Abstract
Motor commands issued by the CNS are based upon memories of past experiences with similar objects, the current state of the hand and arm postures, and sensory input. Thus widespread somatosensory information is available to form precise representations of hand shape on which to base motor commands to match a desired posture or movement. The aim of this study was to examine the extent to which somatosensory information reflecting external influences on independent finger movement is incorporated into the perception of hand shape driving the motor command. To address this issue, a matching task was performed while pairs of fingers in the grasping hand were constrained to move in tandem when grasping familiar objects. The hypothesis was that motor commands would be driven by comparison of the online sensory information from the matching hand to a desired somatosensory state determined by the current somatosensory input from the grasping hand. The results demonstrated that multi-muscle patterns of activation and hand postures were altered with respect to the external constraint on independent finger movement. A secondary aim of this study was to examine the influence of sensory information on the structure of the multi-muscle patterns. The hypothesis was that the same synergies (patterns of activation across muscles) would be used to complete the task but would be rescaled with respect to condition. The results demonstrated that rescaling the patterns of multi-muscle activity from the unconstrained condition could not equivalently represent those from the constrained conditions. Thus it appears that external restriction of independent finger movement was signaled by somatosensory feedback and incorporated into the desired state driving the motor command resulting in selective activation of groups of muscles.
Keywords: Electromyography, finger, grasp, muscle synergies, sensorimotor, somatosensory
1. Grasping and manipulating objects requires a sophisticated level of control needed to form specific postures and apply appropriate forces with our fingers and hands. Therefore, precise motor commands must be issued to coordinate the activation of muscles or groups of muscles to achieve appropriate positioning of the limb and points of contact with the object. These motor commands are shaped by an internal representation of the current state, sensorimotor memories of previous experiences with the object or similar objects (Baugh et al., 2012; Cole et al., 2008; Gordon et al., 1993; Lukos et al., 2013; Parikh and Cole, 2011; Quaney et al., 2003; Wolpert and Flanagan, 2001) and online sensory feedback (for review see Johansson and Flanagan, 2009). The initial commands may be based on predicting elements of fragility, weight, and surface texture of the objects being manipulated (for review see Flanagan et al., 2006). Studies of sequential hand movements by Flanders and colleagues have also supported the idea that a representation of hand shape is continuously updated and maintained by the CNS in order to provide a basis for future motor commands (Jerde et al., 2003; Flanders, 2011). Sensorimotor information acquired by grasping and lifting objects with one hand can be transferred to predictively control grip forces with the other hand (Gordon et al. 1994) although this is disrupted in cerebellar patients (Nowak et al., 2005; Nowak et al. 2009). However, the way in which somatosensory input is incorporated into the motor command is not well understood. Matching tasks where a static grasping posture is pantomimed by the other non-grasping hand have been used to examine what somatosensory input is transferred to the opposite hemisphere and incorporated into the motor command of the non-grasping hand. Pesyna et al. (2011) found that the cutaneous and proprioceptive input specific to a gravitational reference frame, irrespective of grasping forces, was incorporated into the perception of hand shape for a matching task. Interestingly, when the gravitational reference frame of the grasping and matching hand were different, the postures of the finger and wrist joints of the matching hand were altered to keep the imagined object in its proper spatial orientation. Therefore, specific elements of somatosensory information appear to dominate the perception of hand shape while others are ignored. One reason for the difference may be that the influence of gravitational forces is inherent in motor commands and more easily transferred to the other hemisphere while grip forces are initially estimated and then modulated in response to online feedback for object manipulation. Following this line of reasoning, somatosensory information related to other additional external constraints on movement, such as splinting of an injured finger or use of a tool whose function is related to movement of multiple digits together, i.e., scissors, might also be expected to be incorporated into the perception of hand shape.
The aim of this study was to examine the extent to which somatosensory information reflecting external influences on independent finger movement is incorporated into the perception of hand shape driving the motor command. The external restriction of independent finger movement was accomplished by taping two different pairs of fingers together in the grasping (right) hand, requiring them to move in tandem. This constraint creates a novel situation for which sensorimotor memories would not be available to drive motor commands at the matching hand. Therefore, the working hypothesis was that motor commands would be driven by comparison of the online sensory information from the matching hand to a desired somatosensory state determined by the current somatosensory input from the grasping hand. This hypothesis would be supported by distinct differences between control (unconstrained) and tandem conditions in the intrinsic joint angles of the digits (collectively called hand posture) and multi-muscle patterns in the left (matching) hand. Specifically, differences in joint angles and muscle activity of the matching hand should include those for digits not being constrained in the grasping hand, thus demonstrating an overall change in matching hand posture reflecting the constraint at the grasping hand. The alternative to this hypothesis is that the motor commands are driven by sensorimotor memories of grasping these familiar objects under normal (unconstrained) conditions resulting in no differences in hand posture or muscle activity.
A secondary aim of this study was to examine the influence of sensory information on the structure of the multi-muscle patterns. The specific working hypothesis was that the same synergies (patterns of activation across muscles) would be used to complete the task but would be rescaled with respect to condition. If rescaling the activation patterns from the control condition cannot accurately represent the multi-muscle patterns from the tandem conditions, it would support the notion of selective activation of muscles respective of task constraints. The results are discussed with respect to the role of somatosensory input in the motor command and the potential role of sensory input in the flexible activation/coordination of groups of muscles or synergies.
2. Experimental Procedures
Six healthy human subjects (5 right handed, 1 ambidextrous, 3 male, 23± 5 yrs., 1.69 ± 0.13 m) with no known neurological disorders or significant hand injuries were recruited for the study. The experimental protocol was approved by the University of Minnesota’s Institutional Review Board and all subjects gave informed consent prior to the experiment.
2.1 Task
Subjects were asked to grasp 13 objects common to daily use (Fig. 1, left) with their right hand and match this hand posture with their left hand. The objects were selected to provide a wide range of hand shapes and were known to represent distinct areas in Principal Component (PC) space (Pesyna et al. 2011; Santello et al. 1998; Weiss and Flanders 2004). Familiar objects were chosen based on the assumption that an internal memory of hand shape for grasping familiar objects was already formed and would be reliable and useful (Deshpande et al., 2010) for comparing small differences in somatosensory information (Nowak et al., 2004) related to adjustments in hand shape required by constraining pairs of fingers to move in tandem. For consistency of hand posture for a particular object, subjects were instructed to grasp each object the same way throughout the experiment. For each trial, the participant grasped the object presented by the experimenter with their right hand, then closed their eyes and matched the grasping posture with their left hand. Subjects indicated when they had reached a matched hand posture and held that static hand posture for ~ 3 seconds.
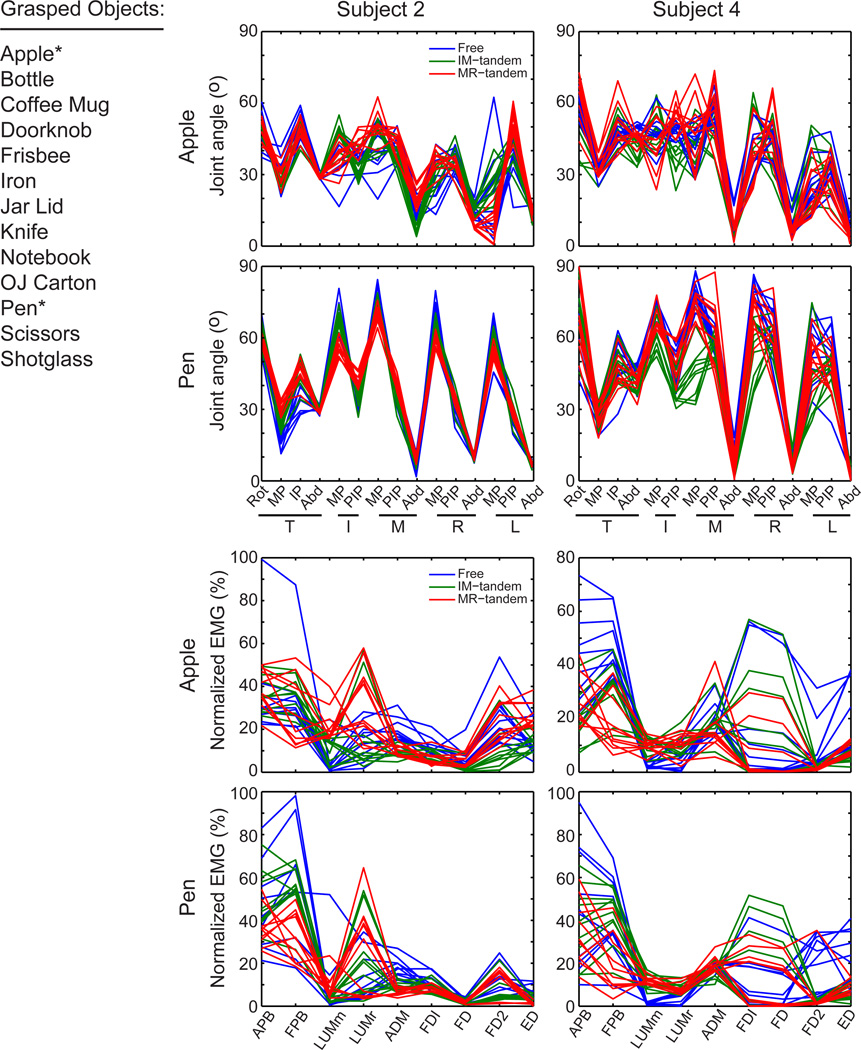
Figure 1. Grasped objects, joints angles and EMG.
A complete list of grasped objects (left; *joint angles and EMG shown for these objects) along with sample joint angle and EMG data from two subjects for all 10 trials for each condition from two objects (right). Line color for each trial is coded according to grasp condition.
The experimental procedure consisted of ten blocks of trials for each grasp condition during which each object (n=13) was presented in pseudorandom order so that each object was grasped ten times for a total of 130 trials performed in each of the three grasp conditions. Rest was given throughout the experiment to prevent fatigue. Subjects grasped the objects freely in the control condition, while two other experimental conditions consisted of constraining independent movement of either the index-middle or middle-ring finger pairs of the right hand such that both fingers moved in tandem (IM-tandem and MR-tandem, respectively). Independent finger movement was constrained by taping the paired fingers around the proximal interphalangeal (PIP) joints which still allowed for tandem movement at the paired PIP joints. Both the index and ring fingers were paired with the middle finger since the pairs move together relatively easily but the index and ring finger differ in their function during object manipulation. While the index finger often opposes the thumb without the use of the other fingers, the ring finger is more coordinated with additional fingers for object manipulation.
2.2 Data acquisition
A custom LabView Virtual Instrument was used to record muscle activity and posture from the left hand for one second while remaining static in the matching posture. Muscle activity was recorded as surface electromyograms (EMGs) using 2-mm diameter bipolar Ag/AgCl surface electrodes (Discount Disposables, St. Albans, VT) placed 10 mm apart (see Fig. 2 of Winges et al., 2007). Custom-made, electrically shielded wire leads were permanently soldered to these electrodes and connected to standard laboratory amplifiers. The ground electrode was placed on the participant’s contralateral wrist. EMG was amplified (× 1,000), band-pass filtered (60–500 Hz), and then sampled at 1,000 Hz.
Figure 2. Joints angles.
Average joint angles across all objects are shown for each subject for the middle (upper left) and ring (lower left) abduction angles for each subject for the control and respective tandem condition. Significant differences from the control condition for the IM-tandem (upper right) and MR-tandem (lower right) condition for each measured joint angle and subject are illustrated using black boxes. The larger black box framing multiple joints indicates the joints belonging to the constrained condition.
Nine channels of muscle activity were recorded; two intrinsic thumb muscles: abductor pollicis brevis (APB) and flexor pollicis brevis (FPB); four intrinsic finger muscles: first dorsal interosseus (FDI), middle and ring lumbricals (LUMm, LUMr, respectively) and adductor digiti minimi (ADM); and three portions of extrinsic hand muscles: a central portions of extensor digitorum (ED) and two portions of the flexor digitorum superficialis (FD, FD2). For each trial, the mean muscle activity was computed from one second of data (1,000 samples) recorded while the participant was statically holding the matched hand posture. Then for each channel, muscle activity was amplitude normalized by subtracting the minimum amplitude then dividing by the maximum amplitude found over the entire experiment for that channel. Therefore, muscle activity in each channel was expressed as a percent of the maximum found over the entire experiment in order to compare across trials, objects, and conditions.
Posture of the left hand was defined by 15 joint angles measured by a left-handed glove with open fingertips; the angular resolution of the glove was <0.5° (Cyberglove, Virtual Technologies, Palo Alto, CA). The recorded joint angles were the metacarpophalangeal (MCP) and proximal interphalangeal joints (PIP) from each digit, abduction (ABD) of the thumb, middle, ring and little fingers, and thumb rotation (ROT). Abduction angles were defined as 0° when digits were completely adducted. (The middle finger abduction angle as defined by the glove is the angle between the index and middle finger.) The glove was calibrated for each participant using a standard set of postures. Joint angle data were recorded at a temporal resolution of 12 ms (~83 Hz) for one second. These data were subsequently averaged to yield a single value for each joint which together represented the static hand posture for each trial.
2.3 Data Analysis
Discriminant analysis was used to examine the extent to which subjects consistently adjusted their hand posture with respect to the grasped object shape and condition. Further, discrimination of muscle activity between objects and conditions would reflect differences in the motor command with respect to online somatosensory information. The “classify” function in Matlab (linear method) quantified the extent to which the hand posture and EMG data could be correctly classified according to matched object or condition. When trials were classified into matched objects, the training group consisted of all other trials within the same grasp condition. When trials were classified into different conditions, the training group consisted of all other trials for the same object in all conditions. Note that the trial being classified was not part of the training group during classification. This analysis was chosen because constraining movement in a pair of digits would be expected to be accommodated by changes in the entire posture of the hand, rather than being restricted to the constrained digits. Paired-sample T-tests were used to examine whether joint angels at the constrained digits differed between the control and tandem conditions.
Principal component (PC) analysis was used to determine how patterns of muscle activity were altered in the tandem conditions and, whether the altered pattern could be well described as a weighted combination of a small number of patterns (i.e. synergies) derived from the control condition. The PC analysis was performed separately for each subject for each grasp condition with an input matrix containing 13 object vectors of nine points of averaged muscle activity, one per EMG channel. The result of the PC analysis was 13 vectors of PC coefficients computed from the 13×13 covariance matrix and a 13×13 matrix of loadings. For a given object, the EMG data could be perfectly reconstructed as the mean plus the weighted sum of each of the 13 PCs (coefficient vectors). The PCs are ranked from highest to lowest according to the amount of variance they account for in the data. An arbitrary cutoff of 80% variance accounted for was used to determine that for each condition only the first three PCs would be included in further analysis. Pearson-r was calculated across coefficients for pairs of PCs derived to match those from different conditions. To determine whether a simple reweighting of the control PC coefficients could provide a good fit for tandem conditions, EMG data from the tandem conditions was regressed onto the PC coefficient vectors from the control condition. For each of the tandem conditions separately, the mean EMG amplitude of each muscle within the tandem condition was subtracted from all trials, and then the remaining EMG for each trial was regressed onto the control-PC1, where the regression coefficients became the new loadings. The next step is to partially reconstruct the tandem EMG using the coefficients from control-PC1 multiplied by the new loadings for PC1. In order to get new loadings for control-PC2, the partial reconstruction was subtracted from the EMG used in the first regression, leaving only the EMG that was not accounted for by PC1. The result was then regressed against control-PC2, where the coefficients of the new regression were the new loadings for PC2. The same process was completed again to get the other new loadings for each control-PC for the tandem condition. The final result was new loadings that were used to reconstruct EMG data from the tandem conditions using the PCs derived from the control condition. This method of finding new loadings was verified by using the same procedure on the control condition and by the perfect reconstruction of the original EMG signals when all PCs were used for the reconstruction. R-squared values representing the goodness of fit were calculated for the fit of reconstructed EMG onto the original EMG using the weightings from the uniquely derived PC1–3 and the control PC1–3. T-tests (P ≤0.05) determined whether the two methods were significantly different from one another.
3. Results
The thirteen objects elicited a range of hand postures and muscle activation patterns from the matching hand. Fig. 1 provides a list of the objects grasped with the right hand and plots of joint angle and amplitude of normalized EMG data for all trials from two different objects and subjects from the left, matching hand. The figure illustrates two common observations among the subjects: 1) the differences between conditions were larger in some joints or muscles than others, and 2) the inter-trial variability was not consistent across joints or muscles. For example, when subject #2 matched the pen grasp the thumb MP joint posture appears to differ between all three conditions while the thumb abduction angle appears to be more consistent across conditions and trials. The corresponding thumb muscle activity (APB, FPB) appears to differ between the tandem conditions while the control (Free) condition is more variable; at the little finger the activity of ADM is more consistent across trials and appears to differ across conditions. When subject #4 matched the pen posture, the MP joints tended to differ across conditions and for the middle MP adopted a more extended posture for the IM-tandem condition. Although variability was observed in both muscle activity and joint rotations, the plots in Fig. 1 illustrate the tendency for condition dependent patterns in both joint angles and muscle activity.
To determine whether subjects had changed hand posture with respect to object shape discriminant analysis was performed on the joint and muscle activity data. On average, 76% ±10% of hand postures and 43±13% of muscle activation patterns could be correctly classified according to the object that was being matched (Table 1 “Matched object”). This was well above chance level (~7.7%) indicating that hand postures and patterns of muscle activation were altered consistently with respect to object. Weiss and Flanders (2004) found a similar difference in hand posture (77–89% correctly classified) and muscle activity (37–49% correctly classified) discriminability when subjects were allowed to lightly grasp objects. Therefore, the extent to which the subjects altered their matching hand’s posture with respect to object shape was similar to a task where the object was lightly grasped. Discriminant analysis was also used to determine whether for each object shape, subjects consistently altered hand posture and muscle activation patterns with respect to condition, i.e. does the constraint imposed on the grasping hand influence the posture and patterns of muscle activity of the matching hand? The results of the analysis shown in Table 1 (“Grasp condition”) reveal that hand posture and patterns of muscle could be correctly classified according to grasp condition at similar rates ranging from 61–92% and 61–87% correctly classified for muscle activity and hand posture, respectively. These rates of discriminability were well above the chance level of ~33.3% indicating that hand posture and muscle activity were altered consistently across objects with respect to grasp condition.
Table 1.
Percent of trials correctly classified into matched object or grasp condition by discriminant analysis on muscle activity (EMG) and hand postures (JT) for each subject
| Matched object | Grasp condition | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | Mean±SD | S1 | S2 | S3 | S4 | S5 | S6 | Mean±SD | |
| EMG | ||||||||||||||
| Free | 48 | 45 | 57 | 32 | 32 | 51 | 44±10 | 76 | 92 | 72 | 88 | 84 | 62 | 79 ±11 |
| IM | 50 | 48 | 51 | 18 | 29 | 58 | 42±15 | 61 | 85 | 62 | 77 | 83 | 61 | 71 ±12 |
| MR | 61 | 45 | 45 | 27 | 22 | 56 | 43±15 | 82 | 87 | 64 | 79 | 85 | 77 | 79 ±8 |
| JT | ||||||||||||||
| Free | 80 | 73 | 89 | 74 | 65 | 74 | 76±8 | 75 | 73 | 77 | 85 | 87 | 83 | 80 ±6 |
| IM | 86 | 70 | 87 | 54 | 71 | 82 | 75±13 | 61 | 75 | 71 | 77 | 85 | 63 | 72 ±9 |
| MR | 79 | 84 | 86 | 67 | 58 | 82 | 76±11 | 70 | 84 | 77 | 73 | 87 | 70 | 77 ±7 |
Chance level was 7.7% for matched objects and 33.3% for grasp condition.
To further examine whether changes in joint angle occurred between the control and each tandem condition, paired sample t-tests were performed on individual joints. Fig. 2 illustrates the average ABD angle for each subject (left) and joints at which the angles in the tandem condition were significantly different from the control condition for each subject (right). Subjects 2–5 all had significantly smaller middle finger abduction angles for both the IM- and MR-tandem compared to control condition, indicating that subjects positioned their index and middle or middle and ring fingers closer together. Subjects 1 and 6 were already using a posture with the fingers close together similar to the other subjects constrained condition so there was no change. The right side of Fig. 2 shows that for each tandem condition, there were significant differences from the control condition for joints involved in the constraint (inside large box) and also at joints of the unconstrained digits.
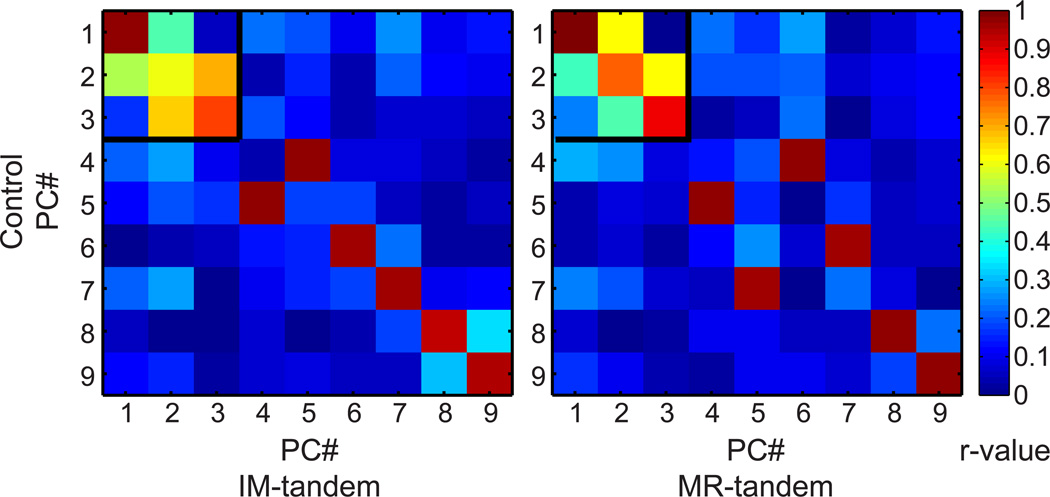
To further assess the influence of grasp condition on multi-muscle activity, principal component (PC) analysis was used to extract patterns of multi-muscle activity for each condition. Note that for a given object, the multi-muscle activity could be reconstructed as the mean EMG for that object plus the object specific weighted sum of each of the 13 PCs (coefficient vectors). For all subjects and conditions, 75% of the variance could be accounted for by the first three PCs (Fig. 3, upper). The coefficients from each condition for PCs 1–3 are shown by subject in the lower portion of Fig. 3. Correlations calculated between PCs confirmed that the first three components in each condition were best matched with PCs 1–3 in the other conditions. (Note that this reordering was only for illustrative purposes and did not affect further analysis.) For most subjects at least one pattern was very similar across conditions while other patterns varied to a larger extent across conditions. For example, subject 3 has a nearly identical coefficient pattern across conditions for PC1 while a distinct change between the control condition and the tandem conditions occurs in PC2; PC3 also appears to be different between the control and tandem conditions although the basic shape of the pattern is present. The r-values for correlations computed between the control PCs and each of the tandem conditions are shown in Fig. 4. Notice that the strongest correlations are within the black box bounding the first three PCs. Therefore, the first three PCs tended to be most highly correlated across conditions indicating that the patterns explaining the most variance within the EMG set may be similar across conditions.
Figure 3. PC analysis.
The cumulative sum of the percent of variance explained (%VAF) for all subjects in each grasp condition (upper) and coefficients for PCs 1–3 (lower) for each grasp condition shown by subject from PC analyses on EMG data
Figure 4. PC correlations between conditions.
Pearson r-values for correlations between PCs derived from the control condition and each of the tandem conditions for subject #3. The color bar (right) maps the colors to the r-value it represents from 0 (dark blue) to 1 (red)
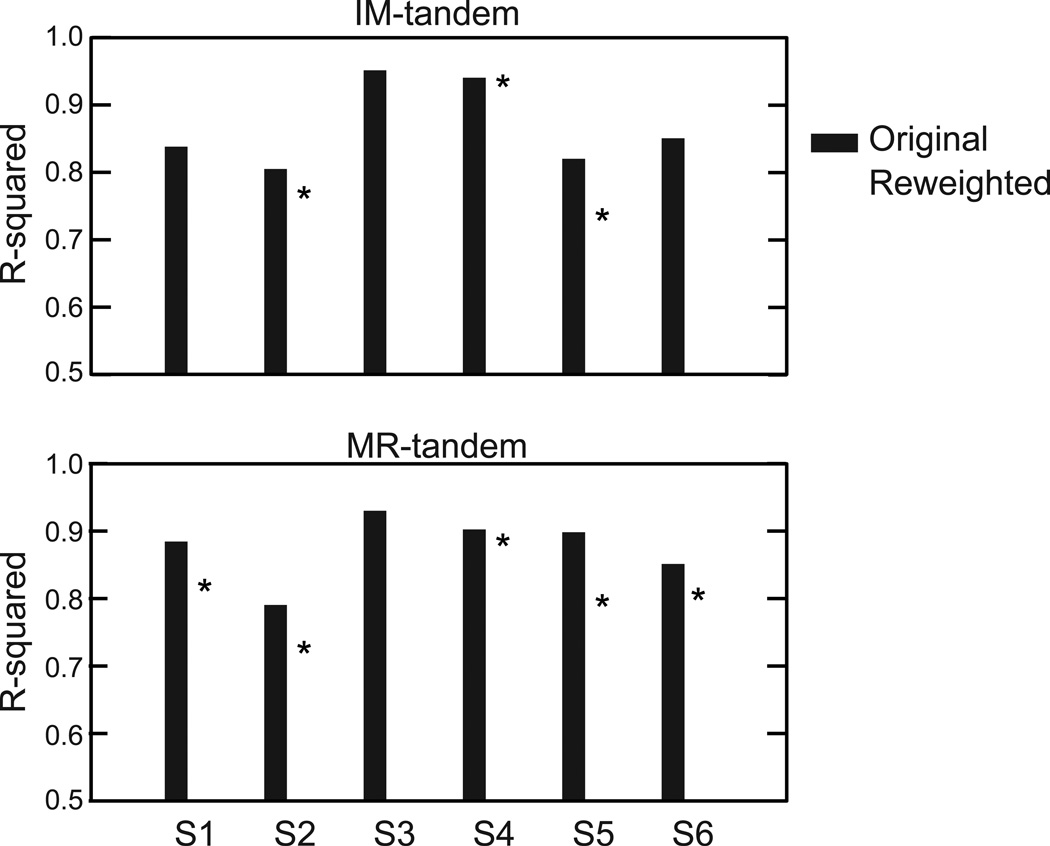
Further analysis on these three primary multi-muscle patterns addressed whether the patterns extracted from the control condition could be rescaled to match the patterns from the tandem conditions. To determine whether the control PCs were capable of representing the tandem EMG data equivalently to their uniquely derived PCs, EMG from the tandem conditions was regressed onto the control PC coefficients to calculate new loadings (see Methods). The new loadings were used to reconstruct the EMG for tandem conditions and then compared to the reconstructions that used the uniquely derived PC coefficients and loadings for the tandem conditions. R-squared values from the reconstructions were compared to determine whether reconstructions using the control PC coefficients were equivalent to those uniquely derived from each tandem condition. The results of this analysis using PC1–3 are shown in Fig 5. For each subject, paired t-tests were used to determine significant differences in R-squared for reconstructions of the tandem conditions. For the IM-tandem condition, three subjects had significantly lower R-squared values from the reconstructions using the control PCs; all except subject 3 had significantly lower R-squared values for the comparison of the MR-tandem condition. For comparison, we also performed this analysis using PCs 1–4 (VAF > 90% in all but two subjects for MR-tandem) which resulted in higher r-squared values but the differences between reconstructions using the uniquely derived or control PCs persisted.
Figure 5. Comparison of original and reweighted fits.
R-squared values for the fit of reconstructed EMG with PC 1–3 onto original EMG using the weightings from the original PCs (black) and the new weightings using PCs
The differences in how well the reweighted PCs fit the tandem data (R2) across subjects may be explained partially by referring to the results of the discriminant analysis. Figure 6 illustrates the relation between discriminability of conditions (% correctly classified) and the difference in fit for the uniquely derived PCs compared to the reweighted control PCs (reweighted – original) for the tandem conditions. Values for the R2 difference were always negative indicating that the fit using reweighted control PCs were never as good as the original for each tandem condition. For both tandem conditions, when subjects had % correctly classified scores of >70%, the fit for the reweighted control PCs was significantly worse than those uniquely derived for the respective condition. The regression lines illustrate the tendency for greater negative R2 differences to be associated with better discriminability between conditions for EMG and posture are shown in each upper panel of Figure 6. For the IM condition, the R2 differences were significantly correlated with the % correctly classified into condition for EMG (r = −0.902, p = 0.014) and posture (r = −0.844, p = 0.035). For the MR condition, the R2 differences were significantly correlated with the % correctly classified into condition for EMG (r = −0.831, p =0.040) but not posture (r = −0.482, p =0.333).
Figure 6. Relation between difference-R2 values and discrimination success.
Difference-R2 (original – reweighted) as a function of % correctly classified into condition by EMG (left) or Posture (right) for the IM and MR conditions (upper). % correctly classified into condition for EMG as a function of Posture (lower)
To examine the relation between condition related differences in posture and EMG, the % of correctly classified cases into condition was plotted for EMG as a function of posture (Fig. 6, lower). There was no significant correlation between the % of correctly classified cases of EMG and posture for the control (r = −0.081, p = 0.879) or MR condition (r = 0.366, p = 0.476), while the IM condition had a significant positive correlation (r = 0.847, p = 0.033). Therefore, differences in posture appear to be reflected in the muscle activity for the IM-tandem condition but not in the control or MR-tandem conditions.
4. Discussion
Somatosensory information gathered at the grasping-hand was sufficient for subjects to produce matching-hand postures that differed across grasped objects and grasp conditions (Table 1). For example, the matched posture differed when an iron was grasped compared to an apple and when the apple or iron was grasped freely or in one of the tandem conditions. Note that matching posture was achieved when the subject’s eyes were closed, excluding the use of online visual feedback to achieve the matched posture; nevertheless the remembered visual information likely contributed since viewing an object for a short time is enough to form motor commands dictating a hand posture specific to the grasped object in adults (Winges et al. 2003). The muscle activity recorded for the matched posture was also specific to the object being grasped. Although the rates of correct classification for EMG from individual trials were about 33% lower on average than those found for hand posture, the difference was consistent with a study by Weiss and Flanders (2004) who reported correct classification rates from 77–89% for grasping posture and 37–49% for EMG. In their study subjects actually wrapped their hand around objects while hand posture and muscle activity were recorded. Subjects in the current study were able to obtain comparable results without contacting the object. In both studies, the lower classification rates for EMG by object reflect the higher inter-trial variability that occurs in the muscle activity compared to joint posture. The redundancy in the neuromuscular system allows for this variability while still achieving the task goals. When hand posture and EMG for each object were discriminated based upon condition, correct classification rates were equivalent for the two measures. Therefore, the influence of constraining pairs of fingers in the grasping hand was appreciable in both hand posture and muscle activity of the matching hand. These results support the working hypothesis that online sensory input from the grasping (right) hand was incorporated into the motor command such that the desired movement was determined by the current somatosensory state of the right hand and mapped onto activation of motor units to achieve the desired hand posture. One limitation to the study was that the actual hand posture and muscle activity used by the grasping hand were not be measured, thus we are unable to make a direct comparison between the matching and grasping hand posture and muscle activity for a measure of accuracy. However, the specific differences between the free and tandem conditions for joint angles such as the middle and ring abduction angles indicate that subjects did not ignore the constrained hand. Additionally, there were significant differences in joint angles of digits in the matching hand outside of those being constrained in the grasping hand (Fig. 2), thus demonstrating an overall change in matching hand posture and reflecting the constraint at the grasping hand. Further, the correct classification of individual trials into their respective conditions also provides some evidence that subjects modified their hand postures with respect to the constraint.
To examine the changing structure of the motor command across conditions, we examined multi-muscle patterns of activation. The working hypothesis that the patterns of muscle activity (EMG amplitude) during the tandem conditions could be represented by rescaling the patterns revealed during the control (unconstrained) condition was not supported. PC analysis defined patterns of EMG amplitude across muscles for each condition. Rescaling the patterns revealed during the control condition did not result in fits equivalent to those uniquely derived for the tandem conditions (Fig. 5). For both muscle activity and posture there was a trend for greater discriminability between grasping conditions (higher classification rate) to be correlated with poorer fits by the reweighted control pattern for the tandem conditions (Fig. 6). For subjects whose matching hand posture and EMG were consistently influenced by the altered somatosensory information from the grasping hand, reflected by the high classification rates into grasp condition (Table 1), the patterns of multi-muscle EMG amplitude from the tandem conditions could not be equivalently represented by a simple rescaling of the control patterns.
4.1 Somatosensory information and the motor command
Many studies have supported the concept of an internal representation of object properties that can be utilized to predictively scale grip forces (Johansson and Westling, 1988; Flanagan and Wing 1997; Flanagan et al., 2006) for grasping and lifting objects; the basis of these internal representations may be mechanical properties of the object such as weight information (Gordon et al., 1994; Chang et al., 2008) or an internal sense of effort defined by previous forces used to manipulate the object (Bensmail et al., 2009, 2010; Quaney et al., 2003). Regardless of what was encoded, there is also support for the concept of an internal model formed in memory that can be updated by comparing actual somatosensory feedback with the expected somatosensory feedback (Nowak et al., 2004; Bensmail et al., 2010). Matching hand postures in the absence of vision would then occur by matching somatosensory information from the grasping hand to guide control of the matching hand. Using a matching task, Pesyna et al. (2011) demonstrated that motor commands to generate the matching hand posture could also be driven by an imagined somatosensory state, i.e. the matching hand posture was influenced by imagined somatosensory conditions such as inertial or spatial properties of the object that were not experienced at the grasping hand.
The current study contributed to this concept by examining the influence of altered somatosensory information on the matching posture and muscle activity. Constraining two digits to move in tandem when grasping familiar objects, results in a situation where the independent movements of the paired digits are limited and the repertoire of hand postures available is subsequently limited. The somatosensory input contains information about these limitations. To create matching hand postures for the tandem conditions, this somatosensory information would be used to update the internal memory model of hand shape by comparing the efference copy of the motor command and the somatosensory feedback from the grasping hand. Since we used familiar objects, the internal memory should have been reliable and useful (Deshpande et al., 2010) for comparing small differences in somatosensory information (Nowak et al., 2004) related to adjustments in hand shape required by constraining pairs of fingers to move in tandem. However, it is possible that the existing internal memory model for grasping these familiar objects would be used to drive the motor command without incorporating the sensory information related to external constraint on independent finger movement to complete the task, albeit presumably not as well.
The results of this study demonstrate that most subjects altered their matching hand postures and muscle activity for each object based on the somatosensory input from the grasping hand. Note that the matching hand was not constrained for any condition so the consistent differences in hand posture and muscle activity between conditions, i.e., discriminability by condition (Table 1), are most simply explained by differences in somatosensory inputs incorporated into the motor command. While biomechanical constraints most certainly influence independent finger movement, this influence would be consistent across conditions since the matching hand was unconstrained. Furthermore, the difference in muscle activity was not equivocally explained by rescaling of a set of muscle patterns used during the unconstrained condition, supporting the notion that motor unit activation was modified to achieve the desired somatosensory condition designated at the grasping hand.
4.2 Synergies and sensory feedback
Many studies have examined patterns of muscle activation using various methods of data reduction to define patterns which may be termed modules, primitives, M-modes that produce specific patterns of coactivation or reciprocal activation of groups of muscles (for recent reviews of muscle synergies, see Giszter and Hart 2013; Santello et al., 2013; Ting and McKay, 2007; Tresch and Jarc, 2009). This view is attractive because it simplifies the control of multiple muscles into patterns of activation which can be easily described for a given class of tasks. The concept of synergies has been applied to both upper and lower limb control (e.g. Berger and d’Avella, 2014; Cheung et al., 2009; Chvatal and Ting, 2013; MacLellan et al., 2014; Ting and Macpherson, 2005; Togo et al. 2012; Torres-Oviedo and Ting, 2010; Zelik et al., 2014). However, the actual structure of the modules or primitives and the flexibility or lack thereof in their combined activation for producing different movements is still a matter of debate (see Giszter et al. 2010; Tresch & Jarc, 2009).
One issue is that these computationally defined modules appear to be task-specific to some extent. For example, in a balance control task a small set of muscle synergies could represent the patterns of muscle activation for some types of stance while others required additional task-specific muscle synergies to be added (Torres-Oviedo and Ting, 2010). MacLellan et al. (2014) have suggested that what appear to be task-specific synergies may actually be a group of primitives that have been adapted by sensory feedback; thus, the selection of muscle synergies is shaped by sensory feedback (Lacquaniti et al. 2012; MacLellan et al. 2014). Santello et al. (2013) also describes a theoretical framework of synergies wherein sensory feedback plays an important role in shaping the coordination pattern of a flexible synergy to accommodate unexpected perturbations, and/or by affecting the state of premotor neurons and spinal motor nuclei. Within the realm of the present study, the altered (from normal) somatosensory information in the tandem conditions could be used to modify control at higher centers, i.e. involvement of specific premotor areas such as the ventral premotor area that has been involved in planning pantomimed grasps (Makuuchi et al. 2012), and/or transferred via corpus callosum to motor areas of the other cortical hemisphere to drive motor commands as observed during grasp and lift tasks (Gordon et al. 1994; Nowak et al., 2005; Nowak et al. 2009). The result being modifications in the muscle synergies that subjects used to match the normal grasp compared to those used to match grasps in the tandem conditions; the difference reflecting the influence of the altered somatosensory information from the grasping hand used to formulate the motor commands to match the posture. Regardless of the mechanism, it is apparent that somatosensory information can be incorporated into the motor command and influences the activation of motor units; if this were not the case a simple rescaling of the muscle patterns would have yielded equivalent fits. Thus it appears that external restriction of independent finger movement was signaled by somatosensory feedback and was incorporated into the desired state driving the motor command which resulted in selective activation of groups of muscles. Therefore, these results provide some support for the neural control of complex hand movements through the use of flexible muscle synergies to accommodate specific task constraints.
Highlights.
Somatosensory information contributes to the perception of hand shape
Muscle synergies depend on available hand postures for object manipulation
External constraints are incorporated into motor commands driving hand posture
Acknowledgements
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-27484.
The author thanks Martha Flanders and Krishna Pundi for assistance on the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baugh LA, Kao M, Johansson RS, Flanagan JR. Material evidence: interaction of well-learned priors and sensorimotor memory when lifting objects. J Neurophysiol. 2012;108:1262–1269. doi: 10.1152/jn.00263.2012. [DOI] [PubMed] [Google Scholar]
- Bensmail D, Sarfeld AS, Fink GR, Nowak DA. Sensorimotor processing in the grip-lift task: the impact of maximum wrist flexion/extension on force scaling. Clin Neurophysiol. 2009;120:1588–1595. doi: 10.1016/j.clinph.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Bensmail D, Sarfeld AS, Fink GR, Nowak DA. Intermanual transfer of sensorimotor memory for grip force when lifting objects: the role of wrist angulation. Clin Neurophysiol. 2010;121:402–407. doi: 10.1016/j.clinph.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Berger DJ, d'Avella A. Effective force control by muscle synergies. Front Comput Neurosci. 2014;8:46. doi: 10.3389/fncom.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Flanagan JR, Goodale MA. The intermanual transfer of anticipatory force control in precision grip lifting is not influenced by the perception of weight. Exp Brain Res. 2008;185:319–329. doi: 10.1007/s00221-007-1156-0. [DOI] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Bizzi E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviors. J Neurophys. 2009;101:1235–1257. doi: 10.1152/jn.01387.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comput Neurosci. 2013;7:48. doi: 10.3389/fncom.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KJ, Potash M, Peterson C. Failure to disrupt the 'sensorimotor' memory for lifting objects with a precision grip. Exp Brain Res. 2008;184:157–163. doi: 10.1007/s00221-007-1088-8. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Hu X, Lacey S, Stilla R, Sathian K. Object familiarity modulates effective connectivity during haptic shape perception. NeuroImage. 2010;49:1991–2000. doi: 10.1016/j.neuroimage.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JR, Bowman MC, Johansson RS. Control strategies in object manipulation tasks. Curr Opin Neurobiol. 2006;16:650–659. doi: 10.1016/j.conb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17:1519–1528. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders M. What is the biological basis of sensorimotor integration? Biol Cybern. 2011;104:1–8. doi: 10.1007/s00422-011-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Hart CB. Motor primitives and synergies in spinal cord and after injury–the current state of play. Ann N Y Acad Sci. 2013;1279:114–126. doi: 10.1111/nyas.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter S, Hart C, Silfies SP. Spinal cord modularity: evolution, development, and optimization and the possible relevance to low back pain in man. Exp Brain Res. 2010;200:283–306. doi: 10.1007/s00221-009-2016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Forssberg H, Iwasaki N. Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia. 1994;32:555–568. doi: 10.1016/0028-3932(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Westling G, Cole KJ, Johansson RS. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophys. 1993;69:1789–1796. doi: 10.1152/jn.1993.69.6.1789. [DOI] [PubMed] [Google Scholar]
- Jerde TE, Soechting JF, Flanders M. Coarticulation in fluent fingerspelling. J Neurosci. 2003;23:2383–2393. doi: 10.1523/JNEUROSCI.23-06-02383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci. 2009;10:345–359. doi: 10.1038/nrn2621. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988;71:59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol. 2012;590:2189–2199. doi: 10.1113/jphysiol.2011.215137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukos JR, Choi JY, Santello M. Grasping uncertainty: effects of sensorimotor memories on high-level planning of dexterous manipulation. J Neurophys. 2013;109:2937–2946. doi: 10.1152/jn.00060.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclellan MJ, Ivanenko YP, Massaad F, Bruijn SM, Duysens J, Lacquaniti F. Muscle activation patterns are bilaterally linked during split-belt treadmill walking in humans. J Neurophys. 2014;111:1541–1552. doi: 10.1152/jn.00437.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M, Someya Y, Ogawa S, Takayama Y. Hand shape selection in pantomimed grasping: Interaction between the dorsal and the ventral visual streams and convergence on the ventral premotor area. Hum Brain Mapp. 2012;33:1821–1833. doi: 10.1002/hbm.21323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Hermsdörfer J, Timmann D, Rost K, Topka H. Impaired generalization of weight-related information in cerebellar degeneration. Neuropsychologia. 2005;43:20–27. doi: 10.1016/j.neuropsychologia.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hufnagel A, Ameli M, Timmann D, Hermsdörfer J. Interhemispheric transfer of predictive force control during grasping in cerebellar disorders. Cerebellum. 2009;8:108–115. doi: 10.1007/s12311-008-0081-5. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Rosenkranz K, Hermsdorfer J, Rothwell J. Memory for fingertip forces: passive hand muscle vibration interferes with predictive grip force scaling. Exp Brain Res. 2004;156:444–450. doi: 10.1007/s00221-003-1801-1. [DOI] [PubMed] [Google Scholar]
- Pesyna C, Pundi K, Flanders M. Coordination of hand shape. J Neurosci. 2011;31:3757–3765. doi: 10.1523/JNEUROSCI.5158-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PJ, Cole KJ. Limited persistence of the sensorimotor memory when transferred across prehension tasks. Neurosci Lett. 2011;494:94–98. doi: 10.1016/j.neulet.2011.02.066. [DOI] [PubMed] [Google Scholar]
- Quaney BM, Rotella DL, Peterson C, Cole KJ. Sensorimotor memory for fingertip forces: evidence for a task-independent motor memory. J Neurosci. 2003;23:1981–1986. doi: 10.1523/JNEUROSCI.23-05-01981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Postural hand synergies for tool use. J Neurosci. 1998;18:10105–10115. doi: 10.1523/JNEUROSCI.18-23-10105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Baud-Bovy G, Jorntell H. Neural bases of hand synergies. Front Comput Neurosci. 2013;7:23. doi: 10.3389/fncom.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophys. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol. 2007;17:622–628. doi: 10.1016/j.conb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo S, Kagawa T, Uno Y. Motor synergies for dampening hand vibration during human walking. Exp Brain Res. 2012;216:81–90. doi: 10.1007/s00221-011-2909-3. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophys. 2010;103:3084–3098. doi: 10.1152/jn.00960.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. 2009;19:601–607. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophys. 2004;92:523–535. doi: 10.1152/jn.01265.2003. [DOI] [PubMed] [Google Scholar]
- Winges SA, Soechting JF, Flanders M. Multidigit control of contact forces during transport of handheld objects. J Neurophys. 2007;98:851–860. doi: 10.1152/jn.00267.2007. [DOI] [PubMed] [Google Scholar]
- Winges SA, Weber DJ, Santello M. The role of vision on hand pre-shaping during reach to grasp. Exp Brain Res. 2003;152:489–498. doi: 10.1007/s00221-003-1571-9. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:R729–R732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Zelik KE, La Scaleia V, Ivanenko YP, Lacquaniti F. Can modular strategies simplify neural control of multidirectional human locomotion? J Neurophys. 2014;111:1686–1702. doi: 10.1152/jn.00776.2013. [DOI] [PubMed] [Google Scholar]