Abstract
Background and Objectives
Staphylococcal food poisoning is a gastrointestinal disease, which is caused by consumption of contaminated food with enterotoxins produced by Staphylococcus aureus (SEs). Milk and its products are known sources of food borne diseases. This study was carried out to evaluate the prevalence of enterotoxigenic S. aureus strains in organic milk and cheese in Tabriz - Iran.
Materials and Methods
A total of 200 samples (100 milk samples and 100 cheese samples) were collected from farms and milk collection points in Tabriz - Iran. The samples were cultured and identified by standard bacteriological methods, then PCR was performed to detect sea gene.
Results and Conclusion
Staphylococcus aureus was found in 27% of all samples (milk and cheese). Results of PCR showed that 12.96% of S. aureus isolates possessed sea gene. It suggested the potential public health threat of S. aureus resulting from contamination of dairy products. So, efforts are required to improve safety standards for preventing staphylococcal food poisoning.
Keywords: Staphylococcus aureus, Enterotoxin, Organic Milk, Cheese
INTRODUCTION
Staphylococcus aureus is an important food-borne pathogen involved in a variety of invasive diseases (1). It produces many toxins as well as non-toxic enzymes and can facilitate bacterial attack and proliferation in the body of host (2). Some strains produce staphylococcal enterotoxins (SEs) that can cause food poisoning if food containing preformed SE is ingested. Symptoms of staphylococcal food poisoning (SFP) have a rapid onset (2 to 6 h) and may include vomiting, stomach pain and diarrhea (3). Enterotoxins from S. aureus strains can be classified into 18 serological types: A-U (except S, F and T)(2,4-9). The SEs are a group of heat stable and pepsin resistant exotoxins encoded by genes in the chromosome, pathogenicity island, phages or plasmids (2). For production of sufficient amount of toxin to cause intoxication symptoms, 105 CFU/ml or CFU/g enterotoxigenic strains of bacteria are needed (10). SEs are low molecular weight proteins (MW 26.900 – 29600 KD) (11). SEA is a leading cause of food intoxication (12) and is an extremely potent gastrointestinal toxin, as little as 100 ng is sufficient to cause symptoms of intoxication (13).
Milk and milk products have frequently been implicated in staphylococcal food poisoning and contaminated raw milk is often involved (2, 3). S. aureus mastitis is a serious problem in dairy production and infected animals may contaminate bulk milk. Human handlers, milking equipment, the environment and the udder and teat skin of dairy animals are other possible sources of bulk milk contamination (3, 14). There are several methods for detection of enterotoxigenic bacteria. PCR is recommended for detection of S. aureus enterotoxin genes (15).
Surveys to detect classical enterotoxins and to identify enterotoxin genes in S. aureus from milk and milk products have been conducted in many counties including Italy, Norway, Turkey, Brazil and Iran (Tehran) (16-20). However, there are no published reports about presence of SEA gene in milk and its products in Tabriz-Iran. Therefore, this study was conducted to investigate the presence of SEA gene of S. aureus in organic milk and cheese using PCR method in Tabriz-Iran.
MATERIALS AND METHODS
Specimen collection
A total of 200 samples (100 milk samples, 100 cheese samples) were collected from milk collection points and food stores in Tabriz, Iran. The samples were collected in sterile containers, immediately kept in an ice box and transported to the laboratory.
Microbiological analysis
The samples were investigated microbiologically for the presence of S. aureus, according to the standard method No. 6806-1 of the Institute of Standards and Industrial Research of Iran (ISIRI). The milk and cheese samples were diluted in ringer and pepton water, respectively. From each diluted solutions produced, 1 ml was transferred to Brain- Heart Infusion broth and incubated at 37°C for 24 h. Baird- Parker agar supplemented with potassium tellurite and egg yolk emulsion was used for isolation of S. aureus. After 24-48 h incubation at 37°C, the BPA plates were analyzed for the presence of S. aureus, which appeared as black colonies with transparent zone. Biochemical confirmation tests (Gram staining, coagulase, catalase, oxidase, VP and growth on mannitol salt agar) were carried out for final identification of the suspected S. aureus isolates.
DNA extraction and PCR experiments
For DNA isolation, biochemically confirmed single colonies of S. aureus isolates on BPA were cultured on LB broth. DNA extraction procedure was done by a commercial kit (Cinna pure kit), according to the supplier’s instructions on the bacterial cells pellet from an overnight culture in LB broth. The primers used for the detection of nuc and sea genes are listed in Table 1.
Table 1.
Primers for the detection of S. aureus nuc and sea genes
Final confirmation of the strains was carried out using PCR with nuc gene. PCR reactions were performed in reaction buffer (10x), MgCl2 (50mM) in a total volume of 25 μl, containing 5 μl of template DNA, 0.75 μl of each primers (10 pm), 0.25 μl of dNTP (10 mM), 0.2 μl of Taq DNA polymerase (5 unit/μl) and 14.05 μl of ddH2O. PCR was performed under the following conditions: initial denaturation at 94°C for 5 min, subsequently followed by 30 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1 min with a final extention of 5 min at 72°C. The amplified products were shown by electrophoresis on 1% gel agarose containing ethidium bromide. Gels were viewed by UV Transillumination and photographed (Fig. 1).
Fig. 1.
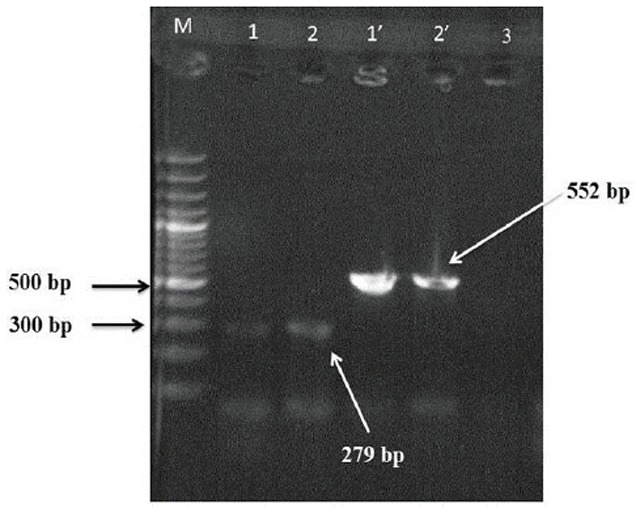
1% Agarose electrophoresis patterns showing PCR products. Lane M, standard molecular size marker (100 bp).
Lanes 1, 1’: positive control.
Lanes 2, 2’: nuc and sea positive isolate.
Lanes 3: negative control.
After final confirmation of the isolates, PCR reaction was performed for sea gene. PCR reaction was conducted like the previous test but with some changes in annealing temperature (50°C for 1 min). The presence of 552 bp amplicon indicates the positive samples for sea gene in these isolates.
RESULTS AND CONCLUSION
In the present study, S. aureus was observed in 54 (27 %) out of 200 samples: 9 from milk and 45 from cheese samples. Total S. aureus counts determined between 102 and 106 CFU/ml in samples. The number of bacteria in contaminated samples by CFU/ml is shown in Table 2. For further molecular confirmation of S. aureus colonies, PCR reaction for nuc gene was performed. All 54 isolates carried nuc gene. DNA from those isolates was examined for the presence of sea gene. Results showed that 7 out of 54 S. aureus isolates harbored sea gene (Table 2).
Table 2.
Prevalence of sea gene in S. aureus isolated from organic milk and cheese.
| Types of samples | Sample size | No. of S. aureus isolates | Distribution CFU/ml | No. of enterotoxigenic S. aureus (sea gene) | |
|---|---|---|---|---|---|
| 102-104 | 104-106 | ||||
| Milk | 100 | 9 | 6(66.6%) | 3(33.3%) | 2 |
| Cheese | 100 | 45 | 26(57.7%) | 19(42.2%) | 5 |
| Total | 200 | 54(27%) | - | - | 7 (12.96%) |
Several researchers have reported different results of the number of bacteria in contaminated dairy products by CFU/ml (10, 20, 21). Variations in S. aureus count in dairy products may depend on sanitary precautions during milk processing chain. The existence of S. aureus in food and dairy products was previously confirmed by Imani Fooladi in Iran (20). SEA is the most common enterotoxin recovered from food poisoning outbreaks in the USA (77.8% of all outbreaks)(24).
In the current study, we used Baird- Parker agar for isolation of S. aureus from dairy products. This method was also employed by Boerema et al. (25), Rall et al. (26), and Ertas et al. (27), in same products. Considering the findings of Brakstad et al.(22), and comparing them to our study, it can be stated that the PCR for amplification of the nuc gene has potential for rapid diagnosis and confirmation of S. aureus isolates. The present study aimed to evaluate the prevalence rate of sea gene in organic milk and cheese in Tabriz, Iran. Outside of Iran, various results have been reported on the presence of S. aureus and its enterotoxins in milk and its products (28-31).
In the current study, we found that 27% of the organic milk and cheese samples were contaminated by S. aureus. PCR results for sea gene, showed that 12.96% of the isolates possessed sea gene. Similar level of incidence was reported by Holeckova et al., (32), Imani Fooladi et al., (20) and El-Jakee et al. (33). In contrast to the above results, Ertas et al. (27) reported lower incidence of sea gene (1.6%). This discrepancy could be attributed to the improvements in the handling and sanitary procedures during milking and its processing. It was concluded that even though the level of microorganisms in dairy products was not sufficient to cause disease, the presence of toxins could be considered a potential risk for public health.
Several conditions such as delay in processing, inadequate refrigeration, poor personal hygiene and post process contamination are associated with staphylococcal growth and enterotoxin production (34). Therefore it is essential to ensure high safety standards for preventing staphylococcal food poisoning. In conclusion, the results of this study provides some important preliminary data about the prevalence of enterotoxigenic S. aureus in organic milk and cheese in Tabriz, Iran. Consumption of organic milk and specially cheese is still widespread and could cause a potential risk to public health, so an effective reduction of contamination levels could be achieved by improving sanitation and hygiene procedures. Further investigations are needed to evaluate the production of other toxins in milk and cheese.
References
- 1.Morandi S, Brasca M, Andrighetto C, Lombardi A, Lodi R. Phenotypic and genotypic characterization of Staphylococcus aureus strains from Italian dairy products. Int J Microbiology. 2009:1–7. doi: 10.1155/2009/501362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinges M, Orwin P, Schlievert P. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jørgensen HJ, Mørk T, Caugant DA, Kearns A, Rørvik LM. Genetic Variation among Staphylococcus aureus Strains from Norwegian Bulk Milk. Appl Environ Microbiol. 2005;71:8352–8361. doi: 10.1128/AEM.71.12.8352-8361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtfreter S, Broker BM. Staphylococcal superantigens: Do they play a role in sepsis? Arch Immunol Ther Exp. 2005;53:13–27. [PubMed] [Google Scholar]
- 5.Letertre C, Perelle S, Dilasser F, Fach P. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus. J Appl Microbiol. 2003;95:38–43. doi: 10.1046/j.1365-2672.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- 6.Omoe K, Hu DL, Takahashi-Omoe H, Nakane A, Shinagawa K. Identification and characterization of a new staphylococcal enterotoxin-related putative toxin encoded by two kinds of plasmids. Infect Immun. 2003;71:6088–6094. doi: 10.1128/IAI.71.10.6088-6094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orwin PM, Fitzgerald JR, Leung DY, Gutierrez JA, Bohach GA, Schlievert PM. Characterization of Staphylococcus aureus enterotoxin. Infect Immun. 2003;71:2916–2919. doi: 10.1128/IAI.71.5.2916-2919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orwin PM, Leung DY, Donahue HL, Novick RP, Schlievert PM. Biochemical and biological properties of staphylococcal enterotoxin K. Infect Immun. 2001;69:360–366. doi: 10.1128/IAI.69.1.360-366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwin PM, Leung DY, Tripp TJ, Bohach GA, Earhart CA, Ohlendorf DH, et al. Characterization of a novel staphylococcal enterotoxin-like superantigen, a member of the group V subfamily of pyrogenic toxins. Biochemistry. 2002;41:14033–14040. doi: 10.1021/bi025977q. [DOI] [PubMed] [Google Scholar]
- 10.Arcuri EF, Angelo FF, Guimarães MF, Talon R, Borges Mde F, Leroy S, et al. Toxigenic status of staphylococcus aureus isolated from bovine raw milk and minas frescal cheese in Brazil. J Food Protect. 2010;73:2225–2231. doi: 10.4315/0362-028x-73.12.2225. [DOI] [PubMed] [Google Scholar]
- 11.Martin MC, Fueyo JM, Gonzales-Hevia JM, Mendoza MC. Genetic procedure for identification of enterotoxigenic strains of Staphylococcus aureus from three food poisoning outbreaks. J Food Microbiol. 2004;94:279–286. doi: 10.1016/j.ijfoodmicro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Archer DL, Young FE. Contemporary issues: disease with a food vector. ClinMicrobiol Rev. 1988;1:377–398. doi: 10.1128/cmr.1.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evenson MI, Hinds MW, Bernstein RS, Bergdoll MS. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int J Food Microbiol. 1988;7:311–316. doi: 10.1016/0168-1605(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 14.Zadoks RN, van Leeuwen WB, Kreft D, Fox LK, Barkema HW, Schukken YH, et al. Comparison of Staphylococcus aureus isolates from bovine and human skin, milking equipment, and bovine milk by phage typing, pulsed-field gel electrophoresis, and binary typing. J Clin Microbiol. 2002;40:3894–3902. doi: 10.1128/JCM.40.11.3894-3902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Belkum A. Molecular diagnostics in medical microbiology: Yesterday, today and tomorrow. Curr Opin Pharmacol. 2003;3:497–501. doi: 10.1016/s1471-4892(03)00108-5. [DOI] [PubMed] [Google Scholar]
- 16.Morandi S, Brasca M, Lodi R, Cremonesi P, Castiglioni B. Detection of classical enterotoxins and identification of enterotoxin genes in Staphylococcus aureus from milk and dairy products. Vet Microbiol. 2007;124:66–72. doi: 10.1016/j.vetmic.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen HJ, Mork T, Hogasen HR, Rovik LM. Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J Appl Microbiol. 2005;99:158–167. doi: 10.1111/j.1365-2672.2005.02569.x. [DOI] [PubMed] [Google Scholar]
- 18.Boynukara B, Gulhan T, Alisarli M, Gurturk K, Solmaz H. Classical enterotoxigenic characteristics of Staphylococcus aureus strains isolated from bovine subclinical mastitis in Van, Turkey. Int J Food Microbiol. 2008;125:209–211. doi: 10.1016/j.ijfoodmicro.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Rall VLM, Vieira FP, Rall R, Vieitis RL, Fernades Jr, Candeias JMG, et al. PCR detection of staphylococcal enterotoxin genes in Staphylococcus aureus strains isolated from raw and pasteurized milk. Vet Microbiol. 2008;132:408–413. doi: 10.1016/j.vetmic.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 20.ImaniFooladi AA, Tavakoli HR, Naderi A. Detection of enterotoxigenic Staphylococcus aureus isolates in domestic dairy products. Iran J Microbial. 2010;2:137–142. [PMC free article] [PubMed] [Google Scholar]
- 21.Gucukoglu A, OnurKevenek T, Uyanik T, Cadirci O, Terzi G, Alisarli M. Detection of enterotoxigenic Staphylococcus aureus in raw milk and dairy products by multiplex PCR. J Food Sci. 2012;77:M620–M623. doi: 10.1111/j.1750-3841.2012.02954.x. [DOI] [PubMed] [Google Scholar]
- 22.Brakstad OG, Aasbak K, Maeland J. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc Gene. J Clin Microbiol. 1992;30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadati M, Barati B, Doroudian M, Shirzad H, Hashemi M, Hosseini SM, et al. Detection of Sea, Seb, Sec, Seq genes in staphylococcus aureus isolated from nasal carriers in Tehran province, Iran; by multiplex PCR. JPS. 2011;2:34–40. [Google Scholar]
- 24.Balabana N, Rasooly A. Staphylococcal enterotoxins. Int J Food Microbiol. 2000;61:1–10. doi: 10.1016/s0168-1605(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 25.Boerema JA, Clemens R, Brightwell G. Evaluation of molecular methods to determine enterotoxigenic status and molecular genotype of bovine, ovine, human and food isolates of Staphylococcus aureus. Int J Food Microbiol. 2006;107:192–201. doi: 10.1016/j.ijfoodmicro.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Rall VL, Vieira FP, Rall R, Vieitis RL, Fernandes A, Candeias JM. PCR detection of staphylococcal enterotoxin genes in Staphylococcus aureus strains isolated from raw and pasteurized milk. Vet Microbiol. 2008;132:408–413. doi: 10.1016/j.vetmic.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Ertas N, Gonulalan Z, Yildirim Y, Kum E. Detection of Staphylococcus aureus enterotoxins in sheep cheese and dairy desserts by multiplex PCR technique. Int J Food Microbiol. 2010;15(142):74–77. doi: 10.1016/j.ijfoodmicro.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 28.De Buyser ML, Dufour B, Maire M, Lafarge V. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int J Food Microbiol. 2001;67:1–17. doi: 10.1016/s0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 29.Kousta M, Mataragas M, Skandamis P, Drosinos EH. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control. 2010;21:805–815. [Google Scholar]
- 30.Kérouanton A, Hennekinne JA, Letertre C, Petit L, Chesneau O, Brisabois A, et al. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int J Food Microbiol. 2007;115:369–375. doi: 10.1016/j.ijfoodmicro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 31.Bingol E, Çetin O, Çolak H, Hampikyan H. Presence of enterotoxin and verotoxin in Turkish cheeses sold in İstanbul. Turk J Vet Anim Sci. 2012;36:424–432. [Google Scholar]
- 32.Hoieckova B, Kalinacova V, Gondol J, Fotta M, Holoda E. Production of enterotoxins by Staphylococcus aureus isolated from sheep milk. Bull Vet Inst. 2004;48:41–45. [Google Scholar]
- 33.El-Jakee J, Zaki E, Randa F. Properties of enterotoxigenic S. aureus isolated from mastitic cattle and buffaloes in Egypt. J Am Sci. 2010;6:170–178. [Google Scholar]
- 34.Wieneke A, Roberts D, Gilbert R. Staphylococcal food poisoning in the united kingdom,1969-90. Epidemiol Infect. 1993;110:519–531. doi: 10.1017/s0950268800050949. [DOI] [PMC free article] [PubMed] [Google Scholar]


