Abstract
AIM: To identify the factors that differentiate acute hepatitis B (AHB) from chronic hepatitis B with acute exacerbation (CHB-AE).
METHODS: From 2004 to 2013, a total of 82 patients (male n = 52, 63.4%; female n = 30, 36.6%) with clinical features of acute hepatitis with immunoglobulin M antibodies to the hepatitis B core antigen (IgM anti-HBc) were retrospectively enrolled and divided into two groups; AHB (n = 53) and CHB-AE (n = 29). The AHB group was defined as patients without a history of hepatitis B virus (HBV) infection before the episode and with loss of hepatitis B surface antigen within 6 mo after onset of acute hepatitis. Biochemical and virological profiles and the sample/cutoff (S/CO) ratio of IgM anti-HBc were compared to determine the differential diagnostic factors.
RESULTS: The multivariate analysis demonstrated that, the S/CO ratio of IgM anti-HBc and HBV DNA levels were meaningful factors. The S/CO ratio of IgM anti-HBc was significantly higher in the AHB group, while the HBV DNA level was significantly higher in the CHB-AE group. The optimal cutoff values of IgM anti-HBc and HBV DNA levels for differentiating the two conditions were 8 S/CO ratio and 5.5 log10 IU/mL, respectively. The sensitivity and specificity were 96.2% and 89.7% for the S/CO ratio of IgM anti-HBc and 81.1% and 72.4% for HBV DNA levels, respectively. The area under receiver operating characteristic curves of both the S/CO ratio of IgM anti-HBc and HBV DNA levels were not significantly different (0.933 vs 0.844, P = 0.105). When combining IgM anti-HBc and HBV DNA, the diagnostic power significantly improved compared to HBV DNA alone (P = 0.0056). The combination of these factors yielded a sensitivity and specificity of 98.1% and 86.2%, respectively.
CONCLUSION: The combination of the S/CO ratio of IgM anti-HBc and HBV DNA levels was a useful tool for differentiating AHB from CHB-AE in patients with positive IgM anti-HBc.
Keywords: Acute hepatitis, Differential diagnosis, Chronic hepatitis, Hepatitis B virus
Core tip: Distinguishing between acute hepatitis B (AHB) and chronic hepatitis B with acute exacerbation (CHB-AE) is important because of the different prognosis and treatment strategy. However, distinguishing AHB and CHB-AE is difficult due to their similar clinical features and serologic profiles, especially in patients with IgM anti-HBc positivity. This is the first study to differentiate between AHB and CHB-AE in a distinct group of subjects with positive IgM anti-HBc. The quantitative determination of IgM anti-HBc was useful for differentiating AHB from CHB-AE in patients IgM anti-HBc positive. The combination of serum IgM anti-HBc ≥ 8 S/CO ratio with HBV-DNA levels < 5.5 log10 IU/mL could effectively distinguish AHB from CHB-AE.
INTRODUCTION
Chronic Hepatitis B virus (CHB) infection has been an important global health problem. Cirrhosis related complications and/or hepatocellular carcinoma (HCC) are found in 25%-40% of the patients with CHB infection[1]. Thus antiviral therapy is indicated for patients with CHB with acute exacerbation (CHB-AE). When adults with an intact immune system become infected with hepatitis B virus (HBV), they usually recover without antiviral therapy[2], and the risk of developing a CHB infection after acute hepatitis B (AHB) is less than 5% in adults[3]. Nonetheless, AHB is worthy of our attention due to recent public health management, such as HBV outbreak[4]. Considering the different prognoses, treatment strategies, and prevention and public health management strategies for these conditions, correct diagnoses are extremely important. However, it is difficult due to the similar clinical features and serologic profiles[2,5]. Little is known about designing a simple assay to distinguish between these conditions.
Immunoglobulin M antibody to the hepatitis B core antigen (IgM anti-HBc) has been considered as a valuable diagnostic marker of AHB[6-8]. However, 20%-27.5% of CHB-AE patients have an IgM anti-HBc positive result with the fully automated, quantitative analysis method, and these patients could be misclassified as AHB[9]. For this reason, the cut-off index value of IgM anti-HBc was suggested to be set at a higher level for the differentiation of AHB and CHB-AE in Taiwanese patients[10]. Some authors suggested measuring IgM anti-HBc by semi-quantitative assays as a meaningful serological marker to monitor the disease activity of CHB and also to reflect the host’s active immune response[11-13].
In Korea, the prevalence of HBV infection is estimated to be 3.7%[14], it is not rare to encounter CHB-AE patients with positive IgM anti-HBc. This study determined the optimal sample/cutoff (S/CO) ratio of IgM anti-HBc by chemiluminescent immunoassay which is recently available semi-quantitative assay that can identify characteristics to discriminate AHB from IgM anti-HBc positive CHB-AE.
MATERIALS AND METHODS
Patients and study design
A total of 82 patients with positive result for IgM anti-HBc upon clinical presentation of acute hepatitis or AE in CHB were retrospectively analyzed at Hallym University Medical Center, Seoul, Korea, from December 2004 to December 2013. All patients were divided into two groups according to their clinical diagnosis, AHB and CHB-AE.
The AHB group was defined patients with the clinical signs or symptoms suggestive of acute hepatitis without a history of HBV infection prior to this episode and with the loss of hepatitis B surface antigen (HBsAg) within 6 mo after onset of acute hepatitis. AE was defined as serum elevation of alanine aminotransferase (ALT) levels more than 10 times the upper limit of normal. Identified CHB patients with AE and positive IgM anti-HBc were recruited as the control group. Patients with other viral infections (hepatitis A virus, hepatitis C virus, human immunodeficiency virus and hepatitis D virus) or other concomitant liver diseases (alcoholic liver disease and autoimmune liver disease) or a recent history of hepatotoxic drugs including herbal medications or HCC were excluded.
The biochemical and virological profiles were compared between the AHB and CHB-AE groups. Then, the parameters with the greatest differences were selected to assess the diagnostic power for differentiating between AHB and CHB-AE. This study was approved by the Investigation and Ethics Committee for Human Research at the Hallym University Medical Center, Seoul, Korea.
Serum assay methodology
Routine biochemical tests were performed using standard laboratory procedures. HBsAg, antibody to HBsAg (anti-HBs), hepatitis B e antigen (HBeAg), and antibody to HBeAg (anti-HBe) were measured using a microparticle enzyme immunoassay (Abbott Laboratories, North Chicago, IL, United States). Serum HBV DNA levels were measured by the VERANT 3.0 assay (Bayer Healthcare, Tarrytown, NY, United States; lower limit of detection 2000 copies/mL) or COBAS TaqMan PCR assay (Roche, Branchburg, NJ, United States; lower limit of detection 60 copies/mL). IgM anti-HBc was performed using the chemiluminescent immunoassay on the Abbott Architect (Abbot GmbH, Wiesbaden Delkenheim, Germany). There is a direct relationship between the amount of IgM anti-HBc in the sample and chemiluminescent reaction measured as relative light units. The presence or absence of IgM anti-HBc in the sample is determined by comparing the chemiluminescent signal in the reaction to the cutoff signal determined from a previous manufacturer’s IgM anti-HBc calibration. According to the product reference, positivity was defined by an S/CO ratio ≥ 1, and an S/CO ratio between 0.5 and 0.999 was categorized as a “gray zone”. In this study, all patients had an S/CO ratio for IgM anti-HBc ≥ 1.
Statistical analysis
The Mann-Whitney U-test for continuous variables and χ2 test for categorical variables were used for the analyses as appropriate. HBV DNA levels were logarithmically transformed for analysis. Multiple logistic regression analysis was used to identify the independent factors that were significantly associated with AHB. Candidate variables with a P-value of less than 0.05 on a univariate analysis were entered into the regression analysis. A P-value of less than 0.05 was considered significant. The Youden’s index was calculated as an index of sensitivity and specificity. To determine the optimal cutoff value of the variables differentiating AHB from CHB-AE, the receiver operating characteristic (ROC) curves were plotted using all possible cutoff values. The area under ROC (AUROC) curves of identified factors were calculated and compared. The Mann-Whitney U-test, χ2 test and multiple logistic regression analysis were performed with SPSS version 16 (IBM corporation, New York, United States). STATA version 11 (StataCorp, Texas, United States) was used to evaluate if the combination of identified factors could be better than either of these factors alone[15,16].
RESULTS
Comparison of clinical features between AHB and CHB-AE groups
A total 82 patients were enrolled and divided into two groups: AHB (n = 53, 64.6%) and CHB-AE (n = 29, 35.4%). The baseline characteristics of both groups are shown in Table 1. Compared to patients in the CHB-AE group, AHB patients had more severe necroinflammation of the liver, which was characterized by higher levels of serum bilirubin and ALT. The S/CO ratio of IgM anti-HBc were significantly higher in AHB group, while the HBV DNA level was significantly higher in the CHB-AE group. The HBeAg status was measured in 80 patients (51 patients in the AHB group; 29 patients in the CHB-AE group). Although the proportion of HBeAg positive patients was not significantly different between the two groups, the HBeAg titers, as reflected by the S/CO ratio, were significantly higher in the CHB-AE group than in the AHB group (415.7 ± 367.8 vs 49.2 ± 60.9, P = 0.001). The alpha fetoprotein (AFP) test was performed in only 54 patients (Thirty-two patients in the AHB group; 22 patients in the CHB-AE group). The CHB-AE group had higher AFP than the AHB group (133.5 ± 395.7 vs 6.7 ± 6.3, P < 0.001).
Table 1.
Comparison clinical features between acute hepatitis B and chronic hepatitis B with acute exacerbation
| Variables | Total (n = 82) | AHB (n = 53) | CHB-AE (n = 29) | P value |
| Age (yr)1 | 41.9 ± 12.7 | 40.2 ± 13.4 | 45.0 ± 11.0 | 0.023 |
| Sex (male, %) | 52 (63.4%) | 30 (56.6%) | 22 (75.9%) | 0.098 |
| WBC (× 103/μL)1 | 6.0 ± 1.9 | 6.1 ± 2.1 | 5.9 ± 1.6 | 0.808 |
| Hb (g/dL)1 | 14.0 ± 1.7 | 13.9 ± 1.8 | 14.2 ± 1.7 | 0.782 |
| Platelet (× 103/μL)1 | 212.1 ± 84.3 | 232.8 ± 85.9 | 174.3 ± 67.4 | 0.001 |
| ALT (IU/L)1 | 1864.2 ± 1397.2 | 2273.2 ± 1373.1 | 1116.8 ± 1118.3 | < 0.001 |
| Total bilirubin (mg/dL)1 | 6.2 ± 5.6 | 7.0 ± 4.6 | 4.7 ± 7.0 | < 0.001 |
| Albumin (g/dL)1 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.5 | 0.151 |
| INR1 | 1.4 ± 0.9 | 1.5 ± 1.1 | 1.3 ± 0.5 | 0.861 |
| HBeAg positivity (+, %)2 | 53 (64.6%) | 31 (60.8%) | 22 (75.9%) | 0.226 |
| HBeAg titer (S/CO)1 | 133.5 ± 261.5 | 49.2 ± 60.9 | 415.7 ± 367.8 | 0.001 |
| IgM anti-HBc titer (S/CO)1 | 16.4 ± 10.7 | 22.3 ± 7.1 | 6.0 ± 6.9 | < 0.001 |
| HBV DNA (log10 IU/mL)1 | 5.3 ± 1.8 | 4.5 ± 1.6 | 6.7 ± 1.4 | < 0.001 |
Mean ± SD;
HBeAg was not checked in two patients of AHB group. AHB: Acute hepatitis B; CHB-AE: Chronic hepatitis B with acute exacerbation; WBC: White blood cell; Hb: Hemoglobin; ALT: Alanine aminotransferase; INR: International normalized ratio; HBeAg: hepatitis B e antigen; IgM anti-HBc: Immunoglobulin M antibody to hepatitis B core antigen; S/CO: Sample/cutoff value; HBV: Hepatitis B virus.
Independent predictor for differentiating between AHB and CHB-AE
A multivariate logistic regression analysis was performed to determine the independent predictors for the discrimination of AHB from CHB-AE using variables that were significant in the univariate analyses. With the multivariate analysis, high IgM anti-HBc titers and low serum HBV DNA levels were identified as independent prediction factors for AHB (Table 2).
Table 2.
Multivariate analysis for predicting acute hepatitis B
| Variables | RR | 95%CI | P value |
| Age (yr) | 0.957 | 0.895-1.023 | 0.197 |
| Platelet (× 103/μL) | 1.001 | 0.988-1.014 | 0.934 |
| Total bilirubin (mg/dL) | 1.071 | 0.927-1.237 | 0.350 |
| ALT (IU/L) | 1.001 | 1.000-1.001 | 0.055 |
| HBV DNA (log10 IU/mL) | 0.323 | 0.135-0.773 | 0.011 |
| IgM anti-HBc titer (S/CO) | 1.293 | 1.125-1.486 | < 0.001 |
AHB: Acute hepatitis B; RR: Relative risk; ALT: Alanine aminotransferase; HBV: Hepatitis B virus; IgM anti-HBc: Immunoglobulin M antibody to hepatitis B core antigen; S/CO: Sample/cutoff value.
Diagnostic values for IgM anti-HBc and HBV DNA for the differentiation of AHB from CHB-AE
To determine the optimal cutoff values for the differentiation of AHB from CHB-AE, ROC curves were plotted (Figure 1). Figure 1 shows that the AUROC of IgM anti-HBc and hepatitis B virus DNA levels for diagnosing AHB were 0.933 (95%CI: 0.869-0.998, P < 0.001) and 0.844 ( 95%CI: 0.757-0.931, P < 0.001), respectively. The best cutoff values for IgM anti-HBc and HBV DNA were 8 S/CO and 5.5 log10 IU/mL, respectively. The sensitivity and specificity at these cutoff values were 96.2% and 89.7% for IgM anti-HBc and 81.1% and 72.4% for HBV DNA, respectively. The AUROC curves of IgM anti-HBc and HBV DNA were not significantly different for differentiating AHB from CHB-AE (0.933 vs 0.844, P = 0.105). To determine if the combination of IgM anti-HBc S/CO ratio and HBV-DNA level was better than either of these markers alone, we created a new variable combining the IgM anti-HBc S/CO ratio and HBV-DNA level (0.2303*IgM anti-HBc - 1.0694*logHBV-DNA), which was made by a logistic regression using the "lroc" function in STATA[15]. The AUROC curve of the combination is shown in Figure 2. The AUROC curves of the combination and HBV-DNA were significantly different for the differentiation of AHB from CHB-AE (combination; 0.960 vs HBV DNA; 0.844, P = 0.0056). There was no significant difference between the combination and IgM anti-HBc (combination; 0.960 vs IgM anti-HBc; 0.933, P = 0.22). When combining the IgM anti-HBc and HBV DNA factors, there was a significant improvement in the diagnostic power compared to HBV DNA alone. The combination of these factors yielded a sensitivity and specificity of 98.11% and 86.2%, respectively.
Figure 1.
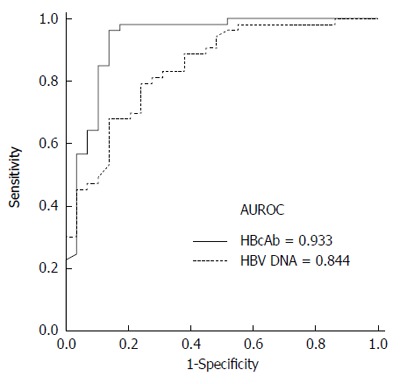
Receiver operating characteristic curves of sample/cutoff ratio of I immunoglobulin M antibodies to the hepatitis B core antigen and hepatitis B virus DNA levels. The area under receiver operating characteristic (AUROC) of IgM anti-HBc and hepatitis B virus DNA levels for diagnosing acute hepatitis B (AHB) were 0.933 (95%CI: 0.869-0.998, P < 0.001) and 0.844 (95%CI: 0.757-0.931, P < 0.001), respectively.
Figure 2.
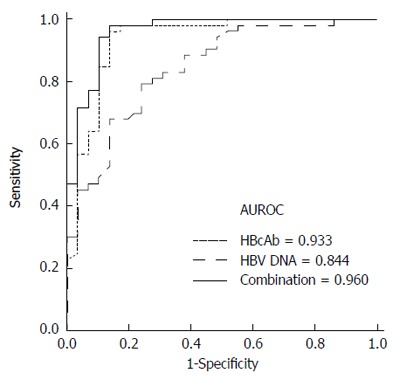
Receiver operating characteristic curves comparing immunoglobulin M antibodies to the hepatitis B core antigen, hepatitis B virus DNA and a combination of immunoglobulin M antibodies to the hepatitis B core antigen and hepatitis B virus DNA in patients with acute hepatitis B vs those with chronic hepatitis B with acute exacerbation. The area under receiver operating characteristic (AUROC) curves for IgM anti-HBc, HBV-DNA and combination are indicated in the inset. P = 0.0056 for HBV DNA vs combination, P = 0.22 for combination vs immunoglobulin M antibodies to the hepatitis B core antigen (IgM anti-HBc).
Diagnostic performance of IgM anti HBc and HBV DNA for differentiating AHB from CHB-AE
Table 3 shows the sensitivity and specificity of IgM anti-HBc alone, HBV-DNA alone and the combination of both factors in differentiating AHB from CHB-AE at various cutoff values. The S/CO ratio of IgM anti-HBc ≥ 8 had better sensitivity, specificity, positive predictive value, and negative predictive values than the other values 7 and 9. Furthermore, the S/CO ratio of IgM anti-HBc ≥ 8 had a better diagnostic performance than HBV-DNA, regardless of the cutoff value chosen.
Table 3.
Diagnostic performance of laboratory tests for acute hepatitis B
| Sensitivity | Specificity | PPV | NPV | |
| IgM anti-HBc (S/Co) | ||||
| ≥ 7 | 98.1 | 82.8 | 91.2 | 96.0 |
| ≥ 8 | 96.2 | 89.7 | 94.4 | 92.9 |
| ≥ 9 | 94.3 | 86.2 | 92.6 | 89.3 |
| HBV DNA (log10 IU/mL) | ||||
| < 5 | 56.6 | 86.2 | 88.2 | 52.1 |
| < 5.5 | 81.1 | 72.4 | 84.3 | 67.7 |
| < 6 | 83.0 | 65.5 | 81.5 | 67.9 |
| Combination1 | 98.1 | 86.2 | 92.7 | 92.6 |
Combination obtained from variable 0.2303*IgM anti-HBc-1.0694*Log HBV-DNA. The values are expressed in percent. PPV: Positive predictive value; NPV: Negative predictive value; IgM anti-HBc: Immunoglobulin M antibody to hepatitis B core antigen; S/CO: Sample to the cutoff value; HBV: Hepatitis B virus.
When the two factors were combined, the sensitivity and specificity increased from 81.1% and 72.4% for HBV-DNA alone to 98.1% and 86.2%, respectively, but when comparing IgM anti-HBc, only the sensitivity increased from 96.2% to 98.1%.
DISCUSSION
In Korea, it is not rare to encounter HBV infected patients with clinical and biochemical features resembling acute hepatitis. Historical information of chronicity or recent HBV exposure would facilitate the differential diagnosis between AHB and CHB-AE. However, this information is not always provided. Discriminating between the two conditions is difficult, especially in patients who are IgM anti-HBc positive.
In this study, an IgM anti-HBc ≥ 8 S/CO ratio and an HBV-DNA level < 5.5 log10 IU/mL aided the differentiation of AHB from CHB-AE in IgM anti-HBc positive patients. The sensitivity and specificity at these cutoff values were 96.2% and 89.7% for IgM anti-HBc, 81.1% and 72.4% for HBV DNA, respectively. These results are consistent with the data from previous studies comparing AHB and CHB-AE patients. AHB patients demonstrate a high titer of IgM anti-HBc and a low level of HBV DNA[17-19]. However, our study recruited only IgM anti-HBc positive CHB-AE patients as a control, unlike previous studies. This prevented the misclassification of the patient groups and enhanced the discrimination between the two groups. Furthermore, the AUROC curves of the combination of the S/CO ratio of IgM anti-HBc and HBV-DNA level was better than each factor alone, a significant difference was shown only with the AUROC curve of the combination and HBV-DNA level alone. In addition to these factors, Han et al[17] suggested that the S/CO ratio of HBeAg could be a diagnostic parameter for distinguishing AHB from CHB-AE. In the present study, similar results were observed between the two groups. Although the proportion of patients with positive HBeAg in the two groups were not significantly different (60.8% vs 75.9%), the HBeAg positive patients from the CHB-AE group had significantly higher S/CO HBeAg ratios than the AHB group (380.8 vs 34.2 S/CO, P = 0.001). A high S/CO IgM anti-HBc ratio, low HBV DNA level and HBeAg titer in the AHB group suggests a more robust immune response. This finding is underpinned by the previous data, the HBcAg effectively activates antigen presenting cells, leading to a strong proliferative T-cell response and triggering the humoral immune response for the production of IgM and IgG anti-HBc. Consequently, the cellular and humoral immune systems suppress HBV replications and clear HBsAg[20].
The level of AFP was higher in the CHB-AE group than the AHB group (24.9 ng/mL vs 5.0 ng/mL, P < 0.001). AFP, a tumor marker for HCC diagnosis and surveillance, can be elevated in a number of nonspecific conditions in patients with cirrhosis or hepatitis[21,22]. Increased serum AFP levels in patients with non-malignant liver disease was suggested to be associated with ongoing liver cell regeneration in response to liver injury[23,24]. Other possible mechanisms include viral derepression of the genome controlling AFP synthesis or an acute-phase reaction to liver injury[25]. However, these mechanisms cannot sufficiently explain why the AFP levels of the CHB-AE group are higher than the AHB group. In CHB-AE, the additive hepatic necrosis in pre-existent altered hepatocyte architecture, such as fibrosis, cirrhosis or liver cell dysplasia, may lead to more severe parenchymal damage leading to greater stimulation and regeneration than with AHB. Further study is necessary to answer this question.
In this study, we defined AHB using the clinical criteria and taking the patient’s history into account. It is very challenging to correctly classify AHB and the first presentation of CHB-AE patients in the clinical settings. Therefore, the classification of patients according to our strict criteria might be the best practical approach to determine chronicity. A small percentage of CHB patients may have been classified as AHB in our study. Considering the natural course of CHB, the possibility of HBsAg loss within 6 months after CHB-AE would be extremely rare and the differences in IgM anti-HBc titer and serum HBV DNA levels between the two groups would decrease if a subset of the CHB-AE patients had indeed been misallocated into the AHB group. Nevertheless, the IgM anti-HBc titers and serum HBV DNA levels remained significant factors for the differentiation of AHB from CHB-AE with a multivariate analysis. This suggests that IgM anti-HBc titers and serum HBV DNA levels are indeed discriminating factors between AHB and CHB-AE, although there is a limitation in the classification of chronicity based on the patient’s medical history.
This is the first study to differentiate between AHB and CHB-AE in a distinct group of subjects with positive IgM anti-HBc. There are some limitations. First, it was retrospectively designed, and some patients were misclassified as mentioned above. Second, the number of patients with CHB-AE (n = 29) was relatively small compared to the patients with AHB (n = 53). Finally, the IgM anti-HBc assay by Abbot Architect is actually a semi-quantitative method, but it is accommodated for the quantitation in this study, and it is unknown whether the formula for IgM anti-HBc S/CO by the Abbot Architect is useful with other commercial IgM anti-HBc kits. Nevertheless, the distinct difference of IgM anti-HBc titers between the two groups was similar to previous studies[10,19].
In conclusion, the quantitative determination of IgM anti-HBc could be a useful, simple tool for differentiating AHB from CHB-AE in IgM anti-HBc positive patients. The combination of a serum IgM anti-HBc ≥ 8 S/CO ratio with an HBV-DNA level < 5.5 log10 IU/mL can effectively distinguish AHB from CHB-AE.
COMMENTS
Background
Distinguishing between acute hepatitis B (AHB) and chronic hepatitis B with acute exacerbation (CHB-AE) is extremely important because of the patients’ different prognoses and treatment strategies as well as different prevention and public health management strategies.
Research frontiers
Immunoglobulin M antibody to hepatitis B core antigen (IgM anti-HBc) is a valuable diagnostic marker of AHB. However, 20%-27.5% of CHB-AE patients are IgM anti-HBc positive according to the fully automated, quantitative analysis method. Thus, these patients could be misclassified as AHB. In this study, the authors demonstrated that the combination of the IgM anti-HBc sample/cutoff (S/CO) value and hepatitis B virus (HBV) DNA level had a good sensitivity and specificity when distinguishing AHB and CHB-AE in patients positive for IgM anti-HBc.
Innovations and breakthroughs
Recent reports comparing AHB and CHB-AE patients indicated that a high titer of IgM anti-HBc and a low level of HBV DNA were the characteristics of AHB patients. However, our study recruited only IgM anti-HBc positive CHB-AE patients as a control, unlike previous studies. Furthermore, the authors created formula to prove that the combination of IgM anti-HBc and HBV DNA levels is a better diagnostic parameter than either of them alone.
Applications
By providing a cutoff value of IgM anti-HBc S/CO values and HBV DNA levels, this study may help physicians differentiate between AHB and CHB-AE in patients who are positive for IgM anti-HBc.
Terminology
HBV infection is one of the causes responsible for acute or chronic hepatitis. Chronic hepatitis is inflammation of the liver that lasts more than six months. IgM anti-HBc is the first antibody observed in the serum of patients with AHB. Therefore, it has been considered to be a diagnostic marker.
Peer-review
The authors compared different cutoff values of the S/CO ratio of IgM anti-HBc and HBV-DNA, and concluded that the S/CO ratio of IgM anti-HBc ≥ 8 with an HBV-DNA level < 5.5 log10 IU/mL can discriminate AHB and CHB-AE. Although this manuscript presents a retrospectively study, the statistical analysis is good.
Footnotes
Supported by Hallym University Medical Center No. HURF-2013-31.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 17, 2014
First decision: September 15, 2014
Article in press: November 11, 2014
P- Reviewer: Hung LY, Komatsu H, Jiang W, MacLachlan JH, Shi ZJ S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211, v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Jindal A, Kumar M, Sarin SK. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int. 2013;33 Suppl 1:164–175. doi: 10.1111/liv.12081. [DOI] [PubMed] [Google Scholar]
- 3.Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 4.Lanini S, Puro V, Lauria FN, Fusco FM, Nisii C, Ippolito G. Patient to patient transmission of hepatitis B virus: a systematic review of reports on outbreaks between 1992 and 2007. BMC Med. 2009;7:15. doi: 10.1186/1741-7015-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orenbuch-Harroch E, Levy L, Ben-Chetrit E. Acute hepatitis B or exacerbation of chronic hepatitis B-that is the question. World J Gastroenterol. 2008;14:7133–7137. doi: 10.3748/wjg.14.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirwan JR. Inappropriate use of tricyclic antidepressants. Br Med J. 1978;2:204. doi: 10.1136/bmj.2.6131.204-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryger P, Mathiesen LR, Aldershville J, Nielsen JO. Presence and meaning of anti-HBc IgM as determined by ELISA in patients with acute type B hepatitis and healthy HBsAg carriers. Hepatology. 1981;1:233–237. doi: 10.1002/hep.1840010307. [DOI] [PubMed] [Google Scholar]
- 8.Papaevangelou G, Roumeliotou-Karayannis A, Tassopoulos N, Stathopoulou P. Diagnostic value of anti-HBc IgM in high HBV prevalence areas. J Med Virol. 1984;13:393–399. doi: 10.1002/jmv.1890130411. [DOI] [PubMed] [Google Scholar]
- 9.Tassopoulos NC, Papatheodoridis GV, Kalantzakis Y, Tzala E, Delladetsima JK, Koutelou MG, Angelopoulou P, Hatzakis A. Differential diagnosis of acute HBsAg positive hepatitis using IgM anti-HBc by a rapid, fully automated microparticle enzyme immunoassay. J Hepatol. 1997;26:14–19. doi: 10.1016/s0168-8278(97)80003-7. [DOI] [PubMed] [Google Scholar]
- 10.Huang YW, Lin CL, Chen PJ, Lai MY, Kao JH, Chen DS. Higher cut-off index value of immunoglobulin M antibody to hepatitis B core antigen in Taiwanese patients with hepatitis B. J Gastroenterol Hepatol. 2006;21:859–862. doi: 10.1111/j.1440-1746.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- 11.Mels GC, Bellati G, Leandro G, Brunetto MR, Vicari O, Borzio M, Piantino P, Fornaciari G, Scudeller G, Angeli G. Fluctuations in viremia, aminotransferases and IgM antibody to hepatitis B core antigen in chronic hepatitis B patients with disease exacerbations. Liver. 1994;14:175–181. doi: 10.1111/j.1600-0676.1994.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 12.Colloredo G, Bellati G, Sonzogni A, Zavaglia C, Fracassetti O, Leandro G, Ghislandi R, Minola E, Ideo G. Semiquantitative assessment of IgM antibody to hepatitis B core antigen and prediction of the severity of chronic hepatitis B. J Viral Hepat. 1999;6:429–434. doi: 10.1046/j.1365-2893.1999.00171.x. [DOI] [PubMed] [Google Scholar]
- 13.Brunetto MR, Cerenzia MT, Oliveri F, Piantino P, Randone A, Calvo PL, Manzini P, Rocca G, Galli C, Bonino F. Monitoring the natural course and response to therapy of chronic hepatitis B with an automated semi-quantitative assay for IgM anti-HBc. J Hepatol. 1993;19:431–436. doi: 10.1016/s0168-8278(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 14.Korean Association for the Study of the Liver. KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol. 2012;18:109–162. doi: 10.3350/cmh.2012.18.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilford JM, Roberson PK, Fiser DH. Using lfit and Iroc to evaluate mortality prediction models. Stata Technical Bulletin. 1995;28:14–18. [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Han Y, Tang Q, Zhu W, Zhang X, You L. Clinical, biochemical, immunological and virological profiles of, and differential diagnosis between, patients with acute hepatitis B and chronic hepatitis B with acute flare. J Gastroenterol Hepatol. 2008;23:1728–1733. doi: 10.1111/j.1440-1746.2008.05600.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar M, Jain S, Sharma BC, Sarin SK. Differentiating acute hepatitis B from the first episode of symptomatic exacerbation of chronic hepatitis B. Dig Dis Sci. 2006;51:594–599. doi: 10.1007/s10620-006-3175-2. [DOI] [PubMed] [Google Scholar]
- 19.Dao DY, Hynan LS, Yuan HJ, Sanders C, Balko J, Attar N, Lok AS, Word RA, Lee WM. Two distinct subtypes of hepatitis B virus-related acute liver failure are separable by quantitative serum immunoglobulin M anti-hepatitis B core antibody and hepatitis B virus DNA levels. Hepatology. 2012;55:676–684. doi: 10.1002/hep.24732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 21.Ruoslahti E, Salaspuro M, Pihko H, Andersson L, Seppälä M. Serum alpha-fetoprotein: diagnostic significance in liver disease. Br Med J. 1974;2:527–529. doi: 10.1136/bmj.2.5918.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen DS, Sung JL. Relationship of hepatitis B surface antigen to serum alpha-fetoprotein in nonmalignant diseases of the liver. Cancer. 1979;44:984–992. doi: 10.1002/1097-0142(197909)44:3<984::aid-cncr2820440328>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Karvountzis GG, Redeker AG. Relation of alpha-fetoprotein in acute hepatitis to severity and prognosis. Ann Intern Med. 1974;80:156–160. doi: 10.7326/0003-4819-80-2-156. [DOI] [PubMed] [Google Scholar]
- 24.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 25.Werner M. Serum protein changes during the acute phase reaction. Clin Chim Acta. 1969;25:299–305. doi: 10.1016/0009-8981(69)90272-1. [DOI] [PubMed] [Google Scholar]


