Abstract
BACKGROUND
Chronic ethanol leads to disruptions in resting EEG activity and in sleep patterns that can persist into the withdrawal period. These disruptions have been suggested to be predictors of relapse. The thalamus is a key structure involved in both normal brain oscillations, such as sleep-related oscillations, and abnormal rhythms found in disorders such as epilepsy and Parkinson’s disease. Previously, we have shown progressive changes in mouse thalamic T-type Ca2+ channels during chronic, intermittent ethanol (CIE) exposures that occurred in parallel with alterations in theta (4–8Hz) EEG patterns.
METHODS
Two groups of eight-week old male C57BL/6 mice were implanted with wireless EEG/EMG telemetry and subjected to 4 weeks of CIE vapor exposure and withdrawal. During the week after the final withdrawal, mice were administered ethosuximide (200 mg/kg) or saline. EEG data were analyzed via discrete Fourier transform and sleep scored for further analysis.
RESULTS
CIE exposure produced changes in the diurnal rhythms of the delta (0.5–4Hz) and theta bands that persisted into a subsequent week of sustained withdrawal. These disruptions were restored with the T-channel blocker ethosuximide. Repeated ethanol exposures preferentially increased the relative proportion of lower frequency power (delta and theta), whereas higher frequencies (8–24Hz) were decreased. The ethanol-induced decreases in relative power for the higher frequencies continued into the sustained withdrawal week for both groups. Increases in absolute delta and theta power were observed in averaged NREM and REM sleep spectral data during withdrawal in ethosuximide-treated animals, suggesting increased sleep intensity.
CONCLUSIONS
These results suggest that persistent alterations in delta and theta EEG rhythms during withdrawal from chronic intermittent ethanol exposure can be ameliorated with ethosuximide and that this treatment might also increase sleep intensity during withdrawal.
Keywords: ethanol withdrawal, electroencephalography, sleep, seizure, T-type calcium channels
Introduction
Preventing relapse after withdrawal from alcohol represents a significant hurdle in overcoming alcohol dependence. Chronic alcohol use leads to disruptions in resting EEG activity and in the sleep patterns of alcoholics that can persist into the withdrawal period, where they have been suggested to be effective predictors of relapse (Bauer, 2001; Brower, 2001; Brower and Perron, 2009). In rodents, repeated ethanol exposures and withdrawals that model the binge/abstain consumption patterns of human alcoholics (Becker and Hale, 1993) have also resulted in similar EEG and sleep alterations (Ehlers and Slawecki, 2000; Veatch, 2006). It is still unresolved whether restoring normal sleep patterns alone can prevent relapse (Friedmann et al., 2008; Brower and Perron, 2009); however, understanding the underlying mechanisms and brain structures involved in progressive EEG and sleep pattern alterations that occur during chronic alcoholism and continue into protracted withdrawal will help provide the basis for additional avenues of adjunct therapies in the treatment of alcohol dependence.
The thalamus is a key brain structure involved in the generation and maintenance of normal brain rhythms during sleep, as well as different states of vigilance (Llinas and Steriade, 2006). Abnormal thalamic activity has also been implicated in several pathological conditions that are marked by abnormal increases in thalamocortical theta coherence (Sarnthein and Jeanmonod, 2007, 2008) and have been described as thalamocortical “dysrhythmias” (Jeanmonod et al., 1996; Llinas et al., 1999). These dysrhythmias are characterized by enhanced activity of T-type Ca2+ channels (Steriade, 2005; Jeanmonod et al., 1996; Nelson et al., 2006), which generate characteristic bursts of action potentials that support intrinsic neuronal oscillations (Huguenard, 1996).
Results from our lab (Graef et al., 2011) have demonstrated that chronic ethanol exposure also produces alterations in thalamic T-type channel expression and function that occur in parallel with disruptions in EEG theta activity. These changes persisted into a subsequent week of sustained withdrawal, but were ameliorated with the relatively selective T-type channel blocker ethosuximide (ETX). Such disruptions are consistent with observations of abnormal EEG activity in alcoholics (Porjesz and Begleiter, 2003; Rangaswamy et al., 2003), including increases in interhemispheric theta coherence that have also been suggested to arise from altered thalamocortical function (Porjesz and Rangaswamy, 2007).
In this study, we expanded upon our previous findings by investigating the effects of chronic intermittent ethanol exposures on the rhythmic patterns and relative power of different EEG power bands and the average spectrum during two types of sleep. We also assessed the efficacy of ETX as a pharmacologic intervention during a subsequent week of sustained withdrawal. We found that chronic ethanol induced disruptions in the diurnal pattern of sleep-related EEG rhythms that continued into the sustained withdrawal week and could be restored to baseline rhythms with ETX. Furthermore, we observed significant increases in the absolute spectral power of these rhythms during REM and non-REM sleep in response to ETX treatment. These results provide new data on changes in important sleep-related rhythms that are elicited by withdrawal from ethanol, and further implicate T-type channel involvement in these CNS alterations.
Materials and Methods
Animals and Experimental Design
All experiments were conducted with the advanced approval of the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine. A total of 18 individually housed, 8-week old male C57BL/6 mice (10 ethanol-exposed, 8 air-exposed) were used in the following experiments. Ethanol was chronically administered via the inhalation route previously described by Becker and Hale (1993). Briefly, all mice were placed in one of two sealed Plexiglas vapor chambers modified after Goldstein (1972) in a room with a 12-hr light/dark schedule with lights on at 5am. Ethanol (95%) was volatilized and delivered to one of the chambers at a rate of 2.0 L/min by a vacuum pump for 16 hrs beginning at the onset of the dark period. This, in combination with air being delivered to both chambers at a rate of 20 L/min, maintained the ethanol concentration in the first chamber in the range of 14–16 mg per L of air (mean ± SEM: 15.6±0.5mg/L). An 8-hr period of abstinence allowed complete clearance of ethanol from circulation prior to the next cycle of intoxication (Becker and Hale, 1993; Becker, 1994). To the second chamber, only room air was delivered. At the beginning of each exposure cycle (5pm), all mice (whether receiving room air or ethanol vapor) were treated with a subcutaneous injection of the alcohol dehydrogenase inhibitor pyrazole (100 mg/kg). Pyrazole is commonly employed in the CIE paradigm to stabilize blood-ethanol concentrations (BECs) over the course of repeated ethanol exposures. Pyrazole was prepared daily by dissolving in sufficient saline to achieve an injection volume of approximately 0.2 mL per animal. BECs achieved under these conditions remained relatively stable from one bout of intoxication to the next. Mean BECs were 221.9±12.7, 189.0±21.0, 149.3±11.2, and 155.5±10.5 mg/dl for weeks 1–4 of exposure, respectively. The mean BEC was 186.0±9.4mg/dl for all four weeks of exposure. BECs for all mice were measured by taking 5μl blood samples from the tail (stored in vials containing 45μl of 6.25% trichloroacetic acid) and analyzed using a NAD-ADH enzyme assay (Diagnostic Chemicals, Oxford, CT).
Data Acquisition
All mice were implanted subcutaneously with telemetric physiologic monitors (Model F20-EET; Data Sciences International (DSI), Arden Hills, MN) that simultaneously record electroencephalogram (EEG), electromyogram (EMG), temperature and activity. Briefly, animals were anesthetized with isoflurane followed by the implantation of electrodes for recording EEG signals and EMG signals. For placement of EEG wire leads, holes slightly larger than the coil diameter of the transmitter lead wire were drilled in the skull 2 mm on either side of midline suture half way between bregma and lambda. The exposed portions of the leads were placed between the skull and underlying dura. Wires were secured to the skull with dental acrylic. EMG leads were placed in the neck muscles and secured with sutures. The signal transmitter body was placed subcutaneously over the dorsal thorax. Mice were allowed to recover from surgery for one week prior to recording. The exposure/recording paradigm for ethanol-exposed mice (n = 10) during the 6-week recording period was as follows: one week of baseline and four weeks of intermittent ethanol exposure (5pm–9am on days 1–5, no exposure days 6 and 7), followed by 1 week of sustained withdrawal. One EEG and one EMG channel were continuously acquired over the entire six-week paradigm using the Dataquest A.R.T. acquisition system (DSI, Arden Hills, MN) at a sampling frequency of 500Hz. The EEG data was then band-pass filtered from 0.5–100Hz and the EMG data filtered from 10–100Hz for analysis. Average activity counts were obtained every 10s. During the sustained withdrawal week, ethanol-exposed mice were subdivided into two groups that received isovolumic (0.2mL) injections of either saline (control group, n = 5) or 250mg/kg ethosuximide (experimental group, n = 5) daily at 5pm (corresponded to time of beginning of ethanol during exposure weeks 1–4) for the first 5 days. Air-exposed mice were subjected to 3 days of baseline recording and 3 days of either saline (control group, n = 4) or ethosuximide (experimental group, n = 4) administration (250 mg/kg, IP, daily at 5pm). Ethosuximide (ETX) was prepared by dissolution in a sufficient volume of normal saline to achieve an injection volume of approximately 0.2 mL per animal.
Cosinor Analysis of EEG
EEG Data were divided into 10-s epochs and analyzed with a conventional Discrete Fourier Transform (DFT) power spectra function using the software program NeuroScore (DSI, Arden Hills, MN). Using a customized MATLAB (The Math Works, Natick, MA) program, epochs were then filtered into five power bands: delta (0.5–4Hz), theta (4–8Hz), alpha (8–12Hz), sigma (12–16Hz) and beta (16–24Hz). Normalized power was obtained by dividing the power in each 10-s epoch by the maximum power within its respective 24-hr period (5pm–5pm) for each frequency band. Normalized power was then further averaged into 1-h bins for Cosinor analysis (Nelson et al., 1979). Relative power for each band was determined by dividing the raw spectral power for that band by the total power for all frequencies analyzed (0.5–25Hz). Diurnal patterns were analyzed with a custom Cosinor analysis function written in MATLAB on all 24hr periods over the course of the six week exposure paradigm for each animal. The Cosinor function, given by the following equation:
determines through least squares approximation the Mesor (midline estimating statistic of rhythm), amplitude (A) and time of peak (Acrophase) values for the fitted cosine function from a predetermined set phase (P). For all animals, a custom MATLAB function was written so that each 24-hr period (5pm–5pm) over the 6-week paradigm was double-plotted and fitted with Cosinor curves using a phase range of 18–32hrs in 10-m increments. A zero-amplitude test was then performed for each fit, using error estimates for the least-squares fit of the amplitude, to determine whether the amplitude of the fitted curve was significantly different from zero. The parameters from the best Cosinor fit, as determined by the lowest p-value, were returned. Data was then grouped and averaged for all 24hr baseline periods, exposure periods (weeks 1–4, days 1–5) and sustained withdrawal periods (week 5, days 1–5), excluding any 24-hr periods that failed the zero-amplitude test (as indicated by the best Cosinor fit having a p-value greater than 0.05). Statistical analysis was performed between weeks both within each treatment group and between treatment groups.
Sleep Scoring
EEG, EMG, and activity data were sleep-scored in 10-s epochs using a customizable rodent sleep scoring algorithm available with NeuroScore. The primary measures employed by this algorithm are the delta and theta power bands, delta ratio, theta ratio, EMG threshold, activity threshold, and EMG and EEG artifact thresholds. These settings were adjusted for the analysis of each week for each animal according to manual verification of randomly selected sleep-scored epochs to account for individual variations in signal strength and noise over time. The algorithm proceeds as follows for each epoch analyzed: Any epoch containing a significant amount of artifact is discarded. If activity or EMG signal exceed their respective thresholds, the epoch is scored as wake. If the first condition is not met and the delta ratio exceeds its threshold, the epoch is scored as NREM sleep. If the previous two conditions are not met and the theta ratio exceeds its threshold, the epoch is scored as REM sleep. If none of the preceding criteria are met, the default scoring is wake. The delta and theta bands remained constant for all animals and all time points at 0.5–4Hz and 6–9Hz, respectively, consistent with previous studies in this model (Veatch, 2006). As compared to the bands used in the analyses above, the theta band was narrowed for the sleep scoring algorithm. This was done to isolate the theta peak observed during REM sleep from any overlap with delta activity and, thus, minimize the number of false positive REM epoch identifications. The delta ratio is defined as the ratio of delta power to the total power in the spectrum from 0.5–25Hz and ranged from 0.35–0.45 in the present analysis. The theta ratio is defined as the ratio of theta power to delta power and ranged from 2–3. The EMG threshold ranged from 10–50μV, depending on the noise in the signal. The activity threshold remained constant across all animals at 0.1 counts per minute. Finally, the EEG and EMG artifact thresholds ranged from 0.5–1.0mV. Sleep epochs were scored as rapid eye movement (REM), non-REM (NREM), wake (W), or artifact, consistent with previous studies in this model (Veatch, 2006).
Spectral Analysis of Vigilance States
The DFT power spectrum of each sleep-scored epoch was exported in 1Hz bins from 1–25Hz in order to analyze the average spectral characteristics of individual vigilance states (NREM, REM, and W) during the sustained withdrawal week. A custom MATLAB function was written to normalize each spectral bin to its maximum value in a 24-hr period. The function then binned the spectra by their associated vigilance state and averaged the normalized power across all epochs for each animal in each week. The end result was an average spectrum for each vigilance state across the sustained withdrawal week.
Statistical Analysis
Statistical analyses were performed both within each treatment group (effects of ethanol and/or withdrawal vs. baseline) and between treatment groups (ETX vs. saline) using either MATLAB or Prism (Graph Pad Software, La Jolla, CA). One- or two-way repeated measures ANOVA was used to assess effects of ethanol, withdrawal, and/or treatment for each group. Post hoc tests were Newman-Keuls, Dunnett, or Bonferroni tests to correct for multiple comparisons. Due to the large number of comparisons being made for the spectral analysis of vigilance states, a two-tailed permutation test was used to compare means between ETX- and saline-treated groups at each 1-Hz frequency band. All statistical tests are specified in the Results section at first use, and then clarified with any change in the choice of test for subsequent comparisons.
Results
Multiple Intermittent Ethanol Exposures and Withdrawals Disrupt Diurnal EEG Patterns
In order to determine the effects of chronic, intermittent ethanol exposures on frequency band patterns, surface EEG for ethanol-exposed mice (n = 10) was continually recorded for one week of baseline, four weeks of multiple intermittent ethanol exposures and one week of sustained withdrawal during which one subgroup (n = 5) received daily injections of ethosuximide (ETX) and the other subgroup (n = 5) received isovolumic injections of saline.
Figure 1A shows that during the baseline week, a diurnal pattern can be seen for theta frequencies (4–8Hz), with lower normalized power exhibited during the animals’ dark (active) period as compared to the light (inactive) period. In order to statistically compare variations in this diurnal rhythm, a Cosinor function was used to fit a cosine curve to each double-plotted 24-hr period for all power bands. The parameters derived from the fitted curves include the Mesor, which gives the estimated midline of the rhythm, the amplitude between the peak and trough of the curve, the time at which the peak of the curve occurs, and the length of the period. Figure 1 illustrates fitted Cosinor curves to the average normalized theta power for the baseline week (A), the fourth week of ethanol (B), and the sustained withdrawal week for both saline (C) and ETX-treated mice.
Figure 1. Cosinor analysis of normalized theta (4–8Hz) EEG band during ethanol exposure and withdrawal.
An average 24-hr theta (4–8Hz) rhythm was compiled by double-plotting the mean of each respective time point during the first five days (M–F) of the baseline week (A), the fourth week of ethanol exposure (EtOH Week 4), and the week of sustained withdrawal with either saline or ethosuximide (ETX) treatment and fitted with a Cosinor function. The values for Mesor, amplitude, period and time of peak for each fitted curve are indicated on the graph. (B) The Mesor was significantly increased, the amplitude was significantly attenuated, and the peak was significantly shifted (p < 0.05 for each parameter) during EtOH Week 4. (C, D) Only the peak was restored in the saline group, whereas the Mesor and amplitude were also restored in the ETX group.
Analysis between the baseline power band rhythms and the fourth week of intermittent ethanol exposures revealed that chronic ethanol significantly increased the Mesor for the theta power band (F2,62 = 34.42, p < 0.001, one-way ANOVA with Newman-Keuls post-hoc), as well as significantly decreasing the Mesor for the beta frequency (F2,47 = 25.97, p < 0.05; see Table 1). Additionally, the amplitude between the peak and trough of the fitted curve was significantly decreased for delta and theta frequencies (F2,47 = 7.762, p < 0.05, and F2,47 = 16.64, p < 0.001, respectively), and the time of the peak was significantly shifted forward in time for the theta, alpha (F2,47 = 3.829, p < 0.05), sigma (F2,47 = 16.18, p < 0.05) and beta (F2,47 = 3.728, p < 0.05) bands (p < 0.05 for each band). Chronic ethanol exposure did not have a significant effect on the average length of the period for any frequency band (see Table 1).
Table 1.
Values from Cosinor analysis for all EEG bands during baseline, fourth week of ethanol exposure and sustained withdrawal week.
| Mesor | Amplitude | Period Length (hrs) | Peak of Rhythm (hr) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power Band | Base | EtOH (Wk4) | WD (saline) | WD (ETX) | Base | EtOH (Wk4) | WD (saline) | WD (ETX) | Base | EtOH (Wk4) | WD (saline) | WD (ETX) | Base | EtOH (Wk4) | WD (saline) | WD (ETX) |
| Delta (0.5–4Hz) | 0.80±0.01 | 0.83±0.03 | 0.84±0.02 | 0.81±0.03 | 0.09±0.01 | 0.07±0.01 * | 0.05±0.01 $ | 0.08±0.01 | 25.0±0.2 | 24.7±0.2 | 23.6±0.5$ | 25.8±0.5# | 17.6±0.6 | 19.7±1.3 | 17.5±1.7 | 16.4±1.1 |
| Theta (4– 8Hz) | 0.77±0.02 | 0.83±0.01 * | 0.85±0.02 | 0.74±0.02 %# | 0.12±0.02 | 0.08±0.01 * | 0.05±0.01 $ | 0.11±0.01 %# | 25.1±0.2 | 24.8±0.2 | 24.7±0.3 | 26.0±0.3# | 17.4±0.7 | 20.4±1.1* | 18.6±0.7 | 18.3±0.5 |
| Alpha (8– 12Hz) | 0.75±0.02 | 0.74±0.01 | 0.84±0.04 | 0.71±0.01 # | 0.14±0.02 | 0.11±0.01 | 0.07±0.02 | 0.12±0.01 | 25.1±0.3 | 25.3±0.2 | 25.8±0.2 | 26.2±0.4 | 18.4±0.5 | 22.0±0.7* | 19.5±0.5% | 19.0±0.6% |
| Sigma (12–16Hz) | 0.75±0.02 | 0.75±0.01 | 0.81±0.03 | 0.74±0.02 # | 0.14±0.02 | 0.12±0.01 | 0.08±0.02 | 0.11±0.01 | 25.3±0.2 | 25.1±0.2 | 25.4±0.3 | 26.2±0.3 | 19.2±0.5 | 22.8±0.4* | 20.1±0.2% | 19.3±0.8% |
| Beta (16– 24Hz) | 0.82±0.02 | 0.77±0.02 * | 0.82±0.05 | 0.82±0.01 | 0.09±0.01 | 0.11±0.01 | 0.05±0.01 % | 0.07±0.01 | 25.2±0.3 | 24.9±0.1 | 24.5±0.2 | 26.3±0.4%# | 19.6±0.6 | 22.5±0.5* | 21.0±1.1 | 19.3±0.8 |
p<0.05, repeated measures ANOVA, with Bonferroni’s post test, compared to baseline (n=10; both treatment groups);
p<0.05, repeated measures ANOVA, with Bonferroni’s post test, compared to baseline (n=5; within treatment group);
p<0.05, repeated measures ANOVA, with Bonferroni’s post test, compared to ethanol week 4 (within treatment group);
p<0.05, unpaired t test (between treatment groups)
These results indicate that multiple intermittent ethanol exposures disrupted diurnal EEG rhythms for the lower delta and theta bands, and shifted the peak of normalized power towards the end of each subsequent withdrawal period for all frequencies greater than 4Hz.
Ethosuximide Restores Altered EEG Patterns during the Sustained Withdrawal Period
Since chronic, intermittent ethanol exposures and withdrawals disrupted diurnal EEG variations seen during the baseline week, we next sought to determine if these changes persisted during the week following the final withdrawal. In addition, we tested the effects of ETX treatment administered daily at the start of the dark period (5pm) during the sustained withdrawal week, corresponding to the beginning of each intermittent bout of ethanol exposure experienced during the previous four weeks.
Figure 1C shows the normalized theta power for saline-treated mice during the sustained withdrawal period (hereafter, referred to as “withdrawal”), while Figure 1D illustrated the normalized power for ETX-treated mice during the same withdrawal week. Cosinor analysis of the theta bands revealed that both the significant increase in the Mesor (F2,62 = 34.42, p < 0.001, one-way ANOVA with Bonferroni post-hoc) and the significant decrease in amplitude (F2,47 = 16.64, p < 0.001) observed during ethanol exposure persisted into withdrawal for saline-treated animals, however this returned to baseline levels for ETX-treated animals (p > 0.05 for both, when compared to baseline; see Table 1). In addition, the average period length of the fitted cosine curves for the delta band during the sustained withdrawal week was significantly reduced compared to the baseline week for saline-treated mice (F2,62 = 21.01, p < 0.05), whereas the average period for the fitted curves for ETX-treated animals was not statistically different from the baseline (p > 0.05).
While unaffected during ethanol exposure, derived values from the fitted cosine curves for the alpha and sigma bands revealed significantly greater Mesor values during the sustained withdrawal week in saline-treated mice as compared to ETX-treated animals (p < 0.05 for both bands, unpaired t-test; see Table 1).
Derived values from the Cosinor analysis on the beta power band revealed a significant decrease in the Mesor during the fourth week of ethanol exposure (F2,47 = 3.728, p < 0.05) that returned to baseline values for both treatment groups. The amplitude was unaffected during ethanol exposure but was significantly reduced during withdrawal regardless of treatment (F2,47 = 11.31, p < 0.05 for both groups), whereas the period length was unaffected during ethanol exposure and withdrawal in saline-treated mice, but significantly increased during withdrawal in ethosuximide (p < 0.01, unpaired t-test).
Ethosuximide Restores Relative Power in Low Frequency Bands during Withdrawal
In addition to alterations in theta rhythms, we also observed changes in the relative power of all frequency bands over the course of ethanol exposure. During the fourth week of exposure, relative power in the lower frequency range (delta and theta; Figure 2A–B) was increased as compared to baseline measures (F4,168 = 8.05, p < 0.01, for delta, two-way repeated measures ANOVA with Dunnett Multiple Comparison test; F4,168 = 78.95, p < 0.001 for theta). Conversely, the higher power bands showed a decrease in relative power, with significant reductions in alpha (F4,168 = 19.05, p < 0.001), sigma (F4,168 = 22.72, p < 0.001) and beta (F4,168 = 36.68, p < 0.001) power during the fourth week of intermittent ethanol exposure (Figure 2C–E).
Figure 2.
Relative power for all power bands during the baseline week and fourth week or intermittent ethanol exposures over an average 24-hr period binned in 4-hr epochs. (A) Relative delta power was significantly increased during the light period after four weeks of intermittent ethanol exposure. (B) Relative theta power was significantly increased throughout both the dark and light periods. (C–E) Relative power in the alpha and sigma bands was decreased in the light period, while the beta and sigma bands showed decreases in the dark period. Dark and light periods indicated by black and white bars at the top of the graph. Ethanol exposure is illustrated by the shaded region. *p < 0.05
During withdrawal, the significant increase in relative delta power seen during the fourth week of ethanol exposure persisted in saline-treated animals (F1,18 = 12.14, p < 0.01); however, this increase was no longer evident for the ETX-treated mice (Figure 3A; p > 0.05). The observed ethanol-induced increases in relative theta power for both saline- and ETX-treated groups returned to values indistinguishable from their baseline week (Figure 3B, p > 0.05 for both bands). Similar to the effect on delta, we observed a significant effect of withdrawal on the relative alpha power in the saline-treated, but not ETX-treated mice (Figure 3C; F1,18 = 9.691, p < 0.01). We also observed a significant main effect of ETX treatment for both delta (F1,36 = 7.317, p < 0.05, two-way ANOVA) and alpha (F1,36 = 5.182, p < 0.05) bands during withdrawal. We did not observe a significant main effect of ETX treatment for the sigma (Figure 3D, p > 0.05) and beta (Figure 3E, p > 0.05) bands, but with both treatment conditions pooled, a significant main effect of withdrawal was seen for both sigma (F1,42 = 6.725, p < 0.05, one-way ANOVA) and beta (F1,42 = 8.275, p < 0.01).
Figure 3. Ethosuximide restores relative delta and alpha power during the sustained withdrawal week.
Relative power for all power bands during the sustained withdrawal week for both treatment groups over an average 24hr period normalized to the baseline and binned in 4hr epochs. Dark and light periods indicated by black and white bars at the top of the graph. There was a significant main effect of ETX treatment during withdrawal for both delta (A) and alpha (C) power bands (p < 0.05 two-way ANOVA, n=4 both groups), as well as a significant main effect of withdrawal in the saline-treated mice (p < 0.01, two-way repeated measures ANOVA, as compared to respective baselines). No significant main effect of ETX treatment was observed for theta (B), sigma (D) or beta (E) bands. However, there was a significant main effect of withdrawal with both treatment groups pooled for the sigma (D) and beta (E) bands (p < 0.05, two-way repeated measures ANOVA, as compared to baseline).
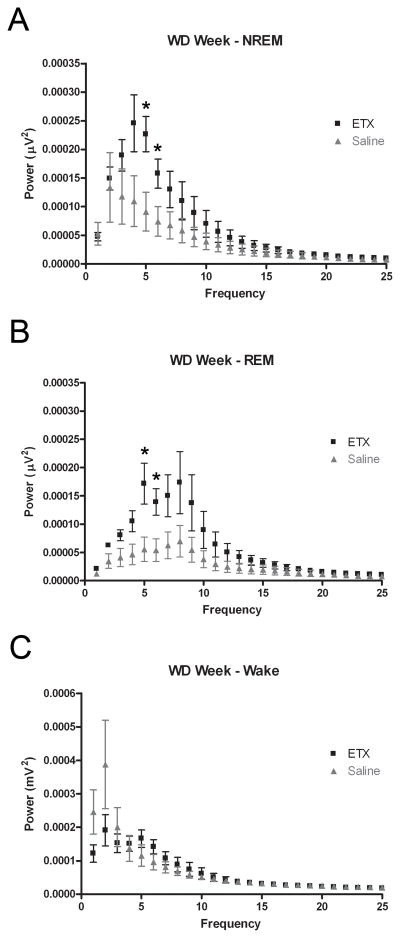
Ethosuximide treatment increases the spectral power of the sleep EEG
Given the importance of the theta rhythm in the scoring of sleep EEG (particularly, in the differentiation between NREM and REM sleep), we assessed the EEG spectra of the individual vigilance states – NREM sleep, REM sleep, and wake – during the sustained withdrawal week. In other studies, increased power in the delta and theta power of sleep spectra have been attributed to increased sleep intensity (Borbély et al., 1981; Finelli et al., 2000) while decreased power has been correlated with decreased quality of sleep in chronic insomnia (Merica, Blois, & Gaillard, 1998).
We found that absolute spectral power was significantly increased in the high delta and low theta ranges of both the NREM and REM sleep spectra of ETX-treated animals, as compared to saline-treated controls during sustained withdrawal. In the NREM spectra (Figure 4A), power was significantly increased in the 4–6Hz range (p < 0.05, permutation test of difference in means). In the REM spectra (Figure 4B), ETX-treated animals exhibited significantly higher power in the 4–6Hz range (p < 0.05) but with increases in the 1–4Hz range (p = 0.0651 for 1–2Hz, p = 0.635 for 2–3Hz, p = 0.683 for 3–4Hz) and the 6–7Hz band (p = 0.0714) that did not reach significance. No significant differences were observed between groups in the wake EEG spectra (p > 0.05 for all 1-Hz frequency bands; see Figure 4C). In a separate experiment, the effects of ETX administration were compared to the effects of saline in a group of ethanol-naïve, air-exposed animals (n = 4 in each group). No significant effects in the NREM sleep or wake spectra were observed in the measure of relative theta in the air-exposed animals (p > 0.05 for all 1-Hz frequency bands; see Figure 5A, C). However, significant increases in the 12–13Hz and 18–19Hz bands (p < 0.05 for both) were detected in the REM spectra of ETX-treated, air-exposed animals (Figure 5B).
Figure 4. Ethosuximide increases spectral power in the high delta and low theta bands of the sleep EEG after ethanol withdrawal.
Average power across all epochs scored as NREM sleep (A), REM sleep (B), or Wake (C) is plotted against frequency (1-Hz bands from 0.5–25 Hz) for each treatment group during the sustained WD Week. (A) Spectral power in the 4–6 Hz range (high-delta to low-theta band) of the NREM sleep EEG is significantly increased in the ETX group, as compared to saline-treated control. (B) Spectral power in the 4–6 Hz range of the REM sleep EEG is significantly increased in the ETX group. (C) No significant differences were detected between the wake EEG spectra of the two groups. One animal from the saline group was excluded from the analysis as an outlier as it demonstrated spectral power an order of magnitude greater than that of all other animals across all frequency bands analyzed in the spectrum. Data are presented as mean ± SD. * p < 0.05, permutation test
Figure 5. Ethosuximide minimally affects the REM sleep EEG spectra in air-exposed mice.
Average power across all epochs scored as NREM sleep (A), REM sleep (B), or Wake (C) is plotted against frequency (1-Hz bands from 0.5–25 Hz) in a cohort of ethanol-naïve animals who received either ethosuximide or saline treatment. No significant differences were detected at any frequency for the NREM sleep and Wake spectra (p > 0.05 for all comparisons, permutation test). Spectral power in the 12–13Hz and 18–19Hz bands was significantly in ETX-treated mice. Data are presented as mean ± SD. * p < 0.05, permutation test
Discussion
In this study, we have demonstrated ethanol-mediated disruptions in both delta and theta diurnal rhythms that persist into withdrawal. These disrupted patterns could be ameliorated with ethosuximide (ETX), a purported T-type Ca2+ channel blocker. In addition, we observed significant ethanol-induced increases in relative delta power and significant decreases in relative alpha power that continued into the sustained withdrawal week that could be restored to baseline levels with ETX. Furthermore, when the absolute power spectra were aggregated according to sleep score, ETX increased power in the delta and theta bands of both the NREM and REM sleep spectra, without any effect on the wake spectrum. The significant ethanol-induced reductions in the higher frequency sigma and beta bands also persisted into withdrawal; however, ETX had no effect on the relative power of these frequencies ranges. Our results suggest that pharmacologically targeting alterations in rhythmic brain patterns that persist beyond the acute withdrawal window could be effective treatments in managing protracted withdrawal symptoms such as sleep disturbances. These types of interventions would be particularly beneficial as adjunctive therapies for treatment-seeking alcoholics since disruptions in sleep have been shown to be effective predictors of relapse (Bauer, 2001; Brower, 2001; Brower and Perron, 2009).
Abnormalities in the EEG of alcoholics have been well documented (for review, see Porjesz and Begleiter, 2003). The most consistent EEG deficiency found among alcoholics has been reduced sensory-evoked or event-related potentials (ERPs). Several studies have shown that the P300, a particular ERP that is believed to reflect the cognitive processing of a sensory stimulus rather than the stimuli’s physical features, is significantly attenuated in alcoholics (Cohen et al., 1997; Rodriguez Holguin et al., 1999). However, studies of resting spectral power have yielded conflicting results, with some showing increases in certain EEG power bands (Rangaswamy et al., 2003, 2005; Feige et al., 2007) and others reporting decreases (Saletu-Zyhlarz et al., 2004; Coutin-Churchman et al., 2006). In general, it appears that alcoholics exhibit both abnormal resting and evoked EEG signals as compared to control subjects.
In contrast to the abundance of investigations of EEG alterations in human alcoholics, there have been considerably fewer studies on chronic ethanol’s effects on EEG bands in rodents. Ehlers and Slawecki (2000) reported that rats chronically exposed to ethanol in a vapor chamber demonstrated decreased spectral power in the delta, theta, alpha and beta bands during a four-hour recording period immediately following six weeks of multiple, intermittent exposures. In addition, they showed that the significant decreases in power remained five weeks later for both the delta and theta band, suggesting alterations in low-frequency EEG power can persist for a prolonged period during withdrawal. Kubota et al. (2002) also reported an overall decrease in spectral power for bands within the 2–25Hz frequency range in rats fed a three-week liquid ethanol diet, however this reduction was no longer apparent one week later. The authors of the latter study attributed this discrepancy to differences in administration methods (ethanol vapor versus liquid diet) and length of exposure (six weeks versus three weeks). In the present study, we did not find any significant differences in absolute power spectra values during our ethanol exposure and withdrawal paradigm until we segregated the data by sleep score. However, we did observe overall shifts in the relative proportion of power among bands that favored increases in lower frequencies and decreases in higher frequency bands. In addition, our results showing that ethanol-induced alterations in delta and theta EEG rhythms continue into the sustained withdrawal week are consistent with the results of Ehlers and Slawecki (2000), which suggested that disruptions in the lower frequency bands are long lasting.
In this study, we treated ethanol exposed animals with a T-type channel blocker, ETX. ETX is a first-choice antiepileptic drug (AED) in the treatment of absence epilepsy, a non-convulsive seizure disorder characterized by generalized 3Hz spike-and-wave discharges (SWDs) recorded on the EEG (for review, see Hughes, 2009). ETX has been shown to inhibit low-threshold T-type Ca2+ currents in both the thalamic ventrobasal nucleus (Coulter et al., 1989) and thalamic reticular nucleus of the rat (Huguenard and Prince, 1994). It is commonly held that ETX produces its antiepileptic effects by reducing T-type channel-mediated thalamic bursts, thereby disrupting the synchronized oscillations within this circuit that underlie SWDs. While some controversy has arisen over the specificity of ETX in blocking T-type currents (see Crunelli and Leresche, 2002), it has been suggested that ETX preferentially inhibits a T-type window current (Gomora et al., 2001) that arises from the interaction of the voltage-dependent activation and inactivation properties of whole-cell T-type current (Hughes et al., 1999; Crunelli et al., 2005).
Previously described experiments from our lab (Graef et al., 2011) demonstrated a progressive, functional upregulation of midline thalamic T-type channels, including alterations in steady-state properties that occurred in parallel with progressive alterations in the theta EEG band during chronic ethanol exposure and withdrawal. It was also noted in that study that disruptions in thalamocortical circuitry can give rise to disruptions in normal thalamocortical rhythms, which have been shown to display a significant increase in medial thalamic T-type channel-mediated bursts, as well as augmented power and thalamocortical coherence in the theta (4–9Hz) band range (Jeanmonod et al. 1996; Llinas et al. 1999; Sarnthein and Jeanmonod 2007, 2008). In addition, increases in resting EEG theta coherence have been reported in alcoholics that were also suggested to arise from altered thalamocortical function (Porjesz and Rangaswamy, 2007). Considering that the persistent, ethanol-mediated alterations in theta rhythms we observed during the sustained withdrawal week could be ameliorated with ETX, it is possible that ETX is exerting its effects by inhibiting this functional increase in midline thalamic T-type channels, thereby restoring normal neural rhythms. Furthermore, it is possible that this effect of ETX is limited to sleep, when T-type channels are most activated, given the increase in sleep intensity observed in NREM and REM sleep spectra, but not the wake spectrum of ETX-treated animals. This beneficial effect of ETX would be in partial agreement with an earlier study where ETX was able to reduced withdrawal signs in ethanol-dependent mice (Kaneto et al., 1986). It is also interesting to note that in addition to its antiepileptic effects, ETX has been shown to be effective in other models of thalamocortical dysrhythmias, including neuropathic pain (Dogrul et al., 2003), Parkinson’s disease (Gomez-Mancilla et al, 1992), and depression (Shaw et al., 2009).
Caution should be used in interpreting the role of thalamic T-type calcium channels from these studies. In our study, ETX was administered systemically and T-type channels are diffusely expressed throughout the nervous system (Talley et al., 1999; Perez-Reyes, 2003). Few studies have characterized the interaction between ethanol and T-type channel expression and function outside of the thalamus (Nordskog et al., 2006; Newton et al., 2008). Additionally, despite known effects on T-type calcium channels (Gomora et al., 2001; Huguenard, 1996; Todorovic & Lingle, 1998), ETX has been suggested to also affect other currents, such as the persistent Na+ current and Ca2+-dependent K+ currents (Leresche et al., 1998; Crunelli and Leresche, 2002). It is important to note that a decrease in T current-mediated burst firing was observed in the latter study. However, both of these studies were carried out at high concentrations of ETX, well above what is considered to be the physiologically relevant range. A separate study of the effects of low concentration ETX confirmed blockade of T-type calcium current at physiologically-relevant concentrations (Gomora et al., 2001). While no studies have investigated the effects of ethanol on persistent Na+ currents, other studies have demonstrated significant modulation of both small (SK) and large (BK) Ca2+-dependent K+ channels during both acute and chronic ethanol exposure (Brodie et al., 2007). Additionally, G-protein mediated inwardly-rectifying K+ (GIRK) channels are also inhibited by ETX, where it has been shown to attenuate the ethanol-induced potentiation of GIRK currents; however, the concentration of ETX used in this study was well above typical therapeutic concentrations (Kobayashi et al., 2009). Future investigations involving localized application of more specific T-type channel inhibitors will clarify the exact role of T-type channels in the ethanol-mediated disruption of EEG rhythms.
In this study, we demonstrated chronic ethanol-induced disruption in both delta and theta EEG rhythms important for normal sleep, as well as relative delta and alpha power, which persisted into the sustained withdrawal week. These effects could be ameliorated with ETX, suggesting a role for T-type Ca2+ channels in underlying these chronic ethanol effects. Since sleep disturbances during withdrawal have been implicated as potential cause for relapse, it will be important to determine if the increased sleep intensity we observed with ETX treatment translates into the restoration of disrupted sleep patterns and sleep-related symptoms associated with protracted withdrawal from chronic ethanol exposure. Moreover, the effects of alcohol withdrawal are complex and these same observations could have implications for other pathology resulting from hyperexcitability in this network, such as alcohol withdrawal-related seizure.
Acknowledgments
Support: F31AA017048, F30AA020159, F32AA017041, T32AA7565, R21EY018159, R01AA016852, R01AA015568, Citizens United for Research on Epilepsy, and the Tab Williams Family Fund.
References
- Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25:332–340. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–495. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Scholz A, Weiger TM, Dopico AM. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res. 2007;31:1625–1632. doi: 10.1111/j.1530-0277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 2009 doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997;21:1398–1406. [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons. Neurosci Lett. 1989;98:74–78. doi: 10.1016/0304-3940(89)90376-5. [DOI] [PubMed] [Google Scholar]
- Coutin-Churchman P, Moreno R, Anez Y, Vergara F. Clinical correlates of quantitative EEG alterations in alcoholic patients. Clin Neurophysiol. 2006;117:740–751. doi: 10.1016/j.clinph.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Block of Thalamic T-Type Ca(2+) Channels by Ethosuximide Is Not the Whole Story. Epilepsy Curr. 2002;2:53–56. doi: 10.1046/j.1535-7597.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Toth TI, Cope DW, Blethyn K, Hughes SW. The ‘window’ T-type calcium current in brain dynamics of different behavioural states. J Physiol. 2005;562:121–129. doi: 10.1113/jphysiol.2004.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105:159–168. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Feige B, Scaal S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31:19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Rose JS, Swift R, Stout RL, Millman RP, Stein MD. Trazodone for sleep disturbance after alcohol detoxification: a double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2008;32:1652–1660. doi: 10.1111/j.1530-0277.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. An animal model for testing effects of drugs on alcohol withdrawal reactions. J Pharmacol Exp Ther. 1972;183:14–22. [PubMed] [Google Scholar]
- Gomez-Mancilla B, Latulippe JF, Boucher R, Bedard PJ. Effect of ethosuximide on rest tremor in the MPTP monkey model. Mov Disord. 1992;7:137–141. doi: 10.1002/mds.870070207. [DOI] [PubMed] [Google Scholar]
- Gomora JC, Daud AN, Weiergraber M, Perez-Reyes E. Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Mol Pharmacol. 2001;60:1121–1132. [PubMed] [Google Scholar]
- Graef JD, Huitt TW, Nordskog BK, Hammarback JH, Godwin DW. Disrupted T-type Ca2+ channel expression and function during ethanol exposure and withdrawal. J Neurophysiol. 2011;105:528–40. doi: 10.1152/jn.00424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav. 2009;15:404–412. doi: 10.1016/j.yebeh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Toth TI, Williams SR, Crunelli V. All thalamocortical neurones possess a T-type Ca2+ ‘window’ current that enables the expression of bistability-mediated activities. J Physiol. 1999;517 (Pt 3):805–815. doi: 10.1111/j.1469-7793.1999.0805s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119 (Pt 2):363–375. doi: 10.1093/brain/119.2.363. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kawatani S, Kaneda H. Differentiation of alcohol and barbital physical dependence. Yakubutsu Seishin Kodo. 1986;6:267–273. [PubMed] [Google Scholar]
- Kobayashi T, Hirai H, Iino M, Fuse I, Mitsumura K, Washiyama K, Kasai S, Ikeda K. Inhibitory effects of the antiepileptic drug ethosuximide on G protein-activated inwardly rectifying K+ channels. Neuropharmacology. 2009;56:499–506. doi: 10.1016/j.neuropharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Leresche N, Parri HR, Erdemli G, Guyon A, Turner JP, Williams SR, Asprodini E, Crunelli V. On the action of the anti-absence drug ethosuximide in the rat and cat thalamus. J Neurosci. 1998;18:4842–4853. doi: 10.1523/JNEUROSCI.18-13-04842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard J-M. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Todorovic SM, Perez-Reyes E. The role of T-type calcium channels in epilepsy and pain. Curr Pharm Des. 2006;12:2189–2197. doi: 10.2174/138161206777585184. [DOI] [PubMed] [Google Scholar]
- Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6(4):305–23. [PubMed] [Google Scholar]
- Newton PM, Zeng L, Wang V, Connolly J, Wallace MJ, Kim C, Shin HS, Belardetti F, Snutch TP, Messing RO. A blocker of N- and T-type voltage-gated calcium channels attenuates ethanol-induced intoxication, place preference, self-administration, and reinstatement. J Neurosci. 2008;28(45):11712–9. doi: 10.1523/JNEUROSCI.3621-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordskog BK, Hammarback JA, Godwin DW. Diurnal gene expression patterns of T-type calcium channels and their modulation by ethanol. Neuroscience. 2006;141:1365–1373. doi: 10.1016/j.neuroscience.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27:153–160. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Scientific World Journal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Rohrbaugh J, O’Connor S, Kuperman S, Reich T, Begleiter H. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003;27:607–615. doi: 10.1097/01.ALC.0000060523.95470.8F. [DOI] [PubMed] [Google Scholar]
- Rodriguez HS, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcohol Clin Exp Res. 1999;23:582–591. [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, Boning J, Saletu B. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol. 2004;39:233–240. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with Parkinson’s disease. J Neurosci. 2007;27:124–131. doi: 10.1523/JNEUROSCI.2411-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with neurogenic pain. Neuroimage. 2008;39:1910–1917. doi: 10.1016/j.neuroimage.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Shaw FZ, Chuang SH, Shieh KR, Wang YJ. Depression- and anxiety-like behaviors of a rat model with absence epileptic discharges. Neuroscience. 2009;160:382–393. doi: 10.1016/j.neuroscience.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28:317–324. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic S, Lingle C. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol. 1998;79:240–52. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30:1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]