Abstract
A cohort of family members with various chronic diseases including Crohn’s disease, asthma, complex regional pain syndrome, hypothyroidism, type 1 diabetes mellitus, and lymphangiomatosis and/or evidence of infection by Mycobacterium avium subsp. paratuberculosis (MAP) are described in this series of case reports. MAP was cultured from the blood of three members affected by the first five diseases and there was accompanying elevated anti-MAP IgG in two members. The patient affected by the sixth disease has a markedly elevated anti-MAP titer. The two patients affected by the first four diseases have been treated with a combination of anti-MAP antibiotics and ultraviolet blood irradiation therapy with resolution of the disease symptomatology and inability to culture MAP in post treatment blood samples. These case reports of patients with MAP infections provide supportive evidence of a pathogenic role of MAP in humans.
Keywords: Crohn’s disease, Complex regional pain syndrome, Lymphangiomatosis, Mycobacterium avium paratuberculosis, Cure
Core tip: Five patients with multiple diseases of unknown etiology were found to have evidence of infection by Mycobacterium avium subsp. paratuberculosis (MAP) including positive blood cultures (except in case 4). Two of the cases (case 1 with Crohn’s disease and asthma and case 2 with complex regional pain syndrome, hypothyroidism and Raynaud’s phenomenon) have been treated with a combination of anti-MAP antibiotics and ultraviolet blood irradiation therapy with resolution of the disease symptomatology and inability to culture MAP in post treatment blood samples. These case reports of patients with MAP infections provide supportive evidence of a pathogenic role of MAP in humans.
INTRODUCTION
In 1998, David Relman described features of a number of poorly understood clinical syndromes that strongly indicate a microbial etiology. His list of chronic inflammatory diseases with possible microbial etiologies included sarcoidosis, inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, Wegener granulomatosis, diabetes mellitus, primary biliary cirrhosis, tropical sprue and Kawasaki disease[1]. He noted that molecular methods of microbial identification offer an alternative when culture based microbial detection methods fail. His prediction regarding the emerging importance of molecular methods has proven correct since the combination of molecular methods of microbial detection and improvements in culture methods has led to advances in the field of paratuberculosis.
Mycobacterium avium subsp. paratuberculosis (MAP) is a bacterium that causes Johne’s disease, a chronic diarrheal wasting disease in cattle[2] and sub-human primates[3] and a chronic wasting disease in sheep and goats[2]. In Johne’s disease, it is well documented that once an animal is infected with MAP, the MAP bacterium grows and multiplies inside the macrophages of the immune system. The organism is excreted in the feces, and to a lesser extent in milk[2]. Outside the host animal, MAP multiplies poorly, but can survive for extended periods in the environment because of its resistance to heat, cold and the effect of drying[2]. This slow-growing bacterium affects the ileum and causes diarrhea and cachexia. There are anecdotal reports of Johne’s disease in which prolonged administration of antibiotics resulted in suppression but not cure of the disease[4].
The viable bacterium has been found in commercially available pasteurized milk[5,6]. Ellingson et al[6] reported that 2.7% of retail pasteurized milk samples purchased in Wisconsin, Minnesota and California contained viable MAP. Because of the presence of this organism in the food supply, it would not be surprising if MAP is widespread in the environment and the human population. The first mass screening study for evidence of MAP infection in humans was done in North India on serum, blood and stool samples submitted from patients with multiple medical conditions including diabetes, liver disorders, anemia, thyroid, tuberculosis, typhoid, abdominal disorders, inflammatory illness and ion imbalance. Singh et al[7] reported that 34% of 23196 serum samples had anti-MAP antibodies (a comparison with normal subjects was not included). The same study showed that 12.7% of 1246 blood samples from normal healthy individuals had IS900 PCR evidence of MAP in their blood and 8.4% of 3093 blood samples from patients with the above listed medical conditions had PCR evidence of MAP.
It has been suggested for years that there may be an association between Crohn’s disease (CD) and Johne’s disease. Dalziel first speculated in 1913 that chronic enteritis, now known as CD, might be caused by MAP[8] and Chiodini first reported the culturing of mycobacteria from the intestinal tissues of CD patients[9]. For many years, the data were conflicting[2,10,11] and the theory that MAP causes CD remains controversial[12-14]. Later on, Hermon-Taylor and others described a case of a boy with cervical lymphadenitis caused by MAP who later developed CD[15]. Recent studies show an increase in the detection and isolation of MAP in adult Crohn’s patients[16] and in children with newly diagnosed CD[17] Meta-analyses by Feller et al[18] and Abubakar et al[19] have concluded that a majority of studies on the association of MAP and CD show that most patients with CD have MAP infection. In 2004, Naser et al[20] reported culturing MAP from the blood of 50% of patients with CD and this work was confirmed in four laboratories including the Centers for Disease Control and Prevention[21,22].
In addition, a large, randomized, double-blind, placebo-controlled study from Australia showed a significant but not lasting response of individuals with CD who were treated with antibiotics against MAP[23] Apparently unaware that antibiotics fail to cure a majority of patients with Mycobacterium avium complex infection (MAC)[24], the authors incorrectly concluded that because they failed to cure patients, CD could not be caused by mycobacterial infection. This study and the conclusions of its authors were significantly flawed[25,26]. The editorial which accompanied the article acknowledged that, “subtherapeutic doses of rifabutin (450 mg), clarithromycin (750 mg) and clofazimine (50 mg) per day were used, whereas the optimal dose of rifabutin, clarithromycin and clofazimine for treatment of M avium complex infections is 600 mg/d, 1000-2000 mg/d, and 100 mg/d, respectively”[27]. A recent meta-analysis of antibiotic trials in CD conducted by Feller and coworkers concludes that a substantial benefit was evident in trials using nitroimidazoles, clofazimine and ciprofloxacin and that a combination of clarithromycin and rifamycin and ciprofloxacin should be studied[28].
Most research attention in inflammatory bowel disease has focused on the genetics of CD rather than the association of the disease with paratuberculosis infection. However, these two areas of research are probably complementary because the genetic mutations, which have been described, may indicate increased susceptibility to MAP infection. Also in cattle, NOD2 mutations are associated with susceptibility to MAP infection[29], and this same mutation has been linked to patients with CD. A 2009 study from China showed that patients with another mycobacterial infection, leprosy, and patients with CD have higher rates of the NOD2, TNFSF15 and IL12B mutations than healthy controls[30,31]. A large meta-analysis genome-wide association study concluded that there is considerable overlap between the susceptibility loci for IBD and mycobacterial infection[32]. In the only simulated human-challenge trial, Israeli researchers showed that fetal human small intestine explants in mice with severe combined immunodeficiency and then inoculated with MAP intraluminally, showed invasion of the goblet cells, tissue damage and inflammation[33].
In 2006, Dow postulated that MAP may be the trigger for type I diabetes mellitus (T1DM)[34] because of the association of T1DM with mutations of the SLC11a1 gene[35]. This gene encodes a membrane protein of the lysozymes of monocytes and macrophages. Mutations in this gene have been associated with susceptibility to infectious diseases including tuberculosis and leprosy and lead to a more hospitable host environment for bacterial survival and replication[36]. Subsequently, Sechi and others reported an association of MAP and T1DM[37-39]. Recently, Naser et al[40] showed that there is a high degree of homology between GAD65 and Hsp65 which supports a mycobacterial role in the immune destruction of the beta cells of the pancreatic islets through molecular mimicry.
Additional findings in T1DM also present in other mycobacterial infections include elevated angiotensin converting enzyme (ACE) levels[41,42] and elevated vascular endothelial growth factor (VEGF)[43]. VEGF has been reported to be elevated in active pulmonary tuberculosis and to decline following successful treatment[44]. Some 24.5% of patients with T1DM have a positive Saccharomyces cerevisiae Antibody (ASCA) test which is similar to the frequency of ASCA positivity in Crohn’s disease[45]. Consumption of milk is a risk factor for the development of T1DM[46,47].
Frau and others have also reported an association of MAP and multiple sclerosis (MS)[48]. Consumption of milk is also a risk factor for the development of MS[49].
The following case reports demonstrate an association of MAP with several of the above described diseases as well as with two diseases which have not yet been linked to MAP. In addition, the diseases which were treated with anti-MAP therapy resolved.
Assays for evidence of MAP infection
Three assays were performed on EDTA blood samples from each patient (Figure 1). The plasma was assayed for antibodies to MAP by ELISA using culture filtrate antigens of MAP strain UCF-4 as described[50].
Figure 1.
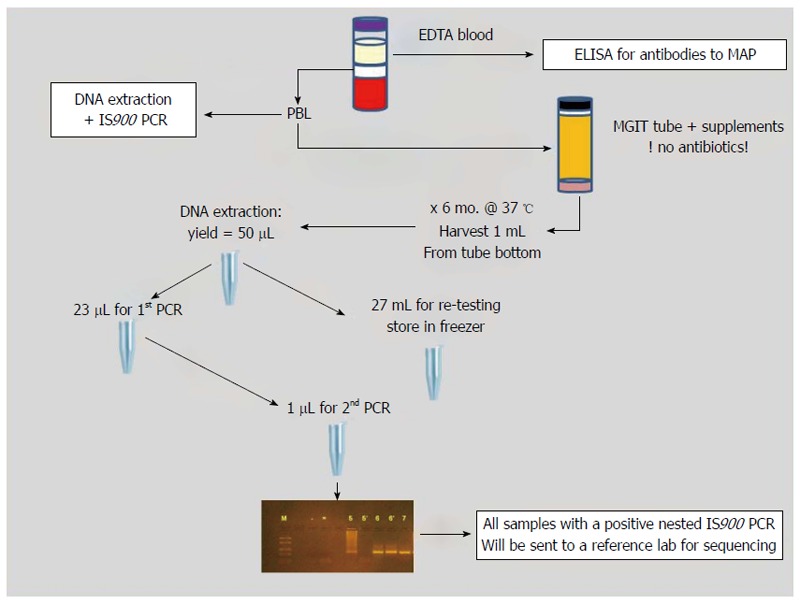
Schematic of sample processing and testing methods.
Peripheral blood leukocytes were harvested and used for DNA extraction followed by IS900 PCR as described[51]. The remainder of the leukocytes were inoculated into BACTEC MGIT ParaTB medium with supplements but without antibiotics and incubated for 6 mo at 37 °C. After incubation, the culture pellets were harvested and subjected to DNA extraction, followed by nested IS900 PCR as described[22]. Subcultures were done on all PCR-positive MGIT cultures to attempt recovery of MAP in pure culture.
Assay for evidence of leprosy
One assay was performed to detect antibodies to M. leprae based on phenolic glycolipid-1 antigen. The assay was performed as described earlier for Para-LP-01 based lipid-ELISA for Johne’s disease[52]. Wells were coated with 100 ng PGL-1 dissolved in iso-propanol and dried. Plates were blocked for one hour at room temperature with 100 μL 3% BSA (in PBS, pH 7.4). ELISA was then performed[52]. One hundred μL of subject serum diluted 1:20 in 10% FBS/PBS (pH 7.4) was added to the wells and incubated for 30 min at room temperature. Plates were washed three times with PBS followed by adding secondary conjugated antibody (sheep anti-human IgH-h+1 HRP conjugated antibody diluted 1:2000 in 10% FBS/PBS). 100 μL of secondary antibody solution were added per well and plates were incubated at room temperature for 30 min. Plates were washed as before and 100 μL of room temperature 3,3′,5,5′-tetramethylbenzidine were added per well. Plates were incubated at room temperature for 10 min. The reaction was stopped by adding 100 μL of 2 mol/L sulfuric acid per well. Plates were read at 450 nm using an iMark Microplate Reader (BioRad).
CASE REPORT
Case 1
At the Children’s Hospital of Wisconsin, Dr. Grzegorz Telega began following a 9-year-old boy who was diagnosed with CD in June 2004. He initially presented in 2004 with persistent diarrhea, weight loss and unexplained fever. His linear growth had slowed considerably. Colonscopy and upper gastrointestinal endoscopy showed multiple aphthous ulcers in the colon, terminal ileum and stomach (Figure 2) and biopsies obtained in the colon and gastric antrum contained the granulomas of CD (Figure 3). His erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) were increased.
Figure 2.
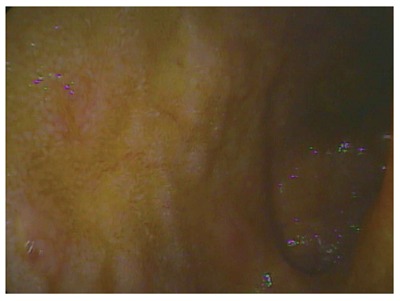
Terminal ileum with multiple ulcers.
Figure 3.
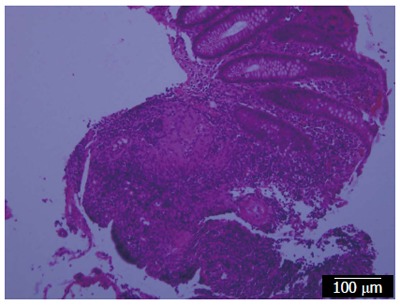
Biopsy from the colon showing a granuloma of Crohn’s disease.
MAP testing was performed on the patient’s blood. The initial sample showed mildly elevated antibody titers to one of the MAP antigens, p35, and after several months of incubation, MAP was grown from the patient’s blood. The second sample drawn more than 3 mo later showed greater elevations of antibodies to both p35 and p36 antigens and also grew MAP (See Table 1 for the summary of MAP testing in this and the subsequent 4 cases). During the 3 mo between the initial and second sample, the patient’s clinical condition steadily worsened with increasing abdominal pain and frequency of diarrhea. At the time of the initial diagnosis, the patient was 4 feet 8.75 inches or in the 95th percentile in stature and weighed 71.8 pounds (75th percentile). Prior to the onset of illness, his weight had previously reached 80 pounds (90th percentile). Initially, in August 2004, the patient received azathioprine and steroids with concurrent antibiotic therapy including clarithromycin and rifabutin, in low doses similar to those used in the Australian trial[23]. Dr. Telega, the pediatric gastroenterologist, prescribed the antibiotics and received consultative advice initially from Dr. Hermon-Taylor and later additionally from Drs. Chamberlin and Borody. The patient also took daily probiotics, which were administered at mid-day. After 7 d of antibiotic therapy, as predicted by Dr. John Hermon-Taylor the patient developed a mild fever that lasted for several days, which Dr. Hermon-Taylor had previously observed in other patients and compared to a Jarish Herxheimer reaction. Because of an elevated ALT and AST, the azathioprine was discontinued in December 2004. The patient responded favorably to the antibiotics for about 8 mo, but by June 2005, he became symptomatic and relapsed (a finding similar to that of the Australian trial). The period of relapse lasted from June 2005 to March 2006 and during this time he remained on low dose antibiotics. A colonoscopy on January 11, 2006 showed multiple aphthous ulcers in the colon and his weight on that day was 77.3 pounds (35 kg). On January 13th a short course of prednisone was initiated at a dose of 10 mg/d. On January 15th, the dose of prednisone was increased to 20 mg/d. By February 11th, his weight was 90 pounds (41 kg).
Table 1.
Summary of Mycobacterium avium subsp. paratuberculosis antibody, PCR and culture results for cases 1 through 5
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| MAP Ab | 5/11/04 | 11/6/12 | 1/14/13 | 3/14/13 | 1/10/13 |
| p35-0.25 | ELISA S/P | ELISA S/P | ELISA S/P | ELISA S/P | |
| p36-0.16 | 1.24 | 0.49 | 1.72 | 0.15 | |
| MAP PCR | 5/11/04 | 11/6/12 | 1/14/13 | 3/14/13 | 1/10/13 |
| negative | negative | negative | negative | negative | |
| MAP culture | 5/11/04 | 11/6/12 | 1/14/13 | 3/14/13 | 1/10/13 |
| positive | negative | negative | negative | positive | |
| MAP Ab | 8/18/04 | 11/20/12 | Month/year- | ||
| p35-0.5 | ELISA S/P | pending | |||
| p36-0.3 | 1.31 | ||||
| MAP PCR | 8/18/04 | 11/20/12 | Month/year- | ||
| negative | negative | pending | |||
| MAP culture | 8/18/04 | 11/20/12 | 1/18/13 | Month/year- | |
| positive | positive | positive | pending | ||
| Anti-MAP | Anti-MAP | ||||
| therapy | therapy | ||||
| started | started | ||||
| 8/19/04 | 12/15/12 | ||||
| MAP Ab | 9/20/04 | 4/17/13 | |||
| p35-0.33 | ELISA S/P | ||||
| p36-0.22 | 1.20 | ||||
| MAP PCR | 9/20/04 | 4/17/13 | |||
| negative | negative | ||||
| MAP culture | 9/20/04 | 4/17/13 | |||
| positive | negative | ||||
| MAP Ab | 7/9/07 | 5/7/14 | |||
| negative | ELISA S/P | ||||
| 1.69 | |||||
| MAP PCR | 7/9/07 | 5/7/14 | |||
| negative | negative | ||||
| MAP culture | 7/9/07 | 5/7/14 | |||
| negative | negative | ||||
| MAP Ab | 5/27/14 | ||||
| ELISA S/P | |||||
| 0.67 | |||||
| MAP PCR | 5/27/14 | ||||
| negative | |||||
| MAP culture | 5/27/14 | ||||
| negative |
MAP: Mycobacterium avium subsp. Paratuberculosis.
In late 2005, in addition to receiving antibiotics, over the course of a three-month period, the patient received a total of 11 once weekly ultraviolet blood irradiation (UVBI) treatments which were performed by Dr. Mitchell Kurk at his office in Long Island, New York. A similar UVBI device has been successfully advanced through phase II clinical trials at the FDA.
In addition to UVBI therapy, on advice from experts, the doses of clarithromycin and rifabutin were increased and ciprofloxacin was added to the regimen. On January 13, 2006, the patient was started on ciprofloxacin at a dose of 125 mg taken twice per day (7 mg/kg per day) and two weeks later this dose was increased to 250 mg taken twice per day (14 mg/kg per day). On February 12, 2006, when the patient weighed 90 pounds (41 kg) the dose of clarithromycin was increased to 750 mg, 500 mg taken in the am and 250 mg taken in the pm (18 mg/kg per day) and the dose of rifabutin was increased to 450 mg taken 150 mg in the am and 300 mg in the pm (11 mg/kg per day). In May 2006, after the patient was in clinical remission, clofazimine (an antibiotic with restricted use in the United States which is used for the treatment of leprosy and Mycobacterium avium complex) was added at a dose of 50 mg taken once daily. The clofazimine was obtained from a source in Australia.
The patient had a history of seasonal (triggered by pollen) asthma beginning at age 3 years and the last episode of asthma he has experienced was in April 2006.
These antibiotics have been used in many prior studies to treat MAP in humans. The doses in this patient were adjusted over time. He received over 4 years of continuous antibiotic therapy until January 2009. From January 2009, he was on cycled therapy of rifabutin, ciprofloxacin and clarithromycin until May 2011. The patient has been in complete remission since April 2006.
Since May 2011, he has received no medications of any type and he has been without any signs or symptoms of CD and is now 5 feet 10.5 inches and 185 pounds (84.1 kg). A follow-up blood culture for MAP in July 2007 failed to recover MAP by culture or detect MAP DNA by PCR and he tested negative for anti- MAP antibody. Currently, he has a normal blood count and is negative for inflammatory markers including ESR and CRP. A colonoscopy and upper gastrointestinal endoscopy in August, 2014 were normal. There are many reports in the literature of patients with CD who have responded favorably to antibiotic therapy[28,53-55].
Case 2
In early 2012, the sibling of case 1, a 23-year-old female began experiencing symptoms initially thought to be carpal tunnel syndrome and by August 2012, developed Raynaud’s phenomenon in both hands. She had a several year history of hypothyroidism and was on thyroid hormone replacement. The symptoms of neuralgia and paresthesia progressively advanced and involved her bilateral hands, elbows, shoulders, neck, legs and feet. By the time she was seen at the Cleveland Clinic Neurological Center for Pain in late November, the physician who examined her noted Raynaud’s phenomenon in both hands and described the purple color change and cold temperature as profound.
Her workup included a normal EMG study, normal CT scan of the brain, and normal values for procalcitonin, ESR, CRP, IL-6, ASCA IgA and IgG, rheumatoid factor, ANA, SS-A/RO, SS-B/LA, SCL, RNP, SM, CCP, JO-1, Centromere antibodies, Anti-Hu TTG-IgA, lyme serology, gliadin peptide IgA and IgG, anti-endomysial IgA, serum MPA IgG, MPA IgA, MPA IgM, MPA kappa, MPA lambda, MPA kappa/lambda ratio, glutamic acid decarboxylase antibody and ganglioside antibody studies. Because of a history of travel to Guatamala 5 years prior to the onset of her illness, the patient’s blood was tested for antibody to M. leprae. The PGL-1 assay was negative. The initial diagnosis at the Cleveland Clinic was hypersensitivity syndrome and the patient was referred to the Cleveland Clinic Neurological Center for Pain where she received the diagnosis of thoracic outlet syndrome with probable evolving complex regional pain syndrome (CRPS).
Recommendations for therapy included physical therapy, muscle relaxants and gabapentin. Gabapentin at the lowest recommended dose made her very dizzy and therefore, she discontinued this medication. The patient obtained multiple sessions of physical therapy which were beneficial and engaged in gradually increasing regular exercise including walking and swimming as tolerated. In December 2012, she could only walk 300 feet or tread water wearing a floatation device for 5 min. The cause of this condition is unknown.
Due to suspicion that CRPS could be a manifestation of a MAP infection, blood samples were tested for evidence of MAP infection; the first blood sample was obtained November 6, 2012 and the second on November 20, 2012. The results of the MAP ELISA assays from both samples showed significantly elevated titers, S/P values of 1.24 and 1.31 respectively, where the positive control serum was from a veterinarian who had accidentally injected himself with the MAP vaccine. The MAP PCR tests were both negative. MAP was detected by culture from the second blood sample. There was rapid progression of clinical disease between November 6, 2012 when her MAP antibody titer was 1.24 and the organism could not be cultured while she had monocytosis and lymphocytosis and November 20, 2012 when her antibody titer increased to 1.31 and the organism could now be cultured while she no longer had monocytosis and lymphocytosis. During this two week period she developed generalized extreme hypersensitivity to minor tactile stimuli. MAP experts were consulted and appropriate antibiotics were prescribed.
Other diagnostic test results included elevated cryoglobulins of 57 (normal 0-50 ug/mL) and ACE level of 59 (normal 8-53 U/L). Cryoglobulins [57-59] and ACE[60,61] are elevated in other mycobacterial infections including tuberculosis and leprosy. Prior to the onset of disease and the initiation of therapy, the patient had persistent relative lymphocytosis and eosinophilia which was present as early as 1997. Relative lymphocytosis has been described in tuberculosis[62].
Neurologic findings are not uncommon in CD[63]. In addition, siblings of patients with CD are at much higher risk of developing CD than the general population[64].
In mid December 2012, the patient was placed on anti-MAP therapy and supplementary Vitamin A and Vitamin D similar to that administered to her brother. Her height and weight are 5 feet 9.5 inches and 150 pounds (68.2 kg), respectively and her antibiotic doses were as follows: Clarithromycin 500 mg twice daily (15 mg/kg per day), rifampin 300 mg twice daily (9 mg/kg per day), levofloxacin 500 mg per day (7 mg/kg per day) and clofazimine 100 mg 3 times per week (4 mg/kg per week). Four days after the initiation of therapy she experienced a mild fever which lasted two days. Dr. Stuart Weg performed 12 UVBI treatments at weekly intervals for 3 mo from January through early April 2013. Previously, Weg speculated that CRPS is due to an infection caused by a cell wall deficient bacterium[65].
Dr. David Haas of the University of Charleston Chemistry Department, confirmed by gas chromatography, mass spectroscopy, ultraviolet absorption spectroscopy and infrared spectroscopy that the clofazimine, which was imported from India, was not a counterfeit drug.
Following the initiation of therapy, she developed monocytosis and the relative lymphocytosis persisted. Since that time, she has shown marked clinical improvement including disappearance of the generalized hypersensitivity, disappearance of the previously grossly visible Raynaud’s phenomenon in her hands, and improved ability to perform motor skills with a reduction in reported pain. By the fall of 2014, she could swim one mile or walk five miles per day. Although her general condition has greatly improved including absence of the generalized extreme hypersensitivity, she still experiences episodes of migratory pain. With treatment of leprosy, reversal reactions and prolonged neuralgia have been observed[66].
Six weeks after beginning the anti-MAP therapy, while still taking supplemental thyroxine, she began experiencing palpitations and it was noted that her TSH had dropped to the low normal range. On the presumption that the palpitations indicated that her thyroid function was recovering, in January 2013, she stopped supplemental thyroxine, has not experienced symptoms of hypothyroidism, and her TSH is now in the normal range. A TSH from May 7, 2014 was 4.06 μIU/mL (reference range 0.350-5.55 μIU/mL). An ACE level from May 7, 2014 was still elevated at 58 U/L and a complete blood count from the same day was normal except for mild monocytosis of 9.3 % (reference range 0%-8%) and eosinophilia of 9.5% (reference range 0%-4%). By October 14, 2014, a complete blood count and differential were normal.
After four months of therapy (April 7, 2013), a follow-up blood culture for MAP showed a minimally decreased MAP ELISA S/P value of 1.2, the MAP PCR test was negative and MAP could not be cultured from this sample. A follow-up cryoglobulin study obtained from April 17, 2013 was negative after 4 h and positive after 72 h. In early January 2014, the patient consulted Dr. Kuruvilla John who has since that time followed her case.
Case 3
Since two siblings had evidence of MAP infections and responded to anti-MAP therapy, other relatives were tested. The paternal uncle of cases 1 and 2, who has longstanding T1DM, is also infected with MAP. In addition, the uncle was found to have elevated ASCA IgA, a serologic marker, which is present in T1DM and CD[45]. The uncle’s MAP serum antibody S/P value was 0.49 (negative). The patient has declined treatment for MAP.
Case 4
The nephew of the mother of cases 1 and 2 has lymphangiomatosis, a disease of unknown etiology. His blood showed a MAP ELISA antibody S/P value of 1.72 (exceptionally high). His MAP PCR and MAP culture results were negative. He had a very elevated VEGF of 506 pg/mL (reference range of 31-86 pg/mL), mild monocytosis of 992 (reference range of 200-950 cells/uL) and a normal neopterin test, ASCA IgA and IgG, and ACE tests. The MAP ELISA study, VEGF and monocytosis in this case suggest a possible mycobacterial causation of lymphangiomatosis and further study is indicated.
Case 5
The father of cases 1 and 2 was tested for MAP infection. After 6 mo of incubation, MAP was grown from his blood. His MAP PCR on PBMCs was negative and his MAP ELISA antibody S/P value was 0.15 (negative). He is healthy but suffered from seasonal asthma (triggered by pollen) at age 12 years and also while living in Germany from 1986 to 1989. In addition, he has rosacea, which was diagnosed by clinical signs and a skin biopsy showing non-caseating granulomas. This condition is treated with a topical ointment containing azelaic acid. In 2004, his blood was found positive for antibodies to p35 and p36 MAP antigens.
Because of the devastating nature of the diseases in case 1 and case 2 and the poor record of efficacy, standard therapies were eschewed. Institutional review board (IRB) approval was not sought since the law allows off label use of FDA approved drugs and also allows the administration of UVBI in New York. IRB approval is generally not required in the care of individual patients. In cases 1 through 3, infectious disease specialists were consulted and informed about the elevated MAP ELISA antibody titers and/or positive MAP cultures but declined to make recommendations regarding therapy.
Additional family members were tested for evidence of MAP infection as well. The mother of case 1 and case 2 was negative for MAP by PCR on PBMC and culture, and had an ELISA S/P of 0.08 (negative). The brother of case 1 and case 2 had a negative MAP PCR and negative culture and ELISA S/P of 0.59 (slightly elevated). The maternal grandfather of case 1 and case 2 had a negative MAP PCR on PBMCs and negative culture and an ELISA S/P of 0.13 (negative). The maternal grandmother (with hypothyroidism) of case 1 and case 2 had a negative MAP PCR on PBMCs and negative MAP culture and an ELISA S/P of 0.0 (negative).
The families of both parents of case 1 and case 2 have a history of susceptibility to mycobacterial infection. Figure 4 which is a family pedigree summarizing the cases and the mycobacterial infection and other disease history in other members.
Figure 4.
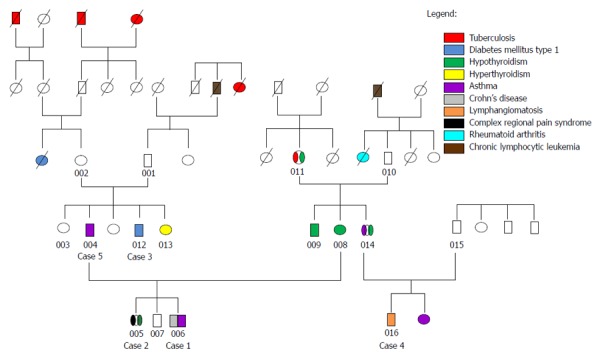
Family pedigree summarizing history of mycobacterial infection and other diseases of cases 1 through 5 and additional family members.
DISCUSSION
The presence of viable MAP in the blood of a majority of CD patients is an important finding which has been previously reported by Naser[20,22]. Some observers ascribe this phenomenon to the “leaky bowel” resulting from mucosal disruption in CD[67]. In case 1, the recovery of the viable organism in the setting of two diseases and the failure to recover the viable organism in the absence of these two diseases argues in favor of a pathogenic role of MAP in these patients. Similarly, in case 2, the recovery of the viable organism in the setting of two other diseases and the failure to recover the organism in the absence of these two other diseases also argues in favor of a pathogenic role of MAP. Furthermore, the recovery of the viable organism in case 2 in which the patient suffered from CRPS cannot be explained by the leaky bowel hypothesis since this patient has not experienced bowel related symptoms. In addition, a pathogenic role of MAP in the human host is likely, considering the zoonotic capacity of slow-growing mycobacteria and because this organism is an obligate pathogen, i.e., one which does not propagate in the environment[68].
A second possible interpretation of the findings in these case reports is that the diseases were not caused by MAP and went into remission spontaneously. In the consideration of the probable events in case 1 and case 2, the percentage of patients who experience long term remissions in CD, CRPS, hypothyroidism and Raynaud’s phenomenon is 10%[69], 74%[70], 62%[71], and 64%[72], respectively. With the assumption that case 1 and case 2 resolved spontaneously, the outcome follows the likelihood function. The probability of spontaneous resolution in case 1 is 0.10 and in case 2 is 0.30 (0.74 × 0.62 × 0.64 = 0.30) and the probability of spontaneous resolution in both patients is 0.10 × 0.30 or 0.03 which is very unlikely. Controlled clinical trials of anti-MAP therapy are necessary to determine whether these case reports are reproducible. Clinical trials have been designed and funding is being sought.
A third possible interpretation of the recovery of MAP from the blood samples in case 1 and in case 2, is that the MAP organism is a contaminant from specimen processing. This interpretation is unlikely since in both cases there are increased antibodies directed against MAP indicating a host response to the organism. The presence of elevated serologic markers which are associated with mycobacterial infection, including CRP in case 1 and ACE in case 2, also weighs against this possibility.
We believe that the profound long lasting remission in case 1 resulted from anti-MAP therapy and is unlikely due to steroid administration, since such remissions rarely result from steroid administration alone. Based on these anecdotal reports, three additional cases of children with CD and MAP infection treated successfully with combination anti-MAP antibiotics and UVBI (personal communication), open label trials in CD, and controlled trials in MAC infection, we recommend the use of three antibiotics including clarithromycin, rifampin and levofloxacin (at 15, 9 and 7, respectively, mg/kg per day) for at least two years in combination with periodic UVBI (if available). However, at this time, the optimal antibiotic combination is unknown.
In cases 1 and 2, the rapid progression of the disease accompanied by an increase in antibodies to MAP antigens between the first two specimens may mirror Johne’s disease in dairy cattle in which the progression in the severity of disease and the degree of mycobacterial colonization coincides with a switch from the TH1 to TH2 type immune response[73].
The presence of the viable bacterium in the blood of an apparently healthy host (case 5) is an interesting finding. Apparently healthy individuals may have less virulent forms of disease such as transient childhood asthma or rosacea as noted in case 5. In addition, if MAP-infected people are followed over a long enough period of time, some may eventually develop one of the diseases traditionally considered autoimmune.
It would not be surprising if there is a population of individuals who are MAP-infected but never develop disease. Mycobacterium tuberculosis, causes active disease in only 10% of infected humans[74]. A similar situation probably pertains to human paratuberculosis, i.e., most MAP infected individuals may never develop disease. Clinically normal cattle with known MAP infection are common suggesting a parallel in the human population[75].
Any theory of causation of the autoimmune diseases must explain two consistent observations for most of these diseases: (1) the north south gradient in geographical distribution of the disease (in the northern hemisphere)[76]; and (2) the predominant female to male ratio in most of these diseases. The first observation is concordant with the worldwide distribution of Johne’s disease[77], the lower levels of Vitamin D in the human host at northern latitudes[78], and the role of Vitamin D in the clinical course of patients with CD and T1DM[79,80]. A possible explanation for the second observation includes reduced host immunity due to the effects of estradiol and/or progesterone[81-83]. Future work may shed light on the immunology of gender differences with these diseases. Because of the known risk of disease progression in CD from birth control medication, women who have been diagnosed and treated for a MAP infection should consider non-hormonal birth control methods.
The optimal hosts for MAP are ruminants; cattle, sheep, and deer, in which, a higher burden of bacteria are generally found than in humans. These animals have a higher body temperature than humans ranging from 100.4 to 102.8, 100.9 to 103.8 to 104 F, for cattle, sheep and deer, respectively[84,85]. These differences in body temperature suggests that the growth of MAP in laboratory culture may be accelerated by raising the incubation temperature to 104 F. Further investigation of this issue is warranted.
If controlled trials of MAP related illnesses confirm the findings of these case reports and the autoimmune diseases can be cured, because the bacterium is present in the food supply, will treated patients redevelop disease on re-exposure to the organism? The precautionary principle should apply and improved food safety and public health measures are necessary to limit human exposure to MAP. Until improved measures are in place, treated and cured patients should probably avoid known sources of MAP which include pasteurized milk and milk products such as yoghurt, cheese and ice cream and undercooked beef.
Open label trials of long-term antibiotic therapy in CD have a significant relapse rate. Adjunctive therapy such as UVBI combined with appropriate antibiotics may be a way to improve therapeutic outcomes. UVBI was developed by Knott[86]. In his article on the development of ultraviolet blood irradiation, he refers to the work of European investigators who “believed that most of the systemic reactions observed following exposure of the skin to ultraviolet rays were due to the influence of the rays upon the blood”[86]. Knott was most likely aware of the work of Finsen who received the Nobel Prize in 1903, for his work showing the beneficial effects of ultraviolet treatments of the skin in patients with lupus vulgaris, i.e., tuberculosis of the skin[87]. The Knott device was used for the treatment of many infections[88-90]. and while exact figures are unavailable, probably thousands of patients were treated with this therapy throughout the United States. Several studies[91,92] as well as three controlled trials from Russia have shown beneficial effects in the treatment of tuberculosis[93-95]. Because the Knott hemo-irradiator predated the advent of the FDA, this device was never FDA approved. However, in recent years, UVBI was advanced successfully through phase II clinical trials for the treatment of hepatitis C infection.
Various studies on UVBI that may explain the benefit of this therapy include the following. Ultraviolet light in the C region (UVC) inactivates bacterial and viral pathogens, present in the blood, which is irradiated. In the case of bacteria and DNA viruses, UVC induces the formation of thymine-thymine dimers, which prevents replication[96]. In the case of RNA viruses, UVC induces the formation of uracil-uracil dimers which also prevents replication[97]. Bacteria including Mycobacterium tuberculosis have UV repair mechanisms and normal lymphocytes also have UV repair mechanisms[98,99].
Because only 200 cc of blood in an average adult (or 4% of the total 5.0 liter blood volume) is treated during a single session, factors other than pathogen inactivation are likely to explain the potential benefit. Ultraviolet light shined on murine fibroblasts results in the formation of hydrogen peroxide and hydroxyl radicals which are also bactericidal and virucidal[100]. Ultraviolet light in the A region and at higher doses and exposure durations causes immune suppression, but ultraviolet light in the B (UVB) region and UVC have been shown to stimulate dendritic cells[101-103]. Hemoglobin which has been irradiated with UVB and UVC wavelengths exhibits fluorescence[104] and the wavelength of light which is emitted, 365 nm, causes the formation of DNA or RNA adducts in riboflavin and other chromophores and these adducts are bactericidal and virucidal[105]. It is now known that in spite of long term treatment of tuberculosis by antibiotics, there are persisters, which are not killed by the drugs[106,107]. Also Mycobacterium avium complex organisms can resist the bactericidal activity of clarithromycin within the phagosomes of macrophages[108]. Viable MAP organisms which have survived the antibiotics by either of these routes and which are within macrophages may not survive ultraviolet irradiation[68]. An in-vitro study showed that monocytes which are irradiated with UVB and then infected with Mycobacterium avium intracellulare (MAI) organisms, efficiently inhibit the intracellular replication of MAI[109]. The authors in this work speculated that the intracellular inhibition of MAI replication in the UV treated macrophages may be due to the induction of intracellular vitamin D production by the UVB.
Vitamin D has been shown to play an important role in the host immune response to mycobacterial infection[110]. Vitamins A and D have been shown to inhibit the growth of MAP in vitro[111]. Vitamin D has also been shown to reduce the proliferation of M. tuberculosis in macrophages[112]. Activated dendritic cells are known to produce Vitamin D[113] and Vitamin D induces the intracellular production of cathelicidin, which is an antimicrobial protein[114]. High levels of Vitamin D have been correlated with a reduced risk of developing multiple sclerosis, and Vitamin D intake is inversely associated with rheumatoid arthritis (another autoimmune condition) and the severity of this latter disease also correlates with Vitamin D levels[113].
Finally, many types of cells including leukocytes and, in particular, monocytes, exposed to ultraviolet light secrete heat shock proteins and these proteins play an important role in the response to infection[115-117].
A small open label trial of UVBI in 4 patients with severe Raynaud’s syndrome showed clinical improvement that lasted for 3 mo in all of the patients and a reduction of mycobacterial heat shock protein antibodies in one of the patients[118].
These case reports support a pathogenic role of MAP in humans. Large controlled trials are indicated for many of the autoimmune diseases associated with MAP infection including CD, T1DM, MS and CRPS using anti-MAP therapy combined with UVBI (perhaps substituting ethambutol for ciprofloxacin)[119] in one arm and combination infliximab and azathioprine in the control arm to determine whether properly dosed anti-MAP therapy is more effective than currently available therapies. RedHill Biopharma Ltd., Tel Aviv, Israel, has initiated phase III clinical trials in Europe and North America to treat CD and MS using a combination therapy of clarithromycin, rifabutin and clofazimine[120]. MAP prevalence studies are indicated in lymphangiomatosis, ankylosing spondylitis, rheumatoid arthritis, hypothyroidism, hyperthyroidism, adrenal insufficiency, systemic sclerosis, Sjogren syndrome, systemic lupus erythematosus, dermatomyositis, psoriasis, sarcoidosis, celiac disease, rosacea, asthma, fibromyalgia, amyotrophic lateral sclerosis, myasthenia gravis, Parkinson’s disease and Alzheimer’s disease.
The results of therapeutic trials should be evaluated with consideration of the success rate in treating Mycobacterium avium complex infections, a mere 42%[27] and that the treatment of Mycobacterium leprae is associated with an absolute relapse rate of 3% and that relapses may occur more than 10 years after multiple drug therapy has concluded[121].
Researchers who explore the role of MAP in the autoimmune diseases should be aware that our current diagnostic tests are crude. The ELISA for serum antibodies to MAP46 was adapted from the cattle assay which has a sensitivity of only 30% to 40% in cattle which are known to be MAP-infected[122]. The suboptimal sensitivity and the variation between current serologic assays for MAP make the diagnosis of MAP infection difficult. However, in the presence of otherwise unexplained autoimmune disease, the occurrence of positive blood cultures for MAP should be a significant finding.
Furthermore, pre-existing therapies for these conditions may hinder culture recovery methods. Many of the currently used immunomodulators have demonstrated bacteriostatic effects on MAP in-vitro[123,124]. When possible, MAP diagnostic testing should be conducted on newly diagnosed patients prior to instituting immunosuppressive therapies which can inhibit the growth of MAP in cultures. While the blood culture method of Naser has been a great advance in the field of human paratuberculosis research, it is positive in 55% of patients with inflammatory bowel disease and in 22% of non-inflammatory bowel disease patients[22], and therefore cannot by itself serve as a discriminator for the presence or absence of disease. Concurrent detection of antibodies directed at MAP will probably be helpful in this regard[50]. MAP cultures should be performed in laboratories with expertise. Parrish et al[125] failed to replicate the blood culture study of Naser et al, but their method did not include egg yolk in the medium (regarded by many as vital for MAP growth) and the cultures were only held for 18 wk (MAP cultures for humans are usually held for at least 6 mo and up to one year).
The current MAP ELISA reacts to both host IgM and IgG. Modifying the assay into its isotype components, i.e., IgM and IgG, may permit a better determination of whether the host response reflects active disease or remote exposure. Further research is necessary in this area.
Should MAP be proven to cause many of the autoimmune diseases, a potential role in carcinogenesis should be explored. Helicobacter pylori is now recognized as playing a major role in the pathogenesis of primary gastric MALT lymphoma and gastric carcinoma[126]. CD patients are known to have increased risk of bowel cancer and lymphoma. Whether this increased risk is due to the immunosuppressive therapies used in this disease or due to infection by MAP is unknown and should be investigated further[127]. In summary, much more must be learned about this elusive and enigmatic organism and about the human diseases with which it is associated.
COMMENTS
Case characteristics
Please summarize main symptoms in one sentence. Case 1 had Crohn’s disease (CD) and experienced abdominal pain, diarrhea and weight loss while case 2 had complex regional pain syndrome (CRPS) and experienced generalized hypersensitivity, neuralgia, paresthesias and Raynaud’s phenomenon.
Clinical diagnosis
Please summarize main clinical findings in one sentence. Case 1 had CD while case 2 had CRPS.
Differential diagnosis
Please summarize thoughts and methods for differential diagnosis in one sentence. The differential diagnosis in case 1 included celiac disease and food allergy while the differential diagnosis in case 2 included multiple sclerosis.
Laboratory diagnosis
Please summarize laboratory testing methods and major findings in one sentence. Case 1 had anemia, elevated erythrocyte sedimentation rate, CRP and WBC while case 2 had elevated angiotensin converting enzyme, cryoglobulins, lymphocyte and eosinophil count and TSH and initially, both patients had blood cultures positive for Mycobacterium avium subsp. paratuberculosis (MAP).
Imaging diagnosis
Please summarize imaging methods and major findings in one sentence. Case 1 had multiple aphthous ulcers on upper gastrointestinal endoscopy and on colonoscopy while case 2 had an unremarkable EMG study.
Pathological diagnosis
Please summarize pathological methods and major findings in one sentence. Case 1 had granulomas in the gastric and colonic biopsies while case 2 had no biopsies.
Treatment
Please summarize treatments and drugs used in one sentence. Both case 1 and case 2 received a combination of periodic ultraviolet blood irradiation (UVBI) and antibiotics which included clarithromycin, rifampin and ciprofloxacin for at least 2 years.
Related reports
Please provide other contents related to the case report to help readers better understand the present case.
Term explanation
Please explain uncommon terms present in the case report. UVBI is ultraviolet blood irradiation which consists of periodic irradiation of approximately 200 cc of patient blood using ultraviolet light in the B and C regions.
Experiences and lessons
Please summarize experiences and lessons learnt from the case in one sentence. Both CD and CRPS, when caused by MAP, resolve when the organism is eradicated from the host.
Peer-review
Please summarize the strengths and weaknesses of the article based on the reviewers’ comments so that readers can obtain objective knowledge from the article. This study is anecdotal and it will be necessary to study large numbers of patients in a controlled trial setting to determine whether these results are reproducible.
Footnotes
Ethics approval: Because of the devastating nature of the diseases in case 1 and case 2 and the poor record of efficacy, standard therapies were eschewed. Institutional review board approval was not sought since the law allows off label use of FDA approved drugs and also allows the administration of UVBI in New York; IRB approval is generally not required in the care of individual patients.
Informed consent: Informed consent was not sought from each of the patients in this series of case reports since each patient was treated individually and did not enroll in a formal study. Unless an operative procedure or blood transfusion is intended, physicians caring for individual patients who are not part of a formal study, do not routinely seek informed consent from their patients.
Conflict-of-interest: Kuenstner and Petrie are shareowners of AVIcure Bioscience, LLC which has a proprietary interest in the UVBI therapy described above; Naser has a proprietary interest (US Patent 7488580 B1) in a MAP test which has been licensed to Quest Diagnostics Inc. Collins is a co-inventor of a MAP serologic assay (US Patent 8158371 B1) and consultant to IDEXX Laboratories, Inc. and Zoetis Diagnostics.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 27, 2014
First decision: November 14, 2014
Article in press: January 8, 2015
P- Reviewer: van der Have M S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Relman DA. Detection and identification of previously unrecognized microbial pathogens. Emerg Infect Dis. 1998;4:382–389. doi: 10.3201/eid0403.980310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 3.McClure HM, Chiodini RJ, Anderson DC, Swenson RB, Thayer WR, Coutu JA. Mycobacterium paratuberculosis infection in a colony of stumptail macaques (Macaca arctoides) J Infect Dis. 1987;155:1011–1019. doi: 10.1093/infdis/155.5.1011. [DOI] [PubMed] [Google Scholar]
- 4.St-Jean G, Jernigan AD. Treatment of Mycobacterium paratuberculosis infection in ruminants. Vet Clin North Am Food Anim Pract. 1991;7:793–804. doi: 10.1016/s0749-0720(15)31085-9. [DOI] [PubMed] [Google Scholar]
- 5.Grant IR, Ball HJ, Rowe MT. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows’ milk from approved dairy processing establishments in the United Kingdom. Appl Environ Microbiol. 2002;68:2428–2435. doi: 10.1128/AEM.68.5.2428-2435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellingson JL, Anderson JL, Koziczkowski JJ, Radcliff RP, Sloan SJ, Allen SE, Sullivan NM. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J Food Prot. 2005;68:966–972. doi: 10.4315/0362-028x-68.5.966. [DOI] [PubMed] [Google Scholar]
- 7.Singh SV, Kumar N, Sohal JS, Singh AV, Singh PK, Agrawal ND, Gupta S, Chaubey KK, Deb R, Dhama K, et al. First mass screening of the human population to estimate the bio-load of Mycobacterium avium subspecies paratuberculosis in North India. J Pub Health Epidemiol. 2014;6:20–29. [Google Scholar]
- 8.Dalziel TK. Chronic interstitial enteritis. Br Med J. 1913;2:1068–1070. [Google Scholar]
- 9.Chiodini RJ, Van Kruiningen HJ, Merkal RS, Thayer WR, Coutu JA. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn’s disease. J Clin Microbiol. 1984;20:966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendoza JL, Lana R, Díaz-Rubio M. Mycobacterium avium subspecies paratuberculosis and its relationship with Crohn’s disease. World J Gastroenterol. 2009;15:417–422. doi: 10.3748/wjg.15.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiappini E, de Martino M, Mangiantini F, Lionetti P. Crohn disease and mycobacterial infection in children: an intriguing relationship. J Pediatr Gastroenterol Nutr. 2009;49:550–558. doi: 10.1097/MPG.0b013e3181b0f908. [DOI] [PubMed] [Google Scholar]
- 12.Cohen RD. Mycobacterium in Crohn’s: something to ruminate about? Gastroenterology. 2005;128:2167–2168. doi: 10.1053/j.gastro.2005.02.069. [DOI] [PubMed] [Google Scholar]
- 13.Shanahan F, O’Mahony J. The mycobacteria story in Crohn’s disease. Am J Gastroenterol. 2005;100:1537–1538. doi: 10.1111/j.1572-0241.2005.50358.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuenstner JT. Mycobacterium avium subspecies paratuberculosis: a human pathogen causing most cases of Crohn’s disease. Am J Gastroenterol. 2006;101:1157–1158; author reply 1158. doi: 10.1111/j.1572-0241.2006.00583_5.x. [DOI] [PubMed] [Google Scholar]
- 15.Hermon-Taylor J, Barnes N, Clarke C, Finlayson C. Mycobacterium paratuberculosis cervical lymphadenitis, followed five years later by terminal ileitis similar to Crohn’s disease. BMJ. 1998;316:449–453. doi: 10.1136/bmj.316.7129.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sechi LA, Scanu AM, Molicotti P, Cannas S, Mura M, Dettori G, Fadda G, Zanetti S. Detection and Isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn’s disease in Sardinia. Am J Gastroenterol. 2005;100:1529–1536. doi: 10.1111/j.1572-0241.2005.41415.x. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood CD, Wagner J, Boniface K, Vaughan J, Michalski WP, Catto-Smith AG, Cameron DJ, Bishop RF. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn’s disease. Inflamm Bowel Dis. 2009;15:1643–1655. doi: 10.1002/ibd.20967. [DOI] [PubMed] [Google Scholar]
- 18.Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, Pfyffer GE, Jemmi T, Baumgartner A, Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]
- 19.Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn’s disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401–410. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 20.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza JL, San-Pedro A, Culebras E, Cíes R, Taxonera C, Lana R, Urcelay E, de la Torre F, Picazo JJ, Díaz-Rubio M. High prevalence of viable Mycobacterium avium subspecies paratuberculosis in Crohn’s disease. World J Gastroenterol. 2010;16:4558–4563. doi: 10.3748/wjg.v16.i36.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naser S, Collins M, Crawford J. Culture of Mycobacterium avium subspecies paratuberculosis (MAP) from the blood of patients with Crohn’s disease: a follow-up blind multi center investigation. Open Inf J. 2009;2:22–23. [Google Scholar]
- 23.Selby W, Pavli P, Crotty B, Florin T, Radford-Smith G, Gibson P, Mitchell B, Connell W, Read R, Merrett M, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology. 2007;132:2313–2319. doi: 10.1053/j.gastro.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Xu HB, Jiang RH, Li L. Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2014;33:347–358. doi: 10.1007/s10096-013-1962-1. [DOI] [PubMed] [Google Scholar]
- 25.Kuenstner JT. The Australian antibiotic trial in Crohn’s disease: alternative conclusions from the same study. Gastroenterology. 2007;133:1742–1743; author reply 1745-1746. doi: 10.1053/j.gastro.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Lipton JE, Barash DP. Flawed Australian CD study does not end MAP controversy. Gastroenterology. 2007;133:1742; author reply 1745–1746. doi: 10.1053/j.gastro.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Peyrin-Biroulet L, Neut C, Colombel JF. Antimycobacterial therapy in Crohn’s disease: game over? Gastroenterology. 2007;132:2594–2598. doi: 10.1053/j.gastro.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M. Long-term antibiotic treatment for Crohn’s disease: systematic review and meta-analysis of placebo-controlled trials. Clin Infect Dis. 2010;50:473–480. doi: 10.1086/649923. [DOI] [PubMed] [Google Scholar]
- 29.Pinedo PJ, Buergelt CD, Donovan GA, Melendez P, Morel L, Wu R, Langaee TY, Rae DO. Association between CARD15/NOD2 gene polymorphisms and paratuberculosis infection in cattle. Vet Microbiol. 2009;134:346–352. doi: 10.1016/j.vetmic.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 30.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 31.Schurr E, Gros P. A common genetic fingerprint in leprosy and Crohn’s disease? N Engl J Med. 2009;361:2666–2668. doi: 10.1056/NEJMe0910690. [DOI] [PubMed] [Google Scholar]
- 32.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golan L, Livneh-Kol A, Gonen E, Yagel S, Rosenshine I, Shpigel NY. Mycobacterium avium paratuberculosis invades human small-intestinal goblet cells and elicits inflammation. J Infect Dis. 2009;199:350–354. doi: 10.1086/596033. [DOI] [PubMed] [Google Scholar]
- 34.Dow CT. Paratuberculosis and Type I diabetes: is this the trigger? Med Hypotheses. 2006;67:782–785. doi: 10.1016/j.mehy.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Satoh J, Kojima Y, Negoro K, Hirai M, Hinokio Y, Kinouchi Y, Suzuki S, Matsuura N, Shimosegawa T, et al. Promoter polymorphism of SLC11A1 (formerly NRAMP1) confers susceptibility to autoimmune type 1 diabetes mellitus in Japanese. Tissue Antigens. 2004;63:231–236. doi: 10.1111/j.1399-0039.2004.000172.x. [DOI] [PubMed] [Google Scholar]
- 36.Jin J, Sun L, Jiao W, Zhao S, Li H, Guan X, Jiao A, Jiang Z, Shen A. SLC11A1 (Formerly NRAMP1) gene polymorphisms associated with pediatric tuberculosis in China. Clin Infect Dis. 2009;48:733–738. doi: 10.1086/597034. [DOI] [PubMed] [Google Scholar]
- 37.Sechi LA, Rosu V, Pacifico A, Fadda G, Ahmed N, Zanetti S. Humoral immune responses of type 1 diabetes patients to Mycobacterium avium subsp. paratuberculosis lend support to the infectious trigger hypothesis. Clin Vaccine Immunol. 2008;15:320–326. doi: 10.1128/CVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosu V, Ahmed N, Paccagnini D, Gerlach G, Fadda G, Hasnain SE, Zanetti S, Sechi LA. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS One. 2009;4:e4386. doi: 10.1371/journal.pone.0004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bitti ML, Masala S, Capasso F, Rapini N, Piccinini S, Angelini F, Pierantozzi A, Lidano R, Pietrosanti S, Paccagnini D, et al. Mycobacterium avium subsp. paratuberculosis in an Italian cohort of type 1 diabetes pediatric patients. Clin Dev Immunol. 2012;2012:785262. doi: 10.1155/2012/785262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naser SA, Thanigachalam S, Dow CT, Collins MT. Exploring the role of Mycobacterium avium subspecies paratuberculosis in the pathogenesis of type 1 diabetes mellitus: a pilot study. Gut Pathog. 2013;5:14. doi: 10.1186/1757-4749-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schernthaner G, Schwarzer C, Kuzmits R, Müller MM, Klemen U, Freyler H. Increased angiotensin-converting enzyme activities in diabetes mellitus: analysis of diabetes type, state of metabolic control and occurrence of diabetic vascular disease. J Clin Pathol. 1984;37:307–312. doi: 10.1136/jcp.37.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dyk DJ, Erman A, Erman T, Chen-Gal B, Sulkes J, Boner G. Increased serum angiotensin converting enzyme activity in type I insulin-dependent diabetes mellitus: its relation to metabolic control and diabetic complications. Eur J Clin Invest. 1994;24:463–467. doi: 10.1111/j.1365-2362.1994.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 43.Chiarelli F, Spagnoli A, Basciani F, Tumini S, Mezzetti A, Cipollone F, Cuccurullo F, Morgese G, Verrotti A. Vascular endothelial growth factor (VEGF) in children, adolescents and young adults with Type 1 diabetes mellitus: relation to glycaemic control and microvascular complications. Diabet Med. 2000;17:650–656. doi: 10.1046/j.1464-5491.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 44.Alatas F, Alatas O, Metintas M, Ozarslan A, Erginel S, Yildirim H. Vascular endothelial growth factor levels in active pulmonary tuberculosis. Chest. 2004;125:2156–2159. doi: 10.1378/chest.125.6.2156. [DOI] [PubMed] [Google Scholar]
- 45.Sakly W, Mankaï A, Sakly N, Thabet Y, Achour A, Ghedira-Besbes L, Jeddi M, Ghedira I. Anti-Saccharomyces cerevisiae antibodies are frequent in type 1 diabetes. Endocr Pathol. 2010;21:108–114. doi: 10.1007/s12022-010-9118-7. [DOI] [PubMed] [Google Scholar]
- 46.Gimeno SG, de Souza JM. IDDM and milk consumption. A case-control study in São Paulo, Brazil. Diabetes Care. 1997;20:1256–1260. doi: 10.2337/diacare.20.8.1256. [DOI] [PubMed] [Google Scholar]
- 47.Virtanen SM, Läärä E, Hyppönen E, Reijonen H, Räsänen L, Aro A, Knip M, Ilonen J, Akerblom HK. Cow’s milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Childhood diabetes in Finland study group. Diabetes. 2000;49:912–917. doi: 10.2337/diabetes.49.6.912. [DOI] [PubMed] [Google Scholar]
- 48.Frau J, Cossu D, Coghe G, Lorefice L, Fenu G, Melis M, Paccagnini D, Sardu C, Murru MR, Tranquilli S, et al. Mycobacterium avium subsp. paratuberculosis and multiple sclerosis in Sardinian patients: epidemiology and clinical features. Mult Scler. 2013;19:1437–1442. doi: 10.1177/1352458513477926. [DOI] [PubMed] [Google Scholar]
- 49.Malosse D, Perron H, Sasco A, Seigneurin JM. Correlation between milk and dairy product consumption and multiple sclerosis prevalence: a worldwide study. Neuroepidemiology. 1992;11:304–312. doi: 10.1159/000110946. [DOI] [PubMed] [Google Scholar]
- 50.Shin AR, Kim HJ, Cho SN, Collins MT, Manning EJ, Naser SA, Shin SJ. Identification of seroreactive proteins in the culture filtrate antigen of Mycobacterium avium ssp. paratuberculosis human isolates to sera from Crohn’s disease patients. FEMS Immunol Med Microbiol. 2010;58:128–137. doi: 10.1111/j.1574-695X.2009.00617.x. [DOI] [PubMed] [Google Scholar]
- 51.Bull TJ, McMinn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P, Rhodes G, Pickup R, Hermon-Taylor J. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. J Clin Microbiol. 2003;41:2915–2923. doi: 10.1128/JCM.41.7.2915-2923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckstein TM, Chandrasekaran S, Mahapatra S, McNeil MR, Chatterjee D, Rithner CD, Ryan PW, Belisle JT, Inamine JM. A major cell wall lipopeptide of Mycobacterium avium subspecies paratuberculosis. J Biol Chem. 2006;281:5209–5215. doi: 10.1074/jbc.M512465200. [DOI] [PubMed] [Google Scholar]
- 53.Gui GP, Thomas PR, Tizard ML, Lake J, Sanderson JD, Hermon-Taylor J. Two-year-outcomes analysis of Crohn’s disease treated with rifabutin and macrolide antibiotics. J Antimicrob Chemother. 1997;39:393–400. doi: 10.1093/jac/39.3.393. [DOI] [PubMed] [Google Scholar]
- 54.Borody TJ, Bilkey S, Wettstein AR, Leis S, Pang G, Tye S. Anti-mycobacterial therapy in Crohn’s disease heals mucosa with longitudinal scars. Dig Liver Dis. 2007;39:438–444. doi: 10.1016/j.dld.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Chamberlin W, Naser SA. Blood cultures of 19 Crohn’s disease patients. Am J Gastroenterol. 2008;103:802–803. doi: 10.1111/j.1572-0241.2007.01612_6.x. [DOI] [PubMed] [Google Scholar]
- 56.Chamberlin W, Ghobrial G, Chehtane M, Naser SA. Successful treatment of a Crohn’s disease patient infected with bacteremic Mycobacterium paratuberculosis. Am J Gastroenterol. 2007;102:689–691. doi: 10.1111/j.1572-0241.2007.01040_7.x. [DOI] [PubMed] [Google Scholar]
- 57.Bonomo L, Dammacco F, Meneghini C, LoSpalluto M. Cryoglobulinemia in lepromatous leprosy: an immune complex phenomenon. Int J Lepr Other Mycobact Dis. 1971;39:554–555. [PubMed] [Google Scholar]
- 58.Vázquez-Escobosa C, Gómez-Estrada H, González-Mendoza A, Barba-Rubio J. Circulating immune complexes in patients with nodular lepromatous leprosy. Arch Invest Med (Mex) 1982;13:181–183 passim. [PubMed] [Google Scholar]
- 59.Teruel JL, Matesanz R, Mampaso F, Lamas S, Herrero JA, Ortuno J. Pulmonary tuberculosis, cryoglobulinemia and immunecomplex glomerulonephritis. Clin Nephrol. 1987;27:48–49. [PubMed] [Google Scholar]
- 60.Lieberman J, Rea TH. Serum angiotensin-converting enzyme in leprosy and coccidioidomycosis. Ann Intern Med. 1977;87:423–425. doi: 10.7326/0003-4819-87-4-422. [DOI] [PubMed] [Google Scholar]
- 61.Fernández Jorge MA, Alonso Mallo E. [Angiotensin-converting enzyme (ACE) in sarcoidosis, tuberculosis, silicosis, and coal mining workers] An Med Interna. 1994;11:588–590. [PubMed] [Google Scholar]
- 62.Read JM. Lymphocytosis: a clinical study from group diagnosis. Boston Med Surg J. 1917;177:691–695. [Google Scholar]
- 63.Elsehety A, Bertorini TE. Neurologic and neuropsychiatric complications of Crohn’s disease. South Med J. 1997;90:606–610. doi: 10.1097/00007611-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Satsangi J, Parkes M, Jewell DP, Bell JI. Genetics of inflammatory bowel disease. Clin Sci (Lond) 1998;94:473–478. doi: 10.1042/cs0940473. [DOI] [PubMed] [Google Scholar]
- 65.McMinn M. Hydrogen peroxide touted for intractable pain, suggesting an infectious component. Anesthesiology News. 1995:4. [Google Scholar]
- 66.Walker SL, Lockwood DN. Leprosy type 1 (reversal) reactions and their management. Lepr Rev. 2008;79:372–386. [PubMed] [Google Scholar]
- 67.Selby WS. Mycobacterium avium subspecies paratuberculosis bacteraemia in patients with inflammatory bowel disease. Lancet. 2004;364:1013–1014. doi: 10.1016/S0140-6736(04)17071-2. [DOI] [PubMed] [Google Scholar]
- 68.Collins MT. Update on paratuberculosis: 1. Epidemiology of Johne’s disease and the biology of Mycobacterium paratuberculosis. Irish Veterin J. 2003;56:565–574. [Google Scholar]
- 69.Markowitz J. The natural history of Pediatric Crohn Disease. Pediatric Inflammatory Bowel Disease. In: Mamula P, Markowitz J, Baldassano R, editors. New York: Springer; 2008. p. 68. [Google Scholar]
- 70.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 71.Meyerovitch J, Rotman-Pikielny P, Sherf M, Battat E, Levy Y, Surks MI. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533–1538. doi: 10.1001/archinte.167.14.1533. [DOI] [PubMed] [Google Scholar]
- 72.Suter LG, Murabito JM, Felson DT, Fraenkel L. The incidence and natural history of Raynaud’s phenomenon in the community. Arthritis Rheum. 2005;52:1259–1263. doi: 10.1002/art.20988. [DOI] [PubMed] [Google Scholar]
- 73.Dennis MM, Reddacliff LA, Whittington RJ. Longitudinal study of clinicopathological features of Johne’s disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis. Vet Pathol. 2011;48:565–575. doi: 10.1177/0300985810375049. [DOI] [PubMed] [Google Scholar]
- 74.Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine, 17th Edition (Harrison’s Principles of Internal Medicine (Single Vol.)) New York: McGraw-Hill Professional; 2008. p. 1342. [Google Scholar]
- 75.Brady C, O’Grady D, O’Meara F, Egan J, Bassett H. Relationships between clinical signs, pathological changes and tissue distribution of Mycobacterium avium subspecies paratuberculosis in 21 cows from herds affected by Johne’s disease. Vet Rec. 2008;162:147–152. doi: 10.1136/vr.162.5.147. [DOI] [PubMed] [Google Scholar]
- 76.Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, Chan AT. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–1692. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tamboli C. A hypothesis for explaining the geographical distribution of Crohn’s disease. Can J Gastroenterol. 1996;10:173–177. [Google Scholar]
- 78.Huotari A, Herzig KH. Vitamin D and living in northern latitudes--an endemic risk area for vitamin D deficiency. Int J Circumpolar Health. 2008;67:164–178. doi: 10.3402/ijch.v67i2-3.18258. [DOI] [PubMed] [Google Scholar]
- 79.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 81.Khalili H, Higuchi LM, Ananthakrishnan AN, Richter JM, Feskanich D, Fuchs CS, Chan AT. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62:1153–1159. doi: 10.1136/gutjnl-2012-302362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, Li J, Fenton A, Williams T, Miller MK, et al. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4:10. doi: 10.1186/2042-6410-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giannoni E, Guignard L, Knaup Reymond M, Perreau M, Roth-Kleiner M, Calandra T, Roger T. Estradiol and progesterone strongly inhibit the innate immune response of mononuclear cells in newborns. Infect Immun. 2011;79:2690–2698. doi: 10.1128/IAI.00076-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trusted medical and veterinary information. Available from: http://www.merckmanual.com.
- 85.Buckhorn Trophy Products. Bio-tec research, Inc. Available from: http://www.deerfood.com.
- 86.Knott EK. Development of ultraviolet blood irradiation. Am J Surg. 1948;76:165–171. doi: 10.1016/0002-9610(48)90068-3. [DOI] [PubMed] [Google Scholar]
- 87.Niels Ryberg Finsen – Biographical. The Nobel Prize in Physiology or Medicine 1903. Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/finsen-bio.html.
- 88.Hancock V. Treatment of blood stream infections with hemo-irradiation. Am J Surg. 1942;3:336–344. [Google Scholar]
- 89.Rebbeck EW. Ultraviolet irradiation of blood in the treatment of Escherichia coli septicemia. Read at the Twenty-first Annual Session of the American Congress of Physical Therapy. Pittsburgh, Pa: American Congress of Physical Therapy; 1942. [Google Scholar]
- 90.Barrett HA. The irradiation of autotransfused blood by ultraviolet spectral energy: results of therapy in 110 cases. Med Clin North Am. 1940;24:723–732. [Google Scholar]
- 91.Sukhodub LF, Tertyshnyĭ NG, Duzhyĭ ID, Pliskachev VM. [Ultraviolet irradiation of blood in patients with pulmonary tuberculosis] Probl Tuberk. 1991;(7):65–68. [PubMed] [Google Scholar]
- 92.Kuvshinchikova VN, Shmelev EI, Mishin VIu. [Effectiveness of extracorporeal ultraviolet blood irradiation in treatment of chronic obstructive bronchitis in pulmonary tuberculosis] Probl Tuberk. 1998;(3):48–50. [PubMed] [Google Scholar]
- 93.Mingalimova RG, Vasil’eva GT, Karzakova LM, Usmanova EM. [Extracorporeal ultraviolet irradiation of blood in combined treatment of patients with pulmonary tuberculosis] Probl Tuberk. 1995;(3):27–28. [PubMed] [Google Scholar]
- 94.Zhadnov VZ, Mishanov RF, Kuznetsov AA, Shprykov AS, Ryzhakova TM. [Effectiveness of chemotherapy in combination with electrophoresis and ultraviolet irradiation of blood in newly diagnosed patients with destructive pulmonary tuberculosis] Probl Tuberk. 1995;(3):20–22. [PubMed] [Google Scholar]
- 95.Shurygin AA. [The efficiency of ultraviolet autologous blood irradiation used in the complex therapy of infiltrative pulmonary tuberculosis in children and adolescents] Probl Tuberk Bolezn Legk. 2009;(9):20–23. [PubMed] [Google Scholar]
- 96.Matsunaga T, Hieda K, Nikaido O. Wavelength dependent formation of thymine dimers and (6-4) photoproducts in DNA by monochromatic ultraviolet light ranging from 150 to 365 nm. Photochem Photobiol. 1991;54:403–410. doi: 10.1111/j.1751-1097.1991.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 97.Miller RL, Plagemann PG. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol. 1974;13:729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darwin KH, Nathan CF. Role for nucleotide excision repair in virulence of Mycobacterium tuberculosis. Infect Immun. 2005;73:4581–4587. doi: 10.1128/IAI.73.8.4581-4587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tuck A, Smith S, Larcom L. Chronic lymphocytic leukemia lymphocytes lack the capacity to repair UVC-induced lesions. Mutat Res. 2000;459:73–80. doi: 10.1016/s0921-8777(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 100.Masaki H, Atsumi T, Sakurai H. Detection of hydrogen peroxide and hydroxyl radicals in murine skin fibroblasts under UVB irradiation. Biochem Biophys Res Commun. 1995;206:474–479. doi: 10.1006/bbrc.1995.1067. [DOI] [PubMed] [Google Scholar]
- 101.Baadsgaard O, Wulf HC, Wantzin GL, Cooper KD. UVB and UVC, but not UVA, potently induce the appearance of T6- DR+ antigen-presenting cells in human epidermis. J Invest Dermatol. 1987;89:113–118. doi: 10.1111/1523-1747.ep12580461. [DOI] [PubMed] [Google Scholar]
- 102.Baadsgaard O, Cooper KD, Lisby S, Wulf HC, Wantzin GL. Dose response and time course for induction of T6- DR+ human epidermal antigen-presenting cells by in vivo ultraviolet A, B, and C irradiation. J Am Acad Dermatol. 1987;17:792–800. doi: 10.1016/s0190-9622(87)70265-5. [DOI] [PubMed] [Google Scholar]
- 103.Schwarz T. Mechanisms of UV-induced immunosuppression. Keio J Med. 2005;54:165–171. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 104.Pan L, Wang X, Yang S, Wu X, Lee I, Zhang X, Rupp RA, Xu J. Ultraviolet irradiation-dependent fluorescence enhancement of hemoglobin catalyzed by reactive oxygen species. PLoS One. 2012;7:e44142. doi: 10.1371/journal.pone.0044142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, Cano M, Dick J, Behrens A. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49:3402–3408. doi: 10.1167/iovs.07-1592. [DOI] [PubMed] [Google Scholar]
- 106.Hu Y, Mangan JA, Dhillon J, Sole KM, Mitchison DA, Butcher PD, Coates AR. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J Bacteriol. 2000;182:6358–6365. doi: 10.1128/jb.182.22.6358-6365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother. 2012;56:2223–2230. doi: 10.1128/AAC.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fréhel C, Offredo C, de Chastellier C. The phagosomal environment protects virulent Mycobacterium avium from killing and destruction by clarithromycin. Infect Immun. 1997;65:2792–2802. doi: 10.1128/iai.65.7.2792-2802.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirando WS, Shiratsuchi H, Tubesing K, Toba H, Ellner JJ, Elmets CA. Ultraviolet-irradiated monocytes efficiently inhibit the intracellular replication of Mycobacterium avium intracellulare. J Clin Invest. 1992;89:1282–1287. doi: 10.1172/JCI115713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greenstein RJ, Su L, Brown ST. Vitamins A & amp; D inhibit the growth of mycobacteria in radiometric culture. PLoS One. 2012;7:e29631. doi: 10.1371/journal.pone.0029631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 113.Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17:6–10. doi: 10.1177/0961203307085879. [DOI] [PubMed] [Google Scholar]
- 114.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agnew LL. Measuring intracellular hsp70 in leukocytes by flow cytometry. Curr Protoc Toxicol. 2011;Chapter 2:Unit2.21. doi: 10.1002/0471140856.tx0221s49. [DOI] [PubMed] [Google Scholar]
- 116.Matsuda M, Hoshino T, Yamashita Y, Tanaka K, Maji D, Sato K, Adachi H, Sobue G, Ihn H, Funasaka Y, et al. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J Biol Chem. 2010;285:5848–5858. doi: 10.1074/jbc.M109.063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 118.Cooke ED, Pockley AG, Tucker AT, Kirby JD, Bolton AE. Treatment of severe Raynaud’s syndrome by injection of autologous blood pretreated by heating, ozonation and exposure to ultraviolet light (H-O-U) therapy. Int Angiol. 1997;16:250–254. [PubMed] [Google Scholar]
- 119.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 120.RedHill Biopharma. Available from: http://www.redhillbio.com.
- 121.Cellona RV, Balagon MF, dela Cruz EC, Burgos JA, Abalos RM, Walsh GP, Topolski R, Gelber RH, Walsh DS. Long-term efficacy of 2 year WHO multiple drug therapy (MDT) in multibacillary (MB) leprosy patients. Int J Lepr Other Mycobact Dis. 2003;71:308–319. doi: 10.1489/1544-581X(2003)071<0308:LEOYWM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 122.Clark DL, Koziczkowski JJ, Radcliff RP, Carlson RA, Ellingson JL. Detection of Mycobacterium avium subspecies paratuberculosis: comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. J Dairy Sci. 2008;91:2620–2627. doi: 10.3168/jds.2007-0902. [DOI] [PubMed] [Google Scholar]
- 123.Greenstein RJ, Su L, Haroutunian V, Shahidi A, Brown ST. On the action of methotrexate and 6-mercaptopurine on M. avium subspecies paratuberculosis. PLoS One. 2007;2:e161. doi: 10.1371/journal.pone.0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin SJ, Collins MT. Thiopurine drugs azathioprine and 6-mercaptopurine inhibit Mycobacterium paratuberculosis growth in vitro. Antimicrob Agents Chemother. 2008;52:418–426. doi: 10.1128/AAC.00678-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parrish NM, Radcliff RP, Brey BJ, Anderson JL, Clark DL, Koziczkowski JJ, Ko CG, Goldberg ND, Brinker DA, Carlson RA, et al. Absence of mycobacterium avium subsp. paratuberculosis in Crohn's patients. Inflamm Bowel Dis. 2009;15:558–565. doi: 10.1002/ibd.20799. [DOI] [PubMed] [Google Scholar]
- 126.Copie-Bergman C, Locher C, Levy M, Chaumette MT, Haioun C, Delfau-Larue MH, Leroy K, Gaulard P, Delchier JC. Metachronous gastric MALT lymphoma and early gastric cancer: is residual lymphoma a risk factor for the development of gastric carcinoma? Ann Oncol. 2005;16:1232–1236. doi: 10.1093/annonc/mdi242. [DOI] [PubMed] [Google Scholar]
- 127.Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in Crohn disease patients. Ann Oncol. 2009;20:574–580. doi: 10.1093/annonc/mdn595. [DOI] [PubMed] [Google Scholar]


