Abstract
Mesalazine is a 5-aminosalicylic acid derivative that has been widely used to treat patients with inflammatory bowel disease. Accumulating evidence indicates that mesalazine has a very low rate of adverse drug reactions and is well tolerated by patients. However, a few cases of pulmonary and cardiac disease related to mesalazine have been reported in the past, though infrequently, preventing clinicians from diagnosing the conditions early. We describe the case of a 32-year-old man with ulcerative colitis who was admitted with a two-month history of persistent fever following mesalazine treatment initiated 14 mo earlier. At the time of admission, mesalazine dose was increased from 1.5 to 3.0 g/d, and antibiotic therapy was started with no improvement. Three weeks after admission, the patient developed dyspnea, non-productive cough, and chest pain. Severe eosinophilia was detected in laboratory tests, and a computed tomography scan revealed interstitial infiltrates in both lungs, as well as a large pericardial effusion. The bronchoalveolar lavage reported a CD4/CD8 ratio of 0.5, and an increased eosinophil count. Transbronchial biopsy examination showed a severe eosinophilic infiltrate of the lung tissue. Mesalazine-induced cardiopulmonary hypersensitivity was suspected after excluding other possible etiologies. Consequently, mesalazine treatment was suspended, and corticosteroid therapy was initiated, resulting in resolution of symptoms and radiologic abnormalities. We conclude that mesalazine-induced pulmonary and cardiac hypersensitivity should always be considered in the differential diagnosis of unexplained cardiopulmonary symptoms and radiographic abnormalities in patients with inflammatory bowel disease.
Keywords: Eosinophilia, Mesalazine, Pericardial effusion, Lung hypersensitivity, Ulcerative colitis
Core tip: We report a case of lung and cardiac hypersensitivity caused by mesalazine therapy in a patient with ulcerative colitis. Despite a few previously reported mesalazine-induced cardiac and pulmonary hypersensitivity cases, both entities are extremely infrequent making it difficult for the clinician to recognize these conditions during their early stages. An early diagnosis of these entities is extremely important, as the treatment consists of mesalazine suspension, usually resulting in a complete resolution of symptoms.
INTRODUCTION
Mesalazine, a 5-aminosalicylic acid derivative, is a medication widely used in the management of inflammatory bowel disease (IBD). The precise mechanism of mesalazine action remains poorly understood. However, it has been proposed that the drug acts locally on the colonic mucosa reducing inflammation through a variety of anti-inflammatory processes. These processes include the inhibition of proinflammatory cytokines (interleukin-1, -2, and -8 and tumor necrosis factor-α), the induction of the proliferator activated receptor-γ gene expression, or mesalazine acting as a potent antioxidant and free-radical scavenger[1]. The use of sulfasalazine in the treatment of IBD has been limited by the side effects, most of them secondary to the sulfapyridine component[2]. On the other hand, the use of mesalazine is usually well tolerated by patients, due to its favorable safety profile. Due to a limited number of cases of mesalazine-induced pulmonary disease and pericardial effusion, it has been difficult for clinicians to diagnose these diseases early. We describe the case of a patient with ulcerative colitis (UC) who, due to mesalazine treatment, simultaneously developed lung disease, pericardial effusion, and severe eosinophilia.
CASE REPORT
A 32-year-old non-smoking man with a 16-mo history of extensive UC treated with mesalazine (1.5 g/d) since the initial UC diagnosis and azathioprine (150 mg/d) for the last 13 mo was admitted to the hospital with a 2-mo history of asthenia, fever and night sweats. Prior to the appearance of the symptoms, UC was in clinical remission. Laboratory tests showed microcytic hypochromic anemia, a normal WBC count, and an increase in the erythrocyte sedimentation rate (91.0 mm/h) and the C-reactive protein level (10.3 mg/dL). Both chest radiograph and electrocardiogram were normal. At the admission, mesalazine dose was increased to 3 g/d. Blood, urine and stool samples were collected for culture prior to a 10-d course of intravenous antibiotic treatment with ciprofloxacin and metronidazole. Nevertheless, the patient continued to be febrile resulting in termination of the antibiotic therapy. Cultures drawn at admission, as well as serologic testing for human immunodeficiency virus, were all negative. A rectosigmoidoscopy showed no evidence of disease activity. A computed tomography (CT) scan of the chest revealed the presence of centrilobular pulmonary nodules in the left lower lobe and lingula, as well as mediastinal and axillary lymphadenopathy.
After a few days of hospitalization, a progressive increase in the WBC and eosinophil counts were detected in peripheral blood. Three weeks after admission, a blood test showed a WBC count of 12.6 × 109/L and a severe eosinophilia of 7.8 × 109/L (62.3%). Immunoglobulins (IgA, IgG and IgM) and complement levels were normal. Rheumatoid factor, anti-citrullinated peptide antibodies, and anti-nuclear antibodies were all negative, while the anti-neutrophil cytoplasmic antibody exhibited a positive cytoplasmic staining pattern (titer, 1:160). During this time, our patient developed clinical symptoms of dyspnea, a non-productive cough, and thoracic pain. A second CT scan was performed, revealing the presence of a patchy ground glass opacification, centrilobular pulmonary nodules extending to both inferior lobes, and a 33.6-mm pericardial effusion not previously present (Figure 1A and B). An echocardiogram showed a large pericardial effusion with no signs of hemodynamic instability. Additionally, pulmonary function testing revealed a marked decrease of 66.8% in the diffusion capacity for carbon monoxide (DLCO). The tuberculin skin test revealed no induration, and the QuantiFERON TB-Gold test was also negative. Bronchoscopy findings reported an inflammatory stenosis of the left principal bronchia. The bronchoalveolar lavage (BAL) showed an eosinophilia of 72.0%, with CD4 and CD8 counts of 29.0 and 56.0%, respectively (CD4/CD8 ratio: 0.52). Transbronchial biopsy examination demonstrated the presence of a dense eosinophilic infiltrate throughout the interstitium, alveolar spaces, and capillaries, consistent with eosinophilic pneumonia, but no indication of necrosis or granulomas (Figure 2).
Figure 1.
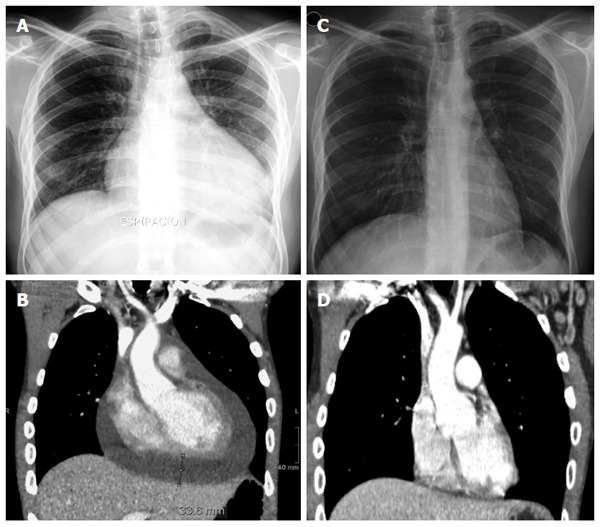
Radiograph of the chest and computed tomography scan before and after mesalazine suspension. A: Chest radiograph showing an enlarged cardiac silhouette due to a cardiac effusion during mesalazine treatment; B: Computed tomography (CT) scan of the chest revealing cardiomegaly due to a large pericardial effusion (maximum width of 33.6 mm) during mesalazine therapy; C: Normal chest radiograph after mesalazine withdrawal; D: CT scan of the chest showing a complete resolution of the pericardial effusion after suspension of mesalazine.
Figure 2.
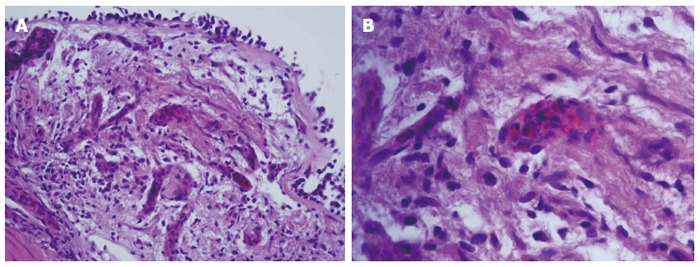
Transbronchial biopsy of the left inferior pulmonary lobe. A: An eosinophilic infiltrate throughout the alveolar septa, alveolar spaces and capillaries (HE stain, × 40); B: An eosinophilic infiltrate in the interior of capillaries (HE stain, × 100).
One month after admission, mesalazine-induced eosinophilic pneumonia, pericardial effusion and severe eosinophilia were suspected. Consequently, mesalazine was withdrawn, and therapy with prednisone was initiated. A few days after discontinuation of mesalazine, our patient had a quick and significant clinical improvement as indicated by normalization of the hemoglobin level and eosinophil count. In addition, a chest radiograph and a CT scan of the thorax revealed a complete resolution of the mediastinal and axillary lymphadenopathy, pericardial effusion, pulmonary nodules and infiltrates (Figure 1C and D). Despite continuous reduction in the DLCO (73.5%) 6 mo after discontinuation of mesalazine, our patient remained completely asymptomatic and capable of conducting a fully active life.
DISCUSSION
Clinicians treating patients with IBD exhibiting pulmonary symptoms face multiple causes for the onset of these symptoms. A respiratory involvement as an extraintestinal manifestation of UC (serositis, sarcoidosis, interstitial lung disease, or pulmonary embolism) should be taken into consideration. Furthermore, lung infections, triggered or worsened by the immunosuppressive medication, as well as pulmonary adverse reactions induced by the drugs used to treat the underlying disease, should also be considered as the underlying cause of the pulmonary symptoms in IBD patients[3].
The use of sulfasalazine has been related to multiple adverse drug reactions (ADRs), hence, mesalazine has become the first-line treatment for the induction and maintenance of remission of mildly to moderately active UC[2]. Although mesalazine is usually well tolerated by patients, some serious ADRs like hepatitis, blood dyscrasias, pancreatitis, and interstitial nephritis have been reported[4]. Mesalazine-induced pulmonary side effects are rare, and its pathogenesis is not well understood. Nevertheless, it is thought that two mechanisms may be responsible for these side effects: (1) a toxicity mechanism that could be dose-dependent; and (2) an immunologic mechanism that might be dose-independent[5,6].
Typical mesalazine-related side effects, which include fatigue, non-productive cough, fever, dyspnea and chest pain[7], usually appear after 1-6 mo of treatment. In very rare cases, patients treated with mesalazine exhibited side effects as early as a few days or several years after the treatment[6]. Laboratory tests may reveal peripheral eosinophilia while pulmonary nodules and an interstitial infiltrate with a ground glass pattern are usually seen in the chest radiograph or CT scan. If performed, the BAL frequently shows an elevated count of eosinophils or lymphocytes, and a reduction of the CD4/CD8 ratio. In addition, pulmonary function tests usually demonstrate a reduced DLCO. Histopathologic findings include interstitial lymphocytic infiltrates, alveolar eosinophilic infiltrates, alveolar fibrosis, and non-necrotizing granulomas[5].
Differentiating mesalazine-induced lung disease from IBD-related pulmonary manifestation, as well as establishing a diagnosis, is challenging. It is known that mesalazine-induced lung disease usually affects the lung parenchyma[8], whereas the IBD-related pulmonary manifestations typically involve the upper respiratory tract[9]. If the diagnosis remains unclear, a lung biopsy should be considered in order to exclude other conditions. Preferably, the presence of eosinophilia in peripheral blood, BAL, or lung tissue should be used as indicators for diagnosing mesalazine-induced lung disease. Otherwise, diagnosis should be based on clinical presentation, exclusion of other causes of lung disease, and a trial of drug discontinuation. One case reported a complete resolution of the symptoms after the reduction of the mesalazine dose[6]. In patients with severe respiratory symptoms or with a lack of improvement after mesalazine withdrawal, glucocorticoid therapy with prednisone (1 mg/kg per day) should be considered. Reintroduction of mesalazine is not usually recommended. Nonetheless, cases have been reported where rechallenge did not produce recurrence of pulmonary symptoms[10-12].
Thirty-eight cases of mesalazine-induced pulmonary disease were found in the literature. The principal characteristics of those cases are summarized in Table 1. There were 18 men (46.2%) and 21 women (53.8%) with a mean age of 42 years (range: 10-72 years) at the onset of symptoms. Thirty-three of them were diagnosed with UC (84.6%) and six had Crohn’s disease (15.4%). Mesalazine dose at the onset of symptoms varied from 750 mg to 4.8 g/d. The time between initiation of mesalazine treatment and the onset of pulmonary symptoms ranged from two days to 4-5 years. The most common symptoms were non-productive cough, fever and dyspnea, which were present in 74.0%, 72.0% and 64.0% of the patients, respectively. Eosinophilia in peripheral blood was reported in 18 patients (46.0%), and pulmonary infiltrates with an interstitial pattern were the most frequent radiologic finding, appearing in 73.0% of patients. Similarly, eosinophilic pneumonia was the most common histologic finding, appearing in 41.0% of the biopsies. Mesalazine was suspended in 38 patients (97.4%), and 23 of them (60.5%) received systemic glucocorticoids as part of the treatment. Rechallenge was tried in seven patients, but recurrence was only seen in four of them (57.1%).
Table 1.
Summary of previously published cases of mesalazine-induced pulmonary hypersensitivity in patients with inflammatory bowel disease
| Ref. | Age (yr)/sex | Disease | Daily dose | Duration of therapy1 | Symptoms | EOS | RP | HF | CD4/CD8 | BAL | DLCO | Steroid therapy | Rechallenge /recurrence |
| Le Gros et al[21] | 54/F | UC | 750 mg | 5 d | T, R | ND | I | ND | Ratio: 0.95 | Mo: 63% L: 35% E: 1.5% | 53% | No | No |
| Welte et al[22] | 67/M | UC | 1 g IR | 10 d | D, DC, R | ND | I | ND | ND | ND | ND | Yes | No |
| Reinoso et al[3] | 64/F | UC | 3.6 g | 2 yr | T, D, DC | ND | I | ND | ND | ND | ↓ | No | No |
| Lagler et al[23] | 66/M | UC | 1.5 g | 3.5 mo | D, DC | No | I | LP | ND | Mo: 30% L: 67% | 54% | Yes | No |
| Honeybourne et al[24] | 30/F | UC | 1.6 g | 7 mo | T, D, DC, CP | Yes 16% | ND | EP | ND | ND | ND | No | No |
| Declerck et al[25] | 45/F | UC | 3 g | 3 mo | D | No | I | ND | ND | Mo: 45% L: 38% E: 11% | ND | No | No |
| Muzzi et al[10] | 60/F | CD | 2.4 g | ND | T, D, DC | No | I | ND | CD4: 56% CD8: 31% Ratio: 1.80 | Mo: 40% L: 55% E: 3% | ND | No | Yes/No |
| Bitton et al[26] | 32/F | UC | 4 g | 9 mo | T, D, DC | Yes 8.9% | ND | LP/IF | ND | ND | ND | Yes | No |
| Sviri et al[27] | 49/M | CD | 3 g | 3.5 mo | T, D, DC | No | I | LP/IF | ND | L: 60% E: 10% | 80% | Yes | Yes/Yes |
| Lázaro et al[28] | 60/M | UC | ND | 4 wk | T, D, DC | Yes | I | IP | ND | Mo: 80% L: 8% E: 10% | 67% | No | No |
| Pascual-Lledó et al[29] | 64/F | CD | 3 g | 2 mo | D, DC, CP | No | I | NL | ND | ND | ND | No2 | No |
| Sesin et al[30] | 72/F | UC | 1.6-2.4 g3 | 2 mo | T, D, CP, PC | No | ND4 | ND | ND | ND | ND | No | No |
| Tanigawa et al[31] | 35/F | UC | 1.5 mg | 40 d | T, DC | Yes | I | EP | CD4: 44% CD8: 34% Ratio: 1.3 | Mo: 44% L: 49% E: 7% | ND | No | No |
| Guslandi et al[32] | 29/F | UC | 3 g | 2 d | D, CP | No | ND | ND | ND | ND | ND | No | Yes/Yes |
| Facchini et al[33] | 15/M | UC | 2.8 g | 4 mo | D, DC | No | A | ND | ND | ND | ND | Yes | No |
| Zamir et al[34] | 23/F | UC | ND | 6 wk | F, DC | Yes | ND | ND | ND | ND | ND | Yes | No |
| Saltzman et al[35] | 53/F | UC | ND | 4 mo | T, DC, R | Yes 27% | A | EP | ND | E: 79% | ND | Yes | No |
| Haralambou et al[36] | 18/F | UC | 1.6 g P.O. + 4 g (enema) | 2 mo | T, D, DC, CP | Yes 88% | I | BO | ND | ND | ND | Yes | No |
| Sossai et al[6] | 70/F | UC | 2.4 g | 3 mo | D, DC | No | I | IP | Ratio: 0.39 | Mo: 40% L: 60% | ND | No | N/A5 |
| Pérez et al[37] | 50/M | UC | 4 g | 2 mo | T, D, DC | ND | ND | EP | ND | ND | ND | Yes | No |
| Foster et al[5] | 44/M | CD | 2.4-4.8 g | 15 mo | T, D, DC | No | A | IP/IF | ND | N: 73% | ND | Yes | No |
| 30/F | UC | 4.8 g | 2 yr | T, D, DC, CP | No | A | IP/IF | ND | N: 43% | ND | No | No | |
| 29/F | UC | 3.6 g | 8 mo | T, D, DC | No | A | IP | ND | ND | 19% | Yes | No | |
| Hakoda et al[38] | 30/M | UC | 2.25 g | 4 wk | T, DC | Yes | ND | EP | ND | ND | ND | Yes | No |
| Actis et al[39] | 57/M | UC | ND | 2 yr | T, D, DC, CP | No | A | ND | ND | ND | ↓ | Yes | No |
| Kohli et al[40] | 10/F | UC | 3.2 g | 2 wk | T, D, DC | Yes 12% | I | IP | ND | ND | ND | Yes | Yes/Yes |
| Katsenos et al[41] | 18/M | UC | ND | 1 yr | T, PC | Yes | I | ND | ND | Mo: 15% L: 20% E: 60% | ND | Yes | No |
| Price et al[11] | 28/F | UC | 1.2 g | 4-5 yr | T, PC | Yes | B | ND | ND | N: 95% | ND | Yes | Yes/No6 |
| Iannone et al[42] | 32/F | UC | ND | 4 mo | T, DC | No | I | ND | ND | ND | 74% | Yes | No |
| Cilloniz et al[43] | 14/M | CD | 3 g | 8 mo | CP | No | I | IP | ND | Mo: 68% L: 27% | 93% | Yes | No |
| Park et al[44] | 35/M | CD | 4 g | 3 mo | T, DC | Yes 32% | I | EP | CD4: 54% CD8: 41% Ratio: 1.3 | L: 31% E: 41% | ND | No | No |
| Shimizu et al[45] | 50/F | UC | ND | 4 wk | T, DC | ND | ND | ND | ND | L: 58% E: 20% | ND | No | No |
| Sposato et al[46] | 42/M | UC | 3.2 g | 8 d | T, CP | Yes 14% | I | ND | ND | Mo: 13% E: 47% N: 34% | ND | Yes7 | No |
| Lamsiah et al[47] | 57/F | UC | ND (enemas) | 3 mo | T, D, DC | Yes | I | ND | ND | ND | ND | Yes | Yes/Yes8 |
| Kevans et al[48] | 17/M | UC | 4 g | 3 mo | D, DC, CP | Yes 23% | A | ND | ND | ND | ND | Yes | No |
| Abraham et al[49] | 65/M | UC | 4.8 g | 2 wk | T, D, DC9 | No | I | LP/IF | ND | ND | ND | Yes | No |
| Kim et al[50] | 30/F | UC | 1 g IR | 19 d | PC | Yes 24% | I | EP | ND | Mo: 73% N: 19% | ND | Yes | No |
| Michy et al[12] | 72/M | UC | ND | 4 mo | D | Yes | ND | EP | ND | L: 23% N: 28% E: 14% | ND | Yes | Yes/No10 |
| Current case | 32/M | UC | 1.5-3 g3 | 14 mo | T, D, DC, CP | Yes 62% | I | EP | CD4: 29% CD8: 56% Ratio: 0.51 | E: 72% | 67% | Yes | No |
Time under mesalazine treatment before symptoms appeared;
Patient was on low-dose Deflazacort during all the course of the pulmonary disease;
Mesalazine dose was increased after patient was admitted;
Bilateral pleural effusion was also noted;
Patient improved after reducing mesalazine dose;
Rechallenge was intended with olsalazine 1.5 g/d, with no recurrence;
Patient had no response to glucocorticoid therapy but showed improvement after mesalazine withdrawal;
After mesalazine removal a second rechallenge with sulfasalazine was intended with no recurrence of symptoms;
Patient required endotracheal intubation for severe respiratory insufficiency;
No recurrence was noted after the reintroduction of mesalazine enemas. A: Alveolar pattern; B: Bronchiectasis; BAL: Bronchoalveolar lavage; BO: Bronchiolitis obliterans; CD: Crohn’s disease; CP: Chest pain; CT: Corticoid therapy; D: Dyspnea; DC: Dry cough; DLCO: Diffusion capacity for carbon monoxide; E: Eosinophils; EOS: Eosinophilia; EP: Eosinophilic pneumonia; F: Female; HF: Histopathologic findings; I: Interstitial pattern; IF: Interstitial fibrosis; IP: Interstitial pneumonitis; IR: Intrarectal; L: Lymphocytes; LP: Lymphocytic pneumonitis; M: Male; Mo: Monocytes; N: Neutrophils; N/A: Not applicable; NC: No change; ND: No data available; NL: Normal; PC: Productive cough; PE: Pleural effusion; R: Rash; RP: Radiologic pattern; T: Fever; UC: Ulcerative colitis.
Cardiac disease as an extraintestinal manifestation of IBD is very rare. When it does occur, acute pericarditis is the most frequent form of presentation[13], but myocarditis, pericardial effusion, and cardiac tamponade have also been described[14,15]. Conversely, most cases of cardiac disease in patients with IBD are drug induced and, even when its pathogenesis is unclear, the consideration is that an idiosyncratic hypersensitivity reaction and a drug-induced lupus-like syndrome mechanism are related[16]. Although most cases of mesalazine-induced cardiac hypersensitivity in IBD patients are not severe, life-threatening complications have been reported[17]. The treatment for the condition consists of mesalazine suspension and administration of non-steroidal anti-inflammatory drugs or corticosteroids, taking into consideration that the former may exacerbate the underlying IBD in some patients[18]. Table 2 summarizes the principal characteristics of previously published cases of mesalazine-induced cardiac hypersensitivity in patients with IBD.
Table 2.
Summary of previously published cases of mesalazine-induced cardiac hypersensitivity in patients with inflammatory bowel disease
| Ref. | Age (yr)/sex | Disease | Daily dose | Duration of therapy | Symptoms | Cardiac disease | Pharmacologic treatment | Rechallenge /recurrence |
| Vayre et al[51] | 53/M | CD | 500 mg | 8 yr | T, CP | AP, PE | Prednisolone | No |
| Ishikawa et al[16] | 17/M | UC | 1.5 g | 2 wk | T, CP | AP, PE | Prednisolone | Yes/Yes |
| Doganay et al[52] | 21/M | UC | 2 g | 10 d | T, D | AM | Budesonide | No |
| García-Morán et al[17] | 39/M | UC | 4 g P.O. + 2 g (enemas) | 2 d | T, CP | AM, AMI | Methylprednisolone | No |
| Martín et al[53] | 22/M | UC | 3 g | ND | CP | AM | Corticosteroids | No |
| Cappell et al[15] | 32/M | UC | ND | 10 yr | T, CP, D | Chronic pericarditis, PT | Prednisone | No |
| Bernal-Sprekelsen et al[54] | 54/M | UC | 1.5 g | 3 wk | T, CP | AP, PE | ASA | Yes/Yes1 |
| Freeman et al[55] | 26/M | UC | 1.6 g | 3 wk | T, CP | AM | Hydrocortisone | Yes/No2 |
| Sierra Ausín et al[56] | 47/M | UC | 3 g P.O. + 1 g (enemas) | 3 wk | T, CP | AP | NSAIDs | No |
| Park et al[57] | 26/M | UC | 2.4 g | 1 mo | T, CP | AM, PE | ASA, prednisolone | Yes/Yes |
| Calafat et al[13] | 37/M | UC | 1 g IR | 1 mo | CP | AP, PE | ASA | No |
| 37/F | UC | 3g | 2 wk | CP | AP, PE | Analgesics | No | |
| Sonu et al[58] | 20/F | UC | Sulfasalazine 2 g + mesalazine (enemas)3 | 3 wk | CP | AM, PT | Ibuprofen and colchicine | Yes/Yes4 |
| Current case | 32/M | UC | 1.5-3 g5 | 14 mo | T, CP, D | AP, PE | Prednisone | No |
After mesalazine suspension a rechallenge with mesalazine 500 mg IR was intended with recurrence of symptoms;
After mesalazine suspension a rechallenge with sulfasalazine was intended without recurrence of symptoms;
Mesalazine dose was not specified;
After both sulfasalazine and mesalazine enemas were suspended, and low-dose balsalazide was initiated with recurrence of symptoms;
Mesalazine dose was increased after patient was admitted. AP: Acute pericarditis; AM: Acute myopericarditis; AMI: Acute mitral insufficiency; ASA: Acetylsalicylic acid; CD: Crohn’s disease; CP: Chest pain; D: Dyspnea; F: Female; IR: Intrarectal; M: Male; ND: No data available; NSAIDs: Nonsteroidal anti-inflammatory drugs; PE: Pericardial effusion; PT: Pericardial tamponade; T: Fever; UC: Ulcerative colitis.
The Naranjo algorithm scale[19] was used to assess the probability of ADRs. The Naranjo algorithm scale is a questionnaire designed to determine the likelihood of whether ADRs are secondary to a drug rather than the result of other factors. ADRs of ≥ 9 points were considered to be definite, probable at 5-8 points, possible at 1-4 points, and doubtful at 0 points. Our patient scored 8 points, suggesting probable ADRs. However, due to the presence of eosinophilia in the peripheral blood, BAL and lung biopsy, the patient’s deteriorating condition following mesalazine treatment followed by a significant improvement after discontinuation of mesalazine therapy, all strongly support our diagnosis, especially since other causes of cardiopulmonary disease were excluded. Azathioprine-induced pulmonary disease has also been described[20], nevertheless, it is by far less frequent and in this case it did not seem to contribute to the patient’s symptoms as he was receiving this medication at same dose for 13 mo prior to admission, throughout his entire hospitalization, and after being discharged. As clinical improvement was documented at the time mesalazine was stopped, we conclude this to be the cause.
Cardiopulmonary toxicity related to mesalazine is extremely infrequent making it difficult for clinicians to recognize and diagnose it in the regular practice. The drug-induced pulmonary and cardiac hypersensitivity should be considered in any IBD patient who develops unexplained lung or cardiac disease while on mesalazine. Early recognition of these ADRs may lead to prompt cessation of the drug, most likely resulting in a complete resolution of the symptoms and radiologic abnormalities.
ACKNOWLEDGMENTS
The authors would like to thank Stacie Griffis, MD and Eleanor Boyce for her help with the translation of the manuscript.
COMMENTS
Case characteristics
A 32-year-old man with ulcerative colitis presented with fever, dyspnea, non-productive cough, and chest pain 14 mo from the initiation of mesalazine treatment.
Clinical diagnosis
Mesalazine-induced eosinophilic pneumonia and pericardial effusion.
Differential diagnosis
Cardiorespiratory involvement as an extra-intestinal manifestation of ulcerative colitis (serositis, sarcoidosis, interstitial lung disease or pulmonary embolism); lung infections; and drug-induced adverse reactions.
Laboratory diagnosis
Microcytic hypochromic anemia, WBC count of 12.6 × 109/L, eosinophilia of 7.8 x 109/L (62.3%), diffusion capacity for carbon monoxide of 66.8%, and a bronchoalveolar lavage that reported an eosinophilia of 72.0%, with CD4 and CD8 counts of 29.0% and 56.0%, respectively (CD4/CD8 ratio: 0.51).
Imaging diagnosis
Computed tomography scan showed the presence of a patchy ground glass opacification, centrilobular pulmonary nodules that extended to both inferior lobes, and a large pericardial effusion of 33.6 mm. An echocardiogram confirmed the presence of a large pericardial effusion without evidence of hemodynamic instability.
Pathological diagnosis
A transbronchial biopsy examination showed the presence of a dense eosinophilic infiltrate throughout the interstitium, alveolar spaces, and capillaries, consistent with eosinophilic pneumonia.
Treatment
Mesalazine was suspended, and therapy with prednisone was initiated. Azathioprine therapy was continued at the same dose, before, during and after hospitalization.
Related reports
Mesalazine-induced pulmonary and cardiac hypersensitivity are extremely infrequent entities, making it difficult for clinicians to recognize them. This diagnosis was supported by the presence of eosinophilia in the peripheral blood, bronchoalveolar lavage, and lung biopsy, the deterioration of our patient after an increment of the mesalazine dose, as well as the improvement of the patient after discontinuation of mesalazine therapy. Nevertheless, elimination of other causes is required prior to establishing mesalazine-induced pulmonary and cardiac hypersensitivity diagnosis.
Experiences and lessons
An early recognition and an extensive diagnostic workup are essential in recognizing mesalazine-induced lung and cardiac hypersensitivity in inflammatory bowel disease patients as drug withdrawal may result in a favorable outcome for the patient.
Peer-review
The authors described an interesting case of a patient with ulcerative colitis who developed lung and cardiac hypersensitivity related to mesalazine therapy. This article highlights the importance of considering drug-induced pulmonary and cardiac hypersensitivity in all inflammatory bowel disease patients who develop unexplained lung or cardiac disease while receiving mesalazine treatment.
Footnotes
Ethics approval: The study was reviewed and approved by the Central University Hospital of Asturias research ethics committee.
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: Sabino Riestra has not received fees for serving as a speaker, a consultant or an advisory board member for any organizations.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 3, 2014
First decision: October 29, 2014
Article in press: January 21, 2015
P- Reviewer: Blonski W, Day AS, Shi RH, Tonelli F, Wittmann T S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Iacucci M, de Silva S, Ghosh S. Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol. 2010;24:127–133. doi: 10.1155/2010/586092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman R, Parkes M. Systematic review: the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;23:841–855. doi: 10.1111/j.1365-2036.2006.02846.x. [DOI] [PubMed] [Google Scholar]
- 3.Reinoso MA, Schroeder KW, Pisani RJ. Lung disease associated with orally administered mesalamine for ulcerative colitis. Chest. 1992;101:1469–1471. doi: 10.1378/chest.101.5.1469. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179–189. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- 5.Foster RA, Zander DS, Mergo PJ, Valentine JF. Mesalamine-related lung disease: clinical, radiographic, and pathologic manifestations. Inflamm Bowel Dis. 2003;9:308–315. doi: 10.1097/00054725-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sossai P, Cappellato MG, Stefani S. Can a drug-induced pulmonary hypersensitivity reaction be dose-dependent? A case with mesalamine. Mt Sinai J Med. 2001;68:389–395. [PubMed] [Google Scholar]
- 7.Schleiermacher D, Hoffmann JC. Pulmonary abnormalities in inflammatory bowel disease. J Crohns Colitis. 2007;1:61–69. doi: 10.1016/j.crohns.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Camus P, Piard F, Ashcroft T, Gal AA, Colby TV. The lung in inflammatory bowel disease. Medicine (Baltimore) 1993;72:151–183. [PubMed] [Google Scholar]
- 9.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011;7:235–241. [PMC free article] [PubMed] [Google Scholar]
- 10.Muzzi A, Ciani F, Bianchini D, Festini G, Volpe C. Adverse pulmonary effects of mesalamine. Chest. 1995;108:1181. doi: 10.1378/chest.108.4.1181-a. [DOI] [PubMed] [Google Scholar]
- 11.Price LC, Poullis A, Grubnic S, Kang JY, Rayner CF. Mesalazine-induced bronchiectasis and eosinophilia in a patient with ulcerative colitis: a case report. J R Soc Med. 2007;100:151–152. doi: 10.1258/jrsm.100.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michy B, Raymond S, Graffin B. [Organizing pneumonia during treatment with mesalazine] Rev Mal Respir. 2014;31:70–77. doi: 10.1016/j.rmr.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Calafat M, Mañosa M, Cabré E, Domènech E. [Acute pericarditis associated with oral or topical mesalazine therapy in patients with ulcerative colitis] Gastroenterol Hepatol. 2014;37:254–255. doi: 10.1016/j.gastrohep.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Jose FA, Heyman MB. Extraintestinal manifestations of inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2008;46:124–133. doi: 10.1097/MPG.0b013e318093f4b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappell MS, Turkieh A. Chronic pericarditis and pericardial tamponade associated with ulcerative colitis. Dig Dis Sci. 2008;53:149–154. doi: 10.1007/s10620-007-9836-y. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa N, Imamura T, Nakajima K, Yamaga J, Yuchi H, Ootsuka M, Inatsu H, Aoki T, Eto T. Acute pericarditis associated with 5-aminosalicylic acid (5-ASA) treatment for severe active ulcerative colitis. Intern Med. 2001;40:901–904. doi: 10.2169/internalmedicine.40.901. [DOI] [PubMed] [Google Scholar]
- 17.García-Morán S, Sáez-Royuela F, Pérez-Alvarez JC, Gento E, Téllez J. Myopericarditis and mitral insufficiency associated with ulcerative colitis treated with mesalazine. Inflamm Bowel Dis. 2006;12:334–335. doi: 10.1097/01.MIB.0000209788.19952.b7. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 19.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 20.Nagy F, Molnar T, Makula E, Kiss I, Milassin P, Zollei E, Tiszlavicz L, Lonovics J. A case of interstitial pneumonitis in a patient with ulcerative colitis treated with azathioprine. World J Gastroenterol. 2007;13:316–319. doi: 10.3748/wjg.v13.i2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.le Gros V, Saveuse H, Lesur G, Brion N. Lung and skin hypersensitivity to 5-aminosalicylic acid. BMJ. 1991;302:970. doi: 10.1136/bmj.302.6782.970-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welte T, Hamm H, Fabel H. Mesalazine alveolitis. Lancet. 1991;338:1273. doi: 10.1016/0140-6736(91)92140-w. [DOI] [PubMed] [Google Scholar]
- 23.Lagler U, Schulthess HK, Kuhn M. [Acute alveolitis due to mesalazine] Schweiz Med Wochenschr. 1992;122:1332–1334. [PubMed] [Google Scholar]
- 24.Honeybourne D. Mesalazine toxicity. BMJ. 1994;308:533–534. doi: 10.1136/bmj.308.6927.533b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Declerck D, Wallaert B, Demarcq-Delerue G, Tonnel AB. [Iatrogenic diffuse interstitial pneumonia linked to 5-aminosalicylate] Rev Mal Respir. 1994;11:292–293. [PubMed] [Google Scholar]
- 26.Bitton A, Peppercorn MA, Hanrahan JP, Upton MP. Mesalamine-induced lung toxicity. Am J Gastroenterol. 1996;91:1039–1040. [PubMed] [Google Scholar]
- 27.Sviri S, Gafanovich I, Kramer MR, Tsvang E, Ben-Chetrit E. Mesalamine-induced hypersensitivity pneumonitis. A case report and review of the literature. J Clin Gastroenterol. 1997;24:34–36. doi: 10.1097/00004836-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Lázaro MT, García-Tejero MT, Díaz-Lobato S. Mesalamine-induced lung disease. Arch Intern Med. 1997;157:462. doi: 10.1001/archinte.1997.00440250122018. [DOI] [PubMed] [Google Scholar]
- 29.Pascual-Lledó JF, Calvo-Bonachera J, Carrasco-Miras F, Sanchez-Martínez H. Interstitial pneumonitis due to mesalamine. Ann Pharmacother. 1997;31:499. doi: 10.1177/106002809703100421. [DOI] [PubMed] [Google Scholar]
- 30.Sesin GP, Mucciardi N, Almeida S. Mesalamine-associated pleural effusion with pulmonary infiltration. Am J Health Syst Pharm. 1998;55:2304–2305. doi: 10.1093/ajhp/55.21.2304. [DOI] [PubMed] [Google Scholar]
- 31.Tanigawa K, Sugiyama K, Matsuyama H, Nakao H, Kohno K, Komuro Y, Iwanaga Y, Eguchi K, Kitaichi M, Takagi H. Mesalazine-induced eosinophilic pneumonia. Respiration. 1999;66:69–72. doi: 10.1159/000029341. [DOI] [PubMed] [Google Scholar]
- 32.Guslandi M. Respiratory distress during mesalamine therapy. Dig Dis Sci. 1999;44:48–49. doi: 10.1023/a:1026641830958. [DOI] [PubMed] [Google Scholar]
- 33.Facchini S, Candusso M, Zennaro F, Ventura A. Clinical quiz. 5-ASA hypersensitivity lung disease. J Pediatr Gastroenterol Nutr. 1999;29:349,357. doi: 10.1097/00005176-199909000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Zamir D, Weizman J, Zamir C, Fireman Z, Weiner P. [Mesalamine-induced hypersensitivity pneumonitis] Harefuah. 1999;137:28–30, 87, 86. [PubMed] [Google Scholar]
- 35.Saltzman K, Rossoff LJ, Gouda H, Tongia S. Mesalamine-induced unilateral eosinophilic pneumonia. AJR Am J Roentgenol. 2001;177:257. doi: 10.2214/ajr.177.1.1770257. [DOI] [PubMed] [Google Scholar]
- 36.Haralambou G, Teirstein AS, Gil J, Present DH. Bronchiolitis obliterans in a patient with ulcerative colitis receiving mesalamine. Mt Sinai J Med. 2001;68:384–388. [PubMed] [Google Scholar]
- 37.Pérez C, Errázuriz I, Brockmann P, González S, Cofré C. [Eosinophilic pneumonia caused by mesalazine. Report of one case] Rev Med Chil. 2003;131:81–84. [PubMed] [Google Scholar]
- 38.Hakoda Y, Aoshima M, Kinoshita M, Sakurai M, Ohyashiki K. [A case of eosinophilic pneumonia possibly associated with 5-aminosalicylic acid (5-ASA)] Nihon Kokyuki Gakkai Zasshi. 2004;42:404–409. [PubMed] [Google Scholar]
- 39.Actis GC, Ottobrelli A, Baldi S, Scappaticci E, Modena V, Fusaro E, Mengozzi G, Rizzetto M. Mesalamine-induced lung injury in a patient with ulcerative colitis and a confounding autoimmune background: a case report. Mt Sinai J Med. 2005;72:136–140. [PubMed] [Google Scholar]
- 40.Kohli R, Melin-Aldana H, Sentongo TA. Mesalamine-induced pneumonitis during therapy for chronic inflammatory bowel disease: a pediatric case report. J Pediatr Gastroenterol Nutr. 2005;41:479–482. doi: 10.1097/01.mpg.0000173601.31647.84. [DOI] [PubMed] [Google Scholar]
- 41.Katsenos S, Psathakis K, Kokkonouzis I, Panagou P, Tsintiris K, Bouros D. Drug-induced pulmonary toxicity in a patient treated with mesalazine and azathioprine for ulcerative colitis. Acta Gastroenterol Belg. 2007;70:290–292. [PubMed] [Google Scholar]
- 42.Iannone P, Lenzi T. An unusual case of pneumonia. Intern Emerg Med. 2008;3:391–393. doi: 10.1007/s11739-008-0146-y. [DOI] [PubMed] [Google Scholar]
- 43.Cilloniz R, Chesrown SE, Gonzalez-Peralta RP. Asymptomatic presentation of mesalamine-induced lung injury in an adolescent with Crohn disease. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.09.2008.0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JE, Hwangbo Y, Chang R, Chang YW, Jang JY, Kim BH, Dong SH, Kim HJ. [Mesalazine-induced eosinophilic pneumonia in a patient with Crohn’s disease] Korean J Gastroenterol. 2009;53:116–120. [PubMed] [Google Scholar]
- 45.Shimizu T, Hayashi M, Shimizu N, Kinebuchi S, Toyama J. [A case of mesalazine-induced lung injury improved by only discontinuation of mesalazine] Nihon Kokyuki Gakkai Zasshi. 2009;47:543–547. [PubMed] [Google Scholar]
- 46.Sposato B, Allegri MP, Riccardi MP, Chigiotti S, Nencioni C, Ricciardi B, Carli T, Cresti A, Perari MG, Migliorini MG, et al. Mesalazine-induced multi-organ hypersensitivity. Clin Drug Investig. 2010;30:413–417. doi: 10.1007/BF03256911. [DOI] [PubMed] [Google Scholar]
- 47.Lamsiah T, Moudden K, Baaj M, Hadri L. [Interstitial pneumonia related to mesalamine] Gastroenterol Clin Biol. 2010;34:224–226. doi: 10.1016/j.gcb.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Kevans D, Greene J, Galvin L, Morgan R, Murray FE. Mesalazine-induced bronchiolitis obliterans organizing pneumonia (BOOP) in a patient with ulcerative colitis and primary sclerosing cholangitis. Inflamm Bowel Dis. 2011;17:E137–E138. doi: 10.1002/ibd.21819. [DOI] [PubMed] [Google Scholar]
- 49.Abraham A, Karakurum A. Acute respiratory failure secondary to mesalamine-induced interstitial pneumonitis. BMJ Case Rep. 2013;2013:bcr2013009834. doi: 10.1136/bcr-2013-009834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JH, Lee JH, Koh ES, Park SW, Jang AS, Kim D, Park CS. Acute eosinophilic pneumonia related to a mesalazine suppository. Asia Pac Allergy. 2013;3:136–139. doi: 10.5415/apallergy.2013.3.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vayre F, Vayre-Oundjian L, Monsuez JJ. Pericarditis associated with longstanding mesalazine administration in a patient. Int J Cardiol. 1999;68:243–245. doi: 10.1016/s0167-5273(98)00367-2. [DOI] [PubMed] [Google Scholar]
- 52.Doganay L, Akinci B, Pekel N, Simsek I, Akpinar H. Mesalazine-induced myopericarditis in a patient with ulcerative colitis. Int J Colorectal Dis. 2006;21:199–200. doi: 10.1007/s00384-004-0706-1. [DOI] [PubMed] [Google Scholar]
- 53.Martín M, Santamarta E, de la Iglesia JM, Saiz A. [Myopericarditis in a patient with ulcerative colitis treated with mesalazine] Med Clin (Barc) 2010;134:43–44. doi: 10.1016/j.medcli.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Bernal-Sprekelsen JC, de las Marinas MD, Salvador A, Landete FJ, Morera FJ. Recurrent pericarditis in a patient with ulcerative proctitis due to mesalazine suppositories. Int J Colorectal Dis. 2010;25:1143–1144. doi: 10.1007/s00384-010-0921-x. [DOI] [PubMed] [Google Scholar]
- 55.Freeman HJ, Salh B. Recurrent myopericarditis with extensive ulcerative colitis. Can J Cardiol. 2010;26:549–550. doi: 10.1016/s0828-282x(10)70470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sierra Ausín M, Rascarachi G, Díez Rodríguez R, Arias Rodríguez L, Del Pozo Maroto E, Muñoz Núñez F. [Mesalazine-induced acute pericarditis] Gastroenterol Hepatol. 2010;33:338–339. doi: 10.1016/j.gastrohep.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Park EH, Kim BJ, Huh JK, Jeong EH, Lee SH, Bang KB, Seol JS, Sung JW, Kim BS, Kang JH. Recurrent mesalazine-induced myopericarditis in a patient with ulcerative colitis. J Cardiovasc Ultrasound. 2012;20:154–156. doi: 10.4250/jcu.2012.20.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonu I, Wong R, Rothenberg ME. 5-ASA induced recurrent myopericarditis and cardiac tamponade in a patient with ulcerative colitis. Dig Dis Sci. 2013;58:2148–2150. doi: 10.1007/s10620-013-2566-4. [DOI] [PubMed] [Google Scholar]


