Abstract
AIM: To evaluate the predictors of 10-year survival of patients with hepatitis C recurrence.
METHODS: Data from 358 patients transplanted between 1989 and 2010 in two Italian transplant centers and with evidence of hepatitis C recurrence were analyzed. A χ2, Fisher’s exact test and Kruskal Wallis’ test were used for categorical and continuous variables, respectively. Survival analysis was performed at 10 years after transplant using the Kaplan-Meier method, and a log-rank test was used to compare groups. A P level less than 0.05 was considered significant for all tests. Multivariate analysis of the predictive role of different variables on 10-year survival was performed by a stepwise Cox logistic regression.
RESULTS: The ten-year survival of the entire population was 61.2%. Five groups of patients were identified according to the virological response or lack of a response to antiviral treatment and, among those who were not treated, according to the clinical status (mild hepatitis C recurrence, “too sick to be treated” and patients with comorbidities contraindicating the treatment). While the 10-year survival of treated and untreated patients was not different (59.1% vs 64.7%, P = 0.192), patients with a sustained virological response had a higher 10-year survival rate than both the “non-responders” (84.7% vs 39.8%, P < 0.0001) and too sick to be treated (84.7% vs 0%, P < 0.0001). Sustained virological responders had a survival rate comparable to patients untreated with mild recurrence (84.7% vs 89.3%). A sustained virological response and young donor age were independent predictors of 10-year survival.
CONCLUSION: Sustained virological response significantly increased long-term survival. Awaiting the interferon-free regimen global availability, antiviral treatment might be questionable in selected subjects with mild hepatitis C recurrence.
Keywords: Hepatitis C, Liver transplantation, Hepatitis C virus recurrence, Antiviral treatment, Ten-year survival
Core tip: The recurrence of hepatitis C virus (HCV) infection after liver transplantation is still a great clinical challenge. Currently, the treatment opportunities are growing with the development of new antivirals; however, in several countries, their availability will not be immediate. The decision to start treatment for HCV recurrence might be difficult in some cases, and the data on the long-term impact are extremely useful in this setting. This study reports the results of 10-year survival analysis on an Italian cohort of liver transplant cases focusing on the differences in outcomes, not only between the treated and not-treated subjects but also in specific subgroups of patients with mild recurrence and those considered too sick to be treated.
INTRODUCTION
End stage liver disease (ESLD) due to hepatitis C virus (HCV) infection is the leading indication for liver transplantation (LT) in Europe and in the United States[1]. The lack of an effective prophylaxis makes the recurrence of the graft virtually universal and chronic hepatitis is found at liver biopsy in the vast majority of cases within one year after LT[2]. Recurrent liver disease is much more aggressive after a LT with respect to the pre-LT period, as progression to cirrhosis at 5 years occurs in 10%-50% of the recipients[2-4]. The efficacy of antiviral therapy with interferon-α (IFN-α), either pegylated (Peg) or not, and ribavirin (RBV) is lower in comparison to non-transplant patients. Moreover, significant concerns remain about potential serious adverse events in the post-LT period, including the risk of rejection[5,6]. Notably, indications for antiviral therapy have changed over the years. In the past, when only non-pegylated interferon was available and the data regarding its efficacy were very limited, antiviral therapy was started when there was evidence of disease progression (increasing fibrosis) at repeated liver biopsies[7]. More recently, after the introduction of pegylated interferon, antiviral therapy was initiated at an earlier stage, when active hepatitis was found at first year liver biopsy[7]. Although widely accepted guidelines for antiviral therapy in a LT setting do not exist[6], in every day practice, the vast majority of patients with HCV-recurrence experience at least one attempt of antiviral therapy. This purposeful approach is likely to become increasingly adopted by currently practicing clinicians according to the availability of shorter and better tolerated antiviral regimens.
By transposing the clinical end points of the antiviral treatment in the immunocompetent patient, published studies[8-10] have focused their attention on the identification of predictors of a virological response in the post-LT phase. However, very interesting studies[7,11-16] attempted to go deeper into the problem and to understand whether antiviral treatment could really change the long-term survival of recipients with HCV-recurrence. In particular, Bizollon et al[14] and Picciotto et al[15] reported the positive role of a sustained virological response (SVR) achievement in the post-LT period while Veldt et al[16] demonstrated that antiviral treatment itself was able to improve overall graft survival of treated patients in respect to the untreated.
In this complex context, direct acting antivirals (DAAs) represent a new era in HCV infection considering the excellent virological response rates. Triple regimens including PegIFN/RBV combined with first generation NS3/4A protease inhibitors (PIs) boceprevir (BOC) and telaprevir (TVR) were proposed, with an increased virological response rate but also with a high rate of adverse events. The second and third wave DAAs comprise new NS3/4A PIs, NS5A inhibitors, and nucleotide and non-nucleotide NS5B polymerase-inhibitors[17]. Among the latter, studies on the treatment of HCV recurrence are producing excellent results[18,19]. Nevertheless, the broad availability of these potent antiviral agents will raise important cost issues, especially, but not only, in developing countries. Thus, the careful identification of predictive factors of long-term overall efficacy is currently required.
In this study, we analyzed the clinical records of transplanted patients with HCV-recurrence followed up by two major Italian Tertiary Hospitals. The aim of this study was to evaluate the determinants of 10-year survival for patients with HCV recurrence and the differences between treated and untreated patients.
MATERIALS AND METHODS
The study population comprised 358 LT patients with established HCV-recurrence followed at the Hepatology Outpatient Clinic of Semeiotica Medica Unit, S. Orsola-Malpighi Hospital, University of Bologna, Italy and at the Department of Hepatology and Gastroenterology, Niguarda Hospital, Milan, Italy.
Patients were transplanted between January 1989 and December 2010, and most (73.5%) were at the Liver Transplant Centre in Milan. Study population characteristics are shown in Table 1.
Table 1.
Study population characteristics n (%)
| n | 358 |
| Gender (M/F) | 281 (78.5)/77 (21.5) |
| Age at LT (yr, mean ± SD) | 52 ± 9 |
| Donor age (yr, mean ± SD) | 53 ± 17 |
| HCV Genotype | |
| 1 | 212 (59.2) |
| 2 | 48 (13.4) |
| 3 | 57 (15.9) |
| 4 | 41 (11.5) |
| Immunosuppression | |
| Cyclosporine | 269 (75.1) |
| Tacrolimus | 83 (23.2) |
| Other regimens | 6 (1.7) |
| Antiviral therapy after LT | |
| Treated | 150 (41.9) |
| Untreated | 208 (58.1) |
HCV: Hepatitis C virus; LT: Liver transplantation.
HCV-recurrence after LT was defined in all cases by the positivity (> 50 IU/mL) of serum HCV-RNA and histological evidence of hepatitis at liver biopsy. Liver biopsies were evaluated by experienced pathologists using Ishak’s scoring system[20].
For patients who underwent antiviral therapy, treatment consisted of IFN-α (either Peg- or not) in association with RBV in all cases with an intended duration of 48 wk of treatment. The type of virological response to treatment was established as a SVR or non-response (NR). SVR was defined as undetectable serum HCV-RNA 24 wk after discontinuation of treatment. The NR group included all non-SVR patients.
Statistical analysis
All sample data were encoded by a physician trained in statistics and data were included in a dedicated database.
Full descriptive statistical analysis was carried out on all evaluated parameters. Data are expressed as the mean ± SD or as median and range, where more appropriate and as indicated. Confidence intervals (CI) are presented whenever appropriate. The significance of differences between variables was calculated with nonparametric tests. A χ2/Fisher’s exact test was used for categorical variables. A Kruskal Wallis’ test was used for continuous variables.
Survival analysis was performed at 10 years after LT using the Kaplan-Meier method, and a log-rank test was used to compare groups. A P level less than 0.05 was considered significant for all tests. Multivariate analysis of the predictive role of different variables on 10-year survival was performed by stepwise Cox logistic regression (variables were entered if P < 0.1 and were removed if P > 0.05). SPSS® software version 17.0 (MJ Norusis, Chicago, United States) was used to perform all statistics.
RESULTS
The 10-year cumulative survival of all patients included in the study was 61.2% (Figure 1A). In the post-LT period, 150 patients (41.9%) were treated with antiviral therapy while 208 were not. Treated and untreated patients had a similar mean age (52 ± 8 years vs 52 ± 9 years, P = NS) and were also comparable for gender, donor age and viral genotype. Regarding the type of immunosuppression, cyclosporine was administered more frequently among untreated patients (Table 2).
Figure 1.
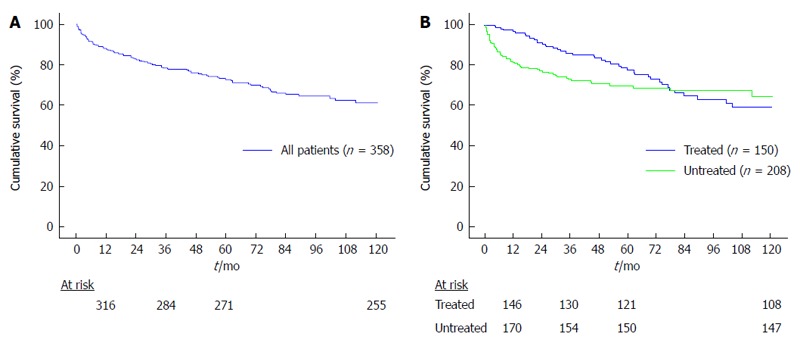
Kaplan-Meyer survival analysis of the entire population (A) and the treated and untreated patients (B).
Table 2.
Characteristics of treated and untreated patients n (%)
| Treated | Untreated | χ2/Mann Whitney-U | |
| n | 150 | 208 | |
| Gender (M/F) | 120/30 | 161/47 | P = NS |
| Age at LT (yr, mean ± SD) | 52 ± 8 | 52 ± 9 | P = NS |
| Donor age (yr, mean ± SD) | 53 ± 17 | 53 ± 17 | P = NS |
| HCV genotype | P = NS | ||
| 1 | 97 (64.7) | 115 (55.3) | |
| 2 | 16 (10.7) | 35 (16.8) | |
| 3 | 25 (16.6) | 35 (16.8) | |
| 4 | 12 (8.0) | 23 (11.1) | |
| Immunosuppression | P = 0.011 | ||
| Cyclosporine | 102 (68.0) | 165 (79.3) | |
| Tacrolimus | 46 (30.7) | 39 (18.8) | |
| Other regimens | 2 (1.3) | 4 (1.9) |
HCV: Hepatitis C virus; NS: Not significant.
Sixty-three of 150 treated patients (42%) achieved a SVR.
NR patients had to reduce more frequently the dosage of antiviral therapy in comparison to patients achieving a SVR (72.4% vs 38.1%), neutron/thrombocytopenia being the main cause of a decrease in both the NR and SVR groups (74.6% vs 83.3%, respectively).
In the intention to treat analysis, the 10-year cumulative survival of treated and untreated patients was not significantly different (59.1% vs 64.7%, P = 0.192; Figure 1B). However, when analyzing the survival functions (Figure 1B), it can be noticed that curves were somehow irregular, especially those representing the survival of untreated patients. This latter curve clearly shows a rapid slope in its first section because some patients were deceased quite early after LT. Indeed, we stratified the study population according to the type of response to antiviral therapy (SVR vs NR) and, for patients who were not treated, according to their clinical status. Thus, we identified 5 different groups of patients: Group A: patients receiving antiviral therapy who achieved a SVR (n = 63); Group B: patients receiving antiviral therapy who were NR (n = 87); Group C: patients untreated with mild recurrence (n = 73); Group D: patients too sick to be treated (n = 35); Group E: patients with clinically relevant comorbidities that contraindicated antiviral therapy (n = 100).
Patients were considered to have a mild recurrence (group C) in the case of a mild transaminase increase (alanine amino transferase < 3x the upper normal limit) and mild fibrosis (Ishak stage < 3) at the first post-LT liver biopsy performed within three years after LT. By definition, these subjects did not show graft malfunction or early complications after LT.
Patients were included in the too sick to be treated group when they were not suitable for antiviral treatment because of graft malfunction and/or early complications after LT.
Patients achieving a SVR showed the best survival time (mean 73 ± 35 mo, median 70, range: 13-120 mo), followed by subjects with mild recurrence (mean: 71 ± 37 mo, median 64, range: 7-120 mo), patients who were NR (mean 57 ± 34 mo, median 52, range: 4-120 mo), patients not treated for comorbidities (mean 48 ± 32 mo, median 36, range: 4-120 mo) and finally, as expected, patients too sick to be treated (mean 4 ± 3 mo, median 2, range: 0-11 mo).
The survival curves of the five groups are reported in Figure 2. Patients who achieved a SVR (group A) had a significantly higher 10-year survival than the NR group (group B; 84.7% vs 39.8%, respectively, P < 0.0001). Conversely, SVR patients (group A) had a 10-year survival rate comparable to that observed in untreated patients with mild recurrence (group C; 84.7% vs 89.3%, respectively, P = 0.639). As expected, patients with the worst prognosis were those included in group D (too sick to be treated).
Figure 2.
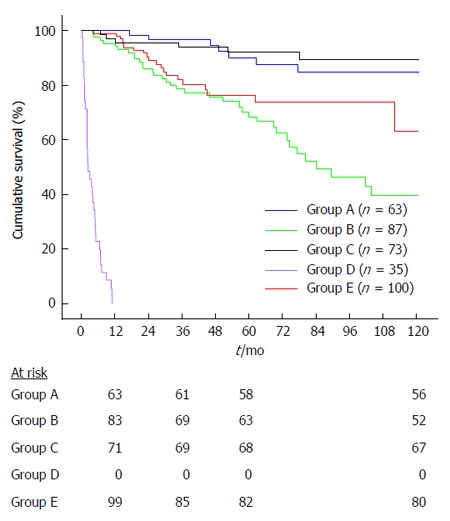
Kaplan-Meyer survival analysis by groups. Group A: patients receiving antiviral therapy who achieved a SVR; Group B: patients receiving antiviral therapy who were NR; Group C: patients untreated with mild recurrence; Group D: patients too sick to be treated; Group E: patients with clinically relevant comorbidities that contraindicated antiviral therapy. SVR: Sustained virological response; NR: Non-response.
The most relevant clinical features of the five proposed groups are reported in Table 3.
Table 3.
Characteristics of patients divided by groups
| Group | A | B | C | D | E | χ2/Kruskall Wallis |
| SVR | NR | Mild recurrence | Too sick | Comorbidity | ||
| n | 63 | 87 | 73 | 35 | 100 | |
| Gender (M/F) | 47/16 | 73/14 | 63/10 | 20/15 | 78/22 | P = 0.007 |
| Age at LT (yr, mean ± SD) | 52 ± 8 | 52 ± 9 | 53 ± 8 | 52 ± 9 | 51 ± 9 | P = NS |
| Donor age (yr, mean ± SD) | 49 ± 17 | 56 ± 15 | 48 ± 17 | 59 ± 15 | 55 ± 17 | P = 0.001 |
| HCV genotype | P = NS | |||||
| 1 | 37 | 60 | 45 | 16 | 44 | |
| 2 | 7 | 6 | 9 | 3 | 13 | |
| 3 | 9 | 13 | 6 | 5 | 14 | |
| 4 | 8 | 5 | 7 | 1 | 10 | |
| Immunosuppression | 48 | 55 | 54 | 24 | 83 | P = NS |
| Cyclosporine | 14 | 31 | 16 | 10 | 13 | |
| Tacrolimus | 1 | 1 | 3 | 1 | 4 | |
| Other regimens | ||||||
| Time between treatment start and LT [mo, median (min-max)] | 20 (1-148) | 16 (1-117) | P = NS | |||
| Treatment Length [mo, median (min-max)] | 11 (1-61) | 6 (1-63) | P = 0.010 |
SVR: Sustained virological response; NR: Non-response; HCV: Hepatitis C virus; NS: Not significant; LT: Liver transplantation.
The groups were comparable for mean age at the time of LT and HCV-genotype. Patients who obtained a SVR (group A) and those who were not treated because of mild recurrence (group C) had a significantly younger donor age when compared to patients who were not treated because they were too sick (group D). In keeping with the available literature showing that cyclosporine may favor a response to anti-viral therapy[21], our patients achieving a SVR were more frequently on cyclosporine (76.19%) than those who did not achieve a SVR (63.21%) although there was no statistically significant difference. As expected, our SVR patients (group A) were treated longer than patients who were NR (group B).
Going deeper into the analysis of patients with a mild recurrence (group C), we further identified a subgroup of 44 patients with mild recurrence and without any relevant comorbidity. Indeed, the results in terms of 10-year survival of this subgroup were the best among the considered groups (95.1%).
Causes of death
Main causes of death are reported in Table 4. Among the 63 patients who achieved a SVR (group A), 7 (11.1%) died within 10 years after LT, but only 2 (28.6%) died because of a liver-related cause. Among the 87 NR patients (group B), 35 (40.2%) died within 10 years after LT and, notably, 22 (62.9%) for a liver-related cause, which was mainly HCV-recurrence (17/22, 77.3%). Of the 73 patients with a mild recurrence (group C), only 6 (8.2%) died within 10 years after LT, but only 3 (50.0%) for liver-related reasons, with 2 of these 3 (66.7%) dying because of HCV-recurrence. All 35 patients who were not treated because they were too sick to be treated (group D) died within 1 year after LT, with the main causes not being liver-related (24/35, 68.6%) and but linked to infections (10/24, 41.7%). Finally, of the 100 patients with relevant comorbidities (group E), 20 (20%) died within 10 years after LT, with half dying because of liver-related causes (10/20, 50%), mainly because of hepatocellular carcinoma recurrence (8/10, 80%).
Table 4.
Causes of death
| Group | A | B | C | D | E |
| SVR | NR | Mild recurrence | Too sick | Comorbidity | |
| n | 63 | 87 | 73 | 35 | 100 |
| No. of deaths at 10 yr after LT, n (%) | 7 (11.1) | 35 (40.2) | 6 (8.2) | 35 (100) | 20 (20) |
| Liver related, n (%) | 2 (28.6) | 22 (62.9) | 3 (50) | 11 (31.4) | 10 (50) |
| HCV recurrence | 1 | 17 | 2 | 3 | 1 |
| HCC | 1 | 4 | 1 | 1 | 8 |
| Primary non function | 0 | 0 | 0 | 6 | 0 |
| Other liver-related | 0 | 1 | 0 | 1 | 1 |
| Non liver-related, n (%) | 5 (71.4) | 13 (37.1) | 3 (50) | 24 (68.6) | 10 (50) |
| Cardiac | 1 | 0 | 0 | 0 | 0 |
| Renal | 0 | 2 | 0 | 0 | 0 |
| Cancer | 0 | 1 | 0 | 3 | 6 |
| Infection | 0 | 6 | 1 | 10 | 3 |
| Vascular | 0 | 0 | 1 | 0 | 0 |
| Other comorbidity | 4 | 4 | 1 | 11 | 1 |
SVR: Sustained virological response; HCC: Hepatocellular carcinoma; NR: Non-response; LT: Liver transplantation.
Multivariate analysis
All the following variables were tested in a univariate analysis: gender, HCV genotype (1 and 4 vs others), type of immunosuppression (cyclosporine vs tacrolimus), donor age (< 53 years vs ≥ 53 years), recipient age (< 52 years vs ≥ 52 years), treatment vs no-treatment and SVR vs NR. The cut-off values of 53 years and 52 years were chosen as the means of both donor and recipient ages, respectively. Only having a SVR and a younger donor age showed a prognostic value (P < 0.1) in the univariate analysis.
In the multivariate analysis, a SVR and donor age < 53 years were confirmed to be independent predictors of 10-year survival (OR = 4.05, 95%CI: 1.81-9.05, P = 0.001 and OR = 3.05, 95%CI: 1.47-6.30, P = 0.001, respectively).
DISCUSSION
Because LT is agreed to be a life-saving therapy for end-stage liver disease, HCV has become one of its leading indications. Re-infection of the graft is virtually universal and HCV recurrence after LT is a “new disease” whose clinical, virological and immunological characteristics are determined by complex, and almost unknown, interactions between patient, graft and the virus. However, it is well known that fibrosis progression is accelerated in HCV recurrence compared with the pre-LT period and a small proportion of patients (approximately 5%) show a fast and aggressive disease after LT[22]. In this context, it can be very complex to decide whether and when it is appropriate to treat the HCV recurrence.
Berenguer et al[7] compared 89 treated patients with 75 matched untreated controls and, among the treated cases, patients who achieved a SVR with subjects who did not. The authors first demonstrated that all the main measures of outcome (mortality, development of cirrhosis and decompensation) were worse for untreated patients compared to patients who were treated and for NR compared to SVR. Our study confirms the findings of Berenguer et al[7] regarding the positive role of SVR. Moreover, it offers an additional analysis focused on the long-term outcome of the broad and heterogeneous population of untreated patients, which has never been thoroughly investigated.
Our data indicate that the treatment itself was not associated with a better long-term outcome. In fact, we showed that treated and untreated patients had a comparable 10-year survival rate. However, the untreated group comprised a very inhomogeneous population. In particular, the reason for the choice not to start antiviral therapy could also be related to conditions different from a mild disease all having a distinct impact on survival, such as a too severe hepatic condition, a comorbidity (of varying severity) or the lack of compliance.
It should be acknowledged that the design of the study from Berenguer et al[7] was more appropriate as the results emerged from the comparison with a population of matched controls. Indeed, we analyzed a heterogeneous group of untreated patients, which comprised three different subgroups: (1) patients with mild HCV recurrence; (2) patients too sick to be treated; and (3) patients with comorbidities. Interestingly, untreated patients with mild HCV recurrence showed a 10-year survival rate that was comparable to treated patients achieving a SVR (89.3% vs 84.7%, respectively). Notably, patients included in the mild recurrence subgroup had no clinically relevant comorbidities nor graft malfunction and/or complications after LT. In fact, these conditions a priori can affect long-term survival.
Our data confirm that a SVR is an independent predictor of 10-year survival as only two SVR patients of 63 died of an HCV-related cause in the follow-up period. Together with achieving a SVR, our study once again confirms the impact of donor age on the severity of HCV-recurrence, the response to antiviral therapy and survival. In fact, it has been largely reported that older donor age negatively affects the progression of fibrosis in transplanted patients[23-27].
Regarding the treatment of HCV recurrence, it is important to consider the oncoming availability of DAAs. Currently, several reports on first wave PI treatment in the post-LT period are available. Recent studies reporting data on the efficacy and safety of BOC/TVR and PegIFN/RBV[28,29] showed encouraging efficacy results but also a complex side effects profile including infections, hematological and dermatological toxicity, renal failure, diabetes, drug-drug interactions with immunosuppressants and severe forms of plasma-cell hepatitis with occasional fatal outcomes[28-32]. The data on triple BOC/TVR based regimens are of great importance considering that the second/third wave DAA cost issue will continue to produce controversy for a certain amount of time in the future. Sofosbuvir, a pan-genotypic inhibitor of polymerase activity, is reported to have a good virological outcome and favorable safety profile when associated with Daclatasvir[33] or PegIFN ± RBV[18,19,34], and also in the treatment of severe HCV recurrence.
The main limitation of our study is the retrospective design. Nonetheless, this approach is a feasible one to analyze the long-term outcomes of transplanted patients. The most important issue emerging from our data is that the antiviral treatment should be undertaken in patients with moderate-severe disease because of the high risk of progression to recurrent cirrhosis, decompensation and death in the time of a few years[2,3]. Evidence has emerged from our study and many others that clearly indicate that achievement of a SVR may avoid this unfortunate course. On the other hand, our data suggest that patients with mild HCV recurrence have a very favorable long-term outcome even if untreated. Interestingly, a prospective randomized trial conducted in Italy confirms, at least partially, the observations emerging from our retrospective cohort[35]. Notably, it appears mandatory to have, in the first three years after LT, a correct histological classification for patients with HCV recurrence.
Our results can be useful in the complex decision-making process regarding whether and when to start antiviral treatment in LT recipients with HCV recurrence. This is even more important today with the availability of new DAAs, whose inappropriate use can dramatically increase costs, adverse event rates, drug-drug interactions and the risk of a virological resistance outbreak. Finally, the results from our cohort reveal that a certain rate of mortality in post-LT HCV recurrence concerns patients with comorbidities that are often considered as contraindications to antiviral treatment. Along with the availability of antiviral agents with a low-toxicity profile, the limitations related to the patients’ eligibility for the treatment are expected to be reassessed. Furthermore, according to recent compassionate program derived results, the antiviral treatment will become increasingly applicable in the so called too sick to be treated population[18]. However, in those cases in whom a severe HCV recurrence is diagnosed, due to the latter’s rapidly progressive nature, the treatment should be started as soon as possible.
Thus, more information from predictive analyses are necessary at this moment because data focused on the long-term effectiveness of antiviral therapy would help with a more feasible guidelines conception, correct clinical approach and rational cost-effectiveness treatment management in the LT population.
In conclusion, awaiting the consolidation of new interferon-free regimens, we suggest that, in carefully selected patients with predictors of long-term favorable outcomes, antiviral treatment might be delayed. Most likely, the development of interferon-free regimens will completely change the approach to HCV in both pre- and post-LT settings. Nevertheless, studies focusing on the mechanisms and factors leading to a mild HCV recurrence will still be extremely useful.
COMMENTS
Background
End stage liver disease due to hepatitis C is a leading indication for liver transplantation, however, infection recurrence is virtually universal. Accepted guidelines on the best timing for starting antiviral treatment do not exist. New direct antivirals represent a new era in hepatitis C virus (HCV) infection considering both the excellent virological response rates and the problems related to side effects and costs. In this complex context, the correct identification of predictive factors of long-term overall efficacy is required.
Research frontiers
It has already been demonstrated that to treat LT patients with hepatitis C recurrence with antiviral therapy leads to a better survival rate, decreasing the probability to develop cirrhosis and decompensation. Moreover, among treated patients, a sustained virological response (SVR) is a strong predictor of a good outcome. On the other hand, among the positive predictors of survival in transplanted subjects, young donor age is certainly the most consolidated predictor. Few data are available on predictors of long-term survival and outcomes of untreated patients.
Innovations and breakthroughs
This study firstly included a large number of patients evaluated with a ten-year analysis. In addition, this study did not only consider treated patients but also analyzed the outcomes of subjects who, for different reasons, did not receive antiviral therapy.
Applications
The most important conclusion emerging from our results is that antiviral treatment should be given to patients with moderate or severe recurrence because of the high risk of progression to recurrent cirrhosis, decompensation and death in a few years. Authors’ data also showed that patients with mild recurrence have a good long-term outcome even if untreated. These results may be helpful in the decision-making process regarding whether and when to start antiviral therapy.
Terminology
Hepatitis C recurrence after liver transplantation was defined by positivity of serum HCV-RNA (> 50 IU/mL) and histological evidence of hepatitis at liver biopsy. A SVR was defined as undetectable serum HCV-RNA 24 wk after discontinuation of antiviral treatment.
Peer-review
The paper is interesting and has a surprising result. To identify a transplanted patient group who will fare even better without treatment than those with a sustained virological response is an important finding and helps to sharpen the discussion about treatment resources with the advent of efficient but expensive direct-acting antivirals.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 8, 2014
First decision: August 27, 2014
Article in press: November 19, 2014
P- Reviewer: Kanda T, Wong T, Wursthorn K S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009;9:1707–1713. doi: 10.1111/j.1600-6143.2009.02702.x. [DOI] [PubMed] [Google Scholar]
- 2.Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673–684. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 4.Guillouche P, Féray C. Systematic review: anti-viral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011;33:163–174. doi: 10.1111/j.1365-2036.2010.04505.x. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee S, Sorrell MF. Controversies in liver transplantation for hepatitis C. Gastroenterology. 2008;134:1777–1788. doi: 10.1053/j.gastro.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Gurusamy KS, Tsochatzis E, Xirouchakis E, Burroughs AK, Davidson BR. Antiviral therapy for recurrent liver graft infection with hepatitis C virus. Cochrane Database Syst Rev. 2010;(1):CD006803. doi: 10.1002/14651858.CD006803.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Palau A, Aguilera V, Rayón JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679–687. doi: 10.1111/j.1600-6143.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 8.Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, Merli M, Strazzabosco M, Tisone G. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46:1009–1017. doi: 10.1016/j.jhep.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, Riely C, Martin P, Teperman L, Jiao J, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005;41:289–298. doi: 10.1002/hep.20560. [DOI] [PubMed] [Google Scholar]
- 10.Cescon M, Grazi GL, Cucchetti A, Vetrone G, Ravaioli M, Ercolani G, Morelli MC, Piscaglia F, Tamè M, Pinna AD. Predictors of sustained virological response after antiviral treatment for hepatitis C recurrence following liver transplantation. Liver Transpl. 2009;15:782–789. doi: 10.1002/lt.21760. [DOI] [PubMed] [Google Scholar]
- 11.Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, Bosch J, Forns X. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746–1756. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Roche B, Sebagh M, Canfora ML, Antonini T, Roque-Afonso AM, Delvart V, Saliba F, Duclos-Vallee JC, Castaing D, Samuel D. Hepatitis C virus therapy in liver transplant recipients: response predictors, effect on fibrosis progression, and importance of the initial stage of fibrosis. Liver Transpl. 2008;14:1766–1777. doi: 10.1002/lt.21635. [DOI] [PubMed] [Google Scholar]
- 13.Belli LS, Burroughs AK, Burra P, Alberti AB, Samonakis D, Cammà C, De Carlis L, Minola E, Quaglia A, Zavaglia C, et al. Liver transplantation for HCV cirrhosis: improved survival in recent years and increased severity of recurrent disease in female recipients: results of a long term retrospective study. Liver Transpl. 2007;13:733–740. doi: 10.1002/lt.21093. [DOI] [PubMed] [Google Scholar]
- 14.Bizollon T, Pradat P, Mabrut JY, Chevallier M, Adham M, Radenne S, Souquet JC, Ducerf C, Baulieux J, Zoulim F, et al. Benefit of sustained virological response to combination therapy on graft survival of liver transplanted patients with recurrent chronic hepatitis C. Am J Transplant. 2005;5:1909–1913. doi: 10.1111/j.1600-6143.2005.00976.x. [DOI] [PubMed] [Google Scholar]
- 15.Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, Lampasi F, Tartaglione MT, Marsilia GM, Calise F, et al. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46:459–465. doi: 10.1016/j.jhep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, Rosen CB, Heimbach JK, Charlton MR. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426–2433. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 17.Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34 Suppl 1:69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afdhal N, Everson G, Calleja JL, McCaughan G, Symonds WT, Denning J, McHutchinson JG, Arterbum S, Charlton M, Reddy R, et al. Sofosbuvir and ribavirin for the treatment of chronic HCV with cirrhosis and portal hypertension with and without decompensation: early virologic response and safety. J Hepatol. 2014;60:S28. [Google Scholar]
- 19.Samuel D, Charlton M, Gane E, Brown R, Curry M, Kwo P, Fontana R, Gilroy R, Teperman L, Muir AJ, et al. P1232 Sofosbuvir and ribavirin for the treatment of recurrent hepatitis c infection after liver transplantation: results of a prospective, multicenter study. J Hepatol. 2014;60:S499. [Google Scholar]
- 20.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 21.Rendina M, Castellaneta NM, Fagiuoli S, Ponziani F, Vigano R, Iemmolo RM, Donato MF, Toniutto PL, Pasulo L, Morelli MC, et al. Acute and chronic rejection during interferon therapy in HCV recurrent transplant patients: results from AISF-RECOLT-C group. J Hepatol. 2011;54:S230. [Google Scholar]
- 22.Crespo G, Mariño Z, Navasa M, Forns X. Viral hepatitis in liver transplantation. Gastroenterology. 2012;142:1373–1383.e1. doi: 10.1053/j.gastro.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl. 2006;12:1192–1204. doi: 10.1002/lt.20865. [DOI] [PubMed] [Google Scholar]
- 24.Rayhill SC, Wu YM, Katz DA, Voigt MD, Labrecque DR, Kirby PA, Mitros FA, Kalil RS, Miller RA, Stolpen AH, et al. Older donor livers show early severe histological activity, fibrosis, and graft failure after liver transplantation for hepatitis C. Transplantation. 2007;84:331–339. doi: 10.1097/01.tp.0000270313.31328.63. [DOI] [PubMed] [Google Scholar]
- 25.Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549–557. doi: 10.1111/j.1600-6143.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 26.Aguilera V, Ponce M, Berenguer M, Moreno R, Rayón JM, Sanjuán F, Prieto M, Mir J. [Old donors in liver transplantation for chronic hepatitis C] Rev Esp Enferm Dig. 2007;99:581–587. doi: 10.4321/s1130-01082007001000004. [DOI] [PubMed] [Google Scholar]
- 27.Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. doi: 10.1016/j.jhep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, Yataco ML, Satyanarayana R, Rosser BG, Vargas HE, et al. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690–700. doi: 10.1002/lt.23669. [DOI] [PubMed] [Google Scholar]
- 29.Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, Si-Ahmed SN, Guillaud O, Antonini TM, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78–86. doi: 10.1016/j.jhep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Tischer S, Fontana RJ. Drug-drug interactions with oral anti-HCV agents and idiosyncratic hepatotoxicity in the liver transplant setting. J Hepatol. 2014;60:872–884. doi: 10.1016/j.jhep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikegami T, Yoshizumi T, Shirabe K, Maehara Y. Frequent plasma cell hepatitis during telaprevir-based triple therapy for hepatitis C after liver transplantation. J Hepatol. 2014;60:894–896. doi: 10.1016/j.jhep.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 32.Coilly A, Sebagh M, Duclos-Vallée JC. Reply to: “Frequent plasma cell hepatitis during telaprevir-based triple therapy for hepatitis C after liver transplantation”. J Hepatol. 2014;60:896–897. doi: 10.1016/j.jhep.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, Symonds WT. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13:1601–1605. doi: 10.1111/ajt.12209. [DOI] [PubMed] [Google Scholar]
- 34.Kim B, Trivedi A, Thung SN, Grewal P. Case report of successful treatment of fibrosing cholestatic hepatitis C with sofosbuvir and ribavirin after liver transplantation. Semin Liver Dis. 2014;34:108–112. doi: 10.1055/s-0034-1371084. [DOI] [PubMed] [Google Scholar]
- 35.Belli LS, Volpes R, Graziadei I, Fagiuoli S, Starkel P, Burra P, Alberti AB, Gridelli B, Vogel W, Pasulo L, et al. Antiviral therapy and fibrosis progression in patients with mild-moderate hepatitis C recurrence after liver transplantation. A randomized controlled study. Dig Liver Dis. 2012;44:603–609. doi: 10.1016/j.dld.2012.01.017. [DOI] [PubMed] [Google Scholar]


