Abstract
Background and Objectives
Treatment of Pseudomonas aeruginosa infections is greatly hampered by innate and acquired antibiotic resistance. The goal of this study was to compure the immunogenicity of conjugates of P. aeruginosa depolymerized alginate-diphtheria toxoid (D-ALGDT) and P. aeruginosa detoxified lipopolysaccharidediphtheria toxoid (D-LPSDT) in mouse model.
Materials and Methods
Alginate and LPS were purified from P. aeruginosa strain PAO1. The resulting depolymerized alginate (D-ALG) and detoxified LPS (D-LPS) were covalently coupled to diphtheria toxoid (DT) as a carrier protein with adipic acid dihydrazide (ADH) as a spacer molecule and carbodiimide as a linker. Sterility, safety and pyrogenicity tests were performed. 30 mice in two groups were immunized intraperitoneally on days 0, 14 and 28 with 10 μg of D-ALGDT and D-LPSDT. Conjugates specific antibody levels were also determined by enzyme-linked immunosorbent assay (ELISA).
Results
The conjugates were non-toxic and non-pyrogenic. Conjugates of D-ALGDT and D-LPSDT were shown to be safe and to elicit total IgG, IgM, IgA, IgG1, IgG2a, IgG2b and IgG3 antibodies in mice. ELISA results indicated that antibodies titer of D-ALGDT was more than D-LPSDT.
Conclusion
Immunization with D-ALGDT showed significant increase in all types of antibodies titers in versus D-LPSDT, suggesting D-ALGDT as a vaccine candidate against P. aeruginosa infections.
Keywords: Pseudomonas aeruginosa, lipopolysaccharide (LPS), alginate (ALG), conjugate vaccine, diphtheria toxoid (DT)
INTRODUCTION
Pseudomonas aeruginosa, a ubiquitous environmental Gram-negative microorganism, is one of the most important opportunistic bacteria in hospital-acquired infections and causes a wide variety of serious infections in individuals with thermal burn, mechanical extensive trauma, cancer, cystic fibrosis and surgical site infections (1-3). Despite considerable advances in antimicrobial therapy, effective treatment and control of P. aeruginosa infections remains a persistent problem, primarily because of the natural resistance of the organism and its remarkable ability to acquire resistance to multiple antimicrobial agents by various mechanisms. As an alternative strategy to prevent P. aeruginosa infections in susceptible populations, effective immunotherapies or vaccines against P. aeruginosa have long been sought for (4). Several P. aeruginosa antigens are used for vaccine development including LPS alone, polysaccharides alginate, extracellular proteins, exotoxin A, and whole killed cell (5). Alginate and LPS are the major surface components and the immunity confers protection (6, 7).
Alginate (ALG) is a mucoid exopolysaccharide produced by P. aeruginosa. Alginate, like LPS, functions as an adhesin, anchoring P. aeruginosa to the colonized respiratory epithelium (7). Because of the association between mucoid P. aeruginosa and the pathogenesis of these infections in cystic fibrosis patients, interest was generated in using Pseudomonas alginate as immunogen to prevent and treat P. aeruginosa infections in this patient population (8). Immunization with alginate antigen gives rise to antibodies that have opsonic activity and lead to clearance of mucoid P. aeruginosa from the respiratory tract in mice and rats (9). Active immunization against alginate is in the process of being optimized, particularly through conjugation with exotoxin A, in order to compensate for the variable efficacy of antibodies produced in response to alginate (7). LPS is the major surface antigen of P. aeruginosa playing an important role in the interaction with the host immune system, as well as it is responsible for determining the various serotypes of P. aeruginosa (10).
This immunogenicity makes them obvious targets for immunotherapy. However, the active immunization elicited by O-antigen based vaccines is lacking in protectiveness. To circumvent this problem, multiple serotype conjugates can be further conjugated with another target such as exotoxin A (7). LPS based conjugate vaccine has been evaluated by many investigators. In general, the conjugate vaccine appears to be recommended for immunization over other immunogens because of its safety and its potential to elicit high quantities of protective antibodies against O-PS antigen, which confer protective immunity against the pathogen (10). In this study, we compared immunogenicity of conjugates composed of D-ALG-DT and D-LPS-DT as P. aeruginosa vaccine candidates.
MATERIALS AND METHODS
Strain and growth conditions
P. aeruginosa strain PAO1 was obtained from the Biologic Research Center, Zanjan Branch, Islamic Azad University, Zanjan, Iran. It was grown on mueller-hinton agar at 37°C for 24 h (11).
Purification of alginate
The mucoid bacteria were cultured on selective medium containing glycerol, dextrose, L-glutamine, Na2HPO4, K2HPO4 and MgSO4.7H2O and incubated at 37°C for 72 h. The bacterial cells were removed by centrifugation at 4000 g, 4°C for 30 min. Alginate was extracted by repeated ethanol precipitation, dialysis and enzymatic digestion. Crude alginate was precipitated from the supernatant by the addition of cold absolute ethanol to a final concentration of 80% (v/v). The precipitate was collected by centrifugation at 4000 g for 30 min. Crude alginate was re-dissolved at 2 mg/ ml in PBS, pH 7.5, supplemented with 0.5% sodium dodecyl sulfate (SDS) and 10 mM CaCl2. Proteinase K was added (100 μg/ml) for 2 h with incubation at 56°C and then kept at 4°C overnight. DNase and RNase were added (each at 100 μg/ml) for 3 h with incubation at 37°C and centrifuged. Alginate was precipitated by the addition of cold absolute ethanol to a final concentration of 80% (v/v). Following centrifugation, the pellet was collected, sterile filtered, and freeze-dried (9, 12).
Purification of LPS
After the growth of bacteria, P. aeruginosa colonies were cultured on nutrient broth in shaker incubator at 37°C for 72 h (13). The biomass was centrifuged at 4000 × g at 4°C for 30 min, sedimented bacteria were collected and used for LPS extraction and purification (14). Extraction of LPS was carried by optimized hot phenol method. The bacterial suspension (20 g cellular wet weight in 68 ml distilled water) was heated at 66°C for 20 min, mixed with 90% phenol and stirred at 66°C for 30 minutes. The mixture was then placed on ice to facilitate the separation of phases and centrifuged at 4000 × g at 4°C for 45 min. The aqueous phase was collected. A second extraction was made on the mixture of phenol and the cellular pellet by addition of cold 95% ethanol and placed at 4°C overnight. After centrifugation, trichloroacetic acid (TCA) (1 g per 10 ml of solution) was added and stirred at 4°C for 30 min. Following centrifugation, the supernatant was dialyzed against distilled water at 4°C until the phenol was completely eliminated and LPS was concentrated by alcohol precipitation. The pellet was then collected by centrifugation at 4000 × g for 45 min, and lyophilized (15-17).
Analysis of alginate and LPS
LPS was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with silver nitrate staining (18). The protein content of alginate and LPS was measured by the method of Bradford, using bovine serum albumin (BSA) as a standard (19). The nucleic acid content of alginate and LPS was measured by the UV absorbance at 260 nm (12).
Depolymerization of alginate and detoxification of LPS
Alginate was depolymerized by controlled heating in dilute acid. Alginate was depolymerized in 1% (vol/vol) acetic acid and heated at 121°C for 15 min. After cooling, the solution was extensively dialyzed against distilled water at 4°C for 1 day with three changes of distilled water and lyophilized (20). LPS was detoxified by the method of alkaline. Pellet of LPS was dissolved in NaOH to a final concentration of 0.2 N and the mixture was heated at 100°C for 2 h. After cooling on ice, the mixture was adjusted to pH 7 with 1 M HCl. LPS was dialyzed against distilled water at 4°C for 2 days with six changes of distilled water. D-LPS was precipitated by the addition of cold absolute ethanol to a final concentration of 80% (v/v) and then placed at 4°C overnight. The suspension was centrifuged at 4000 × g at 4°C for 45 min. The pellet of D-LPS was pooled (18, 21, 22).
Analyses of D-ALG and D-LPS
The content of endotoxin in D-ALG and D-LPS was estimated by Limulus amebocyte lysate (LAL) method (23). Pyrogenicity testing was performed in rabbits (3 per group). D-ALG and D-LPS were injected intravenously into rabbits. Rectal temperatures were measured with indwelling rectal thermostats and recorded every 15 min for 1 h before injection and every 15 min for 3 h after injection (14, 24).
Conjugation of D-ALG to DT and D-LPS to DT
D-ALG (10 mg) was dissolved in 1 ml of distilled water at room temperature, and the pH was brought to 10.5 with 1 N NaOH. Cyanogen bromide (150 ml of a 0.2 g/ml solution in acetonitrile) was added, and pH 10.5 was maintained with 1 N NaOH. The reaction mixture was stirred at 4°C for 10 min. 5 ml of 0.5 M ADH in 0.5 M NaHCO3 was added and the pH was adjusted to 8.5 with 0.1 M HCl. The reaction mixture was kept at 4°C overnight and then dialyzed against distilled water at the same temperature for 24 h with three changes of distilled water. The volume was brought to 3 ml with ultracentrifuged at 4000 x g. ADH-derivatized D-ALG (3 ml) was added to DT (0.5 ml of a 2.5 mg/ ml solution in distilled water) (Biologic Research Center, Zanjan Branch, Islamic Azad University, Zanjan, Iran). The mixture was cooled on ice, and the pH was brought to 5.8 with 0.1 N HCl. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) was added to 0.1 M, and stirring on ice for 4 h, and then placed at 4°C overnight. The mixture was dialyzed against distilled water for 2 days with six changes of the outer fluid and centrifuged at low speed to remove a small amount of precipitate. The conjugate was passed through a Sepharose CL-2B column (1.5 by 90 cm) in 0.2 M NaCl, the void volume peak was collected. Similarly, D-LPS-DT conjugate prepared with ADH as the spacer and EDAC as the linker. The conjugates (D-ALG-DT and D-LPS-DT) were stored at 4°C (23, 25, 26).
Analyses of D-ALG-DT and D-LPS-DT
The protein content of D-ALG-DT and D-LPS-DT was measured by the method of Bradford, using BSA as a standard. The carbohydrate content of conjugates was determined by the method of phenol-sulfuric acid, using glucose as a standard (19). Pyrogenicity testing was performed in rabbits as previously described. The ability of the toxic effect of D-ALG-DT and D-LPS-DT for mice (5 per group) was evaluated by the intraperitoneal injection of graded doses of conjugates. Each mouse received 10 μg/ml from conjugate. Mice were observed daily for 7 days post-injection (9). Sterility testing was performed (27).
Immunization
For evaluation of immunogenicity, 6-8 weeks old female BALB/c mice from the Pasteur Institute of Iran, Karaj, Iran (15 per group) were injected intraperitoneally three times at 2-week intervals with 10 μg of immunogens of the D-ALGDT or the D-LPS-DT. A control group received one injection of 10 μg of normal saline. Five mice were randomly chosen from each group and exsanguinated 2 weeks after each injection, and sera were pooled, sterile filtered and stored at -20°C (28, 29).
ELISA test
Conjugates specific antibody levels in sera were determined using an ELISA for total IgG by indirect ELISA and IgM, IgA, IgG1, IgG2a, IgG2b and IgG3 by antigen mediated ELISA. Each well of the plates was coated with 100 μl of either D-ALG-DT or D-LPS-DT at a concentration of 2 μg/ml in 0.05 M carbonate buffer, pH 9.6 and kept overnight at 4°C. Plates were washed three times with wash buffer (PBS containing 0.05% Tween 20). After a washing, plates were blocked with blocking buffer for 1 h at room temperature. Plates were washed three times with wash buffer. Sera samples were diluted in PBS (1:10), assayed in triplicate and incubated for 2 h at room temperature. Plates were washed three times with wash buffer. Goat anti-mouse antibody was diluted in PBS (1:1000), added, incubated for 1 h at room temperature and plates were washed three times with wash buffer (this step was only in antigen mediated ELISA). Horseradish peroxidase conjugated to goat anti-mouse IgG was diluted in PBS (1:3000), added. After 1 h at room temperature, plates were washed. O-Phenylenediamine dihydrochloride and H2O2 were added as substrate. After 15 min of incubation in the dark, the reaction was stopped with 50 μl H2SO4 and the absorbance was measured at 450 nm (9, 18, 27-31).
Statistical analysis
Comparison of geometric means was performed with the One-Way ANOVA test (Tukey’s test). Values of P<0.01 were considered to be significant. The Statistical Analysis System was used for all data analysis. Statistics were performed with SPSS version 16.
RESULTS
Characterization of D-ALG and D-LPS
Fig. 1 shows the electrophoretic patterns of the LPS bands of P. aeruginosa on an SDS-PAGE. Various characteristics of D-ALG and D-LPS were also shown in Table 1. As can be found from the Table 1, the endotoxin activity assay showed 0.125 EU/ml of D-ALG and D-LPS that were acceptable to use for immunization. D-ALG and D-LPS were non-pyrogenic when tested at a dose of 50 μg/kg and evoked < 0.5°C increase in temperature after 24 h.
Fig. 1.

Silver-stained SDS-PAGE in 14% gel of P. aeruginosa LPS. Lane 1 and 2 were loaded with 10 μg/ml and 5 μg/ml LPS, respectively.
Table 1.
Characteristics of D-ALG and D-LPS
| Composition | ||||
|---|---|---|---|---|
| Protein | Nucleic acid | Endotoxicity | Pyrogenicity* | |
| D-ALG | 1.5 mg/g | 1.4 μg/g | 0.125 EU/ml | 50 μg/kg |
| D-LPS | 1 mg/g | 1.1 μg/g | 0.125 EU/ml | 50 μg/kg |
When administered intravenously to rabbits, 50 μg of D-ALG and D-LPS per kg body weight evoked <0.5°C increase in temperature.
Characterization of D-ALG-DT and D-LPS-DT
D-ALG-DT and D-LPS-DT were isolated by gel filtration, monitoring the elution profile by the UV absorbance at both 210 nm (for the presence of ALG and LPS) and 280 nm (for the presence of DT) and refractive index. Fractions with the maximum absorbance for both 210 and 280 nm (Fig. 2, fractions 72 to 77 and Fig. 3, fractions 72 to 74) were indicated the formation of D-ALG-DT and D-LPS-DT conjugate molecules. Various characteristics of conjugate vaccines are shown in Table 2. The conjugates were non-pyrogenic when tested at a dose of 50 μg/kg and evoked < 0.5°C increase in temperature after 24 h. The conjugates were non-toxic upon intraperitoneal administration to mice. No overt signs of illness and decrease in weight were observed and all mice survived. Mediums were observed after incubation at 37°C for 24 to 48 h. No signs of microorganism growth were observed. Sterility testing showed that the resulting conjugates were sterile. The above results, demonstrating the safety and stability of the conjugate vaccines, led us to evaluate its acceptability and immunogenicity in animals.
Fig. 2.
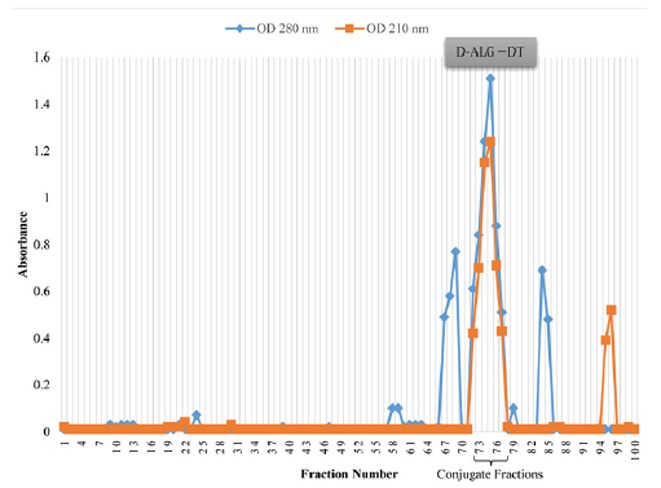
Sepharose CL-2B gel filtration profile of D-ALG conjugated to DT. Fractions were assayed for alginate at 210 nm and at 280 nm for DT.
Fig. 3.
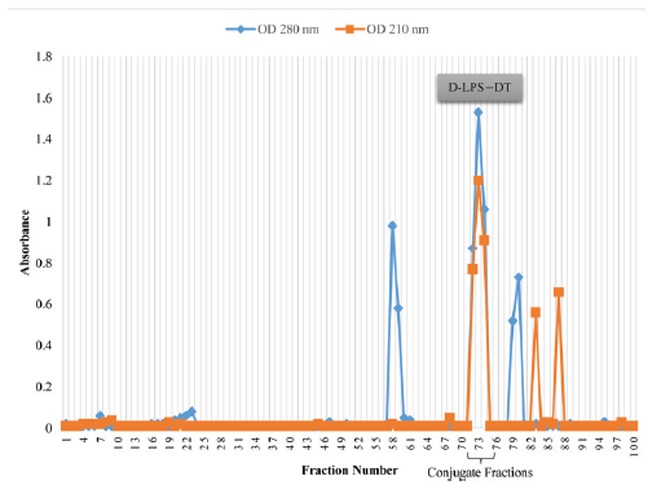
Sepharose CL-2B gel filtration profile of D-LPS conjugated to DT. Fractions were assayed for LPS at 210 nm and for DT at 280 nm.
Table 2.
Characteristics of D-ALG-DT and D-LPS-DT Conjugates
| Composition | ||||
|---|---|---|---|---|
| Protein | Carbohydrate | Pyrogenicity* | Toxicity# | |
| D-ALG-DT | 1.2 mg/g | 0.5 mg/g | 50 μg/kg | 10 μg/ml |
| D-LPS-DT | 1 mg/g | 0.4 mg/g | 50 μg/kg | 10 μg/ml |
When administered intravenously to rabbits, 50 μg of vaccines per kg body weight evoked <0.5°C increase in temperature.
When administered intraperitoneally to mice, 10 μg/ml of vaccines were not observed decrease in weight and mortality.
Comparison of Immunogenicity of D-ALG-DT and D-LPS-DT conjugates
The immunogenicity of the D-LPS-DT and D-ALG-DT conjugate vaccines were analyzed by immunization in mice. Figs. 4, 5 and 6 shows antibody titers in the immunized mice. As shown in these figures, D-ALG-DT displayed higher titers in the IgG and IgM antibodies than D-LPS-DT (P<0.01). The control groups also indicated the lowest antibody titers.
Fig. 4.
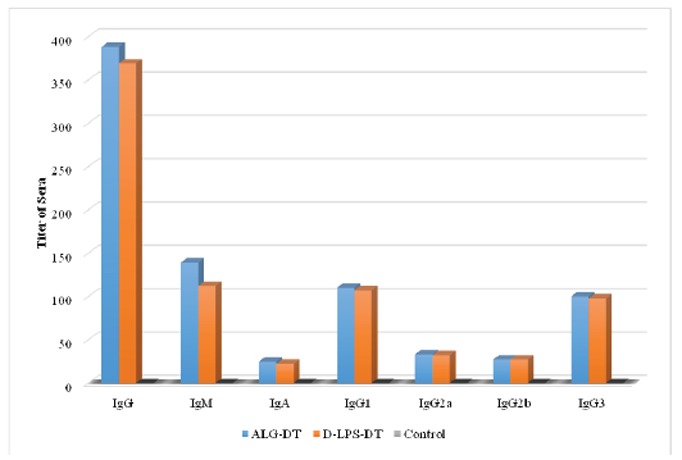
Induction of antibodies in BALB/c mice for two weeks after first injection (Day 14). The results of inductions for all types of antibodies were observed D-ALG-DT>D-LPS-DT.
Fig. 5.
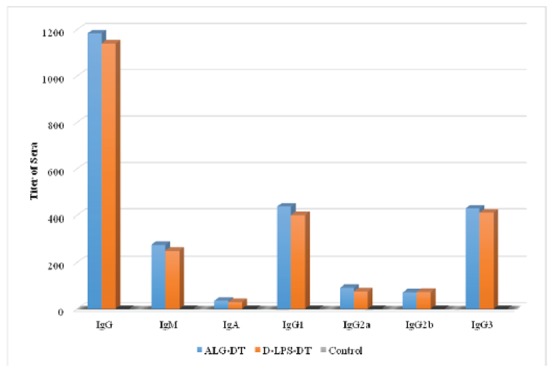
Induction of antibodies in BALB/c mice for two weeks after second injection (Day 28). The results of inductions for all types of antibodies were observed D-ALG-DT>D-LPS-DT. The second immunization with D-ALG-DT and D-LPS-DT conjugates was induced high levels of antibodies in compared to the first immunization.
Fig. 6.
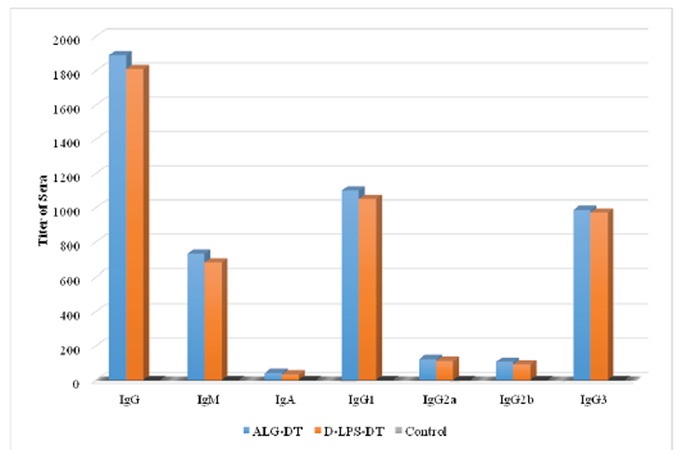
Induction of antibodies in BALB/c mice for two weeks after third injection (Day 42). The results of inductions for all types of antibodies were observed D-ALG-DT>D-LPS-DT. A considerable rise in D-ALG-DT and D-LPS-DT specific antibodies was observed after the third vaccine dose.
DISCUSSION
P. aeruginosa has been considered to be a difficult target for antimicrobial chemotherapy (33). Different approaches have been tested to protect patients with P. aeruginosa infections including passive immunization with monoclonal and polyclonal antibody. In this respect, the conjugate vaccine composed of bacterial antigens and carrier protein is a well-established method because of its safety and its potential to elicit high quantities of protective antibodies (9). Anti-LPS antibody has been shown to be highly protective against P. aeruginosa infections (6). In this study, the LPS and ALG from P. aeruginosa PAO-1 was investigated for the conjugate vaccine preparation. Conjugation of bacterial antigens to carrier proteins has been applied to increase their immunogenicity and create effective vaccines (7). Some proteins such as tetanus toxoid (TT) and bovine serum albumin (BSA) have been used as carrier proteins to conjugation with O-polysaccharide (O-PS) from P. aeruginosa (18). However, in the case of ALG, because of its large molecular mass, conjugating it to carrier proteins to produce immunogenic vaccines has proven to be difficult. Here, we used DT as the carrier because DT is readily available and a part of the pediatric immunizations, which is done within the frame of the Expanded Programme of Immunization of the WHO and UNICEF (17). The DT has been shown to enhance immunogenicity of vaccines when used as the carrier in conjugate vaccine. The detoxified D-LPS and D-ALG were conjugated to DT via the amidation method using EDAC as a linker and ADH as a spacer molecule. The molar ratio of LPS and ALG to DT conjugation was 3:1. Total IgG titers prepared from the immunized mice sera with D-ALG-DT vaccine showed significant rise in comparison to D-LPS-DT. Similar finding has been observed using D-ALG with exotoxin A conjugate derived from P. aeruginosa (9). Briefly, these results showed that the conjugate vaccine based on LPS from P. aeruginosa and diphtheria toxoid raised LPS antibodies. Briefly, our results showed that the conjugate vaccine based on D-ALG from P. aeruginosa and DT raised more antibodies than the D-LPS-DT. In conclusion, our data indicated that the D-ALG-DT can be used as a potential vaccine candidate against P. aeruginosa infections.
Acknowledgments
We thanks from Biologic Research Center of Islamic Azad University of Zanjan Branch.
References
- 1.Peluso L, de Luca C, Bozza S, Leonardi A, Giovannini G, Lavorgna A, et al. Protection against Pseudomonas aeruginosa lung infection in mice by recombinant OprF-pulsed dendritic cell immunization. BMC Microbiol. 2010;10:1–11. doi: 10.1186/1471-2180-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanislavsky ES, Lam JS. Pseudomonas aeruginosa antigens as potential vaccines. FEMS Microbiol Rev. 1997;21:243–277. doi: 10.1111/j.1574-6976.1997.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 3.Priebe GP, Pier GB. Vaccines for Pseudomonas aeruginosa. In: Ellis RW, Brodeur BR, editors. New Bacterial Vaccines. 1. Plenum Publishing; 2003. pp. 260–282. [Google Scholar]
- 4.Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Human Vaccines. 2011;7:999–1011. doi: 10.4161/hv.7.10.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japoni A, Farshad S, Alborzi A. Pseudomonas aeruginosa: burn infection, treatment and antibacterial resistance. Iran Red Crescent Med J. 2009;11:244–253. [Google Scholar]
- 6.Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydrate Research. 2003;338:2539–2547. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Kipnis E, Sawa T, Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Médecine et Maladies Infectieuses. 2006;36:78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Holder IA. Pseudomonas immunotherapy: a historical overview. Vaccine. 2004;22:831–839. doi: 10.1016/j.vaccine.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Kashef N, Behzadian-Nejad Q, Najar-Peerayeh S, Mousavi-Hosseini K, Moazzeni M, Esmaeeli Djavid G. Synthesis and characterization of Pseudomonas aeruginosa alginate–tetanus toxoid conjugate. J Med Microbiol. 2006;55:1441–1446. doi: 10.1099/jmm.0.46696-0. [DOI] [PubMed] [Google Scholar]
- 10.Al-Zeer M, Masoud H. LPS-based conjugate vaccines composed of O-polysaccharide from Pseudomonas aeruginosa IATS 6 and 11 bound to a carrier protein. World J Microbiol Biotechnol. 2007;23:1541–1549. [Google Scholar]
- 11.Aspe M, Jensen L, Melegrito J, Sun M. The role of alginate and extracellular DNA in biofilm-meditated Pseudomonas aeruginosa gentamicin resistance. J Exp Microbiol Immunol. 2012;16:42–48. [Google Scholar]
- 12.Kashef N, Behzadian-Nejad Q, Najar-Peerayen S, Mousavi-Hosseini K, Moazeni M, Rezvan H, Adibi-motlagh B. Preliminary investigation on the isolation of alginate produced by mucoid Pseudomonas aeruginosa. Ann Microbiol. 2005;55:279–282. [Google Scholar]
- 13.Said AA, Livermore DM, Williams RJ. Expression of H 1 outer-membrane protein of Pseudornonas aeruginosa in relation to sensitivity to EDTA and polymyxin B. J Med Microbiol. 1987;24:267–274. doi: 10.1099/00222615-24-3-267. [DOI] [PubMed] [Google Scholar]
- 14.Rezania S, Amirmozaffari N, Tabarraei B, Jeddi-Tehrani M, Zarei O, Alizadeh R, et al. Extraction, purification and characterization of lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J Med Biotech. 2011;3:3–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Shapouri R, Mohabati Mobarez A, Ahmadi H, Tabaraie B, Hosseini Doust R, Norozian D, et al. Optimization of Brucella abortus fermenter cultural conditions and LPS extraction method for antigen production. Res J Microbiol. 2008;3:1–8. [Google Scholar]
- 16.Sharifat Salmani A, Siadat SD, Norouzian D, Ahmadi H, Nejati M, Tabaraie B, et al. Optimization of Brucella abortus S99 lipopolysaccharide extraction by phenol and butanol methods. Res J Biol Sci. 2008;3:576–580. [Google Scholar]
- 17.Renukadevi KP, Angayarkanni J, Karunakaran G. Extraction and characterization of lipopolysaccharide from Serratia rubidaea and its cytotoxicity on lung cancer cell line - NCI-H69. Acta Technica Corvininesis - Bulletin of Engineering. 2012;5:97–101. [Google Scholar]
- 18.Konadu E, Robbins JB, Shiloach J, Bryla DA, Szu SC. Preparation, characterization, and immunological properties in mice of Escherichia coli 0157 0-specific polysaccharide-protein conjugate vaccines. Infect Immun. 1994;62:5048–5054. doi: 10.1128/iai.62.11.5048-5054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson L, Hay FC. Practical immunology. 3. Blackwell Science pub; 1989. [Google Scholar]
- 20.Cryz SJ, JR, Fürer E, Que JU. Synthesis and characterization of a Pseudomonas aeruginosa alginate-toxin A conjugate vaccine. Infection and Immunity. 1991;59:45–50. doi: 10.1128/iai.59.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seid RC, Jr, Sadoff JC. Preparation and characterization of detoxified lipopolysaccharide-protein conjugates. J Biol Chem. 1981;256:7305–7310. [PubMed] [Google Scholar]
- 22.Kabir S. Preparation and immunogenicity of a bivalent cell-surface protein-polysaccharide conjugate of Vibrio cholerae. J Med Microbiol. 1987;23:9–18. doi: 10.1099/00222615-23-1-9. [DOI] [PubMed] [Google Scholar]
- 23.Watson DC, Robbins JB, Szu SC. Protection of mice against Salmonella typhimunium with an O-specific polysaccharide-protein conjugate vaccine. Infect Immun. 1992;60:4679–4686. doi: 10.1128/iai.60.11.4679-4686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein J, Hoffman T, Frasch C, Lizzio EF, Beining PR, Hochstein D, et al. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect Immun. 1992;60:1385–1389. doi: 10.1128/iai.60.4.1385-1389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cryz SJ, Jr, Sadoff JC, Fürer E, Germanier R. Pseudomonas aeruginosa polysaccharide-tetanus toxoid conjugate vaccine: safety and immunogenicity in humans. J Infect Dis. 1986;154:682–688. doi: 10.1093/infdis/154.4.682. [DOI] [PubMed] [Google Scholar]
- 26.Midwinter A, Faine S, Adler B. Vaccination of mice with lipopolysaccharide (LPS) and LPS-derived immuno-conjugates from Leptospira interrogans. J Med Microbiol. 1990;33:199–204. doi: 10.1099/00222615-33-3-199. [DOI] [PubMed] [Google Scholar]
- 27.Cryz SJ, JR, Fürer E, Cross AS, Wegmann A, Germanier R, Sadoff JC. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987;80:51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kossaczka Z, Shiloach J, Johnson V, Taylor DN, Finkelstein RA, Robbins JB, et al. Vibrio cholerae O139 conjugate vaccines: synthesis and immunogenicity of V. cholerae O139 capsular polysaccharide conjugates with recombinant diphtheria toxin mutant in mice. Infect Immun. 2000;68:5037–5043. doi: 10.1128/iai.68.9.5037-5043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu C, Liu B, Watson D, Szu S, Bryla D, Shiloach J, et al. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta RK, Egan W, Bryla DA, Robbins JB, Szu SC. Comparative immunogenicity of conjugates composed of Escherichia coli O111 O-specific polysaccharide, prepared by treatment with acetic acid or hydrazine, bound to tetanus toxoid by two synthetic schemes. Infect Immun. 1995;63:2805–2810. doi: 10.1128/iai.63.8.2805-2810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, Szu SC, Finkelistein RA, Robbins JB. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharide of Vibrio cholerae 01 serotype Inaba bound to cholera toxin. Infect Immun. 1992;60:3201–3208. doi: 10.1128/iai.60.8.3201-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol. 2007;297:277–295. doi: 10.1016/j.ijmm.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13:560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]


