Abstract
Parkinson’s disease (PD) is associated with abnormal synchronization in basal ganglia-thalamo-cortical loops. We tested whether early PD patients without demonstrable cognitive impairment exhibit abnormal modulation of functional connectivity at rest, while engaged in a task, or both. PD and healthy controls underwent two functional MRI scans: a resting-state scan and a Stroop Match-to-Sample task scan. Rest-task modulation of basal ganglia (BG) connectivity was tested using seed-to-voxel connectivity analysis with task and rest time series as conditions. Despite substantial overlap of BG–cortical connectivity patterns in both groups, connectivity differences between groups had clinical and behavioral correlates. During rest, stronger putamen–medial parietal and pallidum–occipital connectivity in PD than controls was associated with worse task performance and more severe PD symptoms suggesting that abnormalities in resting-state connectivity denote neural network dedifferentiation. During the executive task, PD patients showed weaker BG-cortical connectivity than controls, i.e., between caudate–supramarginal gyrus and pallidum–inferior prefrontal regions, that was related to more severe PD symptoms and worse task performance. Yet, task processing also evoked stronger striatal–cortical connectivity, specifically between caudate–prefrontal, caudate–precuneus, and putamen–motor/premotor regions in PD relative to controls, which was related to less severe PD symptoms and better performance on the Stroop task. Thus, stronger task-evoked striatal connectivity in PD demonstrated compensatory neural network enhancement to meet task demands and improve performance levels. fMRI-based network analysis revealed that despite resting-state BG network compromise in PD, BG connectivity to prefrontal, premotor, and precuneus regions can be adequately invoked during executive control demands enabling near normal task performance.
Keywords: Parkinson’s disease, cognitive control, response switching, fMRI, task-related and resting-state functional connectivity, neural compensation
1 Introduction
Parkinson’s disease (PD) is marked by dopaminergic dysfunction in midbrain structures projecting to basal ganglia (BG) and affecting motor functions and cognition via interconnected corticostriatal circuits (Albin et al., 1989; Betchen and Kaplitt, 2003; Damier et al., 1999). Neuroimaging studies in PD have identified neural network changes of BG and motor areas affecting movement behavior, and BG–frontal cortex connectivity affecting cognition (Leisman and Melillo, 2013). Motor impairment is the main clinical feature of PD, but cognitive deficits, particularly those involving mental flexibility, conflict resolution, and executive control (e.g., planning, initiation and monitoring of actions) (Aarsland et al., 2003; Caballol et al., 2007; Dubois and Pillon, 1997; Vandenbossche et al., 2011), are frequently observed in the advanced stages of PD, and can emerge even at the early mild disease stages (Aarsland et al., 2010) and before treatment commences (Cooper et al., 1992). Disturbances of executive functions in PD that involve conflict resolution and task switching (Greenhouse et al., 2013; Obeso et al., 2011) can affect planning and carrying through tasks in daily life (Dirnberger and Jahanshahi, 2013); little is known about their neurofunctional correlates (Aarsland et al. 2003; Caballol et al., 2007; Dubois and Pillon, 1997).
Cognitive functions and action selection are mediated through the BG (Stafford and Gurney, 2007). This set of subcortical nuclei (globus pallidus, caudate, putamen) interacts closely with the thalamus, cerebellum, frontal and premotor cortices (DeLong and Wichmann, 2007; McNab and Klingberg, 2008) and forms a series of anatomically and functionally segregated circuits, and separable cognitive and motor BG loops (Graybiel et al., 1994; Middleton and Strick, 2000; Pasquereau et al., 2007). Dopamine signaling can modulate the dynamic synchronization of neural activity within and between brain structures (Dzirasa et al., 2009). Disruption of these circuits through the degeneration of dopaminergic neurons in the substantia nigra in PD potentially results in widespread changes in brain activity and connectivity (e.g., Costa et al., 2006; Fuentes et al., 2009; Martinu and Monchi, 2013; Poston and Eidelberg, 2012).
Functional connectivity neuroimaging has been used in cognitively unimpaired PD to examine the brain’s intrinsic functional architecture at a resting state. Although unimpaired on behavioral cognitive tests, PD patients demonstrated altered functional brain connectivity at rest that involved bilateral inferior parietal cortices and the right medial temporal lobe (Tessitore et al., 2012). Other studies reported decreased resting-state connectivity in patients with mild PD, relative to controls, from substantia nigra/BG to several cortical regions including supplementary motor area (SMA) and dorsolateral prefrontal cortex (dlPFC) (Wu et al., 2012), and in patients with advanced PD, from the striatum to thalamus, midbrain, pons and cerebellum (Hacker et al., 2012). Notably, Helmich et al. (2010) reported a dissociated striatal connectivity pattern in mild PD, with abnormally weak posterior putamen and abnormally strong anterior putamen connectivity to somatosensory and motor cortical regions. Helmich et al. (2010) speculated that early in the course of PD the cortical sensorimotor system becomes partitioned into different cortico-striatal loops and that the cortico-striatal remapping may also impair cortico-cortical sensorimotor integration function. Greater than normal connectivity may indicate neurofunctional compensation with the anterior putamen compensating for the more-dopamine depleted posterior portions of the striatum (Helmich et al., 2010). Similarly, greater than normal premotor cortical and cerebellar activity was observed in PD patients performing motor tasks and interpreted as compensatory (Catalan et al., 1999; Wu and Hallett, 2005; Wu et al., 2014). In animal models of PD corticostriatal plasticity was observed as sprouting of dopamine terminals (Song and Haber, 2000) and diffusion of dopamine to more distant targets (Stanic et al., 2003; Stafella et al., 2005) enhancing interactions in corticostriatal circuits that are normally segregated (Bergmann et al., 1998). Yet, when a specific pattern of functional connectivity signifies neural coherence necessary for network efficiency, extended activity to other regions can be a failure to keep neural coherence confined to that specific network and result in poor cognitive or motor performance. Such forms of network dedifferentiation have been reported in normal aging (Park et al., 2001) and in PD during the resting state and associated with motor symptoms (Baudrexel et al., 2011; Helmich et al., 2011; Yu et al., 2007). Thus, changes in the dynamic modulation of neural synchrony in frontostriatal and BG-thalamic-motor cortex circuits during rest and task involvement in PD patients may uncover the role of functional subcortico-cortical networking for motor functions and cognition.
To further understanding of the role of BG functional network connectivity in cognitive and motor control function and its modulation in PD, we used a modified Stroop task, i.e., the Stroop Match-to-Sample task (Schulte et al., 2011). Typically, brain areas associated with Stroop conflict resolution are frontoparietal networks, including the dlPFC, anterior cingulate cortex (ACC) (Milham and Banich, 2005) and posterior parietal regions (Liu et al., 2004; Schulte et al., 2009, 2011). These cortical regions are closely connected with the BG forming cortico-BG loops (Arimura et al., 2013) that play an essential role in neuronal computational processes underlying controlled and automatic aspects of motor behavior (Wu et al., 2014) and cognition in PD (Lebedev et al., 2014). The modified Stroop Match-to-Sample task tested motor control in addition to cognitive functions by manipulating response selection demands requiring subjects either to switch responses or to repeat the same response several times in a row. There is evidence that PD patients do well when engaging in more automatic behavior, and that executive functions are especially impaired when automatic behavior needs to be overridden (Cameron et al., 2010). Accordingly, we expected no impairment in PD for task conditions that engage more automatic behavior, i.e., when responses are repeated (vs. switched) in no-conflict trials. However, mild PD patients will be impaired in cognitive conflict trials, when automatic processing of the word’s meaning needs to be overridden for correct color matching. In addition, we expected normal controls to show conflict adaptation effects, expressed as less conflict with repetition, not mild PD patients.
Accordingly, we hypothesized that 1) at the behavioral level, PD patients’ ability to exert cognitive control in a Stroop paradigm would be modulated by response demands such as response switching and repetition. We further assumed that 2) at the neurofunctional level, PD patients, relative to controls, would exhibit weaker BG functional connectivity for motor and cognitive circuits during rest and engage additional areas outside the main regions (of the corticostriatal network) during task processing.
3) Functional compensation versus dedifferentiation hypothesis: In principle, brain networks have the potential to enhance function with redistribution of resources (Schulte et al., 2011), but in mild PD, this likely comes at the expense of usurping functional reserve. We expect compensatory neural network engagement during task processing in mild to moderate PD but not during rest. 3a) Neural compensation: To demonstrate that alternative neural processes are in fact compensatory, any synchronous activity in mild PD not seen in the healthy aging comparison group should be associated with normal level performance (c.f., Davis et al., 2008). 3b) Neural dedifferentiation: By contrast, if extended BG connectivity to additional regions outside the main network correlates with poorer performance and more severe clinical symptoms this would indicate network dedifferentiation and support a pathophysiological view of BG functional circuitry expansion in PD (see e.g., Martinu and Monchi, 2013).
2 Material and Methods
2.1 Participants
Groups comprised 11 patients with mild Parkinson Disease (PD) (Gelb et al., 1999) and 11 age-matched control (CTL) men and women (Table 1). PD participants were recruited from the Valley Parkinson Clinic and through a local Parkinson’s support group; controls were volunteers from the community. Of the 15 patients who contacted us after hearing about the study, 11 completed the study, 1 moved out of the area, and 3 decided not to participate. Subjects had completed at least 8 years of education. All participants underwent a SCID interview, semi-structured health history interview, and general neurological examination. All were free of lifetime schizophrenia or bipolar disorder, neurological illness other than PD, medical conditions potentially affecting the CNS (e.g., stroke, diabetes), or MRI contraindications. PD patient inclusion criteria were the diagnosis of PD from a board certified neurologist, Hoehn and Yahr stages 1–2 (mild to moderate PD), no cognitive dementia (DRS-2; Jurica et al., 2004; cut-off score ≤132/144, Pedraza et al., 2010; for a discussion on cut-off scores for PD see e.g., Fernández de Bobadilla et al., 2013; Pirogovsky et al., 2014). PD patients also underwent assessments of ‘mentation’ (part I), daily living (part II) and motor function (part III) with the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn and Elton, 1987) by trained clinicians. Written informed consent was obtained from all participants, and the Institutional Review Board of SRI International approved the study.
Table 1a.
Demographic and clinical data for the Parkinson’s disease (PD) and control (CTL) groups
| Sex w/m | Age | Educ (yrs) | BMI | BDI | verbal IQ | DRS total | Handedness | PD stage | UDPRS
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||||||
| PD | 6/5 | 63±6 (52–72) | 16±2 (12–18) | 28±4 (22–35) | 8.6±5 (0–15) | 105±9 (90–118) | 138±4 (128–143) | 21±15 (14–67) | 1.5 (1–2.5) | 2.1 (0–3) | 7.8 (2–14) | 9.5 (3–17) |
| CTL | 4/7 | 62±5 (54–73) | 17±2 (13–21) | 25±3 (21–29) | 1.6±2 (0–7) | 113±7 (101–119) | 140±2 (136–144) | 20±7 (14–37) | ||||
| p < | ns1 | ns | ns | .024 | .001 | .024 | ns | ns | ||||
PD=Parkinson’s disease; CTL=healthy controls; w=women, m=men; mean ± standard devastation (range) for education in years (Educ); Body Mass Index (BMI); depressive symptoms (BDI-2; Beck Depression Inventory); PD stage (modified Hoehn and Yahr Staging; Fahn and Elton, 1987); Unified Parkinson’s Disease Rating Scale (UPDRS): mean (range) for 3 subscales: mentation (part I), daily living (part II), motor (part III) ability; verbal IQ (WTAR); Dementia Rating Scale (DRS-2) (Jurica et al., 2004); Handedness inventory (Crovitz and Zener, 1962): scores 14–32 right-handed, 50–70 left-handed; 1 left-handed PD; 1 ambidextrous CTL.
Chi-square statistic
PD and control subjects were matched in age, gender, level of education, and handedness (Table 1). Verbal IQ (WTAR) levels of all participants were within the normal range and, on average, slightly lower in the PD than the control group. Groups did not differ in Dementia Rating Scale (DRS-2) total scores (t(20)=1.43, p=.17). DRS-2 total scores of all participants were within the normal range except for one subject with a total score of 128, who scored low in the sub-category ‘Conceptualization’ (6th–10th age-corrected percentile) (Paolo et al., 1995). Pirogovsky et al. (2014) classified mild cognitive impairment (MCI) in PD for those with complaint in the Unified Parkinson’s Disease Rating Scale (UPDRS Part I, item 1.1.), which assesses the effect of cognitive impairment on daily functioning. This PD patient scored a 0, i.e., no complaint. In addition, as per Movement Disorder Society (MDS) criteria, PD-MCI is diagnosed if there is impairment on at least two neuropsychological tests represented by either two impaired tests in one cognitive domain or one impaired test in two different cognitive domains. Yet, this PD subject had a normal verbal IQ and normal performance level in all other DRS sub-categories ‘Attention, Initiation/Perseveration, Construction, and Memory’ and in the Stroop Match-to-Sample task. Based on these considerations, we included this PD participant in the study.
On average, the PD group had a greater body mass index (BMI) and reported more depressive symptoms (BDI-2) (Beck et al., 1996) than the control group (Table 1a). PD subjects showed mild to moderate symptoms, ranging between 1 (unilateral symptoms) and 2 (bilateral disease, without impairment of balance) on the modified Hoehn & Yahr Staging scale (Fahn and Elton, 1987; Hoehn and Yahr, 1967). Pharmacological treatment was not modified from previous regime during testing (c.f., Baglio et al., 2011). The time since PD diagnosis was on average 3.1 (± 3) years (range: 1 month to 8 years). Medication involved a combination of MAO-B inhibitors, dopamine agonists (DA), and levodopa (l-dopa) in 7 patients, 3 patients were taking MAO-B inhibitors alone, and 1 patient was not treated with any dopamine replacement at the time of the study; yet, at a follow-up phone interview more than a year after the study this patient reported taking DV/L-dopa medication and that the medication was effective in reducing PD symptoms. Levodopa equivalent daily dose (LEDD) was calculated for each patient (Herzog et al., 2003; http://www.birmingham.ac.uk/Documents/college.mds/trials/bctu/PDRehab/Investigators/meetings/2010-2/CSmithLEDReview.pdf) and was on average 473 (± 484 SD) mg (Table 1b). LEDD was not related to UPDRS scores (all p> 0.2) or Hoehn and Yahr staging (Rho=.05, p=0.87) but was to longer duration since PD diagnosis (Rho=.74*, p=0.009).
Table 1b.
Clinical characteristics of the cohort of patients with PD enrolled in the study.
| Subject no. | Age years | Sex | Diagnosis | Years since diagnosis | Lateralization | H & Y score | UPDRS total score | Medication/Treatment | LEDD in mg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | Possible PD | 0.5 | Right | 1.5 | 23 | MAOB | 100 |
| 2 | 72 | F | Possible PD | 1.0 | Right | 1 | 11 | MAOB | 100 |
| 3 | 67 | M | Possible PD | 0.1 | Bilateral | 2 | 34 | - | 0 |
| 4 | 66 | F | Probable PD | 7.0 | Right | 1.5 | 17 | cl-dopa, DA, MAOB | 1175 |
| 5 | 52 | F | Possible PD | 2.0 | Left | 1.5 | 31 | cl-dopa, DA, MAOB | 1500 |
| 6 | 63 | M | Probable PD | 7.5 | Left | 1 | 16 | l-dopa, DA, MAOB | 740 |
| 7 | 58 | M | Probable PD | 4.0 | Right | 1.5 | 27 | cl-dopa, DA, MAOB | 460 |
| 8 | 63 | M | Possible PD | 2.5 | Bilateral | 2 | 20 | cl-dopa, DA, MAOB | 487.5 |
| 9 | 65 | F | Possible PD | 1.5 | Left | 1 | 6 | DA, MAOB | 220 |
| 10 | 69 | F | Possible PD | 0.2 | Right | 1 | 10 | MAOB | 100 |
| 11 | 52 | F | Probable PD | 8.2 | Right | 1 | 18 | cl-dopa, DA, MAOB | 325 |
M= male; F = female; H&Y= Hoehn andYahr Scale; MAOB = Monoamine Oxidase B inhibitor, cl-dopa = Carbidopa-Levodopa, DA = Dopamine Agonists; LEDD = Levodopa equivalent daily dose. Diagnosis has been defined according to Gelb’s criteria (Gelb et al., 1999).
2.2 Stroop Match-to-Sample Task
Subjects performed a Stroop Match-to-Sample task (Schulte et al., 2008, 2009, 2011) in the scanner. Stimuli were created and presented with PsyScope software. Subjects matched the color of a cue stimulus to the color of a Stroop target stimulus. The color cue was presented prior to the Stroop stimulus and either matched or did not match the font color of the Stroop target, which was either congruent (e.g., the word BLUE written in blue font) or incongruent (e.g., the word BLUE written in red font). Subjects were instructed to match the color of the cue to the font color of the Stroop target stimulus. They pressed a YES-key for cue-target color matches and a NO-key for nonmatches using index and middle fingers of their dominant hand, yielding accuracy and reaction time measures. The task used a blocked fMRI design with four blocks requiring response switches (mixed-response blocks) that contained both match (YES-response) and nonmatch (NO-response) trials; and four block requiring response repetitions (same-response blocks) that contained either match trials or nonmatch trials only. Trials presented in same- and mixed-response blocks were the same, only the order of trials differed. Each block had 6 trials; each trial started with a color patch presented for 700 ms, followed by an interstimulus interval of 700 ms, and then the Stroop color-word stimulus was presented for 700 ms; the intertrial interval was 2660 ms. All participants performed a whole task run outside the scanner to ensure that the task instructions were understood and well practiced. Test instructions were further reviewed with the subject by the examiner in a short practice session (8 trials) before entering the scanner and also via the scanner’s intercom system before the onset of the task run. The start of the task was preceded by a countdown of 11 sec. Lengths of scan and number of volumes collected were the same for the task and resting-state fMRI runs.
2.2.1 Behavioral Stroop data and Z-transformation
Errors and reaction times (RTs) were recorded for each trial. Individual mean RTs and SD for correct responses were calculated for each Stroop condition. RT is direct measures of performance that provide information on the effect of an experimental manipulation in milliseconds. To control for task difficulty and individual differences in response latencies (RT) for each participant regardless of diagnosis, we calculated individual z-scores for all Stroop task conditions. Z-scores were obtained by taking the individual’s condition RT means, subtracting the overall mean (collapsed across conditions), and dividing by the standard deviation of the condition mean (Faust et al., 1999).
We used z-transformed reaction time data (z-scores) to test the relationships among Stroop task performance, Parkinson Disease severity, and functional connectivity strength. Z-transformation allowed us to study whether PD severity and brain functional connectivity measures are related to specific component processes of Stroop conflict processing (incongruent–congruent), attentional cueing (cue-target match–nonmatch), and response repetition–switching, thereby controlling for individual difference in overall response latency and condition difficulty (Faust et al., 1999).
2.3 Magnetic Resonance Imaging (MRI)
Structural and functional MR imaging data were acquired using a clinical whole-body GE 3T scanner. Subjects were placed comfortably in the scanner with cushions under neck, knees, and the sides of the head in the 8-channel GE head coil. In addition, a headband is fastened over the forehead as a reminder to keep as still as possible. During the 5-minute resting-state fMRI scan session, participants were instructed to lie relaxed with their eyes open and not to think about anything in particular. A mirror mounted on the head coil enabled subjects to look out of the scanner. During the 5-minute task-activated fMRI scan session, participants performed the Stroop Match-to-Sample task.
2.3.1 Data acquisition and analyses
Subject motion was minimized by following best practices for head fixation, and structural image series were inspected for residual motion. Whole-brain fMRI data were acquired with a T2*-weighted gradient echo planar pulse sequence (2D axial, TE=30ms; TR=2200ms; flip angle=90°; in plane resolution=3.75mm; thick=5mm; skip=0mm; locations=36; FOV=240mm; 1 NEX). Instructions were reviewed with the subject by the examiner via the scanner intercom system before the onset of the task and the resting-state scans. Five dummy scan volumes (not saved to disk) were scanned to establish an equilibrium magnetization prior to task and resting-state image acquisition. A dual-echo FSE (2D axial; TR=5000ms; TE=17/102ms; thick=5 mm; skip=0mm; xy matrix=256; flip angle=90°; locations=36; FOV=240mm; 1 NEX) was used for spatially registering the fMRI data. A field map for correction of spatial distortions in the echo-planar images was generated from a gradient-recalled echo sequence pair (TR=460ms, TE=3/5ms, thickness=5mm, skip=0mm, locations=36).
Image preprocessing was performed using the SPM8 software package (Wellcome Department of Cognitive Neurology; www.fil.ion.ucl.ac.uk/spm/software/spm8/). The fMRI analysis focused on the whole brain. Participants inclusion criteria for fMRI image analysis was head motion <2mm in any direction, and was met by all subjects for both the task-activated and resting-state fMRI scan series. The functional images were subjected to geometric distortion (field map) correction and motion correction. The FSE structural images were co-registered to the mean unwarped and motion-corrected functional image for each subject and functional and structural images were normalized to Montreal Neurological Institute (MNI) space. Functional volumes were smoothed with a Gaussian kernel of 8mm3 (FWHM).
For functional connectivity analysis and group comparison, the SPM-based conn toolbox (www.nitrc.org/projects/conn/) was used. Correlational analyses between the BOLD signal from an a priori selected seed for each network (Raichle et al., 2001; Raichle, 2011) and from every other brain voxel during the entire acquisition condition (135 volumes) provided seed-to-voxel connectivity estimations for each subject. Movement parameters were entered as first-level covariates in the model.
Before averaging individual voxel data, the waveform of each brain voxel was filtered using a bandpass filter (0.0083/s < f < 0.15/s) to reduce the effect of low-frequency drift and high-frequency noise. Several sources of spurious variance along with their temporal derivatives were then removed from the data through linear regression: signal from movement, signal from ventricular regions, and signal from the white matter. Global signal was not included as a regressor, given evidence that this may introduce spurious anticorrelations into the data (Murphy et al., 2009). Because further steps included between-groups comparisons, temporal connectivity maps were generated for each subject across the conditions. These images were then included in a second-level between-groups, random-effects analysis.
2.3.2 Seed region selection
Subcortical seed regions were selected from the aal template (www.fil.ion.ucl.ac.uk/spm/ext/) and included the putamen, caudate nucleus, globus pallidus, and thalamus.
2.3.3 Second-level between-groups, random-effects analysis
Seed-to-voxel connectivity maps for each group were derived via individual time series correlations of activity over 135 time points, an index of synchronous activity, for task and resting-state scans. Within-group analyses describe seed-to-voxel correlations for each group that met criteria for statistical significance of pFWE-corrected < 0.05, with the maps thresholded at the peak level at p = 0.001 with kminimum extent = 50 voxels. For between-group analyses, statistical thresholds for combined peak intensity and spatial extent were set at pFWE corrected < 0.05 (Poline et al., 1997).
2.4 Statistical analyses
To examine whether Parkinson patients differed from controls in Stroop conditions testing cognitive control and response selection demands, we used mixed effects ANOVA with group (PD, CTL) as the between-subject factor and condition (rest, task) as the repeated-measures factor. For correlation analyses, we used 2-tailed Spearman’s Rho correlations. Multiple linear regression analysis was employed to test for the combined effect of PD-related factors such as daily living symptom severity (UPDRS-II), motor symptom severity (UPDRS-III), years since PD diagnosis, and LEDD on Stroop component processes for response repetition and response switching. The alpha level was set to p<0.05 for all statistical tests; results significant at alpha levels FDR-corrected for multiple comparisons (Benjamini et al., 2001) are marked with a star *.
3 Results
3.1 Stroop Match-to-Sample task performance
Both groups showed high accuracy rates and few errors. On average PD committed 7 (6%) and controls (CTL) 3 errors (2%), which was not significantly different (t=1.79, p=0.09). Stroop task-derived response times (RTs) showed a trend to be slower in PD (mean of 910±333 ms) than CTL (mean of 708±68 ms) (t=1.97, p=0.075). Testing in each group whether individuals with faster responses committed more errors (i.e., lax response criterion) (Salo et al., 2001; Schulte et al., 2005), we found the opposite effect, a positive correlation between response time and error count (CTL r=.47, ns; PD r=.64, p=0.034).
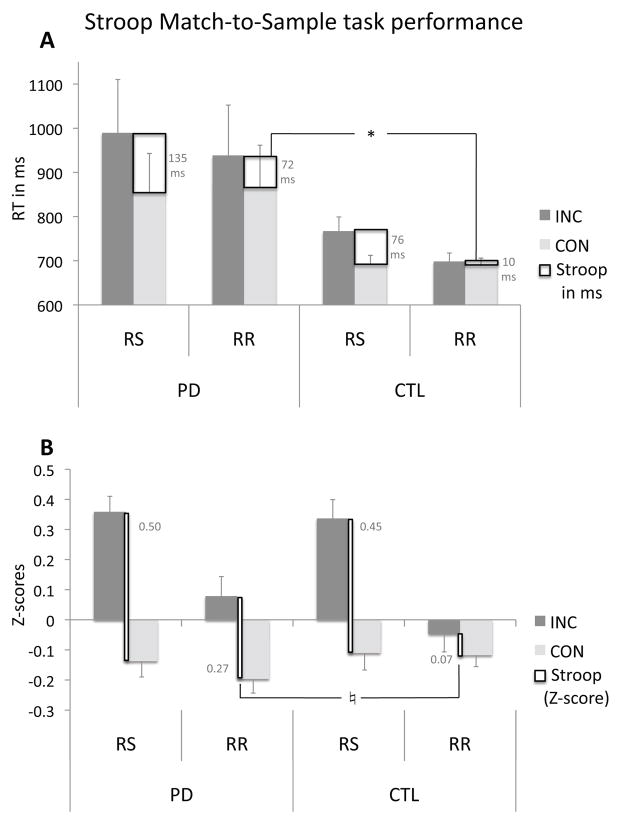
To test for the effect of PD on Stroop interference for the switching and repetition response blocks, reaction time data were submitted into repeated-measures ANOVA with Stroop (incongruent, INC vs. congruent, CON) and motor response (switching, RS vs. repetition, RR) conditions as within-subject factors and group (PD, CTL) as the between-subject factor. Main effects were observed for Stroop and motor-response conditions: longer RTs for incongruent than congruent Stroop words (F(1,20)=22.3, p<0.0001), and longer RTs for response switching than repetitions (F(1,20)=10.66, p=0.004). A Stroop-by-response block interaction indicated greater Stroop effects (incongruent > congruent) for response switches than repetitions (F(1,20)=2.27, p<0.0001). PD showed a trend for greater Stroop effects than controls (F(1,20)=3.23, p=0.068). Following-up on the Stroop-by-response block interaction and based on our hypothesis that cognitive control (Stroop) is modulated by response control demands, we calculated Stroop effects (RTINC minus RTCON) for response switching (RS) and response repetition (RR) conditions, and found similar Stroop-RS effects in both groups (F(1,20)=1.8, ns) but greater Stroop-RR effects in PD than controls (MANOVA; F(1,20)=5.2, p=0.034) that was due to a reduction of interference with response repetition nearly eliminating Stroop conflict in controls (see incongruent condition; INC) (Figure 1A). This analysis was confirmed using Z-transformed scores correcting for individual differences in response latencies (Figure 1B).
Figure 1.
Stroop Match-to-Sample task performance of patients with mild Parkinson’s disease (PD) and healthy controls (CTL). (A) Mean reaction times (RT) and (B) Z-scores and S.E. to incongruent (INC) and congruent (CON) Stroop conditions for response switching (RS) and response repetition (RR). Stroop effects are defined as the difference in RT between incongruent and congruent conditions. Z-scores depict the condition-specific deviation in response latency corrected for each person’s own motoric response speed. Negative values illustrate response facilitation and positive values response slowing. For (B), an ANOVA using Z-scores confirmed the Stroop-by-response block interaction with greater Stroop effects for RS than RR (F(1,20)=15.95, p<0.001). A MANOVA testing group differences for Stroop-RS and Stroop-RR (ZINC minus ZCON) confirmed that both groups had similar Stroop-RS effects (F(1,20)=0.17, ns). The significantly (*) greater Stroop-RR effect in PD than CTL using RT measures (A) emerged as a trend (♮) using Z-scores (F(1,20)=3.18, p=0.09) (B), i.e., when controlling for group differences in motoric slowing and testing interference processing per se.
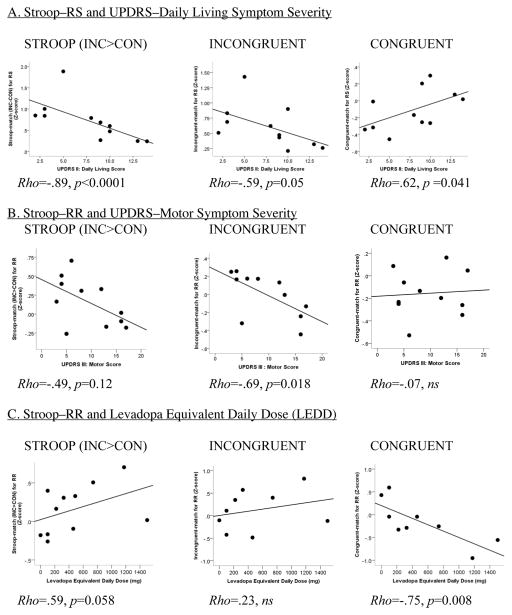
3.1.1 PD Severity, levodopa equivalent daily dose and Stroop task performance
Less severe impairment in daily living (UPDRS-II) was associated with greater Stroop effects during response switching (RS) due to reduced benefit from congruent-match trials (match condition), and less severe motor symptoms (UPDRS-III) correlated with greater Stroop effects during response repetitions (RR) (Table 2). Here, higher LEDD was, as a trend, correlated with the greater Stroop-RR effects for color-match conditions and this was mainly due to greater benefits from response repetition in congruent no-conflict trials, i.e., response facilitation with higher LEDD (Table 2, Figure 2). LEDD was not correlated with overall response latency (Rho=.02, ns), or the number of errors committed (Rho=.52, ns).
Table 2.
Nonparametric correlation analysis (Spearman’s Rho) testing the relations between PD symptom severity, LEDD and Stroop task performance (match condition) in PD patients.
| Stroop-RS | INC-RS | CON-RS | ||
|---|---|---|---|---|
| UPDRS-II | Rho | −.89* | .62 | −.59 |
| p | 0.0001 | 0.041 | 0.056 | |
|
| ||||
| Stroop-RR | INC-RR | CON-RR | ||
|
| ||||
| UPDRS-III | Rho | −.49 | −.69 | −.07 |
| p | 0.12 | 0.018 | ns | |
| LEDD | Rho | .59 | .23 | −.75* 0.008 |
| p | 0.058 | ns | ||
Abbreviations: Parkinson Disease patients (PD), Unified Parkinson’s Disease Rating Scale (UPDRS), part II (daily living), part III (motor symptoms), levodopa equivalent daily dose (LEDD), Reaction time (RT), cue-target color match (M), incongruent (INC), congruent (CON), Stroop=INC-CON, response switching (RS), response repetition (RR).
Figure 2.
Correlation graphs between Parkinson symptom severity sub-scores (A., B.) derived from the Unified Parkinson’d Disease Rating Scale (UPDRS), levodopa equivalent daily dose (LEDD) (C.), and task performance (Z-scores) for Stroop effects (INC - CON), interference processing for incongruent (INC) Stroop stimuli, and benefits from congruent (CON) Stroop stimuli. Match: trials in which the color cue correctly predicted the Stroop word’s font color; nonmatch: trials in which the color cue wrongly predicted the Stroop word’s font color. RR: response repetition blocks of trials; RS: response switching blocks of trials.
Next, we used multiple regression analyses to test for the relative contribution of PD severity factors (UPDRS-II daily living, UPDRS-III motor scores LEDD, years since PD diagnosis, LEDD) to Stroop component processes. A regression model using years since PD diagnosis, UPDRS-II and UPDRS-III scores as factors explained 71% of the variance of Stroop-match effects for response switching (RS) (F(3,7)=5.57, p=0.029). Adding LEDD to the model did not add to the variance explanation of Stroop-RS effects (R2=0.71; F(4,6)=3.58, p=0.08). Regression models testing for the relative contribution of these three factors to component Stroop-RS processes for ‘benefit from congruent match trials’ (ConRS) (F(3,7)=1.73, p=0.25), or ‘interference from incongruent trials’(IncRS) (F(3,7)=2.69, p=0.13) were not significant.
For Stroop-match effects with response repetition (RR), a regression model with UPDRS-III and LEDD as factors explained together 55% of the total variance of Stroop-RR effects (F(2,8)=4.81, p=0.042) with less severe motor symptoms (UPDRS-III, t=−2.52, p=0.036) and as a trend higher LEDD (t=2.12, p=0.067) contributing independently to greater Stroop-RR effects. A regression model with all 4 PD severity factors was not significant (F(4,6)=2.68, p=0.14). Again, regression models testing for the relative contribution of these two factors to component Stroop-RR processes showed a trend for ‘benefit from congruent match trials’ (ConRS) (R2=0.50; F(2,8)=4.01 p=0.062), with LEDD contributing independently (t=−2.82, p=0.023), but not for ‘interference from incongruent trials’ (IncRS) (F(2,8)=2.74, p=0.12).
Thus, within the patient group, those with more severe PD symptoms (UPDRS daily living and motor scores) exhibited smaller Stroop effects. We next tested for group similarities and differences in the functional basal-ganglia networks at rest and during task processing, and whether connectivity differences in PD relative to controls are associated with better or worse task performance.
3.2 Functional Brain Networks
For both, task and rest scans, seed-to-voxel connectivity analyses were performed for each a priori defined seed and group. Task-rest modulation of connectivity was modeled by using the opposite condition (rest/task) as regressor. We tested for group similarities, using conjunction analyses, and group differences, using contrast analyses, in basal ganglia-thalamocortical networks using striatal (caudate and putamen), globus pallidum, and thalamic seeds for whole brain connectivity analysis.
3.2.1 Striatum: Caudate connectivity
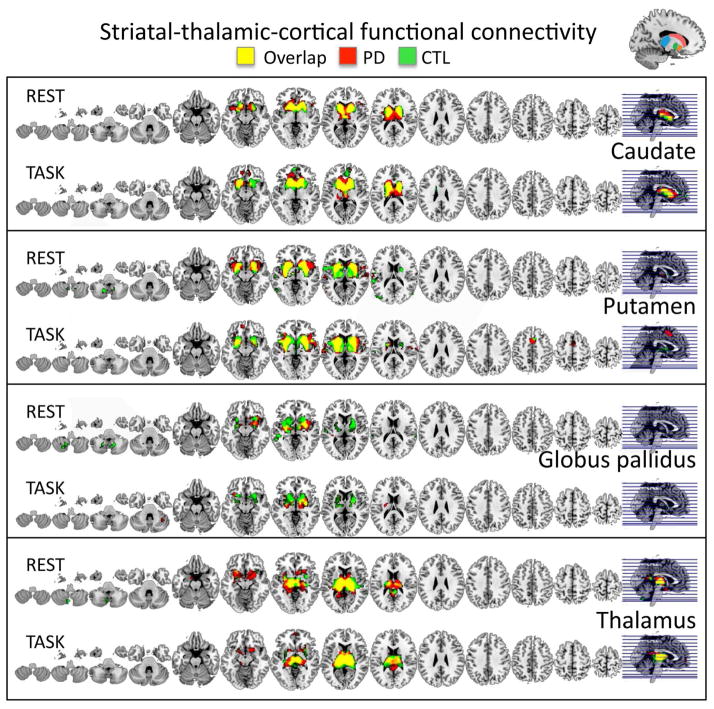
Group similarities (Figure 3, Suppl. Table 1)
Figure 3.
Similarities in functional seed-to-voxel BG and thalamo-cortical connectivity maps for resting-state (REST) and the task-activated (TASK) fMRI runs for PD patients marked in red, and controls (CTL) marked in green, and the groups overlap in yellow.
Both groups showed functional connectivity overlap during both the resting-state and the task between the caudate seed and inferior prefrontal, anterior cingulate, and temporal brain regions including the insula, anterior entorhinal cortex, and temporopolar areas.
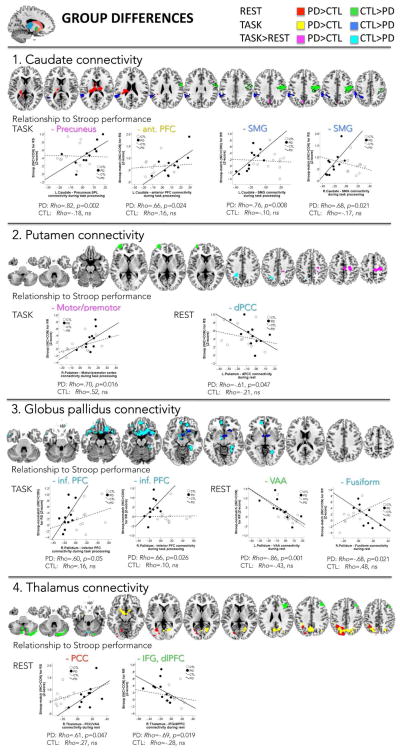
Group differences (Figure 4, Tables 3 and 4)
Figure 4.
Differences in functional seed-to-voxel BG and thalamo-cortical connectivity during the resting-state (REST), the task-activated (TASK) fMRI runs, and task-rest modulation, between PD patients and controls (CTL).
Table 3.
Statistical significance (T-score) of clusters showing group differences in their functional connectivity between the seed and the cluster location in MNI space (X, Y, Z coordinates in mm)
| k | BA/nucleus | X | Y | Z | T-score | |||
|---|---|---|---|---|---|---|---|---|
| CAUDATE | ||||||||
| REST | PD>CTL | Thalamus, claustrum, insula, cingulate cortex | 905 | 13, 29, 30, lateral dorsal and posterior thalamic nuclei | −12 | −22 | 18 | 5.41 |
| −26 | −24 | 22 | 5.36 | |||||
| 16 | −20 | 20 | 4.11 | |||||
| CTL>PD | Premotor, motor, and somatosensory cortices (pre- and postentral gyri) | 830 | 2, 3, 4, 6 | 52 | −14 | 38 | 4.95 | |
| 34 | −12 | 38 | 4.80 | |||||
| 38 | −28 | 40 | 3.42 | |||||
|
| ||||||||
| TASK | PD>CTL1 | Anterior prefrontal and orbitofrontal cortices | 52 | 10, 11 | −12 | 66 | −16 | |
| CTL>PD | Supramarginal gyrus, temporoparietal junction, angular and superior temporal gyrus, insula | 1166 | 13, 22, 40, 39, 42 | −54 | −42 | 18 | 5.94 | |
| −50 | −38 | 52 | 4.81 | |||||
| −60 | −42 | 42 | 4.63 | |||||
|
| ||||||||
| PUTAMEN | ||||||||
| REST | PD>CTL | no suprathrehold voxels | ||||||
| CTL>PD | Anterior prefrontal cortex | 187 | 10 | −32 | 64 | 4 | 6.67a | |
|
| ||||||||
| TASK | PD≠CTL | no suprathreshold voxels | ||||||
|
| ||||||||
| GLOBUS PALLIDUS | ||||||||
| REST | PD>CTL | no suprathreshold voxels | ||||||
| CTL>PD1 | Visual association cortex | 47 | 18, 19 | −38 | −84 | −2 | 5.71 | |
| Cerebellum | 22 | IX | −30 | −52 | −48 | 4.61 | ||
|
| ||||||||
| TASK | PD>CTL | no suprathrehold voxels | ||||||
| CTL>PD | Inferior prefrontal gyrus, subgenual cortex, temporopolar area | 682 | 25, 38, 47 | 18 | 16 | −16 | 4.31 | |
| 10 | 2 | −4 | 4.19 | |||||
| 6 | 8 | −14 | 4.03 | |||||
|
| ||||||||
| THALAMUS | ||||||||
| REST | PD>CTL | Posterior cingulate cortex, visual association areas | 881 | 18, 30 | −26 | −68 | 2 | 4.97 |
| 1 | −32 | −88 | −2 | 4.95 | ||||
| −34 | −94 | −18 | 3.97 | |||||
| Precuneus, inferior parietal lobe | 622b | 7, 40 | −18 | −76 | 42 | 4.70 | ||
| −6 | −86 | 44 | 4.51 | |||||
| −32 | −54 | 42 | 4.12 | |||||
| CTL>PD | Inferior frontal triangular and dorsolateral prefrontal cortices | 754 | 9, 44, 46, 48 | 38 | 24 | 26 | 5.05 | |
| 36 | 30 | 40 | 3.90 | |||||
| Cerebellum | 712 | VIIb, VIII, IX | −6 | −76 | −48 | 5.00 | ||
| −24 | −50 | −50 | 4.06 | |||||
| −2 | −60 | −42 | 3.49 | |||||
| Cerebellum | 721 | Crus2, Crus1 | 24 | −84 | −34 | 4.47 | ||
| 38 | −80 | −42 | 4.16 | |||||
| 16 | −82 | −32 | 4.07 | |||||
|
| ||||||||
| TASK | PD>CTL | Parahippocampal gyrus, hypothalamus, subgenual cortex | 547c | 25, 28, 34 | −10 | 2 | −14 | 6.28 |
| 6 | −2 | −16 | 4.01 | |||||
| 12 | 4 | −14 | 3.64 | |||||
| Precuneus, angular and supramarginal gyri, superior and inferior parietal lobe, dorsal posterior cingulate cortex, extrastriate areas | 2281 | 7, 18, 19, 30, 31, 39, 40 | −22 | −60 | 44 | 4.31 | ||
| −20 | −72 | 46 | 4.19 | |||||
| −6 | −60 | 38 | 3.95 | |||||
| CTL>PD | no suprathreshold voxels | |||||||
For analysis group differences (PD≠CTL), cluster extent threshold at pFWE corrected = 0.05, peak at T(1,20)min=2.53; k = number of synchronously activated brain voxels.
trend for peak pFWE corrected >0.05 <0.1;
cluster extent pFDR corrected < 0.05;
trend for cluster extent pFDR corrected >0.05 <0.1;
peak p=0.001; T(1,20) min = 3.55, k=20.
Abbreviations: Parkinson Disease patients (PD), control participants (CTL), Brodmann area (BA)
Table 4.
Statistical significance (T-score) of clusters showing group-by-condition interactions in their connectivity between the seed and the cluster location in MNI space (X, Y, Z coordinates in mm)
| A) Analysis threshold: peak at T(1,20) min = 2.53; extent at pFWE corrected = 0.05 | |||||||
|---|---|---|---|---|---|---|---|
| k | BA/nucleus | X | Y | Z | T-score | ||
| GLOBUS PALLIDUS – right | |||||||
| T > R CTL>PD | Inferior prefrontal and orbitofrontal gyri, temporopolar area, middle temporal gyrus, subgenual, dorsal anterior cingulate and anterior entorhinal cortices | 2447 | 10, 11, 21, 25, 32, 34, 38, 47 | −2 | 16 | −20 | 6.75 |
| 24 | 24 | −18 | 5.72 | ||||
| 16 | 20 | −18 | 4.96 | ||||
| Fusiform, extrastriate, parahippocampal and inferior temporal gyri, perirhinal and piriform cortices | 477a | 19, 20, 27, 35, 36, 37 | 32 | −38 | −10 | 5.17 | |
| 26 | −54 | −4 | 4.24 | ||||
| 26 | −32 | −16 | 3.03 | ||||
|
| |||||||
| B) Analysis threshold: peak p=0.001, T(1,20) min = 3.55, k min = 60 | |||||||
| CAUDATE – left | |||||||
| T > R PD>CTL | Precuneus, superior parietal lobe | 66 | 7 | 0 | −68 | 36 | 4.41 |
|
| |||||||
| PUTAMEN – left | |||||||
| T > R CTL>PD | Dorsal posterior cingulate cortex | 60 | 31 | −22 | −44 | 36 | 5.58 |
| – right | |||||||
| T > R PD>CTL | Motor and premotor cortices | 80 | 4, 6 | 26 | −22 | 56 | 5.02 |
|
| |||||||
| THALAMUS – left | |||||||
| T > R CTL>PD | Cerebellum | 61 | Crus2, declive | −8 | −72 | −24 | 5.31 |
extent at pFDR corrected < 0.05
Abbreviations: Parkinson Disease patients (PD), control participants (CTL), Task (T), Rest (R), Brodmann area (BA).
During the resting-state, PD showed more connectivity to thalamic and insula regions, and less connectivity to premotor, motor, and somatosensory regions than controls. During task processing, PD exhibited less connectivity to left temporoparietal regions than controls involving among other regions the supramarginal gyrus (SMG) (Figure 4.1). For task>rest, and when using an uncorrected threshold of p<0.001, PD showed more extended caudate–prefrontal cortical connectivity (Table 3) and stronger caudate–precuneus connectivity relative to controls (Table 4).
Putamen connectivity
Group similarities (Figure 3, Supplement Table 2)
Both groups showed putamen connectivity to inferior prefrontal, insula, superior temporal and parietal regions during rest, with additional connectivity to premotor and dorsal frontal regions during task processing.
Group differences (Figure 4, Tables 3 and 4)
During rest, PD showed less putamen connectivity to anterior prefrontal regions than controls (Table 3). For task>rest, and using an uncorrected p-threshold, interactions indicated that PD, relative to controls, had weaker putamen–dorsal posterior cingulate cortex (dPCC) connectivity and stronger putamen–motor/premotor cortical connectivity (Table 4) (Figure 4.2).
3.2.2 Globus pallidus connectivity
Group similarities (Figure 3, Supplementary Table 3)
Testing the functional connectivity of the globus pallidus in PD patients and controls, we found overlapping connectivity for both groups to inferior prefrontal, insula, and entorhinal cortical regions during both rest and task, with additional connectivity to superior and middle temporal regions during rest.
Group differences (Figure 4, Tables 3 and 4)
During task processing, PD showed less pallidum-prefrontal cortical connectivity than controls, also including the subgenual cortex and temporopolar area (Table 3). For task>rest, a significant interaction effect revealed that PD, relative to controls, exhibited weaker pallidum connectivity to inferior prefrontal, anterior cingulate, orbitofrontal, subgenual, and temporal cortices (infPFC), to visual association cortices (VAA) involving fusiform and occipital gyri, and to medial temporal regions including the parahippocampal gyrus, perirhinal and pitiform cortices (Table 4) (Figure 4.3).
3.2.3 Thalamus connectivity
Group similarities (Figure 3, Supplementary Table 4)
Testing the functional connectivity of the thalamus in PD patients and controls, we found overlapping connectivity for both groups to insula, cingulate, and prefrontal cortices during rest and task.
Group differences (Figure 4, Tables 3 and 4)
During task processing, PD, relative to controls, showed more extended thalamus connectivity to posterior parietal cortices and to parahippocampal and subgenual regions, also including the hypothalamus (Table 3). During rest, PD, relative to controls, showed stronger thalamus connectivity to posterior occipital and parietal regions including the posterior cingulate cortex (PCC) and precuneus, and weaker thalamus–prefrontal and thalamus–cerebellar connectivity. For task>rest, and using an uncorrected p-threshold, an interaction indicated weaker thalamus-cerebellum connectivity in PD than controls (Table 4) (Figure 4.4).
3.3 Compensation or dedifferentiation? Relationships to performance
By correlating connectivity strength with performance, we tested the compensation vs. dedifferentiation hypothesis, i.e., whether BG and thalamo-cortical network engagement during task processing in mild PD, that is not observed CTL, is related to better vs. worse performance; and whether less resting-state neural network engagement in mild PD relative to CTL is related to performance decrements and more severe PD symptoms (Figure 3).
3.3.1 Striatum: Caudate
In PD, relative to controls, task-related caudate connectivity correlated with performance: Stronger caudate–precuneus connectivity correlated with larger Stroop effects for response switching blocks (Rho=.82*, p=0.002) due to processing interference from incongruent Stroop words (Rho=.72*, p=0.013). Similar relationships were observed for stronger caudate–prefrontal cortex connectivity that correlated with larger Stroop-match effects during response switching (Rho=.66, p=0.024).
PD as a group showed weaker caudate–SMG connectivity than controls. Within the patient group, those with weaker connectivity had smaller Stroop-match effects (Rho=.66, p=0.024) due to less benefits from correct color cueing in congruent trials during response switching (Rho=−.82*, p=0.002). Also during response repetition, weaker caudate–SMG connectivity correlated with smaller Stroop-match effects (Rho=.66, p=0.024), and here the relationship was driven by less interference from the incongruent word content (Rho=.83*, p=0.002) (Figure 4.1). Further, a moderate relationship was observed between weaker caudate–SMG connectivity and greater UPDRS motor symptom scores (Rho=−.65, p=0.029).
Putamen
Stronger task-related putamen–motor/premotor connectivity in PD correlated with greater Stroop-RR effects (Rho=.70*, p=0.016). During rest, stronger putamen–dPCC connectivity moderately correlated with smaller Stroop-RS effects (Rho=−.61, p=0.047) (Figure 4.2).
3.2.2 Globus pallidus
For task processing, weaker pallidum–inferior PFC connectivity in PD correlated with smaller Stroop-nonmatch effects during response switching (Rho=.60, p=0.05) and repetition (Rho=.66, p=0.026); these relationships were driven by less benefit from congruent trials for response switches (Rho=−.82*, p=0.002) and by less interference from incongruent trials for response repetitions (Rho=.72*, p=0.015). Furthermore, weaker pallidum–PFC connectivity correlated with less UPDRS motor symptoms (Rho=.70*, p=0.016).
For the resting-state, stronger pallidum–VAA connectivity in PD correlated with smaller Stroop-nonmatch effects (Rho=−.86*, p=0.001), and stronger pallidum–fusiform connectivity correlated moderately with smaller Stroop-match effects (Rho=−.68, p=0.021) during response repetitions (Figure 4.3). In addition, stronger pallidum–cerebellar (area IX) connectivity correlated with higher UPDRS daily living (Rho=.71, p=0.015) and motor symptom scores (Rho=.77, p=0.006), and with more depressive symptoms (BDI) (Rho=.80, p=0.003).
For task>rest, stronger pallidum–fusiform connectivity in PD correlated with higher UPDRS daily living (Rho=.65, p=0.030) and motor symptom scores (Rho=.83, p=0.002).
3.2.3 Thalamus
For the resting-state, weaker thalamus–prefrontal connectivity in PD correlated with greater Stroop effects during response repetitions (Rho=−.69, p=0.019) with more interference from incongruent trials (Rho=−.72, p=0.013), and the greater thalamus–PCC connectivity in PD correlated with greater Stroop-match effects during response switching (Rho=.61, p=0.047) due to more interference from incongruent trials (Rho=.69, p=0.019) (Figure 4.4). In addition, stronger task-activated thalamus-precuneus connectivity in PD correlated with higher LEDD (Rho=.72, p=0.013).
3.4 Whole brain analysis of LEDD effects on BG connectivity in PD
Analyzing effect of LEDD on functional connectivity in PD using LEDD as second-level covariate in the within-group whole brain analysis (conn toolbox) revealed effects of LEDD on BG connectivity (Supplement Table 5). Striatum (caudate, putamen): Stronger striatal–prefrontal cortical connectivity (rest and task) and weaker caudate–cerebellar connectivity during task processing was related to higher LEDD. Thalamus: At rest, stronger thalamus–somatosensory and weaker thalamo–prefrontal cortical connectivity was related to higher LEDD. During task processing, stronger thalamus–insula connectivity was associated with higher LEDD.
4 Discussion
PD patients at mild to moderate stages of the disease showed near normal Stroop Match-to-Sample task performance with similar cognitive interference during response switching but greater interference during response repetitions compared with controls. Thus, cognitive performance was influenced by motor response demands, differently in PD from controls. As expected, Stroop effects were greater for response switching than repetition indicating higher conflict for response switching conditions in both groups (Steinhauser and Hübner, 2009); by contrast, response repetition attenuated interference from incongruent trials, almost eliminating Stroop conflict in controls (Figure 1). Such ‘conflict adaptation’ with repetition, a phenomenon previously observed in healthy subjects (e.g., Kim et al., 2014; Wang et al., 2014), was also observed in PD, yet to a significantly lesser extent suggesting alterations in the automatic sources of sequential adaptation for motor response selection with repetition (e.g., Jiménez and Méndez, 2013) in the presence of cognitive conflict. This can be partly explained by general motoric slowing in PD (Figure 1A) and by an impaired adaptation of motor response selection, i.e., less benefit for incongruent trials, when motor commands repeat (Figure 1B).
In the response-switching condition of the Stroop task, a significant negative correlation with the UPDRS-II symptom severity confirms the role of executive functions, as tested with our paradigm, for quality of daily living (Koerts et al., 2011). It is noteworthy that the UPDRS-II represents a good comparison score for the severity of disease in daily life and correlates with the UPDRS-III motor scores OFF medication (Stebbins and Goetz, 1998; Vassar et al., 2012). Together, symptom severity in motor and daily living functions and longer time since diagnosis explained over 70% of the variation observed in PD patients in their ability to exert executive control in a Stroop task with changing response demands (Stroop-RS). Specifically, patients with higher UPDRS-II scores did not show the cognitive benefit pattern that is typically observed when the cue color matches the color of a congruent Stroop word.
With repetitive response demands, over 50% of the variance in Stroop-RR performance in the PD patients was explained by motor symptom severity and LEDD indicating that dopaminergic medication and motor ability together affect automatic response adaptations in cognitive tasks. Automaticity of response selection with repetition is a basic component of motor learning, permits saving of resources (Sanchez-Lopez et al., 2014), and plays an essential role in neuronal computational processes underlying controlled and automatic aspects of motor behavior (Wu et al., 2014) and cognition (Lebedev et al., 2014). PD patients with more severe UPDRS-III motor symptoms showed less interference from incongruent Stroop word information in the easiest task condition, i.e., cue-color match trials with response repetition, which suggests that with more severe motor symptoms less resources were available to process cognitive information such as color-word incongruency in a color matching task (Schulte et al., 2011), potentially due to alterations in basal ganglia–frontal cortical circuitry in PD (Camicioli et al., 2009).
4.2 Basal ganglia-thalamo-cortical functional networks
Overall, our findings show functional task-activated BG network differences in mild PD patients that have behavioral correlates indicative of compensation and resource availability. By contrast, resting-state connectivity pattern in PD, i.e., for the BG (caudate, putamen, and globus pallidus), but not the thalamus, was consistent with a pathophysiological view of intrinsic BG circuitry expansion.
Basal ganglia circuitry
The caudate plays a key role in planning and goal-directed behavior (Grahn et al., 2008; Ryan et al., 2013). In the resting-state, mild PD had more extended caudate functional connectivity to the thalamic and insula regions, and less connectivity to premotor, motor and somatosensory regions, which corresponds to the clinical manifestations of PD (Hacker et al., 2012). However, during the Stroop task, PD patients showed weaker caudate connectivity to left temporoparietal regions and a trend to more prefrontal connectivity compared with controls. The degree of caudate-cortical synchrony during task processing was associated with greater Stroop effects that resulted from greater benefits from congruent information and resource availability for processing interference. Consequently, greater Stroop effects were associated with less severe PD symptoms. Thus, the temporal-to-frontal shift in striato-cortical connectivity during task engagement in mild PD patients may signify a compensatory process that enabled adequate executive control over conflicting response systems (Harrison et al., 2005).
The putamen is relevant for movement regulation (Alexander et al., 1990) but plays also a role in verbal learning, executive functioning, and working memory (Hartberg et al., 2011). The observed weaker putamen–anterior prefrontal cortical connectivity during rest in PD patients could reflect difficulties PD patients have in initiating movements and flexibly changing motor output (Playford et al., 1992). During task processing, however, stronger putamen–motor/premotor cortex connectivity was associated with greater Stroop effects during response repetitions. Considering that greater Stroop effects were related to less PD severity, this result may suggest that at less severe stages of the disease PD patients can adapt frontostriatal cortical networking to accommodate interference processing when resources become available as motor commands repeat.
Within the basal ganglia-cortical network, the globus pallidus is a structure that receives striatal outputs and has been implicated in converging the divergent striatal-cortical signals from the neocortex and sending processed information to frontal cortex areas that have been implicated in motor planning and execution, and cognitive control (Graybiel et al., 1994; Bhatia and Marsden, 1994; Kim et al., 2008; Rodriguez-Oroz et al., 2009). An interaction effect indicated that specifically pallidum connectivity is differently modulated by task–rest conditions in PD than controls. Relative to controls, PD patients demonstrated weaker pallidum connectivity to prefrontal and occipital cortical regions during the task than when resting. Decreased basal ganglia–prefrontal resting-state connectivity was previously observed in PD and associated to more severe disease states (Wu et al., 2012) and in our study, to poorer Stroop task performance. Our results further suggest a role of pallidal resting-state connectivity to cerebellar regions for both disease severity and depressive symptoms, a common symptom in PD (Kirsch-Darrow et al., 2006). Thus, compromised rest-task modulation of pallidum connectivity in mild PD may reflect neural disturbances of wide-spread functional networking that underlie motor symptoms, and can also subserve mood states, and daily living functions. Notably, the intrinsic resting-state patterns of BG network connectivity were more deviant (from controls) in PD patients with greater disease symptom severity; and this stronger resting-state connectivity, i.e., between pallidum–occipital and also putamen–medial parietal cortices, in turn correlated with worse Stroop performance. Here, the association of resting-state connectivity with poorer performance and more severe clinical symptoms supports a pathophysiological view of BG intrinsic circuitry expansion in PD. By contrast, the association of stronger task-evoked BG connectivity to additional regions with milder PD symptoms and better performance suggests a compensatory neural mechanism and brain functional resource availability.
Thalamus circuitry
Within basal ganglia-thalamo-cortical circuits, the thalamus has been considered a ‘center of integration of networks’ (Haber and Calzavara, 2009) with segregated thalamic nuclei projecting to medial prefrontal, amygdala and hippocampus forming limbic-thalamic loops (Amaral and Cowan, 1980; Norita and Kawamura, 1980), to primary motor and sensory cortices forming motor-thalamic loops (Matelli et al., 1989), and to dlPFC, orbitofrontal, and anterior cingulate cortices forming ‘cognitive’ fronto-thalamic loops (Giguerre and Goldman-Rakic, 1988). The resting-state thalamic-cortical connectivity pattern showed stronger thalamus connectivity to posterior occipital and parietal regions, including the PCC/precuneus, a major node of the resting-state self-referential network (Raichle, 2011), and weaker thalamo-prefrontal connectivity in PD than controls, and here the deviation from normal connectivity was associated with better Stroop performance. Thus, the differential thalamo-cortical connectivity during rest in PD relative to healthy controls may reflect a redistribution of intrinsic functional resources that may have played a role in sustaining executive control function (see also, Buhmann et al., 2005; van Nuenen et al., 2012). Specifically, stronger thalamus–PCC connectivity together with weaker thalamus–prefrontal connectivity during the resting-state may reflect a compensatory response within self-referential networks (van Nuenen et al., 2012) with the potential to provide resources for task performance. With its several parallel and segregated motor, limbic, and cognitive loops, and its connectivity to the striatum (Haber and Calzavara, 2009), the thalamus is well positioned to reconcile functional subcortico-cortical networking to compensate for neural compromise in PD.
From an anatomical view, normal motor function is achieved by the interaction of two different basal ganglia–cortical functional loops, a fast ‘direct’ pathway and a slow ‘indirect’ midbrain-striatal-pallidal-thalamo-cortical pathway (Nambu et al., 2002). PD is associated with an up-regulation of the indirect and down-regulation of the direct motor pathway. For example, during rest we observed stronger caudate-thalamocortical connectivity and weaker caudate-motor cortical connectivity, potentially reflecting patterns of ‘up’ and ‘down’ regulation in the synchronization of striatal-thalamic and striatal-motor cortex activity. Yet, differences in functional connectivity may not necessarily indicate differences in anatomical connectivity, and the reduced basal ganglia–cortical connectivity during rest was not restricted to motor cortical regions but involved prefrontal, parietal and extrastriate cortices as well (Boehler et al., 2011; van Nuenen et al., 2012).
During Stroop task processing, thalamus-precuneus connectivity was related to levodopa-equivalent daily dose in PD patients suggesting some influence of dopaminergic medication on the synchronization of activity within thalamic loops. In healthy subjects, dopaminergic medication can both increase and decrease connectivity (Honey et al., 2003), while in mild PD dopaminergic medication was found to decrease striatal–thalamic resting-state connectivity (Kwak et al., 2010). Considering the thalamus as ‘center of integration of networks’ (Haber and Calzavara, 2009) and the precuneus as the functional core of the default mode network that is typically inversely connected to other network regions for rest versus task processing states (Utevsky et al., 2014), it could be speculated that dopaminergic medication can modulate rest–task dynamic synchronization within thalamic loops.
A limitation of our study is that we did not test our PD patients’ off-medication, a state in which decreased functional connectivity in the SMA has been observed in comparison to normal subjects (Wu et al., 2009). Dopaminergic medical treatment, i.e., with levodopa, reduces Parkinson symptoms and provides best possible motor performance levels to patients. Accordingly, increased functional connectivity of the sensorimotor network has been reported, especially in drug-naive PD patients (Esposito et al., 2013). Although we do not claim that simple correlations can grasp the complex effect of dopaminergic medications on the cognitive and sensorimotor networks, we found indication that higher levodopa-equivalent daily dose is related to greater activation synchrony between thalamus–precuneus, which points to a subtle and positive impact of LEDD. The positive effect of LEDD on synchronizing thalamus-somatosensory cortical activity in PD patients was confirmed in an independent whole brain covariate analysis that also revealed an effect of LEDD for stronger striatal–prefrontal synchrony. Behaviorally, LEDD was associated with faster response latencies for the easiest condition during Stroop task performance. Thus, this study provides preliminary evidence for dopaminergic effects on striato-frontal and thalamo-somatosensory network synchrony and behaviorally, for dopaminergic effects on fast response execution for more automatic, i.e., repetitive and no-conflict, processes that do not rely on executive control. Although the brain data reported herein met high statistical standards corrected for multiple comparisons for testing network similarities and differences, a limitation of the current study is the relatively small sample size, a common problem in neuroimaging studies (see also Desmond and Glover, 2002), especially those of neurodegenerative disorders. Although seed-to-voxel connectivity approaches allow examining the dynamic reconfiguration of brain networks, e.g., of the basal ganglia, we cannot rule out that the observed pattern of resting-state and task-activated connectivity dynamics in PD and controls are mediated by other unidentified regions.
Our findings support the view that weak synchrony of resting-state basal ganglia-cortical networks may play an important role in the pathophysiology of motor and cognitive symptoms in PD (Baudrexel at al., 2014; Li et al., 2007). By contrast, for task engagement enhanced BG-cortical connectivity at an early stage of the disease may serve a compensatory role in PD patients (see e.g., Fling et al. 2014) with potential relevance for estimating disease progression in motor and cognitive function. Use of functional MRI with network analysis appears to be a useful method to test the dynamic modulation of network synchronization during rest and in response to cognitive and motoric task demands in PD. Longitudinal studies are warranted to test the predictive value of the observed patterns for disease progression.
Supplementary Material
Acknowledgments
Support: AA018022, AA012388, AA017168, AA010723, AA017923, NIH/NINDS K23 NS075097, NIH/NINDS P50 NS071675, Michael J. Fox Foundation for Parkinson’s disease
Footnotes
Conflict of Interest
Authors Eva M. Müller-Oehring, Edith V. Sullivan, Adolf Pfefferbaum, Neng C. Huang, Kathleen L. Poston, Helen M. Bronte-Stewart, and Tilman Schulte declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Aarsland D, Hutchinson M, Larsen JP. Cognitive, psychiatric and motor response to galantamine in Parkinson’s disease with dementia. International Journal of Geriatric Psychiatry. 2003;18:937–941. doi: 10.1002/gps.949. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neuroscience. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. Review. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. Review. [PubMed] [Google Scholar]
- Arimura N, Nakayama Y, Yamagata T, Tanji J, Hoshi E. Involvement of the globus pallidus in behavioral goal determination and action specification. Journal of Neuroscience. 2013;33:13639–13653. doi: 10.1523/JNEUROSCI.1620-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio F, Blasi V, Falini A, Farina E, Mantovani F, Olivotto F, Scotti G, Nemni R, Bozzali M. Functional brain changes in early Parkinson’s disease during motor response and motor inhibition. Neurobiology of Aging. 2011;32:115–24. doi: 10.1016/j.neurobiolaging.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, Steinmetz H, Deichmann R, Roeper J, Hilker R. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson’s disease. Neuroimage. 2011;55:1728–38. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends in Neurosciences. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Betchen SA, Kaplitt M. Future and current surgical therapies in Parkinson’s disease. Current Opinion in Neurology. 2003;16:487–493. doi: 10.1097/01.wco.0000084227.82329.ae. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Chen LC, Woldorff MG. The role of stimulus salience and attentional capture across the neural hierarchy in a stop-signal task. PLoS One. 2011;6:e26386. doi: 10.1371/journal.pone.0026386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann C, Binkofski F, Klein C, Büchel C, van Eimeren T, Erdmann C, Hedrich K, Kasten M, Hagenah J, Deuschl G, Pramstaller PP, Siebner HR. Motor reorganization in asymptomatic carriers of a single mutant Parkin allele: a human model for presymptomatic parkinsonism. Brain. 2005;128:2281–2290. doi: 10.1093/brain/awh572. [DOI] [PubMed] [Google Scholar]
- Caballol N, Martí MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Movement disorders: Official Journal of the Movement Disorder Society. 2007;22:S358–366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- Cameron IG, Watanabe M, Pari G, Munoz DP. Executive impairment in Parkinson’s disease: response automaticity and task switching. Neuropsychologia. 2010;48:1948–1957. doi: 10.1016/j.neuropsychologia.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WR. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism & Related Disorders. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Research. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain. 1999;122:483–495. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- Cooper J, Sagar H, Doherty S, Jordan N, Tidswell P, Sullivan E. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. A follow-up study of untreated patients. Brain. 1992;115:1701–1725. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52(2):359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. The American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Davis S, Dennis N, Daselaar S, Fleck M, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of Neurology. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. Review. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. Journal of Neuropsychology. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. Journal of Neurology. 1997;244:2–8. doi: 10.1007/pl00007725. Review. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, Gainetdinov RR, Sameshima K, Caron MG, Nicolelis MA. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. Journal of Neuroscience. 2009;29:8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Esposito F, Tessitore A, Giordano A, De Micco R, Paccone A, Conforti R, Pignataro G, Annunziato L, Tedeschi G. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson’s disease by levodopa. Brain. 2013;136:710–725. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS Development Committee. . Unified Parkinson’s Disease Rating Scale. In: Fahn D, Marsden CD, Calne D, Goldstein M, editors. Recent Development in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Maacmillan Healthcare Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and account: Implications for group differences in response latency. Psychological Bulletin. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fernández de Bobadilla R, Pagonabarraga J, Martínez-Horta S, Pascual-Sedano B, Campolongo A, Kulisevsky J. Parkinson’s disease-cognitive rating scale: psychometrics for mild cognitive impairment. Movement Disorders. 2013;28:1376–1383. doi: 10.1002/mds.25568. [DOI] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014;9:e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes R, Petersson P, Siesser W, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science. 2009;323:1578–1582. doi: 10.1126/science.1164901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Archives of Neurology. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86:141–55. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Gould S, Houser M, Aron AR. Stimulation of contacts in ventral but not dorsal subthalamic nucleus normalizes response switching in Parkinson’s disease. Neuropsychologia. 2013;51:1302–1309. doi: 10.1016/j.neuropsychologia.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135:3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Shaw M, Yücel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olver JS, Nathan PJ, Pantelis C. Functional connectivity during Stroop task performance. Neuroimage. 2005;24:181–191. doi: 10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Sundet K, Rimol LM, Haukvik UK, Lange EH, Nesvåg R, Melle I, Andreassen OA, Agartz I. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Prog Neuropsychopharmacol Biological Psychiatry. 2011;35:1122–1130. doi: 10.1016/j.pnpbp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cerebral Cortex. 2010;20:1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Annals of Neurology. 2011;69:269–281. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- Herzog J, Volkmann J, Krack P, Kopper F, Pötter M, Lorenz D, Steinbach M, Klebe S, Hamel W, Schrader B, Weinert D, Müller D, Mehdorn HM, Deuschl G. Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Movement Disorders: Official Journal of the Movement Disorder Society. 2003;18:1332–1337. doi: 10.1002/mds.10518. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SC, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez L, Méndez A. It is not what you expect: dissociating conflict adaptation from expectancies in a Stroop task. Journal of Experimental Psychology. Human Perception and Performance. 2013;39:271–284. doi: 10.1037/a0027734. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Professional manual: Psychological Assessment Resources. 2004. DRS-2 Dementia Rating Scale-2. [Google Scholar]
- Kim SH, Park KH, Sung YH, Lee YB, Park HM, Shin DJ. Dementia mimicking a sudden cognitive and behavioral change induced by left globus pallidus infarction: review of two cases. Journal of the Neurological Sciences. 2008;272:178–182. doi: 10.1016/j.jns.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Gold BT. Conflict adaptation in prefrontal cortex: now you see it, now you don’t. Cortex. 2014;50:76–85. doi: 10.1016/j.cortex.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology. 2006;67:33–38. doi: 10.1212/01.wnl.0000230572.07791.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerts J, Van Beilen M, Tucha O, Leenders KL, Brouwer WH. Executive functioning in daily life in Parkinson’s disease: initiative, planning and multi-task performance. PLoS One. 2011;6(12):e29254. doi: 10.1371/journal.pone.0029254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Müller ML, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson’s disease. Frontiers in Systems Neuroscience. 2010;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Westman E, Simmons A, Lebedeva A, Siepel FJ, Pereira JB, Aarsland D. Large-scale resting state network correlates of cognitive impairment in Parkinson’s disease and related dopaminergic deficits. Frontiers in Systems Neuroscience. 2014;8:45. doi: 10.3389/fnsys.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman G, Melillo R. The basal ganglia: motor and cognitive relationships in a clinical neurobehavioral context. Reviews in the Neurosciences. 2013;24:9–25. doi: 10.1515/revneuro-2012-0067. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deepbrain stimulation. Journal of Neurophysiology. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Martinu K, Monchi O. Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson’s disease: pathophysiology or compensation? Behavoral Neuroscience. 2013;127:222–236. doi: 10.1037/a0031226. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain and Cognition. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Rodríguez-Oroz MC, Obeso JA, Jahanshahi M. Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict-induced slowing in Parkinson’s disease. Experimental Brain Research. 2013;226:451–462. doi: 10.1007/s00221-013-3457-9. [DOI] [PubMed] [Google Scholar]
- Pasquereau B, Nadjar A, Arkadir D, Bezard E, Goillandeau M, Bioulac B, Gross CE, Boraud T. Shaping of motor responses by incentive values through the basal ganglia. Journal of Neuroscience. 2007;27(5):1176–1183. doi: 10.1523/JNEUROSCI.3745-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo AM, Tröster AI, Glatt SL, Hubble JP, Koller WC. Differentiation of the dementias of Alzheimer’s and Parkinson’s disease with the dementia rating scale. Journal of Geriatric Psychiatry and Neurology. 1995;8:184–188. doi: 10.1177/089198879500800308. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues in Clinical Neuroscience. 2001;3:151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza O, Lucas JA, Smith GE, Petersen RC, Graff-Radford NR, Ivnik RJ. Robust and expanded norms for the Dementia Rating Scale. Archives of Clinical Neuropsychology. 2010;25:347–358. doi: 10.1093/arclin/acq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky E, Schiehser DM, Litvan I, Obtera KM, Burke MM, Lessig SL, Song DD, Liu L, Filoteo JV. The utility of the Mattis Dementia Rating Scale in Parkinson’s disease mild cognitive impairment. Parkinsonism and Related Disorders. 2014;20:627–631. doi: 10.1016/j.parkreldis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Annals of Neurology. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Poston KL, Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage. 2012;62:2261–2270. doi: 10.1016/j.neuroimage.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connectivity. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurology. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Thornton JS, Mancini L, Hyare H, Yousry T, Ridgway GR, Zhang H, Modat M, Alexander DC, Rossor MN, Ourselin S, Fox NC. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain. 2013;136:1399–1414. doi: 10.1093/brain/awt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Henik A, Robertson LC. Interpreting Stroop interference: An analysis of differences between task versions. Neuropsychology. 2001;15:462–471. doi: 10.1037//0894-4105.15.4.462. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez J, Fernandez T, Silva-Pereyra J, Martinez Mesa JA, Di Russo F. Differences in visuo-motor control in skilled vs. novice martial arts athletes during sustained and transient attention tasks: a motor-related cortical potential study. PLoS One. 2014;9:e91112. doi: 10.1371/journal.pone.0091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: Evidence from a Stroop Match-to-Sample task. Biological Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Javitz H, Pfefferbaum A, Sullivan EV. Callosal Compromise Differentially Affects Conflict Processing and Attentional Allocation in Alcoholism, HIV, and Their Comorbidity. Brain Imaging and Behavior. 2008;2:27–38. doi: 10.1007/s11682-007-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Vinco S, Hoeft F, Pfefferbaum A, Sullivan EV. Double dissociation between action-driven and perception-driven conflict resolution invoking anterior versus posterior brain systems. Neuroimage. 2009;48(2):381–390. doi: 10.1016/j.neuroimage.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Chanraud S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Age-related reorganization of functional networks for successful conflict resolution: a combined functional and structural MRI study. Neurobiology of Aging. 2011;32:2075–2090. doi: 10.1016/j.neurobiolaging.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. Journal of Neuroscience. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford T, Gurney KN. Biologically constrained action selection improves cognitive control in a model of the Stroop task. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2007;362:1671–1684. doi: 10.1098/rstb.2007.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. European Journal of Neuroscience. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Parish CL, Zhu WM, Krstew EV, Lawrence AJ, Drago J, Finkelstein DI, Horne MK. Changes in function and ultrastructure of striatal dopaminergic terminals that regenerate following partial lesions of the SNpc. Journal of Neurochemistry. 2003;86:329–343. doi: 10.1046/j.1471-4159.2003.01843.x. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Goetz CG. Factor structure of the Unified Parkinson’s Disease Rating Scale: Motor Examination section. Movement Disorders. 1998;13:633–636. doi: 10.1002/mds.870130404. [DOI] [PubMed] [Google Scholar]
- Steinhauser M, Hübner R. Distinguishing response conflict and task conflict in the Stroop task: evidence from ex-Gaussian distribution analysis. Journal of Experimental Psychology. Human Perception and Performance. 2009;35:1398–1412. doi: 10.1037/a0016467. [DOI] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. Journal of Neuroscience. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, Corbo D, Cirillo G, Barone P, Tedeschi G. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79:2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. Journal of Neuroscience. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbossche J, Deroost N, Soetens E, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E. Freezing of gait in Parkinson disease is associated with impaired conflict resolution. Neurorehabilitation and Neural Repair. 2011;25:765–773. doi: 10.1177/1545968311403493. [DOI] [PubMed] [Google Scholar]
- van Nuenen BF, Helmich RC, Buenen N, van de Warrenburg BP, Bloem BR, Toni I. Compensatory activity in the extrastriate body area of Parkinson’s disease patients. Journal of Neuroscience. 2012;32:9546–9553. doi: 10.1523/JNEUROSCI.0335-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar SD, Bordelon YM, Hays RD, Diaz N, Rausch R, Mao C, Vickrey BG. Confirmatory factor analysis of the motor unified Parkinson’s disease rating scale. Parkinson’s Disease. 2012;2012:719167. doi: 10.1155/2012/719167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Chen Z, Zhao G, Hitchman G, Liu C, Zhao X, Liu Y, Chen A. Linking inter-individual differences in the conflict adaptation effect to spontaneous brain activity. Neuroimage. 2014;90:146–152. doi: 10.1016/j.neuroimage.2013.12.055. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neuroscience Letter. 2009;460:6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang J, Wang C, Hallett M, Zang Y, Wu X, Chan P. Basal ganglia circuits changes in Parkinson’s disease patients. Neuroscience Letter. 2012;524:55–59. doi: 10.1016/j.neulet.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P. Attention to Automatic Movements in Parkinson’s Disease: Modified Automatic Mode in the Striatum. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu135. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35:222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.