Introduction
Quantitative electrocenphalography (QEEG) has served as a powerful tool to study both typical and atypical brain development and function, informing the understanding of processes such as perception, cognition, and cortical connectivity (For review see: Saby and Marshall, 2012; Uhlhaas et al., 2010). With a temporal resolution that facilitates quantification of subtle changes in state and function over time, QEEG holds tremendous promise as a quantitative biomarker of clinical phenomenon such as the change in brain function over discrete time points in development (Marshall et al., 2002), the effects of intervention in developmental disorders (Dawson et al., 2012), prediction of functional outcomes (Gou et al., 2011), early disorder detection (Bosl et al., 2011), disease progression (Luckhaus et al., 2008), and subgroup (Clarke et al., 2011) and group (Barry et al., 2010) differences in childhood psychiatric disorders. QEEG holds particular appeal as a metric of individual variability in neurodevelopmental disorders, such as autism spectrum disorder (ASD), where behavioral output is limited and sometimes unable to capture phenotypic and functional heterogeneity (Cantor and Chabot, 2009; Saby and Marshall, 2012).
Scientific merits notwithstanding, it is the practical benefits of QEEG that often motivate its use in the study of developmental populations, as it is non-invasive, less vulnerable to motion artifact, and more readily available in clinical settings (Keil et al., 2014; Saby and Marshall, 2012; Webb et al., 2013). Moreover, cognitive processes such as attention, memory, cognitive inhibition, and feature binding can be characterized without requiring an overt behavioral response. However, these practical benefits also lead to greater challenges in data acquisition and quality, as the target populations of interest (infants, young children, atypically developing children) may also generate the most artifact, resulting in an insufficient amount of useable, clean EEG data (Slifer et al., 2008; Webb et al., 2013). Unlike studies in adults where recording duration often exceed 10 minutes (Barry et al., 2007; Bonfiglio et al., 2013; Hagemann and Naumann, 2001; Leuchter et al., 2012), in infants and children a total of 2 minutes of data are often gathered, with fewer than 30 seconds of clean data remaining after artifact rejection (John et al., 1980; Marshall et al., 2002; Tierney et al., 2012).
Three of the most common internal sources of artifact include eye blinks, saccades, and contraction of face, jaw or neck muscles (electromyographic noise or EMG). Studies in adults have investigated techniques for handling these artifacts individually, such as independent component analysis (ICA; Jung et al., 2000) and regression methods (Gratton et al., 1983). In the study of developmental populations, ICA can be impractical because it requires substantial amounts of data (e.g. at least 10 minutes with 128 channels and a sampling rate of 500 Hz; Onton et al., 2006). When the amount of available data is already limited, there may not be enough artifacts of a given type for clear component selection (Keren, Yuval-Greenberg, and Deouell, 2010). Down sampling the number of channels (e.g. 128 to 64) is one proposed method for overcoming minimum data requirements; however, it does not necessarily make artifact related components more identifiable. Furthermore, component selection in ICA increases in difficult when multiple artifact types exist in the data. Regression methods in either the time or frequency domains can also be problematic because the regression requires removal of electrooculographic (EOG) channels that may also include relevant EEG signals along with the artifact (Jung et al., 2000). When ICA or regression methods are not possible, another traditional strategy for addressing artifacts includes the removal of contaminated sections of data from analysis. This strategy can result in the removal of large quantities of data that may, in fact, contain valuable signal worth preserving.
Little is known about the contribution of artifacts (blinks, saccades, EMG) to the calculation of EEG power in children, despite the fact that developmental populations are the most likely to generate the highest noise:data ratio. Previous studies have provided valuable information about the effect of varying degrees of muscle contraction and its spatial spread on QEEG measurements, but these studies have included only adult subjects (Freeman et al., 2003; Goncharova et al., 2003). Finally, as has been addressed in several papers, the lack of uniformity in methods of data processing and cleaning contributes to difficulties in replicating and comparing findings (Keil et al., 2014; Picton et al., 2000; Pivik et al., 1993; Webb et al., 2013). Such a challenge holds particularly true in studies of infancy and early childhood, where no consistent parameters for data cleaning or processing have been established.
We took a systematic approach to study the potential effects of artifact on EEG power in a cohort of typically developing young children, with the goal of guiding artifact rejection methods in EEG data processing. We focused on three common physiologic artifacts, namely blinks, saccades, and EMG, and we compared the estimation of mean spectral power within these artifacts to the mean spectral power contained in artifact-free data within characteristic frequency bands including theta (4-7 hz), alpha (8-12 hz), beta (13-30 hz), and gamma (35-45 hz). We asked whether certain power types, regions or frequency bands would be more vulnerable to the inclusion of ocular or EMG artifacts.
Methods
Participants
Full ethical approval for the research was obtained through the University of California Institutional Review Board (IRB#:11-000355), and all parents provided written consent for the study. We examined data from 32 typically developing children ages 2 – 6 years old (40% girls; mean age = 52.9 mo.; SD = 13.7 mo) who were recruited as control participants as part of a larger study of young children with ASD. Using birth records provided by the Los Angeles County, families with children in the targeted age range were mailed invitations to participate. Interested families returned a postcard and were later contacted via telephone for a screening interview. During the interview, parents of eligible children were asked about their child's preferences and interests, such as favorite movie or toy. This information was used on the day of the experiment to make the recording session as comfortable and enjoyable as possible for each child. For example, children were offered a snack or shown a favorite video during placement of the EEG net. Children were excluded from the study if they had a history of neurological abnormalities, birth-related complications, developmental delays, need for special school services, uncorrected vision impairment, or a diagnosis of a psychiatric condition such as ADHD, OCD, or bipolar disorder.
On the day of the testing session, each child underwent cognitive assessments as part of the larger study. Tests included the Vineland Adaptive Behavioral Scales (Sparrow et al., 2005) and either the Mullen Scales of Early Learning (Mullen, 1995) or the Differential Abilities Scale-II (Elliott, 1993) depending on the child's chronological age. For the purposes of this study, the cognitive testing results were only used to confirm that the children were age appropriate and not delayed. Any child with a full scale IQ or DQ more than 2 standard deviations below the age expectancy was excluded for this analysis.
EEG recording
EEG data were recorded using a 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR). To improve each child's comfort, four of the electrodes, channels 125-128 had been removed from the net. These electrodes were originally located below and lateral to the eyes (Figure 1). Placement of electrodes conformed to the International 10-20 System (Jasper, 1958). A combination of a Net Amps 300 amplifier and Net Station 4.4.5 software on a Macintosh Pro PC was used to record the EEG (Electrical Geodesics Inc., Eugene, OR). Data were filtered online with an analog band pass elliptical filter between 0.1 to 100 Hz. The high impedance nature of this system allows us to accurately record a child's EEG while keeping impedances below 100 KΩ (Ferree et al., 2001). The EEG was sampled at 250 Hz. Data were referenced online to a vertical reference in a location equivalent to Cz. Two minutes of “resting-state” EEG was recorded while children watched a video of bouncing bubbles in a dark, sound-attenuated room. Children were either seated in a chair or on a caregiver's lap. A video time-locked to the EEG was acquired in order to assist in subsequent data processing.
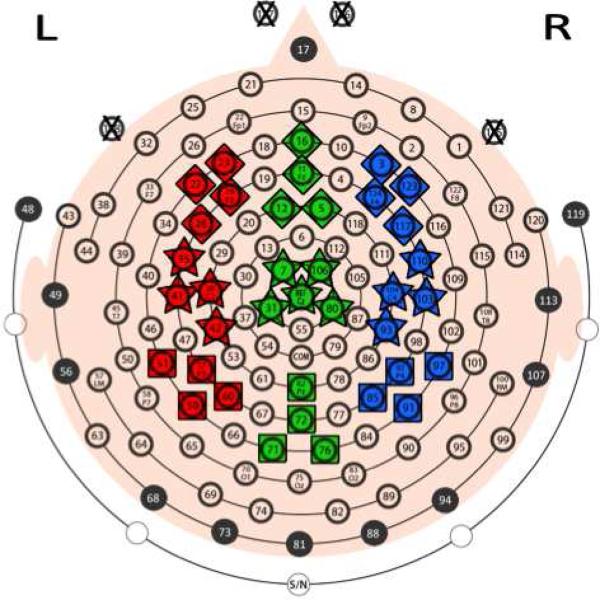
Figure 1. Electrode map.
Nine ROIs were selected to provide maximal spatial coverage of the left (red), midline (green), and right (blue) portions of frontal (diamonds), central (stars), and posterior (squares) scalp regions. All ROIs contain 4 electrodes, except for the midline-central ROI, which contains 5 electrodes. The approximate 10-20 system electrode equivalents and the channel numbers for the regions are: F3 = 23, 24, 27, 28; Fz = 5, 11, 12, 16; F4 = 3, 117, 123, 124; C3 = 35, 36, 41, 42; Cz = 7, 31, 80, 106, Ref; C4 = 93, 103, 104, 110; P3 = 51, 52, 59, 60; Pz = 62, 71, 72, 76; and P4 = 85, 91, 92, 97. Channels 125 – 127 (marked with an X) were originally located below or lateral to the eyes, but were removed to make wearing the net more comfortable for children. Interpolated signals were calculated most often for electrodes located along edge of a net (see 2.3 Data Preprocessing for details).
Data preprocessing
EEG data were processed offline through a series of steps in order to categorize segments of data by artifact: blinks, saccades, EMG, or “Other.” Segments were categorized as “Other” if they contained drift, motion artifact because of net manipulation or pulling, or multiple artifact types. Segments that did not fall into one of these categories were determined to be clean, and hereafter are referred to as “artifact-free” segments.
The full series of processing steps included the following:
We applied a 1-50 Hz band-pass filter with a narrow roll-off (.3 Hz) and strong attenuation (gain = -60 dB).
We segmented the data into 1.024 second sequential, non-overlapping epochs, which resulted in 256 samples per segment. The segment length was chosen to optimize inputs to the Fast Fourier Transform (FFT), which uses inputs of 2n samples (Drongelen, 2007).
We reviewed the filtered file to identify channels with gross abnormalities caused, for example, by an electrode losing contact after recording began. Channels identified as abnormal were then replaced by an interpolated signal using the Net Station software's “Bad Channel Replacement” (BCR) waveform tool, which uses spherical splines to approximate the signal from the remaining electrodes (Fletcher et al., 1996; Perrin et al., 1987; Srinivasan et al., 1996). The electrodes most commonly requiring this interpolation included those located along on the periphery of the net, either seated on the neck or surrounding the ears. No channels in our regions-of-interest (ROI) required bad channel replacement at this step (Figure 1).
We then identified channels whose maximum to minimum voltage exceeded 150 μV within individual segments. Any segment with greater than 15% of electrodes exceeding this threshold, was placed in the “Other” category. These automated rejection criteria are based on common practices in developmental populations (for example, see Jeste et al. 2014)
We manually reviewed all remaining segments and placed them into one of the five previously described categories: blinks, saccades, EMG, other, or artifact-free. A minimum of 30 seconds of clean data was required for inclusion into analysis, and this threshold was based on prior studies in infants and young children (Marshall et al., 2002; Swingler et al., 2011; Tierney et al., 2012). Of the 32 children in the study, 2 subjects were removed from analysis because they provided fewer than 30 segments of clean data, leaving a sample of 30 children (40% girls; mean age = 53.6 mo; SD = 13.3 mo).
Segments underwent an additional BCR operation. In contrast to the first BCR, which replaced a channel's data for the entire recording, this step was performed on a segment-by-segment basis. Per segment, data were interpolated for a maximum of 18 channels (15% of the 124 channels).
The final processing steps before spectral decomposition and frequency band power calculations included (7) baseline correction, (8) re-referencing to the average of all channels, (9) export of Net Station data to Matlab (Mathworks Inc., Natick, MA) and then (10) removal of each segment's DC trend using Matlab's DETREND function.
Several strategies, described below, were used to aid in artifact detection. The most effective strategy (#1) was the traditional method of correlating the expected electrophysiologic signal produced by an artifact with its expected spatial location. This method sometimes leads to missed artifact selection. Therefore other strategies were also employed, as listed below. The strategies listed below are not mutually exclusive. They were used in combination to guide decision making during artifact detection. Finally, when no clear segment categorization could be made using all available resources and strategies, segments were categorized as “Other.” Our purpose in detailing each of these strategies is to a) provide and promote transparency in artifact detection, b) offer strategies based on experience with challenging data, and c) spark a discussion for other methods that can be shared across labs to foster collaborative efforts. The strategies employed include:
Spatial location: An electrode's scalp location was used to identify and distinguish artifacts. For example, the electrophysiological signal produced by blinks should be maximal in electrodes located above the eyes, whereas the signal from saccades should be maximal in lateralized frontal electrodes, with opposite polarities on the left and right. However, we observed several variations of this pattern. For example, the signal produced by a saccade for some individuals only appeared in electrodes ipsilateral to the individual's gaze. Similarly, some individuals would demonstrate a saccade simultaneous to a blink, producing a combined artifact that was more challenging to identify or quantify.
Re-referencing: Temporarily switching the reference electrode within a segment helped to localize the source of an artifact. For example, switching to an average reference of all electrodes can help identify movement artifacts, whereas an average reference of all electrodes outside of the default reference can help identify a poor reference electrode in otherwise clean data. Also, referencing data as linked, bipolar pairs, can be used to enhance the visualization of eye movements.
Filters: Temporary filters can be used to minimize the signal from neural or other sources, while maximizing signals from the artifact in question. For instance, a 1-6 Hz band-pass filter can enhance the visualization of the signal from eye movements by smoothing out a distracting signal from faster sources, such as muscle or alpha generators. Similarly, a high pass filter (e.g. > 20 Hz) can help to distinguish EMG independent of, or related to blink/saccades.
Topologic spectral maps: By combining techniques (1) and (3), this strategy relies on the anatomical location of a signal generator and the characteristic frequency band of an artifact's signal. For example, the signal generated by EMG should be maximal in electrodes adjacent to muscles and an EMG signal should increase power in frequencies above 20 Hz (i.e. beta and gamma bands).
Current Source Density: Calculating the surface Laplacian within a segment enhances information that is maximally specific to each electrode relative to its closest neighbors. Since the surface Laplacian is the second order spatial derivative, this method acts as a spatial band-pass filter that removes shared information. As such, it represents a reference-free method that enhances signals from superficial sources, while minimizing shared signals originating from deeper neuronal sources (Nunez and Srinivasan 2006). This technique can help to identify artifacts that are channel specific (e.g. channel pops) from artifacts whose signal spreads across electrodes (e.g. EMG).
Spectral power calculations
We investigated frequency band power for 9 regions-of-interest (ROI; Figure 1). Regions were selected so that left, midline, and right portions of frontal, central, and posterior scalp were represented during analysis. Each region contained data from four electrodes, except for the midline-central region which contained five. We calculated estimates of the mean power spectral densities (PSD) separately for each artifact type (blinks, saccades, EMG) and for the artifact-free data. Due to the variability in the type of data in the “other” category, we did not include segments categorized as “other” in our analysis. Table 1 provides details on the number of segments per category.
Table 1.
Number of segments by category
| Category | Mean (SD) | Range | Total (%) |
|---|---|---|---|
| Artifact-Free | 75.6 (19.7) | 36 – 113 | 2269 (51.0) |
| Blink | 11.8 (7.3) | 1 – 29 | 353 (7.9) |
| Saccade | 18.6 (14.7) | 2 – 57 | 557 (12.5) |
| EMG | 24.5 (29.2) | 2 – 152 | 735 (16.5) |
| Other | 17.8 (13.8) | 2 – 63 | 534 (12.0) |
| Total | 148.3 (41.0) | 117 – 315 | 4448 |
Mean (with standard deviation in parentheses), range in number of segments contributed by subjects, and the total number of segments per category (with the percent of all segments) for each category. Of note, the relatively high number of saccades is due to the bouncing of bubbles in the video shown during EEG acquisition.
Transformation of the EEG signal from the time-domain to the frequency-domain was accomplished using Welch's method and custom scripts written in Matlab. For each 256-sample segment, FFTs were calculated on 128-point Hamming windows with 50% overlap, yielding a frequency resolution of 0.5 Hz. We calculated absolute and relative power for the theta (4-7 Hz), alpha (8-12 Hz), beta (13-30 Hz), and gamma (35-45 Hz) frequency bands. Absolute power was calculated by summing power estimates at every 0.5 Hz increment within each frequency band. Relative power represents the power contained within a frequency band relative to the total power contained in all bands. As such, we calculated relative power by dividing the absolute power for each band by the total absolute power across all unfiltered frequencies, specifically 1-50 Hz. By averaging across all electrodes within each ROI, final power values used for statistical analysis were obtained for each segment, within each category, and for each subject (Table 1). Values for all power estimates were log transformed to achieve normality.
Statistical analysis
Analysis focused on the log-transformed absolute and relative power estimates of theta, alpha, beta, and gamma bands. As a preliminary analysis to investigate time trends across the 2 minute recording, we plotted all subject-specific response trajectories versus time segments per power band, and fitted population loess smooths. Both population and subject level smooths did not indicate a time trend. However, differences in subject-specific intercepts were apparent. The eight (4Bands * 2Power Types) log-power bands were modeled with a linear mixed model (LMM) using artifact type, region, and their interaction as predictors. Given that we have studied a relatively wide age range in early childhood, we performed a subsequent analysis that include predictors of age and an age*artifact interaction. In adding in these predictors, the statistical comparison of power between artifacts and artifact free segments did not change, suggesting robustness of these comparisons that are independent of the age of the child. Therefore, the results are reported only for the simplified model. A random intercept was included (using SAS PROC MIXED) to account for subject-specific heterogeneity observed in the temporal loess smooths (SAS Institute, Cary, NC). In addition to accounting for within-subject correlations, the LMM allows for unequal numbers of repetitions among subjects. In other words, the LMM controls for different number of segments per category both within (e.g. Subject A: Nartifact-free = 75, NBlink = 10) and between subjects (e.g. Subject B: Nartifact-free = 50, NBlink = 40). Such modeling allowed for the determination of mean power in artifact and artifact-free independent of the amount of data in a given subject. Mean band powers from the artifact-free category and each of the 3 artifact categories were compared for each of the 9 regions and all 8 power bands (using LSMEANS), amounting to 27 tests per model. We have adjusted using SAS PROC MULTITEST for multiple testing in all models (216 tests) controlling the false discovery rate (FDR).
Results
We present the results as comparisons between the mean relative and absolute power for artifact-contaminated and artifact-free segments within each frequency band of interest. These results are independent of the amount of artifact in the recording. Bar graphs in figures 2-4 represent the difference in mean power such that positive and negative values represent higher and lower means for the artifact-contaminated segments compared to the means of the artifact-free segments, respectively. Given the large number of comparisons (2Power Types * 4Frequency Bands * 9ROIs * 3Artifact Types = 216Comparisons), we have limited the narrative of the results to the most clinically meaningful or relevant trends within the corrected significant results.
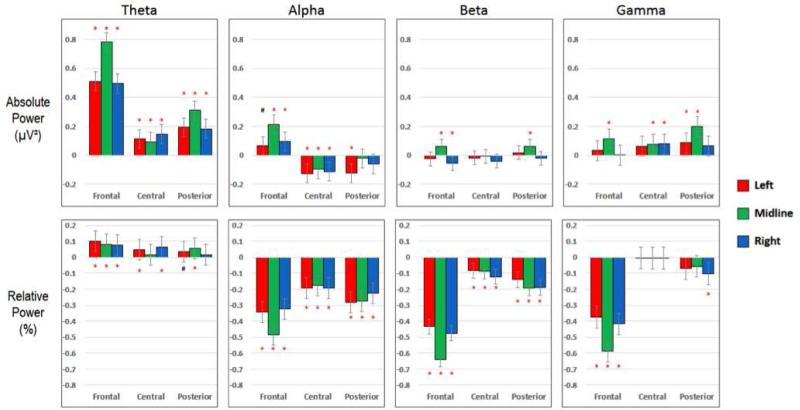
Figure 2. Blinks.
Differences in mean power for the Blink and Artifact-Free categories (i.e. PowerBlink – PowerArtifact-Free). Positive values represent higher power in the Blink category. Figures 3 & 4 display the same information (i.e. PowerArtifact – PowerArtifact-Free) for saccade and EMG artifacts, respectively. Power values were log transformed (see Table 2) prior to calculation of differences. All significant differences (*) are p < 0.05 and corrected for multiple comparisons.
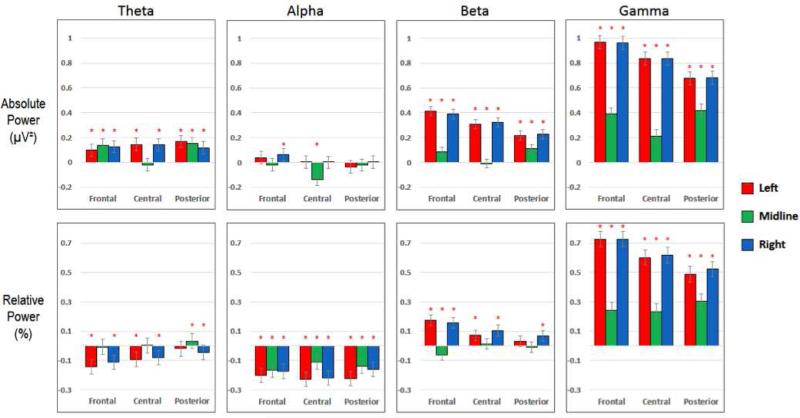
Figure 4. EMG.
Differences in mean power for the EMG and Artifact-Free categories (i.e. PowerEMG – PowerArtifact-Free). See the caption of Figure 2 for details.
Blinks (figure 2)
Mean absolute and relative power was significantly different between blink segments and artifact-free data in all frequency bands. In the theta band, for both absolute and relative power, blink segments had significantly higher power than artifact-free segments, with the most significant differences in frontal regions. In the alpha band, the difference in power varied based on both region and power type. Specifically, the mean relative alpha power in blinks was significantly lower than artifact-free segments across all ROIs, with the mean absolute alpha power in blinks higher in frontal ROIs and lower in central ROIs. Beta power seemed least affected by blinks, particularly in absolute power, although some differences were evidenced in frontal relative beta power between blinks and artifact-free segments. Finally, and somewhat surprisingly, in the gamma band blinks did significantly differ from artifact-free segments. They demonstrated higher mean absolute gamma and lower mean relative gamma power than artifact-free segments. These differences were most prominent in the midline regions.
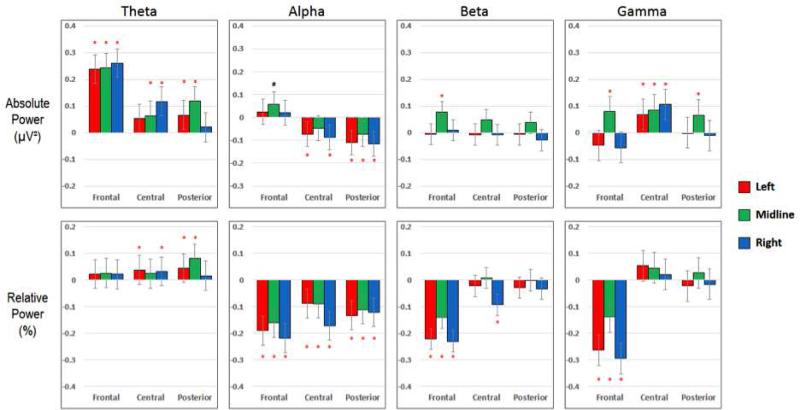
Saccades (figure 3)
Figure 3. Saccades.
Differences in mean power for the Saccade and Artifact-Free categories (i.e. PowerSaccade – PowerArtifact-Free). See the caption of Figure 2 for details.
The mean power differences between saccades and artifact-free segments followed the same general pattern as that of blink segments, with two main distinctions: (1) the magnitude of mean power difference was smaller between saccades and artifact-free segments and (2) significant differences were found in fewer ROIs, particularly central and posterior ROIs. In the theta band, the mean absolute and relative power estimates were higher in saccades than in artifact-free segments. Interestingly, the largest differences in absolute power occurred across frontal regions, but relative theta power in the same areas showed no significant differences. In the alpha and beta band, the differences between Saccade segments and artifact-free segments mirrored those of Blinks. Finally, we found significant differences in mean relative gamma power only in frontal ROI's for saccades compared to artifact-free segments. The mean absolute gamma power, on the other hand, was significantly greater in saccade segments for all midline and central ROIs.
EMG (figure 4)
As expected, EMG segments contained the highest amount of high frequency power ,but they showed significantly different power estimations in all frequency bands compared to artifact-free segments. In the theta band, EMG segments had higher mean absolute power than artifact-free segments in all ROI's except for midline-central, and had higher mean relative power in the posterior regions. In the alpha band, mean relative power in EMG segments was lower in all regions, while the mean absolute power was unaffected. In the beta band, EMG segments had higher mean absolute and relative power than artifact-free segments in all lateral regions, with some smaller differences seen in the midline ROIs. Finally, in the gamma range, EMG segments showed significantly higher mean absolute and relative power than artifact-free segments in all regions, most prominently in the lateral regions.
Discussion
In QEEG studies of young children or developmental populations, participants are often excluded from analysis due to an insufficient amount of artifact-free data. While some studies have suggested that artifacts, such as blinks, may be included in the analyses with the assumption that they do not affect particular types of power estimations or power bands (Grasser et al., 1985; Hagemann and Naumann, 2001; Iacono and Lykken, 1981), there have been no studies that have directly investigated the potential effect of artifacts on EEG power analysis in young children. The present study was motivated by the goal of characterizing and quantifying the potential effect of artifacts, namely blinks, saccades and EMG, on power estimations in young children, when data quantity does not allow for the use of ICA and other blind source separation (BSS) techniques. In order to address this goal, we used a modeling approach that allowed us to compare equal units of artifact to artifact-free data in order to understand differences in power that are independent of the amount of data available. By calculating the difference in mean power, we could then consider whether the inclusion of the artifact within the data could affect the power calculation. In other words, a significant difference in mean power in a specific band between an artifact and artifact-free data would suggest that the addition of that artifact to the artifact-free data would impact the calculation of power in that frequency band. The extent of the effect would depend on the total amount of data gathered and the amount of artifact present.
In this study we have demonstrated that in young children, eye blinks, saccades, and EMG contain spectral components of all frequencies and, therefore, could affect the calculation of mean power of both high and low frequency bands, but that the nature and direction of the differences depends on power type, region, and frequency band of interest. Therefore, data processing and cleaning needs to be sensitive to the oscillations of interest for a given analysis, with the most conservative approach being the removal of all EMG and ocular artifact from data. Unfortunately, such an approach leads us to the problem of excluding participants who provide insufficient clean data. The two most robust findings in our study are the following: (1) First, eye movements contain significantly higher relative and absolute theta power than artifact-free data. This result is particularly relevant for studies that induce lateral eye movements and blinks, such as a dynamic visual stimulus (bubbles) during resting-state recordings. The inclusion of blinks in these data will likely result in a spurious increase in the estimation of theta power . (2) Second, the high frequency nature of EMG activity results in higher beta and gamma power in EMG segments than in artifact-free segments, with the greatest difference found in lateral regions that are nearest to facial and neck muscles. Notably, given the lack of significant difference in absolute alpha power, we would conclude that the alpha band does not seem to be affected by EMG, suggesting that in experiments where head and neck movements are difficult to avoid, absolute power in the alpha band may be a more stable target variable. While these results may be intuitive, a quantification of these power differences can help justify the inclusion or exclusion of certain artifact segments in data processing.
Relative vs. Absolute Power
There is tremendous variability in the literature regarding the choice of power type, with studies targeting absolute (e.g. (Tierney et al., 2012)) or relative power (e.g. (Marshall et al., 2002)), or both (e.g. (Barry et al., 2010)), sometimes without an explicit reason provided for the choice. Justifications in favor of relative power include more robust test-retest reliability and less vulnerability to differences in skull thickness which, in turn, may facilitate the analysis of individual differences in early development (Benninger et al., 1984; John, 1980; Nunez and Shrinivasan, 2006). Absolute power, on the other hand, can be easier to interpret and more intuitive since it reflects the actual power value for one given band, without dependence on power in other ranges (Pizzagalli, 2007). Sometimes, the choice of power type is contingent upon methods used in prior studies, particularly when there is a goal is replication or direct comparison of results.
Our results can contribute to this debate by demonstrating that artifacts may differentially affect absolute and relative power. It is likely that the differential effect on relative and absolute power reflects the difference in the way the two values are calculated, since relative power reflects the power in one frequency band in relation to the power in all other bands in a region. We would recommend that, whenever possible, results of analyses for both absolute and relative power should be reported, with a discussion about the implications of any differences. In instances where results are reported for only one power type, it would be useful to articulate the reasons supporting the choice of one power type over another. One also should also take caution when comparing results from two QEEG studies using different power measurements. General recommendations from our results and experiences are listed in Table 2.
Table 2.
General recommendations for selection of power type and frequency band(s)
| *Whenever possible, authors should report findings for both absolute and relative power. If discrepancies between the two power types are observed, possible reasons and implications should be discussed. |
| *Using careful manual artifact detection methods detailed in this manuscript, it is possible to quantify individual artifact types (blinks, saccades, and EMG) and then to separate them from clean, artifact free, segments of data. The separation of artifacts allows for the flexibility of either including or excluding them based on the type of analyses being performed. |
| *If there is a risk of data contamination due to blinks or saccades, investigations of absolute and relative power in theta and alpha bands, and relative power for the beta and gamma bands, in frontal regions should be avoided (Figure 2 & 3). Posterior gamma power is least likely to be affected by eye movement artifact. |
| *If there is a risk of data contamination due to EMG, investigations of absolute and relative power in absolute and relative beta and gamma bands, in all regions should be avoided (Figure 4). Posterior alpha power is least likely to be affected by EMG artifact. |
| *If the results for only one power type can be reported (e.g. due to space limitations) and multiple frequency bands are compared, absolute power is preferred because the potential effects of artifacts on relative power for each frequency band are more unpredictable. |
| *Artifacts themselves may provide insight into the state of a child which can, in turn, inform indivdiual differences in power. |
Regional effects
Our data show that artifacts might lead to increased or decreased mean power depending on the scalp region. These differences reflect the regional differences in the presence of artifacts such as eye movements and EMG. For instance, an eye blink creates a large amplitude, slow frequency (typically < 5 Hz) deflection in the EEG signal that is most prominent in frontal ROIs (Iacono and Lykken, 1981). Thus, absolute power in the slow theta band is expected to increase especially in frontal ROIs. Even when the mean power measurements in all regions trend in the same direction, the extent to which the regions are affected may not be the same. Such a phenomenon is evidenced in our data by greater differences between EMG and artifact-free data in mean gamma power in lateral ROIs compared to midline ROIs.
Future directions and considerations
EEG power has been a powerful tool to study neurodevelopmental disorders such as ASD or ADHD, as a biomarker that facilitates prediction of diagnosis, clinical stratification, and quantification of changes over development or with treatment. EEG biomarkers are particularly relevant and necessary in populations with such behavioral and cognitive heterogeneity. However, a “stable” electrophysiological signal requires consistent methods in data acquisition and cleaning. Analyses of group differences in EEG power between typical and atypical populations have yielded some promising results in disorders such as autism (Wang et al., 2013). In these group level analyses, it will be critical to measure any possible systematic differences in artifacts between groups. For instance, one might hypothesize that children with delayed development would demonstrate more eye movements or EMG artifact than typically developing children. However, studies do not consistently examine this variable in QEEG studies. Of note, several recent studies in fMRI have shown that head motion leads to systematic biases in the analysis of functional connectivity, and that that children with ASD do generate more motion artifact than typically developing controls (Deen and Pelphrey, 2012; Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012). Such a systematic bias can result in the erroneous appearance of weaker long-range connections in children with ASD. These seminal studies have led to rigorous efforts for consistent motion correction in fMRI data processing across studies and sites in studies of typical and atypical development. Similar considerations will be critical in the handling of EEG artifacts in developmental populations.
Moreover, in the analysis of resting state oscillations, artifacts may, in fact, serve as a proxy for the child's emotional or cognitive state. In a recent behavioral study, Oh et al. found that the spontaneous eye blink rate increased during an attentional task (the Stroop) compared to a resting period, suggesting that blinks may be a proxy for heightened attention or cognitive effort. Whitman et al investigated the effect of EMG on high frequency oscillations in adults and found that cognitive tasks were associated with greater EMG activity, which, in turn, could confound the calculation of gamma power. We would propose that, in children, particularly those with atypical development, there can be tremendous variability in emotional and cognitive states even during “resting state” recordings, with some children requiring additional cognitive control to remain compliant during testing. Such effort or vigilance may be reflected in their power estimations (such as higher frontal theta power), but also may be reflected in a higher blink frequency or greater EMG activity. As we continue to expand our interpretation of resting state oscillations in developmental disorders, we may consider artifacts themselves as a clue to the etiology of the spectral power characterizing subgroups or individuals, and we can quantify their presence and consider them as a biomarker of a child's state before discarding them from data processing.
Conclusion
In this manuscript we have provided considerable detail about data collection, preprocessing, artifact identification (without the use of traditional methods such as ICA), and data analysis, in part to reinforce the need for detailed methodological descriptions that will facilitate common practices across study sites and clinical populations. We highlight the need for transparency regarding the choice of relative or absolute power, regions of interest, and frequency band, as each of these variables are differentially vulnerable to artifacts and, therefore, their interpretation depends on the methods used to identify and remove artifacts. The use of QEEG and, specifically, resting state power, as a biomarker of typical and atypical development holds promise as a tool to better define more subtle differences between individuals and subgroups within clinical populations, where behavioral measures may not be able to capture subtleties in clinical heterogeneity. However, only through rigorous and consistent methods for data processing and artifact removal can we make informed conclusions about individual differences, both cross-sectional and across development.
Acknowledgments
This research was funded by NIMH 5K23MH094517 – 02 (PI Jeste) and the Autism Speaks Weatherstone Predoctoral Fellowship 7845 (McEvoy, Kevin).
References
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Dupuy FE. Resting-state EEG gamma activity in children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2010;121(11):1871–77. doi: 10.1016/j.clinph.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Benninger C, Matthis P, Scheffner D. EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalogr Clin Neurophysiol. 1984;57(1):1–12. doi: 10.1016/0013-4694(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Bonfiglio L, Olcese U, Rossi B, Frisoli A, Arrighi P, Greco G, Carozzo S, Andre P, Bergamasco M, Carboncini MC. Cortical source of blink-related delta oscillations and their correlation with levels of consciousness. Hum Brain Mapp. 2013;34(9):2178–89. doi: 10.1002/hbm.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9:18. doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor DS, Chabot R. QEEG studies in the assessment and treatment of childhood disorders. Clin EEG Neurosci. 2009;40(2):113–21. doi: 10.1177/155005940904000209. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Dupuy FE, Heckel LD, McCarthy R, Selikowitz M, Johnstone SJ. Behavioural differences between EEG-defined subgroups of children with Attention-Deficit/Hyperactivity Disorder. Clin Neurophysiol. 2011;122(7):1333–41. doi: 10.1016/j.clinph.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, Webb SJ. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150–59. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pelphrey K. Perspective: Brain scans need a rethink. Nature. 2012;491(7422):S20. doi: 10.1038/491s20a. [DOI] [PubMed] [Google Scholar]
- Drongelen W. Signal processing for neuroscientists : introduction to the analysis of physiological signals. Elsevier/Academic Press; Amsterdam; Burlington, MA: 2007. [Google Scholar]
- Elliott CD. Differential Abilities Scale (DAS). Child Assessment News. 1993;3:1–10. [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112(3):536–44. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fletcher EM, Kussmaul CL, Mangun GR. Estimation of interpolation errors in scalp topographic mapping. Electroencephalogr Clin Neurophysiol. 1996;98(5):422–34. doi: 10.1016/0013-4694(96)95135-4. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Holmes MD, Burke BC, Vanhatalo S. Spatial spectra of scalp EEG and EMG from awake humans. Clin Neurophysiol. 2003;114(6):1053–68. doi: 10.1016/s1388-2457(03)00045-2. [DOI] [PubMed] [Google Scholar]
- Goncharova II, McFarland DJ, Vaughan TM, Wolpaw JR. EMG contamination of EEG: spectral and topographical characteristics. Clin Neurophsiol. 2003;114(9):1580–93. doi: 10.1016/s1388-2457(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, Benasich AA. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav Brain Res. 2011;220(2):263–70. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser T, Lothar S, Joachim M. The transfer of EOG activity into the EEG for eyes open and closed. Electroencephalography and Clin Neurphysiol. 1985;61:181–93. doi: 10.1016/0013-4694(85)91058-2. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E. The effects of ocular artifacts on (lateralized) broadband power in the EEG. Clin Neurophysiol. 2001;112(2):215–31. doi: 10.1016/s1388-2457(00)00541-1. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Walter C, Naumann E. Skull thickness and magnitude of EEG alpha activity. Clin Neurophysiol. 2008;119(6):1271–80. doi: 10.1016/j.clinph.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Lykken DT. Two-year retest stability of eye tracking performance and a comparison of electro- oculographic and infrared recording techniques: evidence of EEG in the electro-oculogram. Psychophysiology. 1981;18(1):49–55. doi: 10.1111/j.1469-8986.1981.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clin Neurophysiol. 1958;10:371–75. [PubMed] [Google Scholar]
- Jeste SS, Kirkham N, Senturak D, Hasenstab K, Sugar C, Kupelian C, Baker E, Sanders AJ, Shimizu C, Norona A, Paparella T, Freeman S, Johnson SP. Dev Sci. Electrophysiological evidence of heterogeneity in visual statistical learning in young children with ASD. 2014 Jun 11; doi: 10.1111/desc.12188. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210(4475):1255–58. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000;111(10):1745–58. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghofer M, Kappenman ES, Luck SJ, Luu P, Miller GA, Yee CM. Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Pysophysiology. 2014;51(1):1–21. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. Neuroimage. 49(3):2248–2263. doi: 10.1016/j.neuroimage.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Marangell LB, Gilmer WS, Bugoyne KS, Howland RH, Trivedi MH, Zisook S, Jain R, McCracken JT, Fava M, Iosifescu D, Greenwald S. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: results of the BRITE-MD study. Psychiatry Res. 2009;169(2):124–31. doi: 10.1016/j.psychres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics. 2012;9(3):569–87. doi: 10.1007/s13311-012-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaus C, Grass-Kapanke B, Blaeser I, Ihl R, Supprian T, Winterer G, Brinkmeyer J. Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): results of a 1-year follow-up study. Int J Geriatr Psychiatry. 2008;23(11):1148–55. doi: 10.1002/gps.2042. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Monastra VJ, Lubar JR, Linden M, VanDeusen P, Green G, Wing W, Phillips A, Fenger TN. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. Neuropsychology. 1999;13(3):424–33. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. AGS Edition. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neurosci Biobehav Rev. 2006;30(6):808–22. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2nd ed. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr Clin Neurophysiol. 1987;66(1):75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human even-related potentials to study cognition: Recording standards and publication ciriteria. Psychophysiology. 2000;37(2):127–52. [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantatative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–58. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo J, Tassinary LG, Berntson GG, editors. The Handbook of Psychophysiology. Cambridge University Press; Cambridge: 2007. pp. 56–85. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, Marshall PJ. The utility of EEG band power analysis in the study of infancy and early childhood. Dev Neuropsychol. 2012;37(3):253–73. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliot MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer KJ, Avis KT, Frutchey RA. Behavioral intervention to increase compliance with electroencephalographic procedures in children with developmental disabilities. Epilepsy Behav. 2008;13(1):189–95. doi: 10.1016/j.yebeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurphysiol. 2006;23(5):440–55. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales. 2nd ed. Pearson Assessments; Livonia, MN: 2005. [Google Scholar]
- Srinivasan R, Nunez PL, Tucker DM, Silberstein RB, Cadusch PJ. Spatial sampling and filtering of EEG with spline laplacians to estimate cortical potentials. Brain Topogr. 1996;8(4):355–66. doi: 10.1007/BF01186911. [DOI] [PubMed] [Google Scholar]
- Swingler MM, Willoughby MT, Calkins SD. EEG power and coherence during preschoolers’ performance of an executive function battery. Dev Psychobiol. 2011;53(8):771–84. doi: 10.1002/dev.20588. [DOI] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS One. 2012;7(6):e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–38. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, Sweeney JA. Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord. 2013;5(1):24. doi: 10.1186/1866-1955-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Bernier R, Henderson HA, Johnson MH, Jones EJ, Lerner MD, Westerfield M. Guidelines and Best Practices for Electrophysiological Data Collection, Analysis and Reporting in Autism. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO. Scalp electrical recording during paralysis: quantatative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin Neurophysiol. 2007;118(8):1877–88. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–41. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Deouell LY. The broadband-transient induced gamma-band response in scalp EEG reflects the execution of saccades. Brain Topogr. 2009;22(1):3–6. doi: 10.1007/s10548-009-0077-6. [DOI] [PubMed] [Google Scholar]