Abstract
Background
Males show higher rates of attention deficit hyperactivity disorder (ADHD) than do females. Potential explanations include genuine etiological differences or artifact.
Methods
2,332 twin and sibling youth participated in behavioral and cognitive testing. Partially competing models of symptom severity distribution differences, the mean difference and variance difference models, were tested within a randomly selected subsample. The Delta method was used to test for mediation of sex differences in ADHD symptom severity by processing speed, inhibition and working memory.
Results
The combined mean difference and variance difference models fully explained the sex difference in ADHD symptom severity. Cognitive endophenotypes mediated 14% of the sex difference effect.
Conclusions
The sex difference in ADHD symptom severity is valid and may be due to differing genetic and cognitive liabilities between the sexes.
Sex differences in the rate of Attention Deficit Hyperactivity Disorder (ADHD) diagnoses are well documented in the literature. ADHD affects approximately 5–7 percent of the school age population, with a male to female ratio of about 3:1 in community-based samples of youth (Willcutt, 2012). There is disagreement on whether males likewise show more severe inattentive and comorbid neuropsychological symptoms among diagnosed individuals (Elkins, Malone, Keyes, Iacono, & McGue, 2011; Gaub & Carlson, 1997; Gershon, 2002; Kan et al., 2012). For example, in a population based, longitudinal study, affected females had larger effect sizes for behavioral and cognitive functioning deficits, but this could be explained by the better performance of unaffected females compared to unaffected males (Arnett, MacDonald, & Pennington, 2013). Thus, at this point it is unclear whether the behavioral and etiological features of ADHD are the same in both sexes.
The sex discrepancy in ADHD is of interest because it could provide clues to the etiology of the disorder. Additionally, there is little research on females with ADHD (Gaub & Carlson, 1997), which limits our understanding of how they develop and cope with associated symptoms. The present study tests competing explanations for the sex difference in ADHD symptom severity, using a community-based twin sample that is enriched for ADHD symptoms.
Theories on the ADHD Sex Difference
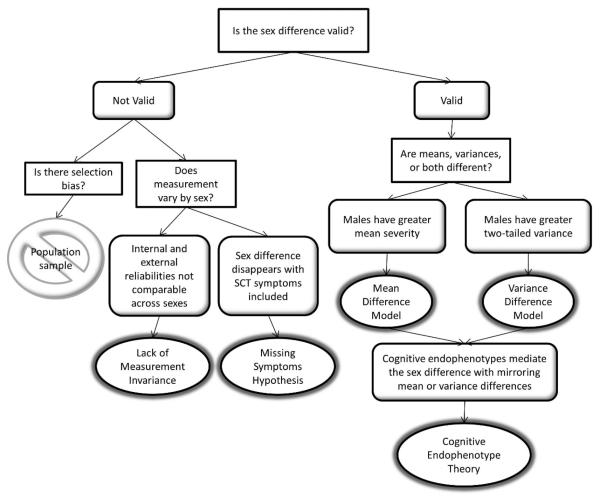
Hypotheses explaining the ADHD sex difference are presented as ovals in the flow chart in Figure 1. The initial question, Is the difference valid (i.e. due to genuine etiologic differences)?, distinguishes two broad categories of hypotheses. If invalid, the three potential explanations include selection bias, lack of measurement invariance across sexes, and missing symptoms. Selection bias has been observed in clinically referred samples (Gershon, 2002). However, our own sample, although overselected for participants with ADHD symptoms, was recruited from community rather than clinical settings. “Case” families were identified based on parent and/or teacher symptom ratings, rather than previous clinical diagnoses, minimizing bias that could result from overparticipation by families with higher rates of ADHD. Further, the results of many other community samples (Graetz, Sawyer, Hazell, Arney, & Baghurst, 2001; Willcutt, 2012; Willcutt, Pennington, Chhabildas, Friedman, & Alexander, 1999) indicate that the gender difference is not due to bias. Thus, selection bias is not considered as a possible explanation in the current study.
Figure 1.
Lack of measurement invariance would mean that ADHD rating scales are not psychometrically equivalent for females and males, such that the measure differs in internal or external validity by sex. Over-referral of males for clinical services in the past could have biased the selection of symptoms that define the diagnostic construct, a possibility that has been supported for conduct disorder, in which relational aggression is more common in females (Zahn-Waxler, Crick, Shirtcliff, & Woods, 2006). In other words, the same underlying liability can be expressed differently as a function of sex. Studies of familial patterns have shown conflicting results regarding rates of psychiatric disorder in relatives of male versus ADHD probands (e.g. Faraone, Biederman, Keenan & Tsuang, 1991; Stawicki & Nigg, 2003). However, measurement invariance for ADHD symptoms across sexes has been established in several community and clinical samples (Collett, Crowley, Gimpel, & Greenson, 2000; Conners, Sitarenios, Parker, & Epstein, 1998; Hudziak et al., 1998; Martel, von Eye, & Nigg, 2010; Reid et al., 2000), suggesting that this is not a valid explanation. We will examine internal and external validities across sex in the current sample to further rule out this hypothesis.
A third possibility is the “missing symptoms” hypothesis. A group of symptoms that was not included in either the DSM-IV or DSM-5 criteria, the Sluggish-Cognitive Tempo (SCT) cluster, presents an alternative, theoretically plausible candidate for ADHD symptoms that are more commonly expressed by females. These symptoms are present more frequently in children with the predominantly inattentive subtype of DSM-IV ADHD, but maintained independence in factor analysis of a community sample (Hartman, Willcutt, Rhee, & Pennington, 2004). While previous research did not find a difference in SCT levels between ADHD males and females in another community sample (Carlson & Mann, 2002), no study that we know of has examined the severity of these symptoms among females who do not already meet criteria for ADHD. The present study will test whether inclusion of these symptoms in the ADHD profile eliminates the sex difference.
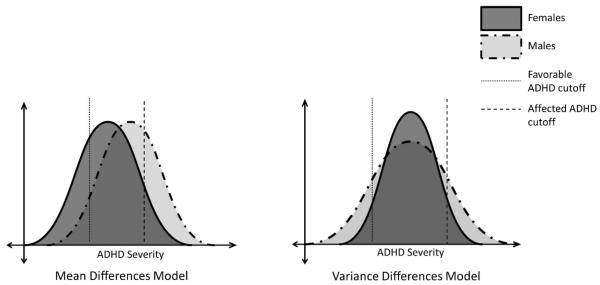
Within the second broad category of hypotheses, which requires the sex difference in ADHD prevalence to be valid (i.e. not an artifact of selection or measurement), two possible explanations are proposed in Figure 1: the Mean Difference Model (MDM), and the Variance Difference Model (VDM). The MDM proposes that the female liability distribution is shifted in the less-affected direction relative to that of males, similar to a multiple threshold model originally described by Carter (1972). Given equivalent variances across sexes and an absolute cutoff for the disorder, fewer females will fall into the affected tail of the overall distribution (Figure 2). Hence, there will be more affected males.
Figure 2.
The other valid explanation that we propose is the VDM, which was tested in reading disorder by Hawke, Olson, Willcutt, Wadsworth & DeFries (2009). These authors found that more variance in males' reading performance accounted for the higher number of males who met criteria for the disorder, despite equivalent mean performance between sexes (see Figure 2). Given the strong comorbidity between reading disorder and ADHD (Pennington, 2002), it is logical that the same explanation could apply to the sex difference in ADHD prevalence. Since the VDM predicts a two-tailed variance difference, we would also expect to see a higher male to female ratio at the non-symptomatic end of the distribution when means are centered.
The MDM and VDM are not mutually exclusive. With variance controlled in the MDM analyses and means controlled in the VDM analyses, there is a possibility that both models explain the sex difference in ADHD to some extent.
Mediation by Cognitive Endophenotypes
We propose that the MDM and VDM apply not only to the symptom distributions, but also to intermediate pathogenetic factors that lie between the etiology and behavior, such as endophenotypes. Cognitive endophenotypes for ADHD are heritable, cognitive risk factors that explain some variance in the disorder and are theoretically more closely tied to the underlying genotype than are the behavioral symptoms of ADHD (Bidwell, Willcutt, DeFries, & Pennington, 2007; Gottesman & Gould, 2003). Youth with ADHD are impaired in a variety of cognitive domains, including IQ, executive functions, and processing speed (For reviews, see Frazier, Demaree, & Youngstrom, 2004; Kofler et al., 2013; Oosterlaan, Logan, & Sergeant, 1998; Pennington, 2002). Although they cannot account for all of the variance in ADHD, cognitive endophenotypes are nonetheless good candidates for explaining individual characteristics of the disorder, including the sex difference.
If we find support for the MDM or VDM at the symptom level, then we also hypothesize that valid cognitive endophenotypes will demonstrate a pattern of male to female ratios that corresponds to the MDM or VDM. Cognitive endophenotypes that display this expected pattern of sex differences will be tested as mediators of the association between sex and ADHD severity.
Method
Participants
Participants were drawn from the Colorado Learning Disabilities Research Center (CLDRC) twin project, which studies the etiology of learning disorders, including ADHD (DeFries et al., 1997). The CLDRC recruits from 27 school districts within a 150-mile radius of the Denver/Boulder area. Case families, in which at least one twin was identified as having a positive history of attention and/or reading problems, were recruited at a rate of about 2:1 relative to control families, in which neither twin was symptomatic. Positive history of ADHD symptoms was defined as at least six attention and/or hyperactivity/impulsivity symptoms indicated by the combined ratings of parents and teachers on a DSM-IV ADHD checklist. Exclusion criteria were pervasive developmental disorder, closed-head injury, full scale IQ less than 70, and rare major gene disorders. Participants recruited prior to the year 1996 were not administered the DSM-IV ADHD inventories and thus excluded from the present analyses. An additional 238 participants tested after 1996 were missing both parent and teacher ADHD ratings. Thus, the final sample in this study consisted of 2,332 twins and siblings, of which 1,579 (67.7%) were recruited as part of a case family.
Participant demographics are described in Appendix A, Table A1. The age of participants ranged from 8 to 19 years old. Average full scale IQ was significantly higher than that of the normative sample (t[2331]=27.452, p<.001), but still within the Average range. As expected, the proportion of participants with behavioral ratings consistent with ADHD (24%) or reading problems (31%) was higher than the current U.S. prevalence (ADHD = 6–7%, RD = 7–10%; Willcutt, 2012; Peterson & Pennington, 2012). Participants meeting research criteria for ADHD did not differ from controls on age (ADHD M=11.46, SD=2.54; Control M=11.68, SD=2.65); however, consistent with previous literature, ADHD participants did have significantly lower IQ scores than controls (M(SD)=103.31(11.90) versus M(SD)=108.68(13.03), respectively). The disorder classifications in this study are likely over-inclusive relative to clinical standards, since we depended solely on symptom criteria and did not consider degree of impairment, consistency across situations, or age of onset.
Procedures
Cognitive testing took place during two six-hour days of testing typically scheduled within one month of each other. Parents and youth who were 18 years or older provided consent prior to testing, and minor youth provided written assent. Procedures were in compliance with the institutional review boards at the University of Denver and University of Colorado, Boulder. Neuropsychological testing was administered by trained examiners with at least a bachelor's degree in psychology and prior experience working with children. Examiners were blind to participants' case or control designation. Behavioral questionnaires were mailed to teachers and received within a year of testing.
Measures
ADHD
Parents and teachers completed the Disruptive Behavior Rating Scale (DBRS), a DSM-IV ADHD checklist. Items were rated on a four-point Likert scale ranging from “0 = never or rarely” to “3 = very often.” Parent- and teacher-ratings were averaged to form inattentive, hyperactive/impulsive, and total ADHD severity scores. When teacher scores were not available, parent ratings were used (9.1%), and vice versa (1.8%). The “and/or” rule was applied to identify participants who met diagnostic criteria for ADHD: symptoms rated as “often” or “very often” by the teacher and/or the parent were considered positive. Participants with at least six positive symptoms in either the inattentive or hyperactive/impulsive subscales, or both, were classified as meeting research criteria for ADHD.
Sluggish Cognitive Tempo
Following previous research (Hartman et al., 2004), five items were selected for inclusion in the SCT symptom cluster: sluggish/slow to respond, seems to be “in a fog,” drowsy or sleepy, easily confused and daydreams/stares into space. Items were rated on the same scale as the DBRS and administered to parents and teachers as part of the same questionnaire packets.Parent- and teacher-ratings were again averaged, except when only one responder was available.
Cognitive Endophenotypes
Candidate endophenotypes were selected for strong, previously established associations with ADHD. Additionally, given the high comorbidity between ADHD and RD, and the overselection of participants with reading problems in the current sample, phonemic awareness (PA), the primary endophenotype for RD, was also included for consideration. Factor scores were calculated for each of the cognitive endophenotype constructs using theoretically-driven neuropsychological tests. Individual measures comprising each factor have been described in previous research (Bidwell et al., 2007; McGrath et al., 2011; Pennington et al., 2012; Willcutt, Pennington, Olson, Chhabildas, & Hulslander, 2005) and are detailed in Appendix A. Each composite for a given construct comprised several indicators and the model fits were in the acceptable range (see Appendix A, Table A2).
Preliminary Analyses
Cognitive and behavioral measures were winsorized to within three standard deviations of the mean. These corrections affected fewer than 3.5% of participants on each variable. The Gordon Diagnostic System (Gordon, 1983) Vigilance Commission Errors variable was further log transformed to reduce skew and kurtosis to acceptable values (.443 and −.308, respectively). All other variables were within acceptable skew and kurtosis ranges (absolute value < 2). Next, cognitive variables (except for Wechsler tests) were standardized within the sample by regressing raw scores on the participant's age and squared age. Wechsler test scale scores were transformed into z scores using the sample means and standard deviations. Mplus 6.0 was used to compute two-level confirmatory factor analysis, from which factor scores were computed for the cognitive composites. Family was modeled at level two in order to control for non-independence of sibling and twin data. When two subtests of the same test were given (e.g. Trails A and B), these scores were permitted to covary in the model. Additionally, the Wechsler Object Assembly and Block Design subtests were permitted to covary in the nonverbal reasoning factor as this significantly improved the model fit. All model fits were acceptable, with at least two of the following fit statistics: non-significant chi-square, CFI>.90, RMSEA<.08 (see Appendix A, Table A2).
Results
Sex Differences in ADHD
To confirm that ADHD was more prevalent in males in our sample, we selected one random participant from each family (“subsample”; n=1,074) for group comparison analyses. The subsample was comparable to the full sample on all demographic variables (see Appendix A, Table A1). As expected, males had more severe scores than females on the inattention (male M(SD) = 8.44(6.40); female M(SD) = 5.15(5.31); t(1072)=9.21, p<.001), hyperactivity/ impulsivity (male M(SD) = 5.24(5.07); female M(SD) = 3.19(3.92); t(1072)=7.44, p<.001) and total ADHD (male M(SD) = 13.72(10.40); female M(SD) = 8.34(8.49); t(1072)=9.32, p<.001) scores than females. Likewise, more males met research criteria for ADHD (χ2[1]=64.09, p<.001), with a male to female ratio similar to that in the general population, at 2.43:1. The results were the same when parent- and teacher-ratings were tested separately. The subsequent analyses tested explanations of this sex difference in ADHD symptoms.
Measurement Invariance Hypothesis
We examined internal and external reliabilities across sexes to test the theory that lack of measurement invariance explained the sex difference in ADHD symptoms. Lower-bound internal reliability was compared across sexes by examining monozygotic twin correlations for ADHD severity scores (Byrne et al., 2007). As expected, twin correlations were comparable across both males and females for total ADHD (male r=.73, p<.001; female r=.77, p<.001), inattention (male r=.69, p<.001; female r=.69, p<.001) and hyperactivity/impulsivity (male r=.72, p<.001, female r=.78, p<.001) subscales. Fisher r to z transformations confirmed that the correlation coefficients were not statistically different for each comparison. Using the subsample, gender invariance in external validity was examined next by correlating total ADHD severity scores with each of the cognitive endophenotypes, within sex. Each of the correlations was comparable across males and females. Z statistics were not statistically significant, with absolute values ranging from .09 to 1.01. These results, in conjunction with previously published literature, support measurement invariance of the 18 DSM-IV ADHD symptom criteria across sexes.
Missing Symptoms Hypothesis
To test the hypothesis that females' lower ADHD symptom severity could be due to a difference in symptom expression, severity of SCT items was compared across sexes within the subsample. Contrary to the missing symptoms hypothesis, males' SCT severity (M=1.78) was higher than females' (M=1.23; t[765]=4.00, p<.001), although the SCT sex effect was smaller than that of standard ADHD symptoms (male M=13.72; female M=8.34; t[1004]=9.26, p<.001). Males also showed worse severity when SCT and standard ADHD symptoms were combined (male M=15.01; female M=9.47; t[783]=7.63, p<.001). Thus, we concluded that the sex difference in ADHD symptoms is valid and proceeded to test the two partially competing models for this valid sex difference.
Mean Difference Model
The MDM specifies that males will show more severe symptoms than will females. To test this initial hypothesis, we transformed the ADHD severity scores so that males and females in the subsample would have equivalent variances (i.e. standard deviation = 1) by dividing scores by their respective group standard deviations. As expected, t-tests revealed that males had significantly higher total ADHD (t(1072)=5.51, p<.001), inattention (t[1072]=5.72, p<.001) and hyperactivity/impulsivity (t[1072]=3.60, p<.001) scores when variances were equivalent across groups. Further, with variances equated, males were more prevalent in the affected 10th percentile (χ2(1)=13.74, p<.001) and females were more prevalent in the favorable (i.e. non-symptomatic) 10th percentile (χ2(1)=22.72, p<.001) of the distribution. The results were consistent when the 25th percentiles were applied as cutoffs. The male to female ratios in the affected 10th and 25th percentiles (1.85:1 and 1.64:1, respectively) were about half the sex ratio of participants meeting research criteria for ADHD.
Variance Difference Model
To test the VDM hypothesis that a sex difference in symptom variance explains males' worse ADHD severity, Levene's test for variance differences was run on the subsample. We first subtracted male and female mean severities from the respective individual scores so that the groups would have equivalent means, i.e. zero. Males had significantly more variance in inattention (F[1, 1072]=30.29, p<.001), hyperactivity/impulsivity (F[1, 1072]=45.36, p<.001), and total ADHD (F[1, 1072]=36.62, p<.001) severities. Consistent with the VDM, more males than females fell into both the affected (χ2(1) = 11.84, p=.001) and favorable 10th percentiles (χ2(1) = 126.14, p<.001) when means for both groups were equated. Results were replicated using the 25th percentiles as cutoffs. Again, the male to female ratios in the affected 10th and 25th percentiles were approximately half that of those who met research criteria, at 1.78:1 and 1.22:1, respectively.
Combined MDM and VDM
Next, we tested both the MDM and VDM simultaneously within the Subsample, with the hypothesis that the combined models would fully explain the full sex discrepancy in ADHD symptom severity. We standardized the total ADHD severity scores within sex, such that male and female groups each had a mean of zero and standard deviation of one. The number of males and females who fell into the affected extreme (i.e. z score > 1) was not significantly different (χ2(1)=1.82, p=.177) with a male:female ratio of 0.91:1. At the favorable end (z score < −1), a significant difference remained (χ2(1)=104.27, p<.001), with 90 males and zero females in that tail.
Cognitive Endophenotype Hypothesis
We next tested whether the results of the MDM and VDM were replicated using cognitive endophenotypes, and whether such endophenotypes mediated the sex difference in symptoms. Within the subsample, candidate endophenotypes were tested for 1) a negative correlation with total ADHD severity, 2) a sex main effect, wherein males performed more poorly than females once variances were equated, and 3) a variance difference once means were equated. Endophenotypes that fit the first two criteria, consistent with the MDM, included inhibition (INH) and processing speed (PS). Endophenotypes that fit the first and third criteria, consistent with the VDM, were PS and working memory (WM). See Appendix A, Table A3.
Two-level mediation analyses were computed using the entire sample, with family at level two to control for non-independence of sibling data. Mediation models included sex as the independent variable, ADHD severity as the outcome, and PS, INH and WM as mediating variables. MPlus 6.0 was used to run the Delta method of testing for mediation (MacKinnon, 2008). The results supported partial mediation of total ADHD severity, with a standardized total indirect effect (i.e. via PS, INH and WM) of .04 (p<.001), which constituted 14% of the total effect (.29, p<.001). PS and INH contributed greater indirect effects than did WM, although all endophenotypes were independently significant (Table 1). Results were similar for inattention and hyperactivity/impulsivity subscales.
Table 1.
Standardized Effects in Mediation Models
| Total ADHD |
Inattention |
Hyperactivity/Impulsivity |
|
|---|---|---|---|
| Processing Speed | .03*** | 04*** | .01** |
| Inhibition | .02*** | .02*** | .02*** |
| Working Memory | .01* | .01* | .00 |
| Total Indirect | 04*** | .05*** | .03** |
| Total Indirect + Direct | .29*** | .29*** | .23*** |
p<05,
p<.01,
p<.001
Discussion
The MDM and VDM were both supported as explanations for the sex differences in ADHD symptom severity. The MDM specifies that, assuming equivalent variances across sex, males have a shifted distribution relative to females, such that the male mean is closer to the diagnostic threshold. In contrast, the VDM specifies that males have a greater variance than females, so that more males fall into the extreme tails, assuming equivalent means. When both the MDM and VDM assumptions were met, the number of extreme affected males and females was comparable, with a ratio of nearly 1. Each model partially explained, and the combined models fully explained, the sex discrepancy at the affected tail.
Our community based sample was overselected for participants with a history of ADHD symptoms, and we used the “and/or” rule to classify participants as case individuals. This resulted in a higher rate of ADHD symptoms in our sample relative to population estimates. Alternative approaches for recruiting and classifying participants might change our results. However, the sex ratio in our study was comparable to population ratios, suggesting that the sex difference is consistent regardless of the diagnostic algorithm. Nonetheless, the current results should be interpreted specifically as explanations for the sex difference in ADHD symptom severity, rather than the rate of clinical diagnosis.
We attempted to get one step closer to the polygenic liability for ADHD by testing a mediation model using cognitive endophenotypes that demonstrated distribution patterns consistent with either the MDM or the VDM. Our mediation model supported PS, INH and WM as partial mediators of the association between ADHD severity and sex in the overall sample. These neurocognitive mediators accounted for 14% of the overall association, which is moderate, given that ADHD symptoms are estimated to be about 70% genetic in origin (Pennington, 2002). INH, which was consistent only with the MDM, had a greater partial mediation effect than did WM, which was consistent only with the VDM. PS had the largest effect and was consistent with both models. These results strengthen the results supporting both models, but also suggest that mean differences in underlying cognitive processes may contribute to the sex difference in ADHD symptoms more than do variance differences.
Because cognitive endophenotypes are genetically correlated with ADHD symptoms, (Willcutt et al., 2010), these results suggest at least a partial genetic etiology for the differential liability of ADHD in males and females that is due to sex differences in neuropsychological processes. This is consistent with the findings from our previous research that reading disability and ADHD share cognitive deficits that are primarily due to genetic influences (Willcutt et al., 2010).
Although we did not have power to test it in this sample, we expect that both the MDM and VDM correspond to patterns of sibling differences. At the affected end of the distribution, we would expect that females' siblings would have more severe symptoms than affected males' siblings, because in both models affected females are more distant from the population mean in terms of standard deviations. Consistent with this, tests of the polygenic multiple threshold model for total ADHD symptoms (Rhee, Waldman, Hay, & Levy, 1999) and individual ADHD symptom dimensions (Rhee & Waldman, 2004) have both found that siblings of females with ADHD had more symptoms than did siblings of ADHD males. In contrast, the MDM and VDM sibling predictions diverge at the favorable end of the distribution. The MDM would predict that siblings of favorable males would be less symptomatic, due to high loading for favorable polygenes, while the VDM would predict the opposite, because favorable females are less common than favorable males in the VDM.
The distinction at the favorable end of the distribution is difficult to test in this study in part because we used the DBRS, which only measures ADHD symptoms at the dysfunctional end of the distribution. Measurement of favorable attention and impulse regulation is curtailed (Arnett et al., 2013). This is a possible explanation for the fact that a sex difference remained in the favorable tail of the distribution even when both the MDM and VDM assumptions were met. Thus, a measure that captures variance at both ends of the ADHD symptom distribution, such as the SWAN (Swanson et al., 2006) rating scale, would be useful for testing sibling predictions and replicating our results.
Conclusion
Higher ADHD symptom severity for males relative to females can be explained by the MDM and VDM. The sex difference in ADHD symptom severity is partially moderated by PS, INH and WM, three commonly identified cognitive endophenotypes for ADHD that themselves exhibit a corresponding sex difference (females outperform males). Since these endophenotypes are genetically correlated with ADHD, the sex difference in ADHD may be genetically mediated, but our study did not directly test that possibility.
Appendix A
Table A1.
Demographics
| Variable | Subsample n=1,074 |
Full Sample n=2,332 |
|---|---|---|
| Mean Age (SD) | 11.63 (2.70) | 11.63 (2.63) |
| % Female | 52% | 50% |
| % Monozygous Twins | 31% | 32% |
| % Siblings of Twins | 9% | 8% |
| % Primary Caregiver Caucasian | 87% | 88% |
| Median Years of Mother's Educationa | 14 | 14 |
| Median Years of Father's Educationb | 12 | 12 |
| Mean Full Scale IQ (SD) | 107.84 (12.72) | 107.37 (12.97) |
| % History of Reading Problems in School | 29% | 31% |
| % Meeting Symptom Criteria for ADHD | 23% | 24% |
14 years=Associate's Degree or Equivalent
12 years=High School Graduate or Equivalent
Table A2.
Individual Measures of Composites and Model Fits of Confirmatory Factor Analyses
| Composite | Measures | X2(df, n) | P | CFI | RMSEA |
|---|---|---|---|---|---|
| Processing Speed (PS) |
Wechsler Symbol Search & Coding B; Colorado Perceptual Speed (DeFries et al., 1997), Identical Pictures (French, Ekstrom, & Price, 1963), Trails A & B (Reitan, 1971) |
41.97(8, 2332) | <.001 | .990 | .043 |
| Working Memory (WM) |
Wechsler Digit Span & Arithmetic; Sentence Span (Siegel & Ryan, 1989), & Counting Span (Case, Kurland, & Goldberg, 1982) |
7.26(2, 2332) |
.027 | .997 | .034 |
| Phoneme Awareness (PA) |
Phoneme Deletion 1 and 2 (Olson, Forsberg, Wise, & Rack, 1994), Weighted Pig Latin (Olson, Wise, Conners, Rack, & Fulker, 1989), Lindamood Auditory Conceptualization Test (Lindamood & Lindamood, 1971) |
1.30(1, 2145) |
.254 | 1.000 | .012 |
| Verbal Reasoning (VR) |
Wechsler Information, Similarities, Vocabulary, & Comprehension |
28.38(2, 2332) |
<.001 | .993 | .075 |
| Nonverbal Reasoning (NVR) |
Wechsler Block Design, Object Assembly, Picture Completion, & Picture Arrangement |
.28(1, 2332) | .594 | 1.000 | .000 |
| Inhibition (INH) |
GDS Vigilance Commission Errors (Gordon, 1983); Stop Signal Task (Logan, 1994); Stroop Color Word Interference (Golden, 1978) |
0.00(0, 2146) |
<.001 | 1.000 | .000 |
| Standard Deviation of Reaction Time (SDRT) |
Stop Signal Task (Logan, 1994) | n/a | n/a | n/a | n/a |
| Risk | Cambridge Gambling Task (CGT; Rogers et al., 1999) Overall Proportion Net & Risk Taking |
0.00(0, 244) | <.001 | 1.000 | .000 |
| Monitor | CGT Delay Aversion, Quality of Decision Making, & Risk Adjustment |
0.00(0, 244) | <.001 | 1.000 | .000 |
Note: The Wechsler test version varied depending on testing date and participant age. Sixty-one percent of participants completed subtests from the Wechsler Intelligence Scale for Children, Revised (WISC-R; Wechsler, 1974); 36% the Wechsler Intelligence Scale for Children, Third Edition (WISC-III; Wechsler, 1991); 1% the Wechsler Adult Intelligence Scale, Revised (WAIS-R; Wechsler, 1981); and 2% the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 1997). The symbol search subtest always originated from the WISC-III battery and was administered to 70% of participants. The Stop Signal Task (Logan, 1994) standard deviation of reaction time (SDRT) was a single measure and thus not transformed into a factor score.
Table A3.
Associations Between Cognitive Endophenotypes, ADHD and Sex
| Cognitive Endophenotype |
Correlation with Total ADHD |
Mean Sex Difference | Variance Difference |
|---|---|---|---|
| PS | r=−.32, p<.001 | t(1072) = 5.50, p<.001†, d=.27 | F(1, 930) = 6.79, p=.009 |
| WM | r=−.22, p<.001 | t(1050) = −3.03, p=.003†, d=−.14 | F(1, 1072) = 5.57, p=.018 |
| PA | r=−.22, p<.001 | t(976) = −0.51, p=.611, d=−.03 | F(1, 976) = 1.60, p=.206 |
| VR | r=−.16, p<.001 | t(1072) = −3.90, p<.001, d=−.20 | F(1, 1072) = 3.23, p=.072 |
| NVR | r=−.06, p=.063 | t(1072) = −3.97, p<.001, d=−.16 | F(1, 1072) = .06, p=.812 |
| INH | r=−.34, p<.001 | t(977) = 3.26, p=.001, d=−.12 | F(1, 977) = 2.80, p=.095 |
| SDRT | r=−.28, p<.001 | t(930) = −2.36,p=.018, d=.16 | F(1, 930) = 1.60, p=.207 |
| Risk | r=−.16, p=.090 | t(111) = −2.50, p=.014, d=−.46 | F(1, 111) = 1.21, p=.275 |
| Monitor | r=−.10, p=.292 | t(111) = 3115, p=.002, d=39 | F(1, 111) = .72, p=.400 |
Note: P-values are two-tailed.
T-test statistic reported using unequal variances assumed. Bold italics indicate significant results in the expected direction.
References
- Arnett AB, MacDonald B, Pennington BF. Cognitive and behavioral indicators of ADHD symptoms prior to school age. Journal of Child Psychology and Psychiatry. 2013 doi: 10.1111/jcpp.12104. doi:10.1111/jcpp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett AB, Pennington BF, Friend A, Willcutt EG, Byrne B, Samuelsson S, Olson RK. The SWAN captures variance at both the negative and positive ends of the ADHD symptom dimension. Journal of Attention Disorders. 2013;17(2):152–162. doi: 10.1177/1087054711427399. doi:10.1177/1087054711427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, DeFries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Samuelsson S, Wadsworth S, Hulslander J, Corley R, DeFries JC, Olson RK. Longitudinal twin study of early literacy development: Preschool through Grade 1. Reading and Writing. 2007;20(1–2):77–102. [Google Scholar]
- Carlson CL, Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, predominantly inattentive type. Journal of Clinical Child and Adolescent Psychology. 2002;31(1):123–129. doi: 10.1207/S15374424JCCP3101_14. [DOI] [PubMed] [Google Scholar]
- Carter CO. The inheritance of common congenital malformation. Progress in Medical Genetics. 1965;IV:59. [PubMed] [Google Scholar]
- Carter CO. Sex-linkage and sex-limitation. In: Ounsted C, Taylor DC, editors. Gender differences: Their ontogeny and significance. Churchill Livingstone; Edinburgh and London: 1972. [Google Scholar]
- Case R, Kurland M, Goldberg J. Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology. 1982;33:386–404. [Google Scholar]
- Collett BR, Crowley SL, Gimpel GA, Greenson JN. The factor structure of DSM-IV attention deficit-hyperactivity symptoms: Confirmatory factor analysis of the ADHD-SRS. Journal of Psychoeducational Assessment. 2000;18:361–373. [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised conners' parent rating scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Filipeck PA, Fulker DW, Olson RK, Pennington BF, Smith SD, Wise BW. Colorado learning disabilities research center. Learning Disabilities. 1997;8:7–19. [Google Scholar]
- Doyle AE, Ferreira MA, Sklar PB, Lasky-Su J, Petty C, Fusillo SJ, Faraone SV. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: Suggestive linkage to 3q13. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:1399–1411. doi: 10.1002/ajmg.b.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, Malone S, Keyes M, Iacono WG, McGue M. The impact of attention-deficit/hyperactivity disorder on preadolescent adjustment may be greater for girls than for boys. [null] Journal of Clinical Child and Adolescent Psychology. 2011;40:532–545. doi: 10.1080/15374416.2011.581621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Keenan K, Tsuang MT. A family-genetic study of girls with DSM-III attention deficit disorder. American Journal of Psychiatry. 1991;148:112–117. doi: 10.1176/ajp.148.1.112. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- French JW, Ekstrom RG, Price LA. Manual for a kit of reference tests for cognitive factors. Educational Testing Service; Princeton, NJ: 1963. [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: A meta-analysis and critical review. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(8):1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Golden JC. Stroop color and word test. Stoelting; Chicago: 1978. [Google Scholar]
- Gordon M. The gordon diagnostic system. Gordon Systems; DeWitt, NY: 1983. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Graetz BW, Sawyer MG, Hazell PL, Arney F, Baghurst P. Validity of DSM-IV ADHD subtypes in a nationally representative sample of australian children and adolescents. Journal of American Academy of Child and Adolescent Psychiatry. 2001;40(12):1410–1417. doi: 10.1097/00004583-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Willcutt EG, Rhee SH, Pennington BF. The relation between sluggish cognitive tempo and DSM-IV ADHD. Journal of Abnormal Child Psychology. 2004;32(5):491–503. doi: 10.1023/b:jacp.0000037779.85211.29. [DOI] [PubMed] [Google Scholar]
- Hawke JL, Olson RK, Willcut EG, Wadsworth SJ, DeFries JC. Gender ratios for reading difficulties. Dyslexia. 2009;15(3):239–242. doi: 10.1002/dys.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak JJ, Heath AC, Madden PF, Reich WR, Bucholz KK, Slutske W, Todd RD. Latent class and factor analysis of DSM-IV ADHD: A twin study of female adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(8):848–857. doi: 10.1097/00004583-199808000-00015. [DOI] [PubMed] [Google Scholar]
- Kan KJ, Dolan CV, Nivard MG, Middledorp CM, vanBeijsterveldt CEM, Willemsen G, Boomsma D. Genetic and environmental stability in attention problems across the lifespan: Evidence from the netherlands twin register. [null] American Academy of Child and Adolescent Psychology. 2012;52:12–25. doi: 10.1016/j.jaac.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review. 2013 doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lindamood CH, Lindamood PC. Lindamood auditory conceptualization test. Reading Resources Corporation; Boston: 1971. [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user's guide to the stop signal paradigm. In: Dagenbach D, Carr T, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Lawrence Erlbaum Associates; New York: 2008. [Google Scholar]
- Martel MM, von Eye A, Nigg JT. Revisiting the latent structure of ADHD: Is there a `g' factor? Journal of Child Psychology and Psychiatry. 2010;51(8):905–914. doi: 10.1111/j.1469-7610.2010.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath L, Shanahan M, Santerre–Lemmon L, Barnard H, Willcutt E, Olson R, Pennington B. A multiple deficit model of reading disability and attention-deficit/hyperactivity disorder: Searching for a shared cognitive deficit. Journal of Child Psychology and Psychiatry. 2011;557 doi: 10.1111/j.1469-7610.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R, Forsberg H, Wise B, Rack J. Measurement of word recognition, orthographic, and phonological skills. In: Lyon GR, editor. Frames of reference for the assessment of learning disabilities: New views on measurement issues. Paul H. Brookes; Baltimore, MD: 1994. pp. 243–277. [Google Scholar]
- Olson R, Wise B, Conners F, Rack J, Fulker D. Specific deficits in component reading and language skills: Genetic and environmental influences. Journal of Learning Disabilities. 1989;22:339–348. doi: 10.1177/002221948902200604. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD+ CD, anxious, and control children: A meta-analysis of studies with the stop task. Journal of Child Psychology and Psychiatry. 1998;39(3):411–425. [PubMed] [Google Scholar]
- Pennington BF. The development of psychopathology: Nature and nurture. Guilford Press; New York, NY: 2002. [Google Scholar]
- Pennington BF, Santerre-Lemmon L, Rosenberg J, MacDonald B, Boada R, Friend A, Olson RK. Individual prediction of dyslexia by single versus multiple deficit models. Journal of Abnormal Psychology. 2012;121(1):212. doi: 10.1037/a0025823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. Developmental dyslexia. The Lancet. 2012;379(9830):1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Trail making test results for normal and brain-damaged children. Perceptual and Motor Skills. 1971;33:575–581. doi: 10.2466/pms.1971.33.2.575. doi:10.2466/pms.1971.33.2.575. [DOI] [PubMed] [Google Scholar]
- Reid R, Riccio CA, Kessler RH, DuPaul GJ, Power TJ, Anastopoulos AD, Noll MB. Gender and ethnic differences in ADHD as assessed by behavior ratings. Journal of Emotional and Behavioral Disorders. 2000;8(1):38–48. [Google Scholar]
- Rhee SH, Waldman ID. Etiology of sex differences in the prevalence of ADHD: An examination of inattention and hyperactivity-impulsivity. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2004;127B:60–64. doi: 10.1002/ajmg.b.20131. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID, Hay DA, Levy F. Sex differences in genetic and environmental influences on DSM–III–R attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 1999;108(1):24. doi: 10.1037/0021-843X.108.1.24. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel LS, Ryan EB. The development of working memory in normally achieving and subtypes of learning disabled children. Child Development. 1989;60(973):980. doi: 10.1111/j.1467-8624.1989.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Stawicki J, Nigg JT. Familial psychiatric disorders in child DSM-IV ADHD: moderation by child gender. Ann N Y Acad Sci. 2003;1008:293–6. doi: 10.1196/annals.1301.035. [DOI] [PubMed] [Google Scholar]
- Swanson J, Shuck S, Mann M, Carlson C, Hartman K, Sergeant J, McCleary R. Categorical and dimensional definitions and evaluations of symptoms of ADHD: The SNAP and SWAN rating scales. University of California, Irvine; Irvine: 2006. [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United states, 2003–2011. Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1016/j.jaac.2013.09.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the wechsler intelligence scale for children-revised. The Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D. Manual for the wechsler adult intelligence scale-revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. The wechsler intelligence scale for children. third edition The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-III. The Psychological Corporation; New York: 1997. [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics. 2012;9:490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Chhabildas NA, Friedman MC, Alexander J. Psychiatric comorbidity associated with DSM-IV ADHD in a nonreferred sample of twins. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(11):1355. doi: 10.1097/00004583-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27(1):35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Betjemann RS, McGrath L, Chhabildas N, Olson RK, DeFries J, Pennington BF. Etiology and neuropsychology of comorbidity between RD and ADHD: The case for multiple-deficit models. Cortex. 2010;46(10):1345. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Crick NR, Shirtcliff EA, Woods KE. The origins and development of psychopathology in females and males. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology volume 1: Theory and method. 2nd ed. John Wiley & Sons Inc; Hoboken, NJ: 2006. pp. 76–138. [Google Scholar]