Abstract
As humans and climate change alter the landscape, novel disease risk scenarios emerge. Understanding the complexities of pathogen emergence and subsequent spread as shaped by landscape heterogeneity is crucial to understanding disease emergence, pinpointing high-risk areas, and mitigating emerging disease threats in a dynamic environment. Tick-borne diseases present an important public health concern and incidence of many of these diseases are increasing in the United States. The complex epidemiology of tick-borne diseases includes strong ties with environmental factors that influence host availability, vector abundance, and pathogen transmission. Here, we used 16 years of case data from the Minnesota Department of Health to report spatial and temporal trends in Lyme disease (LD), human anaplasmosis, and babesiosis. We then used a spatial regression framework to evaluate the impact of landscape and climate factors on the spread of LD. Finally, we use the fitted model, and landscape and climate datasets projected under varying climate change scenarios, to predict future changes in tick-borne pathogen risk. Both forested habitat and temperature were important drivers of LD spread in Minnesota. Dramatic changes in future temperature regimes and forest communities predict rising risk of tick-borne disease.
Keywords: annaplasmosis, babesiosis, climate change, ixodes scapularis, landscape epidemiology, lyme disease, spatial model, tick-borne pathogens, tick-borne disease
Introduction
The extent and rate of spread of emerging diseases has important consequences for public health, as well as animal management and conservation. Pathogens vectored by arthropods spread at a rate affected by fine-scale factors including habitat, host ecology, human movement, and broad-scale factors such as temperature and precipitation. Understanding the environmental drivers of disease spread is essential for effective management of disease risks. Vector-borne pathogens illustrate the complexities of eco-epidemiological processes: not only must a pathogen transmit to a host, but also there must be suitable environments to support both vector and host populations, and to facilitate contact between species.
Lyme disease (LD) is caused by the spirochete Borrelia burgdorferi which is primarily vectored by Ixodes scapularis (blacklegged ticks) in North America. LD provides a classic demonstration of the linkage between environmental factors and epidemiological processes (Ostfeld et al. 1995). LD exhibits a complex ecology in which landscape composition and configuration shape the mammalian communities available for tick feeding (Allan et al. 2003; Ostfeld et al. 2006), and climatic factors influence the survival, life cycle, and phenology of ticks as well as that of potential host communities (Gatewood et al. 2009; Ogden et al. 2004). Since LD was first recognized in the mid-1970s (Steere et al. 1977), it has become the most common vector-borne illness in the USA (Killilea et al. 2008). I. scapularis is also the primary vector of several less common, but emerging, pathogens including the agents of human anaplasmosis (HA, Anaplasma phagocytophilum), babesiosis (Babesia spp.), and a form of human ehrlichiosis (HE, Ehrlichia muris-like agent; Pritt et al. 2011). Thus, the ecology of I. scapularis, tick hosts, and pathogens is crucial for multiple public health concerns.
The impact of global climate change on the risk of infectious diseases is a hotly debated topic in both ecology and public health (Altizer et al. 2013; Dobson and Randolph 2011; Harvell et al. 2009; Randolph 2010). In the case of arthropod-vectored pathogens, mounting evidence suggests that climate change will alter disease dynamics by creating hospitable climate conditions for survival and expansion of vector populations (Lindgren et al. 2000; Ogden et al. 2006), and by allowing accelerated pathogen development (Ogden et al. 2008), and facilitating pathogen transmission cycles (Rogers and Randolph 2006). The prolonged multi-host feeding behavior of ticks makes pathogen transmission highly dependent on developmental rate and seasonal synchrony of tick life stages which are both subject to climate drivers (Gatewood et al. 2009; Ogden et al. 2008; Randolph 1998). Further, because questing I. scapularis withstand a relatively narrow range of temperature and humidity conditions, climate may strongly influence host-seeking behavior (Perret et al. 2000; Schulze and Jordan 2003; Vail and Smith 2002), where questing is curtailed in hotter or more arid conditions (Schulze and Jordan 2003; Schulze et al. 2001). Climate may also limit tick populations through limits imposed on host populations (Lewellen and Vessey 1998; Ogden et al. 2006).
Expansion of tick populations and tick-borne pathogens (TBP) have been associated with anthropogenic land use change (Harrus and Baneth 2005) and changing climate (Altizer et al. 2013; Githeko et al. 2000; Randolph 2010). Minnesota (MN) sits near the western edge of I. scapularis’ range and the Midwestern focus of LD (Diuk-Wasser et al. 2010; Hamer et al. 2010), and large-scale modeling suggests increasing risk of LD across the USA (Estrada-Pena 2002), and Canada (Ogden et al. 2013). Within MN, I. scapularis has expanded northward since 2000 (MDH, unpublished data). Additionally, recent investigations indicate northern range expansions of the white-footed mouse (Peromyscus leucopus), an important tick and pathogen host (Simon et al. 2014). The establishment of both host and vector populations provides conditions for TBP emergence.
While much TBP research has focused on the origin of LD in the Northeast USA, the Upper Midwest remains an understudied, but important system, in terms of public health and landscape epidemiology of TBP. The incidence of reported LD and HA cases has increased nationally since the 1990s, and incidence in Minnesota (MN) has consistently been in the top 20% nationwide (Bacon et al. 2008; Demma et al. 2005). Babesiosis, which was not nationally notifiable until 2011, occurs less frequently but is increasingly reported (Diuk-Wasser et al. 2014; Krause et al. 2003). MN provides a natural case study for understanding impacts of landscape gradients and changing climate on TBP. The state spans nearly 6 latitudinal degrees covering over 650 km north to south. Parkland and mixed forest ecozones mark distinct landscapes providing varied tick habitat. Due to its northern continental location, MN is expected to experience climate change pressures that will alter this landscape and the composition of both plant and animal communities (Pryor et al. 2013).
In this study, we combine public health and environmental data to analyze factors shaping the spread of TBP in MN as a means to understand current disease risk, predict future spread of disease, and aid public health planning. Our first objective was to present public health data describing the emergence and spread of TBP in MN (1996– 2011). Next, we constructed a multivariate landscape epidemiological model to discern the impacts of spatial, climatic, vegetative cover, and host-related risk factors in TBP spread. Finally, we used the fitted model in combination with projected changes in climate and forest cover, to predict changes in TBP risk in MN by the year 2100. Understanding the dynamic nature of environmental risk factors will help shape public health actions and inform future strategies to mitigate TBP risk.
Methods
Study Area and TBP Data
The study area covered the state of Minnesota, USA. We examined TBP incidence from1996 to 2011 using surveillance data provided by the Minnesota Department of Health (MDH). The first LD cases in Minnesota were reported in the early 1980s in east-central counties, with the presumed origin in Pine County (based on early case numbers, and proximity to LD prevalence in neighboring Wisconsin; Schmid et al. 1985) (Fig. 1). Disease surveillance and reporting methods for LD, HA, and babesiosis did not change substantially between 1996 and 2011. Reports meeting surveillance case definitions (Council of State and Territorial Epidemiologists 1997) for confirmed (clinical diagnosis and laboratory detection of agent) LD or babesiosis and confirmed or probable (clinical diagnosis only) HA were used to calculate disease incidence.
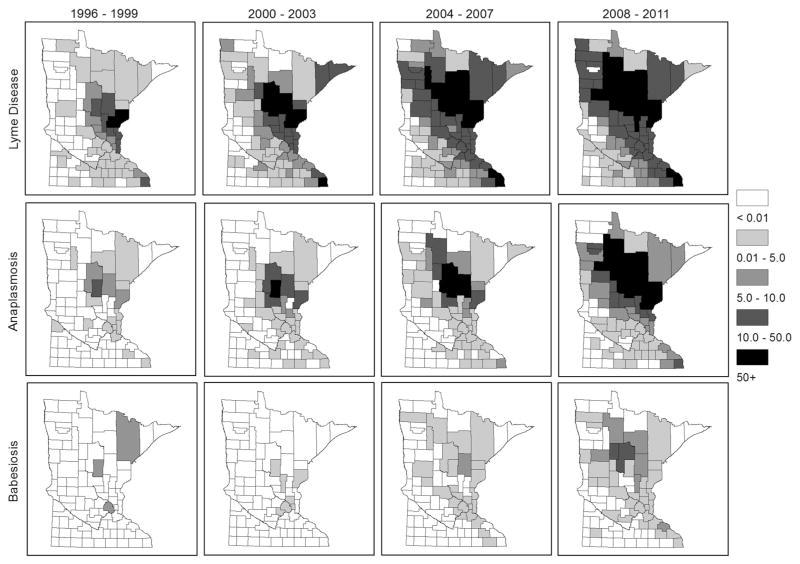
Figure 1.
Incidence of Lyme disease, human anaplasmosis, and babesiosis increased across Minnesota from 1996 to 2011. Data are based on cases reported to the Minnesota Department of Health, summarized by county. The color legend represents cases per 100,000 county residents.
We report case data at the county level to maintain patient privacy. County-specific incidence was based on county of case residence at the time of illness onset, and is presented as incidence per 100,000 county residents (to account for variation in county populations from approximately 4,000 to over 1,000,000; U.S. Census Bureau 2010). Summary statistics are presented for LD, HA, and babesiosis. Because LD was the most prevalent, and thus provided the most data, it was the focus for disease risk modeling. The limited number of HA and babesiosis cases per time point was insufficient for reliable inference from statistical models. However, we expect our landscape epidemiological model for LD to be generally applicable to the public health management of I. scapularis-vectored TBP.
Environmental Data
We constructed a multivariate risk model based on disease spread, vector habitat conditions, host communities, and climate factors. Distance (DIST) and angle (ANGL) from LD origin were measured from the centroid of county i to the centroid of Pine County, to account for the process of disease diffusion under which it would be expected for the disease agent to take longer to reach areas far from the origin.
Because LD risk is closely tied to landscape characteristics, we evaluated the impact of predominant landcover types in MN (based on level 2 classifications in the MN GAP dataset (Minnesota Department of Natural Resources 2013). Each landcover type was quantified as the percent of land occupied within each county based on 30 m2 pixels using the Zonal Histogram tool in ArcGIS 10 (ESRI Inc., Redlands, CA). Deciduous forest habitat (DEC, approximately 18.88% of MN landcover) is crucial habitat for both small and large mammal hosts for I. scapularis, and is related to tick abundance (LoGiudice et al. 2003). Lower LD risk may be expected as coniferous forests (CON, 8.21%) replace deciduous. Urban landcover (URB, 1.87%) may be an important indicator of habitat fragmentation and potential for human contact with ticks. The adaptability of both deer and mice to small patches of habitat, combined with high human densities, can create high TBD risk in urban/suburban areas (Allan et al. 2003; Lewellen and Vessey 1998; Nupp and Swihart 1998). Agricultural lands and grasslands (AGG, 53.84%) typically provide poor habitat for tick populations even when adequate host populations are present. This may be due to microclimate factors that are less favorable for tick survival and questing compared to forested areas (Guerra et al. 2002; Sumilo et al. 2006). Water bodies and wetlands (WET, 9.57%) might provide an index of local humidity and soil moisture conditions that would be important for tick questing, and may indicate higher human exposure to ticks through outdoor recreation (Schwartz and Goldstein 1990).
White-tailed deer are crucial for adult tick feeding and reproduction, making deer populations key drivers of tick density on the landscape; accordingly, deer have been closely associated with tick distributions (Duffy et al. 1994; Leger et al. 2013; Wilson et al. 1985). We used the annual deer harvest (DEER) in each county as a measure of potential adult-tick host populations to support tick populations (data from Minnesota Department of Natural Resources 2013). While small mammals also constitute critical host populations for immature tick stages, statewide data were limited. We expect that the above landscape variables will provide a valuable proxy for small mammal habitat impacting tick populations.
We also evaluated several climatic factors likely to influence the life cycles and host-seeking activity of ticks and the incidence of LD (compiled from all weather stations in Minnesota from 1996 to 2011; MN State Climatology Office 2013). Temperature is an important factor driving tick survival and development (Lindsay et al. 1995). We measured average yearly minimum (Tmin) and maximum (Tmax) temperatures. Additionally, degree days above freezing (DD > 0) has been shown to be a useful index of climate impacts on tick life cycles whereby insufficient DD > 0 may depress development to a degree that few larvae develop quickly enough to quest within the year they hatch, thus limiting populations when many ticks deplete their energy reserves and die before finding a host (Lindsay et al. 1995; Ogden et al. 2005). We evaluated the effect of DD > 0 as both a numerical and a categorical variable to represent threshold effects on tick growth cycles (cold: fewer than 2,900 DD > 0, fair: 2,901–3,200 DD > 0, and ideal: greater than 3,200 DD > 0; values after Ogden et al. 2005). Previous studies have found precipitation to be a crucial climate factor affecting tick populations (Jones and Kitron 2000; McCabe and Bunnell 2004). We calculated average annual precipitation (PREC). Average annual snowfall was measured separately (SNOW), to provide an indication of winter severity and snow cover (Ogden et al. 2013).
Environmental Risk Factor Model
To capture the spatial and temporal complexities of LD epidemiology in MN, we used a multi-faceted modeling approach. We first summarized temporal trends in LD incidence using response feature analysis, which is an effective way to distill relevant aspects of a response variable over time or across longitudinal data (Matthews 2005). For each county, we calculated the slope of annual LD incidence regressed against time (years). This slope captured the rate of disease increase, providing an informative measure of risk. We would expect that high-risk areas would have high disease incidence in any given year and exhibit rapid rates of incidence increase (County-level LD risk measures correlated closely whether based on slope or annual incidence; r = 0.82). We then used this slope as the response variable for our landscape epidemiological models.
Disease spread is an inherently spatial process, particularly early in the emergence of a disease (Hess et al. 2002). Epidemiological data are likely to exhibit spatial autocorrelation brought about by endogenous (disease spread locally through contact between neighbors) as well as exogenous factors (neighboring areas may be subject to similar environments) (Lawson 2013). Thus, we implemented a spatial simultaneous autoregressive (SAR) model. The autoregressive term (ρ) in the SAR model lets us acknowledge that disease incidence is not independent in each county, and the ρ parameter quantifies the dependence on incidence in neighboring counties (Waller and Gotway 2004). SAR models are particularly well suited to capture spatial patterns in non-uniform areal data typical of county-level analyses (Wall 2004). Further, because we are specifically characterizing the spatial dependence structure in the data (as opposed to simply detrending spatial patterns or assigning random effects), this model is well suited to replicate the spatial structure in the predictive models used in further steps in the analysis. We fit a landscape epidemiological model in the form:
where yi is the response variable (rate of disease increase) at county i, and ρ is the coefficient of autocorrelation in the response. Wij is a spatial weighting matrix wherein we considered counties as neighbors to those with centroids within 120 km (ensuring that even the largest counties were neighbors with adjacent counties), and yj is the response in the neighboring counties. β is the matrix of regression coefficients describing the effects of environmental variables X, and ε is the random error term. Models were fit using the lagsarlm routine in the spdep package in R (Bivand et al. 2011). We selected between competing models based on Akaike Information Criterion (AIC) weights (Burnham and Anderson 2002). We used an iterative approach, first selecting from univariate models for each variable. We chose the most informative variables representing climate, landscape, LD origin, and host populations based on AIC weights. We also requiring that variables retained have regression coefficients significantly different from 0 (P vaules ≤ 0.05). Further, we eliminated collinear variables based on variance inflation factors (using a conservative threshold of 5.0; see Robinson and Schumacker 2009). We then compared models combining the selected factors and interactions among them, again using AIC weights to determine the most informative model.
Future Risk Prediction Model
Climate change has been recognized as a driving factor influencing vector-borne diseases. As such, it is important not only to understand the disease risk in current landscapes, but also to assess potential risk under future scenarios. After identifying risk factors under current conditions, we applied models of climate and landscape change to predict changes in TBP risk. To estimate the range of realistic future LD risk, we based predictive models on scenarios at the extremes of climate predictions from the Intergovernmental Panel on Climate Change (IPCC). A low climate change case (CClow) was based on the B1 scenario emphasizing carbon emissions decrease and predicting a 1.1–2.9°C rise in global temperatures by 2100 (Nakicenovic et al. 2000); MN temperatures would rise 0.5–2.5°C in this scenario (Hayhoe et al. 2010). A high climate change case (CChigh) was based on the A1FI scenario in which carbon emissions increase and global temperatures rise 2.4–6.4°C (Nakicenovic et al. 2000); up to 8°C in MN (Pryor et al. 2013).
To predict the future distribution of forest habitat important to I. scapularis, we used tree habitat occupancy models from the Tree Atlas project of the US Forest Service (Prasad et al. 2007-ongoing). This model predicts the range suitable for numerous tree species based on climate, elevation, land use, and soil properties to determine the habitat occupied by individual tree species, then used predicted changes in climate and disturbance patterns to predict the range suitable for each species by 2100 (Iverson et al. 2008). To predict the potential future deciduous forest landcover, we combined Tree Atlas predictions for suitable tree habitat for all deciduous species in MN. The same was done for coniferous species. The general prediction was for replacement of coniferous forests with deciduous forests as climate warms and precipitation and disturbance regimes change. We used Tree Atlas predictions calculated under both the B1 (CClow) and A1FI (CChigh) climate change scenarios.
Results
TBP Data Description
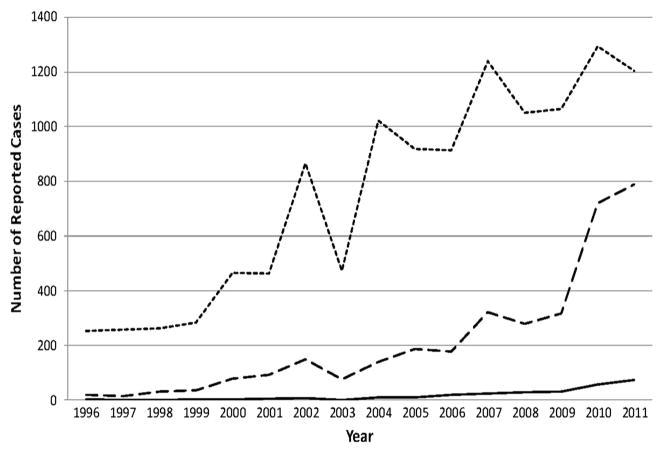
From 1996 through 2011, 15,715 cases of I. scapularis-transmitted disease were reported in Minnesota residents, comprising 12,024 (77%) confirmed LD cases, 3,422 (22%) confirmed and probable HA cases, and 269 (2%) confirmed babesiosis cases (Fig. 1). Of these, 262 patients met case definitions for more than one disease. The number of I. scapularis-transmitted diseases in Minnesota increased from 278 total cases (5.8 cases per 100,000 population) in 1996 to 2,063 total cases (39.7 cases per 100,000 population) in 2011, a 742% increase over the 16-year period (Fig. 2). Although HA was reported less frequently than LD, it accounted for an increasing proportion of I. scapularis-transmitted disease cases, rising from 7% of total cases in 1996 to 38% of total cases in 2011. Babesiosis is still rare by comparison. A strain of human ehrlichiosis (E. muris-like agent) has also been recently reported in MN during the study period (Pritt et al. 2011), but was not included in the current analysis due to rarity. Increases in LD and other tick-borne diseases were not spatially uniform across MN, and showed a northwesterly geographic expansion trend (Fig. 1).
Figure 2.
Total reports of tick-borne diseases increased 742% in Minnesota from 1996 to 2011. Lines show Lyme disease (dotted), human anaplasmosis (dashed), and babesiosis (solid). Data are based on total cases (state-wide) reported to the Minnesota Department of Health.
Environmental Risk Factor Model
The best model for LD increase in MN incorporated spatial autocorrelation, angle from outbreak origin, climate factors, and tick habitat, (Fig. 3):
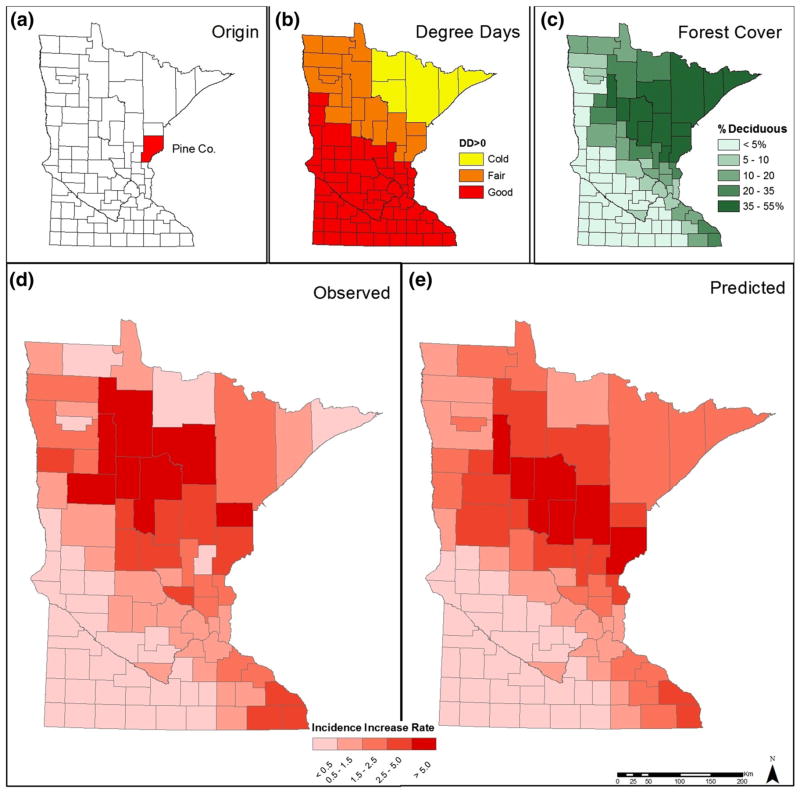
Figure 3.
Origin, Degree Days, and Forest Cover were the landscape and climate factors identified as drivers of tick-borne risk in MN (top row). Observed data (bottom left) were used to fit a landscape epidemiological model; predicted values (bottom right).
The SAR modeling framework was important for accounting for the spatially dependent nature of our epidemiological data; evidenced by significance of the ρ parameter, and tests of residual autocorrelation (lmmorantest in spdep; Bivand et al. 2011) indicate that accounting for spatial pattern directly in the model is essential to avoid autocorrelation in the residuals. All models incorporating some combination of DD > 0 and DEC provided strong explanatory power (Table 1), indicating their importance as drivers of TBP risk in MN. Variables were scaled so that parameter estimates could be compared (Table 2). Our model indicates that DD > 0(categorical) had the strongest effect on the increasing risk of LD in MN. Counties with ideal or fair temperature conditions experienced 3- to 4-U increase in LD risk compared to counties with cold temperature conditions (DD < 0 below 2900). For each unit of increase in deciduous forest cover (DEC) LD risk increased over 1.5 U. Risk increased at a lower, but significant rate, in counties at a northwesterly angle from the county of LD origin. The model-predicted LD risk under current environmental conditions was strongly correlated with observed risk values, though spatial smoothing was evident in the model-derived values (Fig. 3d–e). Recalling that the response variable is the slope of LD increase over a 16 year period in each county, the model-derived risk measures indicate a rate of increase in annual incidence per 100,000 residents.
Table 1.
The Best Model of Lyme Disease Risk in MN Included Climate, Landscape, and Spatial Factors (★★)
| Candidate models | AIC | ΔAIC | AICwt |
|---|---|---|---|
| O | 333.79 | 49.93 | 6.9E-12 |
| C | 329.30 | 45.44 | 6.5E-11 |
| L | 307.67 | 23.81 | 3.2E-06 |
| H | 313.36 | 29.50 | 1.9E-07 |
| O + C | 331.22 | 47.36 | 2.5E-11 |
| O + L | 309.41 | 25.55 | 1.4E-06 |
| O + H | 310.36 | 26.50 | 8.5E-07 |
| C + L | 285.18 | 1.32 | 0.248 |
| C + H | 311.45 | 27.59 | 4.9E-07 |
| L + H | 300.71 | 16.85 | 1.1E-04 |
| O + C + L | 283.86 | 0.00 | 0.480★★ |
| O + C + H | 312.57 | 28.71 | 2.8E-07 |
| O + L + H | 300.58 | 16.72 | 1.1E-04 |
| C + L + H | 287.12 | 3.26 | 0.094 |
| O + C + L + H | 285.85 | 1.99 | 0.177 |
| Model component | Variable |
|---|---|
| Origin (O) | Angle from Pine Co. |
| Climate (C) | DD > 0 (categorical) |
| Landscape (L) | % Deciduous Forest |
| Hosts (H) | Deer Harvest/square km |
AIC weights indicate some support for any model including at least climate and landscape (bold). The best variable for each model component was chosen from preliminary examination of univariate models.
Table 2.
Regression Parameters (β) for the Fitted Landscape Epidemiological Model Show That Temperature Conditions (DD > 0) and Deciduous Forest Habitat (DEC) had the Strongest Effects on Lyme Disease Risk in MN from 1996 to 2011
| Variable | β | P value |
|---|---|---|
| Spatial autocorrelation | ||
| ρ | 0.518 | 0.002 |
| Proximity | ||
| ANGL | 0.409 | 0.057 |
| Landscape | ||
| DEC | 1.548 | 6.8E-14 |
| Climate | ||
| DD > 0 (poor) | 0 | na |
| DD > 0 (fair) | 3.213 | 1.5E-07 |
| DD > 0 (good) | 4.224 | 4.4E-08 |
Spatial autocorrelation (ρ) and angle from outbreak origin (ANGL) were also significant factors.
Future Risk Prediction Model
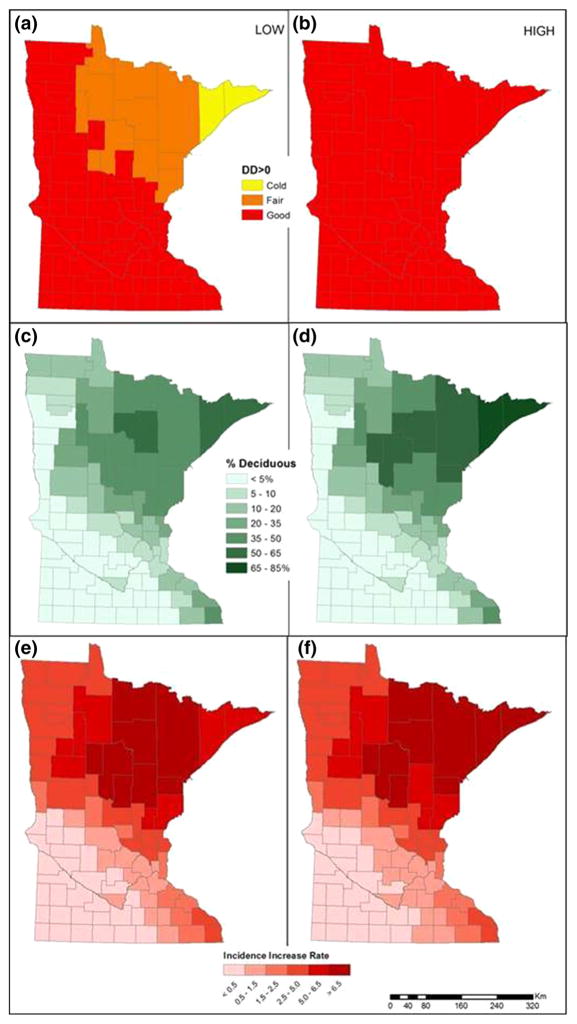
Predictions of future LD were similar under both climate change scenarios. LD risk increased overall and most substantially for northeastern counties, where future models predict transition from coniferous to more deciduous forest, and where cold counties transitioned to fair or ideal temperature regimes for ticks. Under the CClow scenario, DD > 0 in the average county increased by 166 degree days (a 4.8% increase), leaving only two counties in the cold and 11 counties in the fair DD > 0 categories (Fig. 4a). The average county gained 1.29% DEC landcover (Fig. 4c). Under the CChigh scenario, the average county experienced an increase of 1,166 in DD > 0, representing a 34.3% increase from observed levels (Fig. 4b). Under this scenario, all 87 counties had climates in the ideal DD > 0 category. The average county gained 1.94% DEC landcover (Fig. 4d). Note that the overall proportion of forest cover in MN was predicted to change little; the majority of DEC increase was due to replacement of declining CON forests. While the model based on current environmental conditions yielded a maximum LD risk estimate of 6.38 (increase in incidence per 100,000 residents) in the highest risk counties, the CClow scenario predicted up to 8.86 (Fig. 4e), and the CChigh scenario predicted up to 8.95 with more counties in the highest risk levels (Fig. 4f). Southeast counties with little forested habitat maintained the lowest levels of TBP risk in MN.
Figure 4.
Lyme disease risk factors and predicted incidence increase change under climate change predictions for MN by 2100 (based on IPCC scenarios B1 left, and A1Fl right). Datasets reflecting changes in DD > 0 (a, b) and deciduous forest distribution (c, d) were used to model potential changes in LD risk (e, f) (baseline values in Fig. 3.).
Discussion
The incidence of reported I. scapularis-transmitted diseases in Minnesota increased markedly from 1996 through 2011. By examining landscape factors in a multiple regression framework, we were able to show that both landcover and climate conditions played important roles in the distribution of TBP risk across Minnesota. Further, by applying our landscape epidemiological model to future conditions projected under climate change scenarios, we were able to estimate the future expansion of TBP risk.
Increasing Incidence of TBP in MN
The increased incidence of LD, concomitant with HA and babesiosis, suggests a true increase in I. scapularis-vectored pathogens in MN, rather than disease reporting issues. Although MDH surveillance methods did not change substantially during this time, increased physician awareness or diagnostic testing for HA and babesiosis could partially explain why the incidence of these diseases rose at a faster rate than LD. However, the distinct spatial expansion of all of the diseases indicates a likely transmission increase rather than a disease reporting issue.
Counties at the highest risk for LD were northwest of MN’s LD origin. We note that, while early case reports suggest that LD first became established in the Pine County area of MN, we cannot confirm the actual entry point of Borrelia-infected ticks. However, epidemiological data suggest that this area of high and early incidence is a useful reference point for modeling efforts. Additionally, a number of LD cases from urban counties (Ramsey and Hennepin) may have been exposed to ticks while traveling to central Minnesota or western Wisconsin for recreation, while residents of rural counties were more likely to have been both exposed and diagnosed in their county of residence (based on case history interviews, MDH unpublished). Thus, for the vast majority of the MN landscape, the county-of-residence data adequately characterize TBP exposure and project minimal bias on the spatial risk analysis. If data are biased by reporting location, that bias likely leads to under-estimation of risk in those counties identified as high risk.
Risk Model Performance and TBP Risk Factors
Models combining spatial patterns, climate factors, and landcover characteristics dramatically outperformed models relying on either landscape or climatic factors alone, demonstrating the complexity of the TBP ecology in MN. Deciduous forest habitat is consistently noted as a key factor in supporting the vertebrate host and tick communities necessary for LD and other TBP cycles (Ostfeld et al. 2006). Warmer areas within forested ecoregions were identified as higher LD risk in our model, similar to previous studies (Ogden et al. 2004). Temperature factors, and DD > 0 specifically, have been linked to tick survival, life stage development, and synchronous feeding of larvae and nymphs (Gatewood et al. 2009; Lindsay et al. 1998; Ogden et al. 2005; Simon et al. 2014). While humidity is also important to support tick populations and questing (Schulze et al. 2001), neither precipitation nor wetland variables were included in our best model. It is likely that county-level summaries of these variables do not adequately represent the microclimate relevant to questing ticks. Additionally, although host populations are essential for parasites, the deer harvest variable was not included in the best model, likely because it contributed little that was not accounted for by forest cover. In addition to relatively static landscape characteristics, spread of LD is also influenced by host-facilitated tick dispersal (Hamer et al. 2010; Leger et al. 2013), and long-distance dispersal is likely associated with deer as well as migratory bird hosts (Brinkerhoff et al. 2009).
We would expect our model findings to be applicable to other ixodid tick-vectored pathogen systems. As climate and forest conditions grow more favorable to I. scapularis populations in MN, we can expect that risk of HA and babesiosis will rise in a fashion similar to that modeled for LD, and continuing the trend observed since 1996. In fact, other research has suggested more generally that warming climate may favor emergence of several TBD (Kurtenbach et al. 2006; Ogden et al. 2008; Woolhouse and Gowtage-Sequeria 2005).
Predictive models come with important caveats. Here, we considered only environmental variables; human reactions to environmental risks, future alterations in population trends, land use, recreation, or other human behaviors are beyond the scope of our analysis, but could impact human exposure to TBP risks. Further, diagnosis and reporting of TBP are imperfect and incomplete data can impact risk estimates (Pfaffle et al. 2013). While we used landscape proxies of potential host communities, additional complex interactions among host species are likely to impact establishment of tick and TBP communities (Levi et al. 2012; Simon et al. 2014). Additionally, host movement is not captured here, but may impact the introduction and spread of TBP (Brinkerhoff et al. 2009; Gatewood et al. 2009; Ogden et al. 2013). Finally, cascading effects of climate change and landscape change are likely to lead to interactions and threshold effects that may not be well explained by linear models. Future research incorporating observational and experimental study of climate impacts on habitat composition and host communities could clarify some of these uncertainties.
Conclusions
Our research represents a strong combination of climate and landscape predictions to demonstrate the likely increase in TBP risk with climate change. The greatest increases in risk were in forested regions that are currently cold enough to provide poor conditions for tick developmental cycles. The projected increases in deciduous forest cover were small compared to predicted temperature changes, but resulted in additional impacts on the future disease risk. Results add to the growing body of research suggesting important disease implications of warming climate. Both empirical-(Diuk-Wasser et al. 2010) and simulation-based (Ogden et al. 2005) models suggest that warming climate will favor tick survival, population expansion, and host feeding. Climate change scenarios (A2 and B2) have also been used to predict increases in suitable tick range (Ogden et al. 2008), and recent observations support such predictions; tick population expansions in North America and Europe have been associated with warming climate (reviewed in: Pfaffle et al. 2013).
The predicted increase of TBP under a warming climate and an increasingly deciduous landscape provides advanced warning that will be important for public health education and intervention. It is our hope that this landscape epidemiological model has the potential to inform long-term planning of disease surveillance and prevention efforts by public health agencies.
Acknowledgments
We thank the graduate student team at MDH for conducting TBP case follow-up. This work was supported by the University of Minnesota Institute on the Environment and Cooperative Agreements (U50/CCU510333-06). Partial support for UGM came from NIH Grant (R01AI042792).
References
- Allan BF, Keesing F, Ostfeld RS. Effect of forest fragmentation on Lyme disease risk. Conservation Biology. 2003;17:267–272. [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease–United States, 1992–2006. Department of Health & Human Services, Centers for Disease Control and Prevention; 2008. [PubMed] [Google Scholar]
- Bivand R, Altman M, Anselin L, et al. R Package ‘spdep’: spatial dependence: weighting schemes, statistics and models. 2011 http://cran.r-project.org/web/packages/spdep/index.html.
- Brinkerhoff RJ, Folsom-O’Keefe CM, Tsao K, Diuk-Wasser MA. Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen. Borrelia burgdorferi Frontiers in Ecology and the Environment. 2009;9:103–110. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: a Practical Information-Theoretic Approach. New York, NY: Springer; 2002. [Google Scholar]
- Council of State and Territorial Epidemiologists. Case definitions for infectious conditions under public health surveillance. MMWR. 1997;46(RR-10) [PubMed] [Google Scholar]
- Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001–2002. The American Journal of Tropical Medicine and Hygiene. 2005;73:400–409. [PubMed] [Google Scholar]
- Diuk-Wasser MA, Liu Y, Steeves T, et al. Monitoring human Babesiosis emergence through vector surveillance New England, USA. Emerging Infectious Diseases. 2014;20:225–231. doi: 10.3201/eid2002.130644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vourc’h G, Cislo P, et al. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecology and Biogeography. 2010;19:504–514. [Google Scholar]
- Dobson AD, Randolph SE. Modelling the effects of recent changes in climate, host density and acaricide treatments on population dynamics of Ixodes ricinus in the UK. Journal of Applied Ecology. 2011;48:1029–1037. [Google Scholar]
- Duffy DC, Campbell SR, Clark D, DiMotta C, Gurney S. Ixodes scapularis (Acari: Ixodidae) deer tick mesoscale populations in natural areas: effects of deer, area, and location. Journal of Medical Entomology. 1994;31:152–158. doi: 10.1093/jmedent/31.1.152. [DOI] [PubMed] [Google Scholar]
- Estrada-Pena A. Increasing habitat suitability in the United States for the tick that transmits Lyme disease: a remote sensing approach. Environmental Health Perspectives. 2002;110:635. doi: 10.1289/ehp.110-1240908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h Gl, et al. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Applied and Environmental Microbiology. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bulletin of the World Health Organization. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Guerra M, Walker E, Jones C, et al. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the North Central United States. Emerging Infectious Diseases. 2002;8:289–297. doi: 10.3201/eid0803.010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, Hickling GJ. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. EcoHealth. 2010;7:47–63. doi: 10.1007/s10393-010-0287-0. [DOI] [PubMed] [Google Scholar]
- Harrus S, Baneth G. Drivers for the emergence and reemergence of vector-borne protozoal and bacterial diseases. International Journal for Parasitology. 2005;35:1309–1318. doi: 10.1016/j.ijpara.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E. Climate change and wildlife diseases: when does the host matter the most? Ecology. 2009;90:912–920. doi: 10.1890/08-0616.1. [DOI] [PubMed] [Google Scholar]
- Hayhoe K, VanDorn J, Croley T, II, Schlegal N, Wuebbles D. Regional climate change projections for Chicago and the US Great Lakes. Journal of Great Lakes Research. 2010;36:7–21. [Google Scholar]
- Hess G, Randolph S, Arneberg P, et al. Spatial aspects of disease dynamics. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP, editors. The Ecology of Wildlife Diseases. Oxford, UK: Oxford University Press; 2002. pp. 102–118. [Google Scholar]
- Iverson LR, Prasad AM, Matthews SN, Peters M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. Forest Ecology and Management. 2008;254:390–406. [Google Scholar]
- Jones C, Kitron U. Populations of Ixodes scapularis (Acari: Ixodidae) are modulated by drought at a Lyme disease focus in Illinois. Journal of Medical Entomology. 2000;37:408–415. doi: 10.1603/0022-2585(2000)037[0408:POISAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Killilea ME, Swei A, Lane RS, Briggs CJ, Ostfeld RS. Spatial dynamics of Lyme disease: a review. EcoHealth. 2008;5:167–195. doi: 10.1007/s10393-008-0171-3. [DOI] [PubMed] [Google Scholar]
- Krause PJ, McKay K, Gadbaw J, et al. Increasing health burden of human babesiosis in endemic sites. The American Journal of Tropical Medicine and Hygiene. 2003;68:431–436. [PubMed] [Google Scholar]
- Kurtenbach K, Hanincova K, Tsao JI, et al. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nature Reviews Microbiology. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- Lawson AB. Statistical Methods in Spatial Epidemiology. New York, NY: Wiley; 2013. [Google Scholar]
- Leger E, Vourc’h G, Vial L, Chevillon C, McCoy KD. Changing distributions of ticks: causes and consequences. Experimental and Applied Acarology. 2013;59:219–244. doi: 10.1007/s10493-012-9615-0. [DOI] [PubMed] [Google Scholar]
- Levi T, Kilpatrick AM, Mangel M, Wilmers CC. Deer, predators, and the emergence of Lyme disease. Proceedings of the National Academy of Sciences USA. 2012;109:10942–10947. doi: 10.1073/pnas.1204536109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellen RH, Vessey SH. The effect of density dependence and weather on population size of a polyvoltine species. Ecological Monographs. 1998;68:571–594. [Google Scholar]
- Lindgren E, Talleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environmental Health Perspectives. 2000;108:119. doi: 10.1289/ehp.00108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, et al. Survival and development of the different life stages of Ixodes scapularis (Acari: Ixodidae) held within four habitats on Long Point, Ontario, Canada. Journal of Medical Entomology. 1998;35:189–199. doi: 10.1093/jmedent/35.3.189. [DOI] [PubMed] [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, et al. Survival and development of Ixodes scapularis (Acari: Ixodidae) under various climatic conditions in Ontario, Canada. Journal of Medical Entomology. 1995;32:143–152. doi: 10.1093/jmedent/32.2.143. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JN. Summary Measures Analysis of Longitudinal Data. New York: Wiley; 2005. [Google Scholar]
- McCabe GJ, Bunnell JE. Precipitation and the occurrence of Lyme disease in the northeastern United States. Vector-Borne and Zoonotic Diseases. 2004;4:143–148. doi: 10.1089/1530366041210765. [DOI] [PubMed] [Google Scholar]
- Minnesota Department of Natural Resources. [Accessed May 2013]; http://deli.dnr.state.mn.us/data_catalog.html.
- MN State Climatology Office. [Accessed May 2013]; http://climate.umn.edu/
- Nakicenovic N, Alcamo J, Davis G, et al. Special Report on Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change. Richland, WA: Pacific Northwest National Laboratory, Environmental Molecular Sciences Laboratory; 2000. [Google Scholar]
- Nupp TE, Swihart RK. Effects of forest fragmentation on population attributes of white-footed mice and eastern chipmunks. Journal of Mammalogy. 1998;79:1234–1243. [Google Scholar]
- Ogden N, Bigras-Poulin M, Hanincova K, et al. Projected effects of climate change on tick phenology and fitness of pathogens transmitted by the North American tick Ixodes scapularis. Journal of Theoretical Biology. 2008;254:621–632. doi: 10.1016/j.jtbi.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Ogden N, Bigras-Poulin M, O’callaghan C, et al. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. International Journal for Parasitology. 2005;35:375–389. doi: 10.1016/j.ijpara.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Ogden N, Lindsay L, Beauchamp G, et al. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. Journal of Medical Entomology. 2004;41:622–633. doi: 10.1603/0022-2585-41.4.622. [DOI] [PubMed] [Google Scholar]
- Ogden N, Maarouf A, Barker I, et al. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. International Journal for Parasitology. 2006;36:63–70. doi: 10.1016/j.ijpara.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Leighton PA. Predicting the rate of invasion of the agent of Lyme disease Borrelia burgdorferi. Journal of Applied Ecology. 2013;50:510–518. [Google Scholar]
- Ostfeld R, Cepeda O, Hazler K, Miller M. Ecology of Lyme disease: habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecological Applications. 1995;5:353–361. [Google Scholar]
- Ostfeld R, Keesing F, LoGiudice K. Community ecology meets epidemiology: the case of Lyme disease. In: Collinge S, Ray C, editors. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford, UK: Oxford University Press; 2006. pp. 28–40. [Google Scholar]
- Perret J-L, Guigoz E, Rais O, Gern L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland) Parasitology Research. 2000;86:554–557. doi: 10.1007/s004360000209. [DOI] [PubMed] [Google Scholar]
- Pfaffle M, Littwin N, Muders SV, Petney TN. The ecology of tick-borne diseases. International Journal for Parasitology. 2013;43:1059–1077. doi: 10.1016/j.ijpara.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Prasad AM, Iverson LR, Matthews S, Peters M. A Climate Change Atlas for 134 Forest Tree Species of the Eastern United States [database] Delaware, OH: Northern Research Station, USDA Forest Service; 2007. [Accessed May 2014]. http://www.nrs.fs.fed.us/atlas/tree. [Google Scholar]
- Pritt BS, Sloan LM, Johnson DKH, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. New England Journal of Medicine. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor S, Barthelmie R, Schoof J. High-resolution projections of climate-related risks for the Midwestern USA. Climate Research. 2013;56:61–79. [Google Scholar]
- Randolph S. Ticks are not insects: consequences of contrasting vector biology for transmission potential. Parasitology Today. 1998;14:186–192. doi: 10.1016/s0169-4758(98)01224-1. [DOI] [PubMed] [Google Scholar]
- Randolph SE. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Veterinary Parasitology. 2010;167:92–94. doi: 10.1016/j.vetpar.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Robinson C, Schumacker RE. Interaction effects: centering, variance inflation factor, and interpretation issues. Multiple Linear Regression Viewpoints. 2009;35:6–11. [Google Scholar]
- Rogers D, Randolph S. Climate change and vector-borne diseases. Advances in Parasitology. 2006;62:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- Schmid GP, Horsley R, Steere AC, et al. Surveillance of Lyme disease in the United States, 1982. Journal of Infectious Diseases. 1985;151:1144–1149. doi: 10.1093/infdis/151.6.1144. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA. Meteorologically mediated diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. Journal of Medical Entomology. 2003;40:395–402. doi: 10.1603/0022-2585-40.4.395. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW. Effects of selected meteorological factors on diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) Journal of Medical Entomology. 2001;38:318–324. doi: 10.1603/0022-2585-38.2.318. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Goldstein MD. Lyme disease in outdoor workers: risk factors, preventive measures, and tick removal methods. American Journal of Epidemiology. 1990;131:877–885. doi: 10.1093/oxfordjournals.aje.a115578. [DOI] [PubMed] [Google Scholar]
- Simon JA, Marrotte RR, Desrosiers N, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evolutionary Applications. 2014;7:750–764. doi: 10.1111/eva.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Snydman DR, et al. An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis & Rheumatism. 1977;20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- Sumilo D, Bormane A, Asokliene L, et al. Tick-borne encephalitis in the Baltic States: identifying risk factors in space and time. International Journal of Medical Microbiology. 2006;296:76–79. doi: 10.1016/j.ijmm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. [Accessed May 2013];Data generated using American FactFinder. 2010 http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml.
- Vail SG, Smith G. Vertical movement and posture of blacklegged tick (Acari: Ixodidae) nymphs as a function of temperature and relative humidity in laboratory experiments. Journal of Medical Entomology. 2002;39:842–846. doi: 10.1603/0022-2585-39.6.842. [DOI] [PubMed] [Google Scholar]
- Wall MM. A close look at the spatial structure implied by the CAR and SAR models. Journal of Statistical Planning and Inference. 2004;121:311–324. [Google Scholar]
- Waller LA, Gotway CA. Applied spatial statistics for public health data. New York, NY: Wiley; 2004. [Google Scholar]
- Wilson ML, Adler GH, Spielman A. Correlation between abundance of deer and that of the deer tick, Ixodes dammini (Acari: Ixodidae) Annals of the Entomological Society of America. 1985;78:172–176. [Google Scholar]
- Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerging Infectious Diseases. 2005;11:1842. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]