pattern in Resting-State Default Mode Network Connectivity in First-Episode, Treatment-Naive Major Depression Patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. http://dx.doi.org/10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


