SUMMARY
Background
Risks and benefits of protease inhibitor (PI) (telaprevir or boceprevir) triple therapy in hepatitis C virus (HCV)-infected patients with mildly decompensated cirrhosis, including those wait-listed for liver transplantation (LT), are incompletely known.
Aim
To assess virological responses and safety of PI triple therapy in patients with mildly decompensated Child-Pugh (CP) CP ≥6 vs. compensated (CP = 5) cirrhosis.
Methods
Multicentre cohort of 160 adults with cirrhosis treated with peginterferon/ribavirin (peg-IFN/RBV) plus telaprevir (69%) or boceprevir (31%), comparing outcomes between those with CP = 5 and CP ≥6.
Results
Patients, 47% with CP ≥6 cirrhosis (CP range 6–10), received PI triple therapy for a targeted duration of 48 weeks. The cohort was median age 59 years, 32% female, 59% genotype 1a, 35% previous null/partial responders. Sustained virological response at 12 weeks (SVR12) was achieved by 35% of patients with CP ≥6 vs. 54% of those with CP = 5 (P = 0.02). CP = 5, achievement of rapid virological response and genotype 1b/other, independently predicted SVR12. Compared to those with CP = 5, patients with CP ≥6 had more peg-IFN dose reductions, eltrombopag use, transfusions and hospitalisations to manage adverse events (all P < 0.05). Overall, 67 (42%) discontinued treatment early. Nine wait-listed patients were treated for a median of 97 days (IQR 60–160) prior to liver transplantation and five achieved post-LT SVR.
Conclusions
In the presence of mild decompensation (Child-Pugh ≥6), SVR12 rates with protease inhibitor triple therapy are significantly reduced and adverse events increased. Thus, treatment with protease inhibitor triple therapy, if judged as necessary, should be undertaken with close monitoring and awareness of the significant risks.
INTRODUCTION
Hepatitis C virus (HCV) infection affects an estimated 4.1 million Americans of whom an estimated 3.2 million have chronic hepatitis.1, 2 Most of those infected with HCV acquired the infection 30–40 years ago3, 4 and thus are expected to be at increased risk of HCV-related cirrhosis, hepatocellular carcinoma and liver-related death over the next decade.4 Thus, anti-viral therapy in HCV-infected patients with cirrhosis is especially relevant. Achievement of sustained viral clearance in those with advanced fibrosis significantly reduces the risk of liver complications.5 For the estimated 185 million people globally with HCV infection,6 many of whom have advanced fibrosis, defining risk and benefit of available treatment options is critically important.
With the approval in 2011 of the first direct acting anti-viral drugs for HCV, telaprevir and boceprevir, the triple-drug regimen of peginterferon (peg-IFN), ribavirin (RBV) with either telaprevir or boceprevir became the new standard of care for treatment of patients with genotype 1 HCV infection. However, all the registration trials for telaprevir and boceprevir triple therapy excluded patients with cirrhosis with Child-Pugh (CP) score above 5. There is no published United States (US) experience using protease inhibitors combined with peg-IFN and RBV for the treatment of decompensated cirrhosis. The French Compassionate Use of Protease Inhibitors in Viral C Cirrhosis (CUPIC) study, a large compassionate access experience of protease inhibitor triple therapy in patients with cirrhosis, included 489 patients with CP class A and 8 patients with CP class B cirrhosis.7 Indeed, caution in the use of protease inhibitor (PI) (telaprevir or boceprevir) triple therapy has been advised due to concerns of the effects of hepatic impairment on telaprevir and boceprevir pharmacokinetics and potential risk of increased adverse side-effects,8, 9 especially in the face of portal hypertension.10 While better tolerated therapy including interferon-free therapies for patients with genotype 1 are anticipated in the near future in the US, PI triple therapy with telaprevir and boceprevir, will remain the mainstay of HCV treatment in other countries for a more prolonged period of time. Thus, detailed information on the risks and benefits of PI triple therapy in patients with cirrhosis and mild decompensation remains extremely relevant.
In this US multicentre cohort study, we evaluated the real-life effectiveness and safety of protease inhibitor (telaprevir or boceprevir) triple therapy in HCV genotype 1-infected patients with cirrhosis with compensated and mildly decompensated liver disease.
PATIENTS AND METHODS
Study design
This retrospective multicentre cohort study included genotype 1 HCV-infected adult patients (18 years of age or older) with cirrhosis who received PI (telaprevir or boceprevir) triple therapy since PI approval (June 2011) through October 2013 in three different clinical settings: the University of California at San Francisco (UCSF), the San Francisco Veterans Affairs Medical Center (SFVAMC) and Kaiser Permanente Northern California (KPNC). UCSF is an academic, tertiary care referral centre that treats San Francisco patients as well as patients referred from northern and central California. SFVAMC is a Veterans Affairs hospital that functions as the HCV treatment referral centre for veterans living in northern and central California. KPNC is an integrated health care delivery organisation with over 3.2 million members in the San Francisco and Sacramento greater metropolitan areas. The membership is representative of the area’s total insured population except for persons with extremes in income.11, 12 The institutional review boards at each of the participating study centres approved this study. Patient demographic, virological and clinical data, including medication dosing were collected by individual health record review and/or programmed capture from health plan databases.
Treatment Regimens
Treatment included peg-IFN α2a of 180 µg/week or α2b 1.5 µg/kg/week with RBV 1000 mg (<75 kg)– 1200 mg (≥75 kg) daily adjusted to maximum tolerability, plus telaprevir (750 mg three times daily or 1125 mg twice daily) or boceprevir (800 mg three times daily) for an intended total treatment period of 48 weeks. Telaprevir was given with peg-IFN/RBV for 12 weeks and boceprevir was given with peg-IFN/RBV for 44 weeks. Lead-in of at least 4 weeks of peg-IFN/ RBV were used in patients receiving boceprevir and in patients receiving telaprevir in whom there were concerns regarding tolerability of peg-IFN/RBV. Standard treatment guidelines for dosing, duration and stopping rules were used.8, 9, 13 Patients in the cohort who were on the waiting list for liver transplantation were treated with PI triple therapy prior to LT with the primary goal of preventing recurrent HCV post-LT. Growth factors were used to manage cytopenias prior to and during treatment, at the discretion of the treating physician.
Primary predictor and study endpoints
Patients with baseline CP ≥6 (decompensated cirrhosis) were compared to those with baseline CP = 5 (compensated cirrhosis). The primary virological outcome was achievement of SVR12, defined as an undetectable HCV viral load 12 weeks (±2 weeks) after completion or early discontinuation of PI triple therapy. Secondary virological outcomes included achievement of rapid virological response (RVR), defined as an undetectable HCV viral load at 4 weeks (±1 week) of triple therapy, extended rapid virological response (eRVR), defined as an undetectable HCV viral load at weeks 4 and 12 weeks (±1 week) of triple therapy, and end of treatment response (EOTR), defined as an undetectable HCV viral load at the completion of therapy. Plasma HCV RNA levels were quantified by assays providing lower limits of quantification ranging from 615 to 43 IU/mL. Lower limits of detection of tests used to define ‘undetectable’ levels were 10 IU/mL or lower at all sites. The primary safety outcome was early discontinuation of treatment due to an adverse event. Secondary safety outcomes included peg-IFN/RBV dose reductions, growth factor usage, transfusions, discontinuation of treatment due to virological failure, hospitalisation due to therapy-related adverse events and mortality.
Patient population
The target population was patients with cirrhosis undergoing PI triple therapy with the accessible population including those treated at UCSF, SFVAMC and KPNC. Cirrhosis was defined by the presence of at least two of the following five criteria: (i) radiographic (ultrasound, computed tomography, magnetic resonance imaging) evidence of liver nodularity, (ii) radiographic evidence of portal hypertension, (iii) platelet count less than 120 thousand (K)/mm3 secondary to liver disease (i.e. no other identifiable cause), (iv) endoscopic evidence of varices or portal hypertensive gastropathy, or (v) liver biopsy with Ludwig-Batts stage 3 or 4 fibrosis. Patients without baseline laboratory data to calculate CP score within 3 months prior to initiation of anti-HCV therapy were excluded.
Of the 181 patients with cirrhosis who underwent PI triple therapy between June 2011 and October 2013 at the three sites, 21 (12%) were excluded due to the lack of CP score criterion yielding 160 total patients in the study cohort. Among those excluded, twenty were eligible for SVR12 of which 11 (55%) achieved the outcome. There were no statistically significant differences noted in the available baseline characteristics among those excluded compared to those included. However, one patient who was excluded died in week 14 of treatment from complications of sepsis.
Statistical analysis
All 160 patients were included in the analysis of the safety outcomes. A total of 147 (92%) patients were eligible for the virological outcomes with 60 weeks follow-up after treatment start (48 weeks treatment plus 12 weeks follow-up). Virological outcomes were analysed using an intention-to-treat approach. When assessing predictors of early treatment discontinuation due to adverse events, the comparator group was all others. For those who discontinued therapy early, MELD increase from baseline of ≥2 upon discontinuation was defined as worsening decompensation; these data were unavailable from one site.
Median with interquartile range (IQR), range and proportions, were used for descriptive statistics as appropriate. For comparisons of those patients with CP = 5 vs. ≥6, the Chi-square test was used for dichotomous variables, and the Mann–Whitney U-test was used for continuous variables. Exact methods were used, as appropriate.
Logistic regression was used to examine predictors of primary virological and safety outcomes, with P < 0.05 defined as statistically significant. The primary predictor of interest was baseline CP score (CP ≥6 vs. CP = 5). For both primary virological and safety outcomes, baseline characteristics including age, gender, race/ethnic group, HCV genotype 1a (vs. 1b or other), previous null or partial response to peg-IFN/RBV treatment (vs. treatment-naïve or relapsers to peg-IFN/RBV treatment), interleukin-28B (IL28B) genotype CC (vs. CT/TT), baseline laboratory indices [bilirubin, creatinine, international normalised ratio (INR), albumin and platelet count], baseline MELD, use of telaprevir (vs. boceprevir), baseline HCV viral load (VL), and presence of varices were examined in regression models. For the primary virological outcome, achievement of RVR was also examined in regression models separately. Those covariates with P < 0.20 were evaluated in multivariable models. Models were built using backward elimination of covariates, using a P < 0.05 as the criterion for inclusion in the final model. The primary predictor, baseline CP score (CP ≥6 vs. CP = 5), was forced into all models. Multicollinearity within the models was examined using the variance inflation factor (VIF). If the VIF was >10, then multicollinearity was established and the offending covariate was removed from the model. Optimisation of baseline bilirubin, INR, albumin and platelet count as a predictor of SVR12 was examined post hoc using receiver operator curves (Figure S1). The optimal point that maximised sensitivity and specificity within the cohort for each covariate (bilirubin of 1.2 mg/dL, INR of 1.2, albumin of 4.0 g/dL and platelet count of 115K/mm3) was included in univariate and multivariate analyses of SVR12.
Clinical site was identified as a proxy variable for on-treatment differences in peg-IFN dose reductions, RBV dose reductions, epotein-alfa use, granulocyte colony-stimulating factor use and eltrombopag use between sites. Thus, all final models were adjusted for clinical site.
Statistical analyses were performed using STATA version 13 software (Stata Corporation, College Station, TX, USA).
RESULTS
A total of 160 patients with cirrhosis were included in the study; 75 (47%) had a CP ≥6. The median age of the cohort was 59.0 years (IQR: 54.4–62.5), 32% were female, 9% were Black, 59% had genotype 1a, 29% had IL28B-CC (available in 73 patients) and 35% had a previous null or partial response to peg-IFN/RBV. Compared to patients with compensated cirrhosis (CP = 5), those with mildly decompensated cirrhosis (CP ≥6, range: 6–10) were older, had higher baseline bilirubin and INR and lower baseline albumin and platelet count. The median baseline MELD scores in patients with compensated and decompensated cirrhosis were 7 (IQR: 6–8) and 9 (IQR: 7–11) respectively. Baseline hepatocellular carcinoma (HCC) and listing for liver transplantation prior to starting treatment were more frequent in the CP ≥6 group (Table 1).
Table 1.
Characteristics of study cohort undergoing PI* triple therapy
| Characteristic at start of treatment | Compensated cirrhotics, CP = 5 (n = 85) |
Mildly decompensated cirrhotics, CP ≥6 (n = 75) |
P-value |
|---|---|---|---|
| Age, yrs, median (IQR) | 58.2 (53.6–61.5) | 60.0 (56.4–63.2) | 0.02 |
| Male, no (%) | 59 (69) | 50 (67) | 0.71 |
| Race/ethnicity, no. (%)† | 0.08 | ||
| White, Non-Hispanic | 38 (47) | 25 (34) | |
| Hispanic | 30 (37) | 29 (40) | |
| Black | 7 (9) | 7 (10) | |
| Asian | 6 (7) | 6 (8) | |
| Other | 0 (0) | 6 (8) | |
| Genotype 1a (vs. 1b or other), no. (%) | 49 (58) | 45 (60) | 0.76 |
| Previous treatment | |||
| Null/partial responders (vs. treatment naïve/relapser), no (%) | 29 (34) | 27 (36) | 0.80 |
| Bilirubin, mg/dL, median (IQR) | 0.7 (0.6–0.9) | 1 (0.7–1.8) | <0.0001 |
| INR, median (IQR) | 1.1 (1.0–1.1) | 1.2 (1.0–1.3) | 0.0004 |
| Albumin, g/dL, median (IQR) | 4 (3.8–4.2) | 3.3 (3.1–3.6) | <0.0001 |
| Platelet count, K/mm3, median (IQR) | 151 (108–190) | 92 (69–124) | <0.0001 |
| Creatinine, mg/dL, median (IQR) | 0.84 (0.70–0.97) | 0.8 (0.69–0.91) | 0.27 |
| MELD, median (IQR) (range) | 7 (6–8) (6–23)‡ | 9 (7–11) (6–20) | <0.0001 |
| Telaprevir (vs. boceprevir), no. (%) | 56 (66) | 55 (73) | 0.31 |
| Baseline VL, log international units, median (IQR) | 6.15 (5.79–6.49) | 5.97 (5.70–6.33) | 0.10 |
| HCC, no (%) | 3 (4) | 11 (15) | 0.02 |
| Nonbleeding or bleeding varices (vs. no varices), no. (%) | 4 (5) | 26 (35) | <0.0001 |
| Listed for liver transplant, no. (%) | 3 (4)§ | 22 (29) | <0.0001 |
| Time on triple therapy, days, median (IQR) | 257 (168–336) | 182 (88–332) | 0.10 |
| Peg-IFN/RBV lead-in, no. (%) | 32 (38) | 28 (37) | 0.50 |
| Peg-IFN α2a (vs. a2b), no. (%) | 80 (94) | 69 (92) | 0.76 |
| IL28B genotype CC (vs. CT/TT), no. (%)¶ | 12 (34) | 9 (24) | 0.32 |
| Site, no (%) | 0.005 | ||
| #1 | 31 (36) | 46 (61) | |
| #2 | 15 (18) | 11 (15) | |
| #3 | 39 (46) | 18 (24) |
CP, Child-Pugh; HCC, hepatocellular carcinoma; IL28B, interleukin-28B 12979860 polymorphism; INR, international normalised ratio; IQR, interquartile range; MELD, model for end-stage liver disease; peg-IFN, peginterferon; PI, protease inhibitor; RBV, ribavi-rin; VL, viral load.
Telaprevir or boceprevir.
Race/ethnicity data available in 154 patients.
One patient with compensated cirrhosis was treated while on dialysis.
All patients with compensated cirrhosis listed for liver transplant had concomitant HCC.
Available in 73 patients.
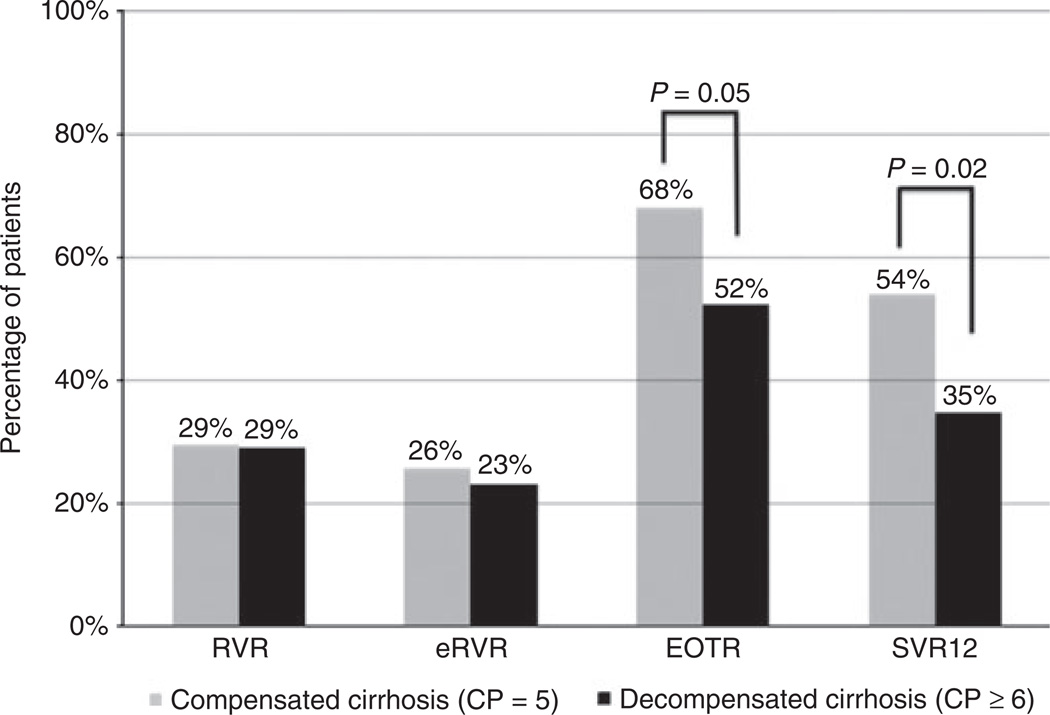
Virological outcomes
The overall SVR12 rate was 45%; 54% (42/78) in those with compensated cirrhosis and 35% (24/69) in those with mildly decompensated cirrhosis (P = 0.02). Rates of RVR and eRVR were similar between groups, with significant differences in response only seen at EOTR and SVR12 (Figure 1). Among those who achieved EOTR, the overall relapse rate was 20%; 17% (9/53) in those with compensated cirrhosis and 25% (9/36) in those with mildly decompensated cirrhosis (P = 0.42). In univariate analysis, SVR12 was associated with compensated cirrhosis (CP = 5), genotype 1b or other, previous HCV treatment-naïve or relapser status, IL28B-CC, lower baseline bilirubin, INR and MELD, higher baseline albumin and platelets, absence of varices and achievement of RVR (Table 2, Table S1). Choice of protease inhibitor was not associated with SVR12 in univariate analysis (P = 0.46, Table S1). In multivariate models, bilirubin <1.2 mg/dL, INR <1.2, albumin ≥4.0 g/dL, platelet count ≥115K/mm3 and MELD were found to be collinear with CP ≥6 group (VIF >10 for all) likely because all are markers of liver disease severity. Therefore, multivariate models excluded baseline bilirubin, INR, albumin, platelet count and MELD as covariates (Table 2). Mildly decompensated cirrhosis, genotype 1a and achievement of RVR were factors significantly associated with achievement of SVR12 in multivariate models. When included, platelet count ≥115K/mm3 was also significantly associated with SVR12 in multivariate modelling (OR = 5.01, 95% CI: 1.80– 13.98) while baseline bilirubin, INR and albumin were not.
Figure 1.
Virological outcomes of patients with compensated and mildly decompensated cirrhosis with telaprevir or boceprevir triple therapy.
Table 2.
Univariate and multivariate predictors of SVR12 in HCV genotype 1 patients with cirrhosis undergoing PI* triple therapy
| All SVR12 eligible patients (n = 147) |
||||||
|---|---|---|---|---|---|---|
| Univariate† |
Multivariate 1‡,§ |
Multivariate 2†¶ |
||||
| Covariate | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Mildly decompensated cirrhosis (CP ≥6) | 0.46 (0.23–0.89) | 0.02 | 0.32 (0.15–0.68) | 0.003 | 0.35 (0.16–0.75) | 0.007 |
| Genotype 1a (vs. 1b or other) | 0.52 (0.27–1.02) | 0.06 | 0.44 (0.21–0.89) | 0.02 | 0.47 (0.22–0.97) | 0.04 |
| Previous null/partial responde (vs. treatment naïve or relapser) | 0.56 (0.28–1.12) | 0.10 | – | – | – | – |
| Bilirubin start treatment <1.2 mg/dL | 0.48 (0.21–1.06) | 0.07 | – | – | – | – |
| INR start treatment <1.2 | 0.49 (0.24–0.99) | 0.05 | – | – | – | – |
| Albumin start treatment ≥4.0 g/dL | 2.4 (1.23–4.69) | 0.01 | – | – | – | – |
| Platelet count start treatment ≥115K/mm3 | 4.90 (2.03–11.84) | <0.0001 | – | – | – | – |
| MELD start treatment, per 1 | 0.87 (0.76–0.99) | 0.04 | – | – | – | – |
| Nonbleeding or bleeding varices (vs. no varices) | 0.51 (0.22–1.23) | 0.14 | ||||
| IL28B genotype CC (vs. CT/TT) (n = 73)** | 5.85 (1.47–23.3) | 0.01 | – | – | – | – |
| RVR | 3.75 (1.77–7. 9 7 ) | <0.001 | – | – | 3.12 (1.33–7.37) | 0.009 |
CP, Child-Pugh; IL28B, interleukin-28B 12979860 polymorphism; INR, international normalised ratio; MELD, model for end-stage liver disease; RVR, rapid virological response; SVR12, sustained virological response at 12 weeks.
Telaprevir or boceprevir.
Univariate results of variables with P < 0.20. Univariate analysis for every variable examined is presented in Table S1 [age, gender, race/ethnic group, HCV genotype 1a (vs. 1b or other), previous null or partial response to peg-IFN/RBV treatment (vs. treatment naïve or relapsers to peg-IFN/RBV treatment), IL28B genotype CC (vs. CT/TT), baseline laboratory indices (bilirubin, creatinine, INR, albumin and platelet count), baseline MELD, baseline mildly decompensated cirrhosis CP >6 (vs. CP = 5), use of telaprevir (vs. boceprevir), baseline HCV viral load, presence of varices, and achievement of RVR].
Adjusted for centre effect.
Multivariate model 1 created with only baseline covariates.
Multivariate model 2 created with baseline covariates and inclusion of RVR.
Excluded from presented multivariate modelling because of limited sample size.
While IL28B genotype was available in only 73 patients precluding its use in multivariate models, CC status was significantly associated with SVR12 in multivariate models adjusting for genotype 1a, decompensated cirrhosis and centre effect (OR = 5.96, 95% CI: 1.25–28.48).
Lack of RVR was highly predictive of non-SVR12 among patients with mildly decompensated cirrhosis. Among the 49 (71%) patients with mildly decompensated cirrhosis who did not achieve RVR, only five (10%) went on to achieve SVR12 yielding a negative predictive value of RVR for SVR12 of 90% (95% CI: 80–99%). Among the 55 (71%) of compensated cirrhotics who did not achieve RVR, 27 (49%) went on to achieve SVR12 yielding a negative predictive value of RVR on SVR12 of only 51% (95% CI: 40–62%).
In a subgroup analysis limited to patients with mildly decompensated cirrhosis, SVR12 was associated with Hispanic ethnicity (vs. non-Hispanic, White) (OR = 4.85, P = 0.02), genotype 1b or other (OR = 1.95, P = 0.19), IL28B-CC (OR = 13.5, P = 0.03) and achievement of RVR (OR =9.1, P < 0.0001) in univariate analysis (Table S4). Multivariate analysis adjusting for centre effect could not be completed due to limited sample size.
Safety Outcomes
Patients with mildly decompensated cirrhosis more frequently received peg-IFN dose reductions, eltrombopag, transfusions and hospitalisations due to an adverse event (Table 3). While rates of early treatment discontinuation for any reason were similar between the two groups, patients with mildly decompensated cirrhosis who discontinued therapy early were more likely to experience subsequent worsening of liver status as evidenced by an increase in MELD by at least 2 points (Table 3). Among those who discontinued treatment early for virological failure, most were for futility rules vs. virological breakthrough. Among those who discontinued treatment early due to an adverse event (n = 31), six (23%) did due to liver decompensation while five (16%) did due to cytopenias (Table S2). Median time on triple therapy was 74 days (IQR: 29–161) among patients who discontinued treatment early due to adverse events. Five patients, maintained on treatment for median 223 days (IQR: 161–258), who stopped therapy early due to adverse event achieved SVR12.
Table 3.
Safety outcomes for patients with cirrhosis undergoing PI* triple therapy
| Safety outcome | Compensated Cirrhosis, CP = 5 (n = 85) |
Decompensated Cirrhosis, CP ≥6 (n = 75) |
P-value |
|---|---|---|---|
| RBV dose reduction, no. (%) | 58 (68) | 52 (69) | 0.88 |
| RBV minimum dose, mg/day, median (IQR) | 600 (600–1000) | 600 (200–1000) | 0.05 |
| Peg-IFN dose reduction, no. (%) | 21 (25) | 41 (55) | <0.0001 |
| Peg-IFN minimum dose, µg/week, median† | 180 (158–180) | 135 (90–180) | <0.0001 |
| Growth factor use, no. (%) | |||
| Epotein-alfa | 48 (56) | 47 (63) | 0.43 |
| Granulocyte colony-stimulating factor | 23 (27) | 28 (37) | 0.16 |
| Eltrombopag | 2 (2) | 19 (25) | <0.0001 |
| Received transfusion(s) during treatment, no. (%) | 7 (8) | 18 (24) | 0.006 |
| Adverse event requiring hospitalisation, no. (%) | 10 (12) | 18 (24) | 0.04 |
| Early treatment discontinuation, no. (%) | 32 (38) | 34 (45) | 0.32 |
| Early treatment discontinuation due to adverse event, no. (%)‡ | 12 (14) | 19 (25) | 0.07 |
| Early treatment discontinuation due to virological failure, no. (%) | 20 (24) | 15 (20) | 0.94 |
| Futility rule | 14 (16) | 8 (11) | 0.52 |
| Breakthrough | 6 (7) | 7 (9) | 0.48 |
| MELD increase ≥2 upon early treatment discontinuation, no. (%)§ | 2 (13) | 11 (52) | 0.02 |
CP, Child-Pugh; IQR, interquartile range; MELD, model for end-stage liver disease; peg-IFN, peginterferon; RBV, ribavirin.
Telaprevir or boceprevir.
Among those receiving peg-IFN a2a.
List of adverse events leading to treatment discontinuation is presented in Table S2.
Early treatment discontinuation due to either adverse event or virological failure; analysis excluded one site where MELD score upon early treatment discontinuation was unavailable.
In univariate analysis, mildly decompensated cirrhosis (CP ≥6), nonwhite race, genotype 1a, lower baseline albumin and higher baseline creatinine and MELD were associated with early treatment discontinuation due to adverse event (Table 4, Table S3). Choice of protease inhibitor was not associated with early treatment discontinuation due to adverse event in univariate analysis (P = 0.83, Table S3). In multivariate models, baseline albumin and MELD were found to be collinear with CP ≥6 group (VIF >10 for all) likely because both are markers of liver disease severity. Therefore, multivariate models excluded baseline albumin and MELD as covariates (Table 4). Only baseline mildly decompensated cirrhosis was significantly associated with early treatment discontinuation due to an adverse event in multivariate models (Table 4). When included, baseline MELD per point (OR = 1.23, 95% CI: 1.05–1.43) was significantly associated with early discontinuation in multivariate modelling while baseline albumin was not.
Table 4.
Univariate and multivariate predictors of early treatment discontinuation due to adverse event(s)
| All patients (n = 160) |
||||
|---|---|---|---|---|
| Univariate* |
Multivariate† |
|||
| Covariate | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Decompensated cirrhosis (CP ≥6) | 2.06 (0.93–4.60) | 0.08 | 3.08 (1.28–7.42) | 0.01 |
| Race/ethnicity | ||||
| White, nonhispanic | Reference | Reference | – | – |
| Hispanic | 2.04 (0.74–5.61) | 0.17 | ||
| Black | 3.20 (0.79–12.99) | 0.10 | ||
| Asian | 2.67 (0.58–12.25) | 0.21 | ||
| Other | 4.00 (0.61–25.96) | 0.15 | ||
| Genotype 1a (vs. 1b or other) | 1.94 (0.83–4.53) | 0.13 | – | – |
| Albumin start treatment, per 1 g/dL | 0.58 (0.28–1.17) | 0.13 | – | – |
| Creatinine start treatment, per 1 mg/dL | 1.30 (0.89–1.92) | 0.18 | – | – |
| MELD start treatment, per 1 | 1.09 (0.96–1.24) | 0.17 | – | – |
CP, Child-Pugh; MELD, model for end-stage liver disease.
Univariate results of variables with P < 0.20. Univariate analysis for every variable examined is presented in Table S3 [age, gender, race/ethnic group, HCV genotype 1a (vs. 1b or other), previous null or partial response to Peg-IFN/RBV treatment (vs. treatment naïve or relapsers to peg-IFN/RBV treatment), IL28B genotype CC (vs. CT/TT), baseline laboratory indices (bilirubin, creatinine, INR, albumin and platelet count), baseline MELD, baseline mildly decompensated cirrhosis CP ≥6 (vs. CP = 5), use of telaprevir (vs. boceprevir), baseline HCV viral load and presence of varices].
Adjusted for centre effect.
In a subgroup analysis limited to patients with mildly decompensated cirrhosis, early treatment discontinuation due to an adverse event was associated with higher MELD score at baseline (OR = 1.10, P = 0.19) in univariate analysis (Table S5). Multivariate analysis adjusting for centre effect could not be completed due to limited sample size.
Liver Transplant Recipients
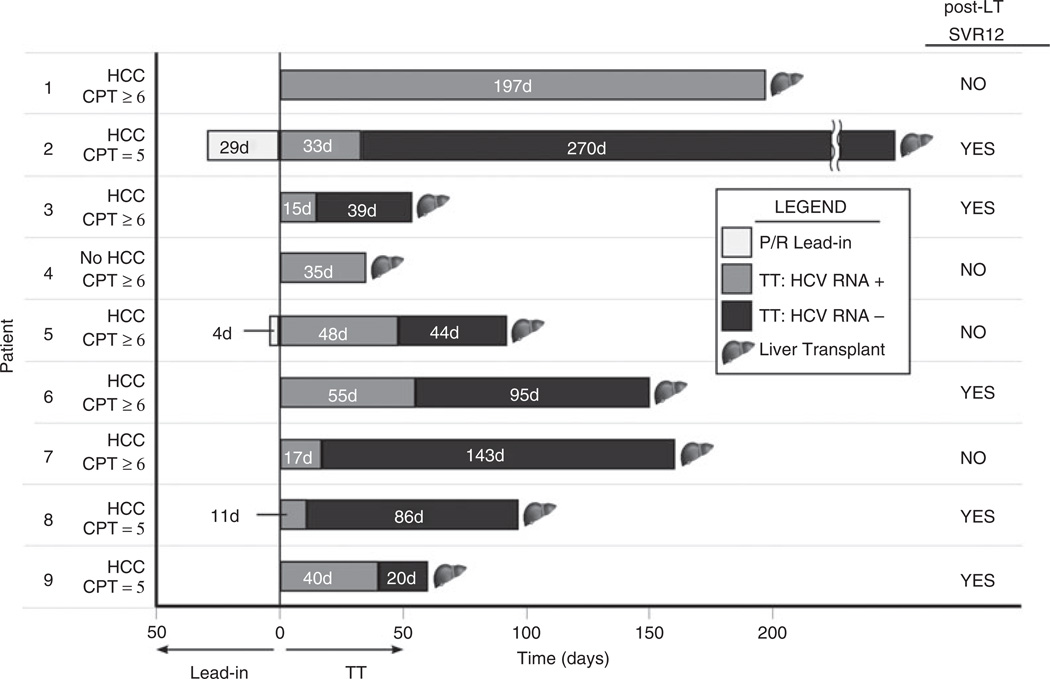
Nine patients (median age 64.7 years. 33% female, 66% genotype 1a, 43% (3/7) IL28B-CC and 33% previous null/partial responders) undergoing LT were treated for a median of 97 days (IQR: 60–160) prior to surgery. Six patients had mildly decompensated cirrhosis (CP ≥6) and eight had concomitant HCC. Seven achieved undetectable HCV RNA on treatment prior to LT and five had a SVR12 post-LT (Figure 2). Median time on triple therapy was similar between those who achieved post-transplant SVR12 compared to those who did not (97 vs. 126 days), as was the median time of HCV RNA negativity prior to LT (86 days vs. 94 days).
Figure 2.
Outcomes of patients receiving telaprevir or boceprevir triple therapy prior to liver transplantation. Graphical representation of the nine wait-listed patients treated prior to liver transplantation. The x-axis is time in days with lead-in to the left of the vertical line and triple therapy to the right. The number 1–9 along the y-axis identifies each patient. Also along the y-axis, each patient is identified as having HCC vs. no HCC and as having compensated (CP = 5) or mildly decompensated (CP ≥6) cirrhosis. All nine patients were treated with telaprevir-based triple therapy. On the right, we represent those that achieved post-LT SVR12 with ‘YES’ and those who did not with ‘NO’.
DISCUSSION
Capturing treatment outcomes from three diverse treatment settings (university, VA and health maintenance organisation), we provide real-life estimates of the effectiveness and safety of triple therapy in patients with mildly decompensated cirrhosis. We show that the effectiveness of current triple therapy in such patients was reduced, with only 35% achieving SVR12 compared to 53% among patients with well-compensated cirrhosis. Moreover, the rate of discontinuation was high in patients with mildly decompensated cirrhosis (47%), reflecting both poor response to therapy (met futility rules or experienced breakthrough) and reduced tolerability. However, even among patients who successfully completed therapy, SVR12 rates differed between patients with compensated (81%) and mildly decompensated (66%) cirrhosis, suggesting that the underlying severity of liver disease affects likelihood of achieving sustained viral clearance. Our results suggest that risks associated with treatment using PI (telaprevir or boceprevir) triple therapy likely outweigh potential benefit in patients with mildly decompensated cirrhosis. However, if treatment is undertaken in this group, use of early stopping rules may be of particular merit as they can reduce the risk of adverse consequences in those with low likelihood of SVR. In our study, failure to achieve RVR was highly predictive of nonSVR, with a negative predictive value of 89%. Using repeated HCV RNA testing of week 4 samples may in fact increase the negative predictive value to 100%.14 Using lack of RVR as a stopping rule has been proposed in the treatment of nonge-notype 1 HCV infections with a resultant economic and safety benefit.15 These safety benefits may be even greater in the context of treating patients with mildly decompensated cirrhosis with PI triple therapy.
Across a variety of HCV treatments, patients with cirrhosis have a lower response than patients without cirrhosis.16–23 The reasons for this differential rate of response are not known. Previous studies have shown that increased portal hypertension, measured directly using the hepatic vein pressure gradient, independently predicted non-SVR possibly due to altered pharmacokinetics of drug uptake, distribution and metabolism.24 Another study showed increased intrahepatic resistance with increased shear in the hepatic sinusoids that dampens the chemoat-traction of T lymphocytes and the interaction of T lymphocytes and HCV-infected hepatocytes, an interaction that appears crucial for viral clearance.25 These and other factors may explain the lower treatment response among patients with cirrhosis, factors that may be more exaggerated in patients with cirrhosis and decompensation.
Our results in patients with compensated cirrhosis are similar to those reported in the CUPIC study and a recent study published by Bichoupan et al., with SVR12 in 54% vs. 40% vs. 40% and early discontinuation due to adverse events in 14% vs. 11.7% vs. 20% respectively.7, 26, 27 In contrast, Ogawa et al. reported SVR12 in 69.6% of patients with advanced fibrosis, a rate that is higher than our results likely because of inclusion of only genotype 1b infections.28 However, we found the safety of PI triple therapy in patients with mildly decompensated cirrhosis was worse than patients with compensated cirrhosis with 25% discontinuing treatment early due to adverse effects and a higher proportion of patients requiring drug dose reductions, growth factor use, transfusions and hospitalisations due to adverse events. Indeed, CP ≥6 at baseline was the only independent predictor of treatment discontinuation due to adverse events. Haematological complications represent the most frequent serious complication with PI triple therapy and the frequency of complications parallels the severity of the underlying liver disease and portal hypertension.29–32 Management of these cytopenias contributes considerably to the complexity and cost of treatment.
We found that genotype 1a (vs. 1b or other) HCV infection was negatively associated with SVR12. A differential rate of SVR by HCV sub-genotype has been seen with telaprevir or boceprevir triple therapy in compensated cirrhotics26 as well as with other PI triple therapies.33 This highlights the specificity of these new direct anti-viral agents.
Development of liver decompensation, liver-related mortality and overall mortality is slowed and possibly prevented with successful HCV treatment in patients with compensated cirrhosis.34 Although data are lacking, similar benefits may be expected by successful HCV treatment in patients with mildly decompensated disease and these patients are an important group for treatment interventions.35 Clearly IFN-free therapies offer much in terms of safety and are highly desirable for patients with decompensated disease. However, in non-US countries, telaprevir or boceprevir triple therapy is likely to remain the standard of care for treatment of genotype 1 HCV infections for some years. Our results highlight the modest SVR12 rates achievable and the significant risk for adverse events in patients with mildly decompensated cirrhosis. Consequently, treatment in these patients should be very selective. Patients with favourable response characteristics such as those with IL28B-CC genotype or HCV genotype 1b may be best candidates. RVR is the strongest on-treatment predictor and stopping treatment in those who fail to achieve RVR is an extremely important means of minimising risk and maximising benefit. Ultimately, the severity of liver disease, as reflected by MELD or CP, strongly influences the risks on treatment. This risk is best mitigated by close monitoring and treatment in experienced centres, preferably with liver transplantation available as potential rescue therapy.
Of the nine wait-listed patients undergoing triple therapy pre-LT, 67% remained HCV RNA negative at 12 weeks post-LT. This post-LT SVR rate is encouraging given that prior studies reported only 24–30% of waitlisted patients with genotype 1 treated with peg-IFN/ RBV pre-LT achieved sustained viral clearance post-LT.36, 37 Moreover, the duration of therapy needed to achieve these post-LT outcomes was only ~3 months, minimising the likelihood of adverse events. The optimal duration of PI triple therapy and time of HCV RNA negativity prior to LT cannot be discerned from our series, but the majority (80%) of patients who achieved post-LT SVR12 were negative for at least 5.5 weeks pre-LT. Most recently, preliminary data on noninterfer-on-based pre-LT anti-viral therapy with sofosbuvir and ribavirin in wait-listed patients with compensated cirrhosis reported a 64% success in preventing post-LT HCV recurrence.18 Looking to the future, shorter duration of therapy and greater safety and tolerability with interferon-free regimens are expected.
There are some limitations of our study. First, due to its retrospective nature and reliance on medical records from routine clinical practice, factors of potential importance in predicting response to therapy, such as IL28B status, are lacking in all patients. However, in the specific case of IL28B, a significant association between CC status and SVR12 was found among the subset of patients with IL28B available. Importantly, inclusion of this predictive factor did not alter the strength or significance of other correlations we observed. Second, given the inclusion of three centres with multiple treating physicians, there was likely variability in how peg-IFN/RBV dose reductions were made, as well as thresholds for growth factor use or discontinuing therapy due to adverse events. However, we believe capturing the real-life clinical application of PI triple therapy in different clinical settings is a strength that increases the generalisability of our findings.
In summary, we show that PI triple therapy in patients with mildly decompensated cirrhosis promises only modest SVR12 rates with significant risk for adverse events. Thus, treatment of these patients needs very careful consideration, with a weighing of risks and benefits. Additionally, in patients with mildly decompensated cirrhosis who undergo PI triple therapy, lack of RVR should be strongly considered as an early stopping rule. Overall, these results point to the importance of obtaining ‘real world’ outcomes on the risk vs. benefit of recently approved treatments to better inform clinical decisions and of the high need for safer and more effective therapies in patients with decompensated cirrhosis.
Supplementary Material
ACKNOWLEDGEMENT
Declaration of personal interests: MPP discloses clinical trial research support from Roche and Merck. NT discloses research support from Gilead, Genetech/Roche, Vertex, Novartis, Eisai and AbbVie and has served as consultant for Bristol-Myers Squibb. None of these companies supported the current study.
Declaration of funding interests: VS was supported in part by an NIH-funded post-doctoral research training program grant (T32 DK060414). MMM discloses research support from Vertex, Merck, and Gilead. HY and JC contributed in the context of AM’s VA Merit Funding (1I01 CX000295-01A1). At Kaiser Permanente, this work was supported by The Permanente Medical Group, and a portion of data collection by a contract from Vertex Pharmaceuticals Incorporated. At University of California San Francisco and at the San Francisco Veterans Affairs Medical Center, this work was supported by the Liver Center Grant (NIH P30 DK 026743).
Footnotes
AUTHORSHIP
Guarantor of the article: Norah A. Terrault.
Author contributions: VS designed the research study, collected and analyzed the data, and wrote the first draft of the manuscript. MMM contributed to the analysis of the data and manuscript writing. HSY, LC, EW, RCM, VAS, MPP and JC all contributed to the data collection and analysis. AM contributed to the design of the research study and writing of the paper. NAT designed the research study, and contributed to data analysis and paper writing. All authors approved the final version of the manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Figure S1. Receiver operator curves for prediction of baseline bilirubin on achievement of non-SVR12 (Panel A), of baseline INR on achievement of non-SVR12 (Panel B), of baseline albumin on achievement of SVR12 (Panel C) and of baseline platelets on achievement of non-SVR12.
Table S1. Univariate predictors of SVR12 in HCV genotype 1 patients with cirrhosis undergoing PI triple therapy.
Table S2. Adverse events leading to early discontinuation of PI triple therapy.
Table S3. Univariate predictors of early treatment discontinuation due to adverse event(s).
Table S4. Univariate predictors of SVR12 in HCV genotype 1 patients with mildly decompensated cirrhosis (Child-Pugh ≥6) undergoing PI triple therapy.
Table S5. Univariate predictors of early treatment discontinuation due to adverse event(s) among patients with mildly decompensated cirrhosis (Child Pugh ≥6).
REFERENCES
- 1.Edlin BR. Hepatitis C screening: getting it right. Hepatology. 2013;57:1644–1650. doi: 10.1002/hep.26194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA. 1990;264:2231–2235. [PubMed] [Google Scholar]
- 4.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 5.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 6.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 7.Hezode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Product Information. Incivek [telaprevir] Cambridge: MVPIM; [Google Scholar]
- 9.Product Information. Victrelis [boceprevir] Whitehouse Stataion, NJ: Whitehouse Station NMC, Inc; May, 2011. [Google Scholar]
- 10.Saxena V, Terrault N. Hepatitis C virus treatment and liver transplantation in the era of new antiviral therapies. Curr Opin Organ Transplant. 2012;17:216–224. doi: 10.1097/MOT.0b013e3283534d64. [DOI] [PubMed] [Google Scholar]
- 11.Gordon N. Similarity of the adult Kaiser Permanente membership in northern California to the insured and general population in northern California: statistics from the 2007 California Health Interview Survey. Internal Report: Kaiser Permanente Division of Research. 2012 [Google Scholar]
- 12.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee HS, Chang MF, Pocha C, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol. 2012;107:669–689. doi: 10.1038/ajg.2012.48. [DOI] [PubMed] [Google Scholar]
- 14.Maasoumy B, Cobb B, Bremer B, et al. Detection of low HCV viraemia by repeated HCV RNA testing predicts treatment failure to triple therapy with telaprevir. Aliment Pharmacol Ther. 2014;39:85–92. doi: 10.1111/apt.12544. [DOI] [PubMed] [Google Scholar]
- 15.Bunchorntavakul C, Chavalitdhamrong D, Tanwandee T. Hepatitis C genotype 6: a concise review and response-guided therapy proposal. World J Hepatol. 2013;5:496–504. doi: 10.4254/wjh.v5.i9.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawitz E, Poordad F, Brainard D, et al. Sofosbuvir in combination with pegIFN and ribavirin for 12 weeks provides high SVR rates in HCV-infected genotype 2 or 3 treatment experienced patients with and without compensated cirrhosis: results from the LONESTAR-2 study. Program and abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 1–5, 2013; Washington, DC LB4. [Google Scholar]
- 17.Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 18.Curry M, Forns X, Chung R, et al. Pretransplant sofosbuvir and ribavirin to prevent recurrence of HCV infection after liver transplantation. Program and abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 1–5, 2013; Washington, DC. Abstract 213. [Google Scholar]
- 19.Jacobson I, Ghalib R, Rodriguez-Torres M, et al. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naive and prior null responder patients: the COSMOS study. Program and abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 1–5, 2013; Washington, DC. Abstract LB3. [Google Scholar]
- 20.Zeuzem S, Dusheiko G, Salupere R. Sofosbuvir + ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE trial. Program and abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases; November 1–5, 2013; Washington, DC. Abstract 1085. [Google Scholar]
- 21.Lawitz E, Ghalib R, Rodriguez-Torres M, et al. Suppression of viral load through 4 weeks post-treatment results of a once-daily regimen of simeprevir + sofosbuvir with or without ribavirin in hepatitis C virus GT 1 null responders. Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. Abstract 155LB. [Google Scholar]
- 22.Iacobellis A, Siciliano M, Annicchiarico BE, et al. Sustained virological responses following standard anti-viral therapy in decompensated HCV-infected cirrhotic patients. Aliment Pharmacol Ther. 2009;30:146–153. doi: 10.1111/j.1365-2036.2009.04025.x. [DOI] [PubMed] [Google Scholar]
- 23.Carrion JA, Martinez-Bauer E, Crespo G, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: a retrospective study. J Hepatol. 2009;50:719–728. doi: 10.1016/j.jhep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Reiberger T, Rutter K, Ferlitsch A, et al. Portal pressure predicts outcome and safety of antiviral therapy in cirrhotic patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2011;9:602–608. e1. doi: 10.1016/j.cgh.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Zhu H, Tu Z, Xu YL, Nelson DR. CD8+ T-cell interaction with HCV replicon cells: evidence for both cytokine- and cell-mediated antiviral activity. Hepatology. 2003;37:1335–1342. doi: 10.1053/jhep.2003.50207. [DOI] [PubMed] [Google Scholar]
- 26.Fontaine H, Hezode C, Dorival C, et al. ANRS CO20 CUPIC study group. SVR12 rates and safety of triple therapy including Telaprevir or Boceprevir in 221 cirrhotic non-responders treated in the French early access program (ANRS CO20-CUPIC). 48th Annual Meeting of the European Association for the Study of the Liver; April 24–28, 2013; Amsterdam, Netherlands. abstract 60. [Google Scholar]
- 27.Bichoupan K, Schwartz JM, Martel-Laferriere V, et al. Effect of fibrosis on adverse events in patients with hepatitis C treated with telaprevir. Aliment Pharmacol Ther. 2014;39:209–216. doi: 10.1111/apt.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa E, Furusyo N, Nakamuta M, et al. Telaprevir-based triple therapy for chronic hepatitis C patients with advanced fibrosis: a prospective clinical study. Aliment Pharmacol Ther. 2013;38:1076–1085. doi: 10.1111/apt.12494. [DOI] [PubMed] [Google Scholar]
- 29.Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130:225–230. doi: 10.1053/j.gastro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Peck-Radosavljevic M, Wichlas M, Pidlich J, et al. Blunted thrombopoietin response to interferon alfa-induced thrombocytopenia during treatment for hepatitis C. Hepatology. 1998;28:1424–1429. doi: 10.1002/hep.510280535. [DOI] [PubMed] [Google Scholar]
- 31.Schmid M, Kreil A, Jessner W, et al. Suppression of haematopoiesis during therapy of chronic hepatitis C with different interferon alpha mono and combination therapy regimens. Gut. 2005;54:1014–1020. doi: 10.1136/gut.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317–323. doi: 10.1097/00042737-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Larrey D, Lohse AW, de Ledinghen V, et al. Rapid and strong antiviral activity of the non-nucleosidic NS5B polymerase inhibitor BI 207127 in combination with peginterferon alfa 2a and ribavirin. J Hepatol. 2012;57:39–46. doi: 10.1016/j.jhep.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104:1147–1158. doi: 10.1038/ajg.2009.31. [DOI] [PubMed] [Google Scholar]
- 35.Rowe IA, Houlihan DD, Mutimer DJ. Despite poor interferon response in advanced hepatitis C virus infection, models of protease inhibitor treatment predict maximum treatment benefit. Aliment Pharmacol Ther. 2012;36:670–679. doi: 10.1111/apt.12018. [DOI] [PubMed] [Google Scholar]
- 36.Forns X, Garcia-Retortillo M, Serrano T, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389–396. doi: 10.1016/s0168-8278(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 37.Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57:1752–1762. doi: 10.1002/hep.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.