Abstract
We previously demonstrated that mutation of the staphylococcal accessory regulator (sarA) in a clinical isolate of Staphylococcus aureus (UAMS-1) results in an impaired capacity to form a biofilm in vitro (K. E. Beenken, J. S. Blevins, and M. S. Smeltzer, Infect. Immun. 71:4206-4211, 2003). In this report, we used a murine model of catheter-based biofilm formation to demonstrate that a UAMS-1 sarA mutant also has a reduced capacity to form a biofilm in vivo. Surprisingly, mutation of the UAMS-1 ica locus had little impact on biofilm formation in vitro or in vivo. In an effort to identify additional loci that might be relevant to biofilm formation and/or the adaptive response required for persistence of S. aureus within a biofilm, we isolated total cellular RNA from UAMS-1 harvested from a biofilm grown in a flow cell and compared the transcriptional profile of this RNA to RNA isolated from both exponential- and stationary-phase planktonic cultures. Comparisons were done using a custom-made Affymetrix GeneChip representing the genomic complement of six strains of S. aureus (COL, N315, Mu50, NCTC 8325, EMRSA-16 [strain 252], and MSSA-476). The results confirm that the sessile lifestyle associated with persistence within a biofilm is distinct by comparison to the lifestyles of both the exponential and postexponential phases of planktonic culture. Indeed, we identified 48 genes in which expression was induced at least twofold in biofilms over expression under both planktonic conditions. Similarly, we identified 84 genes in which expression was repressed by a factor of at least 2 compared to expression under both planktonic conditions. A primary theme that emerged from the analysis of these genes is that persistence within a biofilm requires an adaptive response that limits the deleterious effects of the reduced pH associated with anaerobic growth conditions.
Staphylococcus aureus is a prominent human pathogen that causes a wide variety of infections. Of particular interest in our laboratory are musculoskeletal infections including those associated with orthopedic implants. The hallmark characteristic of these infections is formation of a biofilm, which consists of multiple layers of bacteria encased within an exopolysaccharide glycocalyx. The presence of this glycocalyx protects the enclosed bacteria from host defenses and impedes delivery of at least some antibiotics (64). Moreover, bacteria within biofilms adopt a phenotype that confers intrinsic resistance to many antibiotics. For example, the reduced growth rate of biofilm-associated bacteria limits the efficacy of antibiotics that target cell wall biosynthesis, while the reduced oxidative metabolism limits the uptake of aminoglycosides (33, 64, 65). Consequently, biofilm-associated infections are recalcitrant to antimicrobial therapy and often require surgical intervention to debride infected tissues and/or remove colonized implants.
The formation of three-dimensional biofilms is a complex process that can be subdivided into the relatively distinct phases of attachment, accumulation, maturation, and dispersal (10). With respect to staphylococcal biofilms, the primary emphasis so far has been placed on the attachment and accumulation phases, which appear to be mediated by different types of adhesins. More specifically, a group of surface-exposed proteins collectively referred to as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (48) appear to be the primary determinants responsible for the initial attachment to both native tissues and biomaterials, while the accumulation phase appears to be dependent on polysaccharide adhesins that promote adhesive interactions between bacterial cells (26). Although a number of candidate polysaccharides have been described, there is an emerging consensus that the primary determinant of the accumulation phase of staphylococcal biofilm formation is the polysaccharide intercellular adhesin (PIA), production of which is dependent upon the genes within the icaADBC operon (28). Composition studies have demonstrated that PIA consists of polymeric N-acetylglucosamine, and for this reason it has also been referred to as PNAG (40).
The ica operon was first identified in Staphylococcus epidermidis (28) and has been studied most extensively in that species. However, it is also present and appears to serve the same function in S. aureus (14). Most S. aureus strains appear to contain the entire ica operon (14, 22, 53), although there are reports to the contrary (3), and it is clear that there are strain-dependent differences with respect to the overall capacity to form a biofilm in vitro (5, 14, 53). The ica operon is subject to phase variation in S. epidermidis (75), and a number of studies have indicated that expression of ica in both S. epidermidis and S. aureus is also subject to environmental regulation. Perhaps most importantly, McKenney et al. (42) demonstrated that PNAG production in S. aureus is enhanced during in vivo growth. Rachid et al. (52) subsequently demonstrated that expression of ica is at least partially controlled by the stress response transcription factor σB. In addition, anaerobic growth was found to induce expression of the ica operon and PIA production in both S. epidermidis and S. aureus (15).
Recently, Conlon et al. (12) reported that icaR, which is located immediately upstream of the ica operon, encodes a repressor that is important for the environmental regulation of ica expression in S. epidermidis. However, studies done with S. aureus have demonstrated that regulation of ica expression and the ability to form a biofilm also involve regulatory elements other than σB and IcaR (66). Included among these additional regulatory loci are the accessory gene regulator (agr) and the staphylococcal accessory regulator (sarA). The agr locus encodes a two-component quorum-sensing system that modulates production of a regulatory RNA molecule (RNAIII) in a density-dependent manner. Induction of RNAIII synthesis results in reduced production of surface proteins (e.g., MSCRAMMs) and a concomitant increase in production of exotoxins (4, 45). Production of δ-toxin, which is encoded within the RNAIII locus, has been negatively correlated with biofilm formation (69, 70). This suggests that strains expressing agr at high levels would have a reduced capacity to form a biofilm, which is consistent both with our results (5) and results from other laboratories (70).
The sarA locus encodes a 14.5-kDa DNA-binding protein (SarA) that is required at least under some growth conditions for maximum expression from the agr and RNAIII promoters (29). This would imply that mutation of sarA would limit production of RNAIII and thereby enhance the ability to form a biofilm. However, recent reports have confirmed that mutation of sarA results in a reduced capacity to form a biofilm (5, 66). SarA also regulates expression of other genes in an agr-independent manner (6, 19, 72, 74), and Valle et al. (66) recently demonstrated that mutation of sarA results in reduced transcription of the ica operon and a reduced capacity to produce PNAG. They also suggested that SarA may promote biofilm formation in an indirect manner by suppressing transcription of a repressor of PNAG synthesis or a protein involved in the turnover of PNAG.
The persistence of bacteria within a biofilm also requires an adaptive response appropriate for the sessile lifestyle. The availability of complete bacterial genome sequences has facilitated the use of microarray technologies to identify genes that are differentially expressed by biofilm-encased bacteria. Using an array representing 99% of the Bacillus subtilis genome, Stanley et al. (63) identified 519 genes that were differentially expressed in biofilms as opposed to planktonic cultures. Similarly, Schembri et al. (59) found that 5 to 10% of the genes in the Escherichia coli genome were differentially expressed in biofilms, depending on which planktonic growth condition was used as a reference. Included among these genes were 30 of the 65 genes previously reported to be under the regulatory control of the general stress response regulator rpoS (36). Schembri et al. (59) subsequently demonstrated that an E. coli rpoS mutant was incapable of forming a biofilm. However, Whiteley et al. (71) found that expression of rpoS was repressed in Pseudomonas aeruginosa biofilms and that a P. aeruginosa rpoS mutant formed a more extensive biofilm than the corresponding wild-type strain. While these results confirm that biofilms represent a unique growth state by comparison to planktonic cultures, they also suggest the existence of species-specific pathways that contribute to biofilm formation and maintenance of the sessile lifestyle. To date, no comprehensive transcriptional analysis of S. aureus biofilms has been reported. However, Prigent-Combaret et al. (51) demonstrated that biofilm-encased E. coli encounter high osmolarity, oxygen limitation, and higher cell density than cells grown under planktonic conditions, and all of these factors are known to influence gene expression in S. aureus (11, 45).
To further investigate these issues, we generated sarA and ica mutations in a clinical isolate of S. aureus (UAMS-1) and examined their relative capacity to form a biofilm both in vitro and in vivo. We also used a custom-made Affymetrix GeneChip representing the combined genomes of six strains of S. aureus (N315, Mu50, COL, NCTC 8325, EMRSA-16 [strain 252], and MSSA-476) to investigate differential gene expression in a mature S. aureus biofilm.
MATERIALS AND METHODS
Bacterial strains.
The experiments described here focus on the S. aureus clinical isolate UAMS-1. This strain was cultured from the bone of a patient suffering from osteomyelitis and was previously shown to form a biofilm both in vitro (5) and in vivo (21). The UAMS-1 sarA mutant was generated by transduction as previously described (7). Φ11-mediated transduction from a derivative of S. aureus SA113 carrying an ica::tet mutation (14) was used to generate a UAMS-1 ica mutant. Transductants were confirmed by Southern blotting using probes corresponding to the sarA and ica loci (6, 7).
Detection of PNAG production.
To assess the production of PNAG in S. aureus clinical isolate UAMS-1 and its sarA and ica mutants, cultures were grown in tryptic soy broth with the appropriate antibiotic. After overnight incubation, the optical density at 560 nm (OD560) was determined, and an equal number of cells (2 to 5 ml of each culture grown overnight) was harvested by centrifugation. Cells were resuspended in 50 μl of 0.5 M EDTA (pH 8.0) and boiled for 5 min. After cellular debris was removed by centrifugation, a 40-μl aliquot of the supernatant was incubated for 30 min with 10 μl of proteinase K (20 mg/ml) at 37°C to reduce nonspecific background levels. After the addition of 10 μl of Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl [pH 7.4]), 8 μl of each extract was spotted onto a nitrocellulose membrane using a BIO-dot microfiltration apparatus (Bio-Rad Laboratories, Inc., Hercules, Calif.). After drying, the presence of PNAG in the extract was assessed using the WesternBreeze chemiluminescence immunodetection kit (Invitrogen Corp., Carlsbad, Calif.) and anti-PNAG antiserum (kindly provided by Kimberly Jefferson, Channing Laboratory, Harvard Medical School).
Planktonic culture conditions.
Because biofilm formation by strain UAMS-1 in vitro is dependent on supplementation of the medium with 0.5% glucose and 3.0% sodium chloride (5), these supplements were also added to the medium used for planktonic culture. Specifically, 5-ml samples of cultures grown overnight in tryptic soy broth at 37°C with constant aeration were used to inoculate 250 ml of fresh biofilm medium to an OD560 of 0.05. Cultures were incubated with aeration at 37°C. Aliquots were then removed at the mid-exponential (OD560 = 1.0) and stationary (OD560 = 3.5) growth phases. Aliquots were immediately mixed with an equal volume of ice-cold acetone-ethanol (1:1) and stored at −20°C prior to RNA extraction.
Biofilm cultures.
Biofilms were generated in disposable flow cells (Stovall Life Science, Greensboro, N.C.) as previously described (5). Briefly, flow cells were precoated overnight at 4°C with 20% human plasma diluted in carbonate buffer (pH 9.6). The inlet side of the flow cell was then connected to a sterile reservoir filled with biofilm medium. The outlet side was connected to a waste reservoir. Tubing upstream of each individual cell was injected with 0.5 ml of the appropriate culture grown overnight. After the flow of medium was started and bacteria were allowed to enter the flow cell, the flow was stopped and the chamber was incubated inverted at 37°C for 1 h. After the flow cell was set upright, nonadherent bacteria were flushed by adjusting the flow rate to 0.5 ml/min, which is sufficient to replace the volume of the flow cell once every minute. Cells harvested after 1 week by aspiration from the downstream side of the flow cell were immediately mixed with acetone-ethanol as described above.
Assessment of biofilm formation.
Biofilm formation in vitro was assessed using a microtiter plate assay and flow cells as previously described (5). The murine model of catheter-associated biofilm formation described by Kadurugamuwa et al. (31) was used to assess biofilm formation in vivo. Briefly, 20- to 30-g female BALB/c mice (Charles River, Wilmington, Mass.) were anesthetized with ketamine (100 mg/kg of body weight) and xylazine (5 mg/kg), their flanks were shaved, and the skin was cleansed with Betadyne and alcohol. Under aseptic conditions, a 1-cm segment of 14-gauge Teflon intravenous catheter was implanted subcutaneously. The wound was closed with sutures and then cleansed with a Betadyne rinse. Infection was induced approximately 1 h after the implantation procedure by injecting 105 CFU of the test strain into the lumen of the catheter. In some cases, mice were coinfected by injection of a mixture containing 105 CFU of UAMS-1 and 105 CFU of either the sarA or ica mutant. Mice were euthanized 10 days postinfection. The catheters were removed aseptically and washed with phosphate-buffered saline. Catheters were then placed in 10 ml of sterile phosphate-buffered saline and sonicated for 1 min to remove adherent bacteria. The number of bacteria in the sonicate was then determined by plating on tryptic soy agar. To confirm the identity of recovered bacteria and to determine the proportion of UAMS-1 versus the corresponding sarA and ica mutants in coinfection experiments, colonies obtained on tryptic soy agar were transferred to the appropriate selective medium and scored for growth.
RNA isolation and cDNA labeling.
Aliquots of cells harvested from flow cells and stored as described above were pelleted by centrifugation at 7,500 × g for 10 min at 4°C. Each pellet was washed in an equal volume of TES buffer (150 mM NaCl, 78 mM disodium salt EDTA, 100 mM Tris [pH 7.5]) and resuspended to a concentration of 109 CFU per ml in TES buffer containing 100 μg of lysostaphin (Ambicin L; AMBI, Inc., Lawrence, N.Y.) per ml. Samples were incubated at 37°C for 1 h prior to applying the equivalent of 1010 CFU to a Qiagen RNeasy Maxi column. Total bacterial RNA was isolated according to the manufacturer's directions (Qiagen, Inc., Valencia, Calif.). After purification, contaminating DNA was removed with RNase-free DNase I (10 U/40 μg of total bacterial RNA at 37°C for 20 min). RNA was then repurified using RNeasy Mini columns (Qiagen, Inc.). The amount of recovered RNA was determined spectrophotometrically, and the absence of DNA was verified by PCR using primers (Table 1) corresponding to the collagen adhesin gene (cna). Samples were then stored at −80°C.
TABLE 1.
Sequences of primers and TaqMan probes used in this study
| Primer or probea | Oligonucleotide sequence (5′→3′) |
|---|---|
| cna-F | CAAGCAGTTATTACACCAGACGG |
| cna-R | CACCTTTTACAGTACCTT |
| arcA-F | GTGGTTGACTCATACATCTAGGGC |
| arcA-R | AGACCAGGCGTTGTAGTGACTTA |
| arcA-P | CCCACGTCCACGTACCAGCTCGCT |
| pyrR-F | TTGATGATGTGCTGTATACTGG |
| pyrR-R | CGAATTGGTAACTCACGATGT |
| pyrR-P | CGGTTCGTGCTTCACTTGATGCT |
| ureA-F | CATTTTACACAACGAGAGCAAG |
| ureA-R | ACGTGCTTTACGACGACG |
| ureA-P | CAACTTCCGCCGCCACTACAATCA |
| gyrB-F | AGTAACGGATAACGGACGTGGTA |
| gyrB-R | CCAACACCATGTAAACCACCAGAT |
| gyrB-P | CCGCCACCGCCGAATTTACCACCA |
| spa-F | GTAACGGCTTCATTCAAAGTCT |
| spa-R | TCATAGAAAGCATTTTGTTGTTCT |
| spa-P | AAAGACGACCCAAGCCAAAGCACT |
Forward (F) and reverse (R) primers and the Taqman probe (P) for the ORFs are shown.
RNA was converted to cDNA, and microarray analysis was performed according to the manufacturer's instructions (Affymetrix expression analysis technical manual, Affymetrix, Inc., Santa Clara, Calif.) for antisense prokaryotic arrays. Briefly, 10 μg of total RNA that had been mixed with random hexamer primers (Invitrogen) was denatured at 70°C for 10 min and allowed to anneal at 25°C for 10 min. cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) in 1× first-strand synthesis buffer, dithiothreitol, deoxynucleoside triphosphates, and SUPERase-In (Ambion, Inc., Austin, Tex.). The mixture was incubated at 25°C for 10 min, 37°C for 60 min, and 42°C for 60 min. The reaction was stopped by incubating for 10 min at 70°C prior to degrading the RNA with 1 N NaOH for 30 min at 65°C and neutralizing with 1 N HCl. The cDNA was purified using a QIAquick PCR purification kit (Qiagen) and fragmented with DNase I in One-Phor-All buffer (Amersham Biosciences, Piscataway, N.J.). DNase I was inactivated by heating the reaction mixture for 10 min at 98°C. The fragmented cDNA products were labeled with biotin on the 3′ terminus using the Enzo BioArray terminal labeling kit with biotin ddUTP (Affymetrix, Inc.).
DNA microarray hybridization and analysis.
Labeled cDNA (1.5 μg) was hybridized to custom-made S. aureus GeneChips and detected according to the manufacturer's instructions for antisense prokaryotic arrays (Affymetrix, Inc.). The GeneChip used in these experiments included 7,723 qualifiers representing the consensus open reading frame (ORF) sequences identified in the genomes of the S. aureus strains N315, Mu50, COL, NCTC 8325, EMRSA-16 (strain 252), and MSSA- 476, as well as novel GenBank entries and N315 intergenic regions greater than 50 bp, After hybridization and staining, the arrays were scanned using the Agilent GeneArray laser scanner (Agilent Technologies, Palo Alto, Calif.). The data from duplicate experiments was normalized and analyzed using GeneSpring version 5.1 gene expression software (Silicon Genetics, Redwood City, Calif.). Genes were considered to be induced in a biofilm if they were determined to be present by Affymetrix algorithms in the biofilm condition and they were transcribed at a level at least twofold higher than the corresponding planktonic growth condition. Genes were considered downregulated in a biofilm if they were determined to be present in either planktonic condition and had an expression level no more than half of that observed in the corresponding planktonic growth condition. Differential expression in biofilms was judged to be significant on the basis of statistical analysis, namely, the t test with a P value of ≤0.05.
Real-time PCR.
To confirm the results of our microarray data, the relative expression levels of the arcA, pyrR, ureA, and spa genes in each growth condition were also determined by real-time PCR. Briefly, DNase-treated RNA was reverse transcribed using the iScript cDNA synthesis kit as described by the manufacturer (Bio-Rad Laboratories). A portion (1/20th) of each reaction mixture was then used for real-time PCR using an iCycler iQ real-time PCR detection system, gene-specific primers, and TaqMan probes corresponding to each ORF, and the iQ supermix (Bio-Rad). The sequences of the primers and TaqMan probes are shown in Table 1. Relative expression levels were determined by comparison to the level of gyrB expression in the same cDNA preparations.
RESULTS
Mutation of sarA, but not ica, results in a reduced capacity to form a biofilm in vitro.
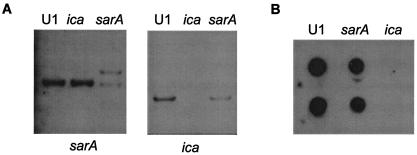
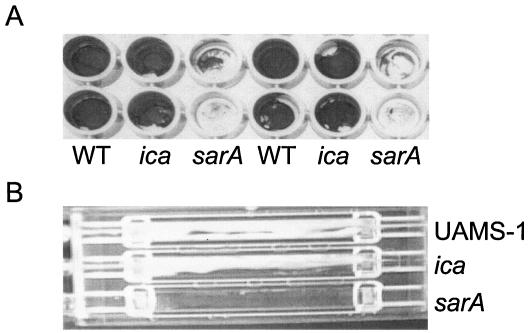
In a previous report from our laboratory, we demonstrated that mutation of sarA in clinical isolates of S. aureus results in a reduced capacity to form a biofilm (5). Although our experiments did not address the mechanistic basis for this, Valle et al. (66) also observed that mutation of sarA results in a reduced capacity to form a biofilm and concluded that this was due to the impact of SarA on production of PIA (also known as PNAG). To more definitively address the role of PNAG in biofilm formation by our clinical isolates, we generated an S. aureus UAMS-1 ica mutant and assessed its ability to form a biofilm in vitro. Mutation of the ica locus was confirmed by Southern blotting (Fig. 1A), and the inability of the ica mutant to produce PNAG was confirmed by immunoblotting using PNAG-specific antisera (Fig. 1B). We found that mutation of ica, and the resulting inability to produce PNAG, had little impact on biofilm formation (Fig. 2). In contrast, mutation of sarA resulted in a reduced capacity to form a biofilm. The relative capacities of the UAMS-1 sarA and ica mutants to form a biofilm were evident both in our microtiter plate assay (Fig. 2A) and in flow cells (Fig. 2B). Under in vitro growth conditions, mutation of sarA resulted in reduced production of PNAG (Fig. 1B), which is consistent with the results of Valle et al. (66). However, the results observed with our ica mutant make it difficult to conclude that this would explain the biofilm-deficient phenotype of the UAMS-1 sarA mutant.
FIG. 1.
Confirmation of S. aureus UAMS-1 sarA and ica mutants. (A) Genomic DNA isolated from UAMS-1 (U1) and its corresponding ica or sarA mutants was digested with HpaI and blotted with probes corresponding to an internal fragment of the ica operon or the sarA locus. (B) Dot blot analysis of PNAG expression in S. aureus strain UAMS-1 and its corresponding ica and sarA mutants. Duplicate samples prepared as described in Materials and Methods were spotted onto membranes and analyzed using antiserum raised against S. aureus PNAG.
FIG. 2.
Biofilm formation in S. aureus UAMS-1 sarA and ica mutants in vitro. Biofilm formation under static (A) and flow (B) conditions was assessed as described in Materials and Methods. WT, wild type.
Mutation of sarA, but not ica, results in a reduced capacity to form a biofilm in vivo.
The results discussed above suggest that ica is not required for biofilm formation in at least some strains of S. aureus and that the reduced capacity of a UAMS-1 sarA mutant to form a biofilm in vitro is not a function of the impact of the sarA mutation on expression of the ica operon or production of PNAG. However, in S. aureus, it is well established that expression of the ica locus is tightly regulated and that it is preferentially expressed under in vivo conditions (42). This leaves open the possibility that the results we observed in vitro do not reflect the situation observed in vivo.
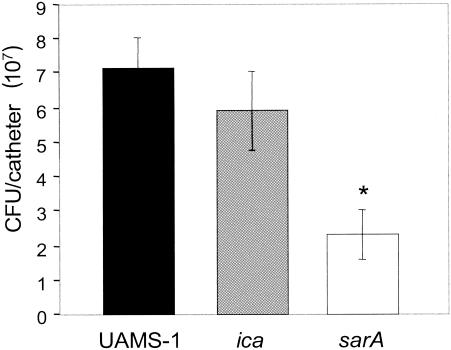
To examine this issue directly, we assessed the relative abilities of S. aureus UAMS-1 and its sarA and ica mutants to form a biofilm in vivo using a murine model of catheter-associated biofilm formation (31). The average numbers of bacteria obtained from explanted catheters at 10 days postinfection were 7.1 × 107 CFU per catheter in mice infected with UAMS-1 and 5.9 × 107 CFU per catheter in mice infected with the ica mutant (Fig. 3). In contrast, we recovered only 2.3 × 107 CFU per catheter from mice infected with the UAMS-1 sarA mutant. Although the sarA mutant was capable of colonizing the implanted catheter, the reduced recovery observed with the sarA mutant was statistically significant compared to the recovery for both UAMS-1 (P = 0.001) and its ica mutant (P = 0.022).
FIG. 3.
Biofilm formation in S. aureus UAMS-1 sarA and ica mutants in vivo. Bacteria were recovered from implanted catheters after 10 days in vivo. The number of bacteria recovered from the catheters was determined by plate count as described in Materials and Methods. The value that was statistically significantly different (P < 0.05) from the values for UAMS-1 and the ica mutant is indicated by the asterisk.
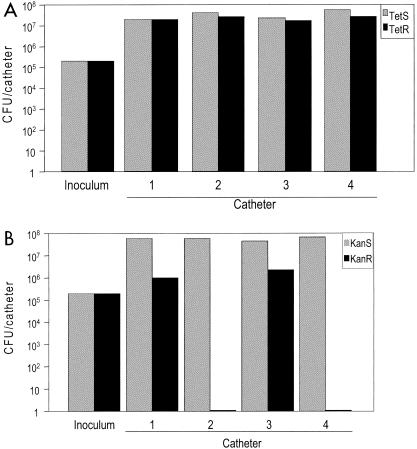
To further investigate the relative capacities of S. aureus UAMS-1 and its ica and sarA mutants to form a biofilm in vivo, we also performed experiments in which catheters were coinfected with equivalent numbers of both UAMS-1 and its ica mutant or UAMS-1 and its sarA mutant (in both cases, total inoculum of 2 × 105 CFU). In mice coinfected with UAMS-1 and its ica mutant, we recovered 3.4 × 107 CFU per catheter of the wild-type strain and 2.3 × 107 CFU per catheter of the ica mutant (Fig. 4A). These results confirm our in vitro experiments and demonstrate that UAMS-1 and its corresponding ica mutant have an equivalent capacity to form a biofilm not only in vitro but also in vivo. In contrast, when we examined mice coinfected with UAMS-1 and its sarA mutant, the number of wild-type cells recovered was similar to the number found in previous experiments (4.9 × 107 CFU), but the number of the UAMS-1 sarA mutant we recovered had decreased to an average of only 8.0 × 105 CFU per catheter (Fig. 4B). These results also confirm the results of our in vitro experiments. Moreover, the reduced recovery of the sarA mutant in coinfection experiments relative to in vivo experiments in which the sarA mutant was introduced without competition from the wild-type strain also indicates that the wild-type strain has a competitive advantage that further limits the capacity of a sarA mutant to form a biofilm in vivo.
FIG. 4.
Biofilm formation in coinfection experiments with S. aureus UAMS-1 and its sarA and ica mutants in vivo. Catheters were coinfected with equal numbers of UAMS-1 and its ica mutant (A) or UAMS-1 and its sarA mutant (B). The total number of bacteria in the inoculum and the total number recovered from each catheter was determined by plate count on nonselective medium. The number of bacteria resistant to tetracycline (A) or kanamycin (B) was subsequently determined by transferring individual colonies to selective media. Resistance to tetracycline and kanamycin is indicative of the ica and sarA mutations, respectively. Abbreviations: TetS and TetR, tetracycline sensitive and resistant, respectively; KanS and KanR, kanamycin sensitive and resistant, respectively.
Transcriptional profiling in S. aureus UAMS-1 planktonic cultures.
Taken together, the results discussed above confirm that a UAMS-1 sarA mutant has a reduced capacity to form a biofilm and that this is not a function of the impact of sarA on expression of the ica operon or production of PNAG. Moreover, the results observed with our in vitro models were consistent with the results observed in our in vivo model. Because sarA is a global regulator of gene expression in S. aureus, this suggests that other elements of the sarA regulon are also important in biofilm formation both in vitro and in vivo. Presumably, these elements could be identified by defining transcriptional changes observed within S. aureus biofilms and correlating these changes with experiments aimed at defining the sarA regulon. Because comprehensive transcriptional profiling of an S. aureus sarA mutant has been reported (19), we focused our efforts on defining the transcriptional changes that occur when UAMS-1 is grown within a biofilm.
Because biofilm formation by S. aureus UAMS-1 requires supplementation of the medium with both glucose and salt (5), we also added these supplements to the medium used for planktonic culture. We first wanted to investigate whether the current paradigm (e.g., preferential expression of surface proteins during the exponential phase, followed by a shift to exoprotein production in the postexponential phase) was altered by supplementation of the medium. As expected, we found that expression of genes encoding protein A, clumping factor B, collagen adhesion, coagulase, and fibronectin-binding protein (spa, clfB, cna, coa, and fnb, respectively) were upregulated in the exponential versus stationary phase (6, 44, 45) (Table 2). In contrast, expression of the gene (clfA) encoding a second fibrinogen-binding protein (ClfA) was upregulated in the postexponential phase. This is consistent with previous reports examining the temporal expression of clfA (72, 73). Expression of the secreted proteins was also elevated in stationary phase as expected. Specific examples include the genes encoding several cysteine proteases, the Clp proteinase, alpha-toxin, and the genes within the accessory gene regulator (agr) operon (sspA, sspB, sspC, clpC, hla, RNAII, and RNAIII, respectively). Taken together, these results confirm that our growth conditions accurately reflect the transition between the exponential and stationary growth phases and that this transition is not dramatically altered by supplementation of the medium in a manner that promotes biofilm formation.
TABLE 2.
Selected genes differentially expressed in exponential-phase versus stationary-phase cells
| N315 ORFa | Common namea | Functiona | ERb |
|---|---|---|---|
| N315-SA0144 | cap5A | Capsular polysaccharide synthesis enzyme | 0.312 |
| N315-SA0145 | cap5B | Capsular polysaccharide synthesis enzyme | 0.306 |
| N315-SA0146 | cap5C | Capsular polysaccharide synthesis enzyme | 0.223 |
| N315-SA0147 | cap5D | Capsular polysaccharide synthesis enzyme | 0.244 |
| N315-SA0148 | cap5E | Capsular polysaccharide synthesis enzyme | 0.206 |
| N315-SA0149 | cap5F | Capsular polysaccharide synthesis enzyme | 0.204 |
| N315-SA0150 | cap5G | Capsular polysaccharide synthesis enzyme | 0.187 |
| N315-SA0155 | cap5L | Capsular polysaccharide synthesis enzyme | 0.277 |
| N315-SA0156 | cap5M | Capsular polysaccharide synthesis enzyme | 0.232 |
| N315-SA0157 | cap5N | Capsular polysaccharide synthesis enzyme | 0.267 |
| N315-SA0158 | cap5O | Capsular polysaccharide synthesis enzyme | 0.262 |
| N315-SA0159 | cap5P | Capsular polysaccharide synthesis enzyme | 0.267 |
| N315-SA0899 | sspC | Cysteine protease | 0.400 |
| N315-SA0900 | sspB | Cysteine protease precursor | 0.393 |
| N315-SA1007 | α-Hemolysin precursor | 0.327 | |
| N315-SA1842 | agrB-c | Accessory gene regulator B | 0.449 |
| N315-SA1843 | agrC-124c | Accessory gene regulator C | 0.269 |
| N315-SA1844 | agrA | Accessory gene regulator A | 0.289 |
| N315-SA2336 | clpC | ATP-dependent Clp proteinase chain | 0.284 |
| agrB-3 | 99.5% protein IDc to AgrB | 0.410 | |
| agrC-3 | 99.5% protein ID to AgrC | 0.364 | |
| agrC-3c | 99.5% protein ID to AgrC | 0.431 | |
| agrD-3 | 100% protein ID to AgrD | 0.389 | |
| cap8H | 100% protein ID to Cap8H capsular polysaccharide synthesis enzyme | 0.176 | |
| cap8I | 100% protein ID Cap8I capsular polysaccharide synthesis enzyme | 0.227 | |
| cap8J | 100% protein ID Cap8J capsular polysaccharide synthesis enzyme | 0.204 | |
| cap8K | 100% protein ID to Cap8K capsular polysaccharide synthesis enzyme | 0.226 | |
| 86.7% protein ID to ClfA fibrinogen-binding protein A | 0.476 | ||
| sspA | 99.4% protein ID to glutamic acid-specific protease prepropeptide truncated alpha-toxin | 0.333 | |
| 0.311 | |||
| N315-SA0107 | spa | Immunoglobulin G-binding protein A precursor | 5.084 |
| N315-SA2423 | clfB | Clumping factor B | 2.744 |
| clfB | 93.7% protein ID to ClfB (clumping factor B) | 3.479 | |
| cna | 98.7% protein ID to MW2612 collagen adhesin precursor | 7.974 | |
| coa | 99.8% protein ID to Coa coagulase | 3.675 | |
| fnb homolog | 87.8% protein ID to Fnb | 3.975 | |
| spa | 5.007 |
Based on the published sequence of S. aureus strain N315. For genes not present in N315, the gene name and description given are from the S. aureus strain COL genome, available from The Institute for Genomic Research website (http://www.tigr.org) or by the putative function.
Normalized values based on the expression ratio (ER), defined as the expression level in exponential-phase cells/expression level in stationary-phase cells.
ID, identity.
Transcriptional profiling of S. aureus UAMS-1 biofilms.
We identified a total of 580 genes that were expressed in an altered fashion when UAMS-1 was harvested from a mature biofilm. The greatest distinction, at least in terms of overall numbers of differentially expressed genes, was between the biofilm mode of growth and the exponential growth phase of planktonic culture (Table 3); however, a significant number of genes were also differentially expressed in comparison to stationary-phase cultures (Table 4). These findings clearly imply that S. aureus biofilms represent a unique growth condition by comparison to both exponential- and stationary-phase planktonic cultures. Indeed, we identified 48 genes whose expression was enhanced at least twofold in a biofilm in comparison to both exponential- and stationary-phase planktonic cultures (Table 5). These 48 genes included 30 genes located in six independent clusters as determined by their N315 ORF numbers. Included among these linked genes were the arginine deiminase cluster (arc; N315-SA2424-SA2428), a potassium-specific transport system (kdp; N315-SA1879-SA1881), the pyrimidine biosynthesis operon (pyr; N315-SA1041-SA1049), and the urease operon (ure; N315-SA2081-2088). Interestingly, only one gene in the ica locus (icaD) was found to be significantly upregulated in a biofilm, and this was limited to the comparison between biofilms and the stationary phase of planktonic growth (Table 4). This is consistent with a recent report concluding that ica expression is associated with the initial colonization of S. epidermidis in a foreign body infection model but not with its persistence (67).
TABLE 3.
Genes differentially expressed in a biofilm versus exponential growth phase
| N315 ORFa | Common namea | Producta,b | ERc |
|---|---|---|---|
| Cell envelope and cellular processes | |||
| N315-SA1960 | mtlF | PTS system, mannitol-specific IIBC component | 5.46 |
| N315-SA1882 | kdpD | Sensor protein KdpD | 5.13 |
| N315-SA2311 | Similar to NAD(P)H-flavin oxidoreductase | 2.71 | |
| N315-SA1156 | ABC transporter (ATP-binding protein) homolog | 2.68 | |
| N315-SA0724 | Similar to cell division inhibitor | 0.496 | |
| N315-SA2253 | opp-1C | Oligopeptide transporter putative membrane permease domain | 0.490 |
| N315-SA0567 | Similar to iron(III) ABC transporter permease protein | 0.474 | |
| N315-SA2216 | Similar to ABC transporter, ATP-binding protein | 0.471 | |
| N315-SA0980 | Similar to ferrichrome ABC transporter | 0.471 | |
| N315-SA0981 | Similar to ferrichrome ABC transporter | 0.468 | |
| N315-SA0592 | tagA | Teichoic acid biosynthesis protein | 0.462 |
| N315-SA1935 | hmrA | Similar to amidase | 0.442 |
| N315-SA1169 | γ-Aminobutyrate permease | 0.425 | |
| N315-SA0243 | tagB | Similar to teichoic acid biosynthesis protein B | 0.423 |
| N315-SA0110 | sirB | Lipoprotein | 0.399 |
| N315-SA2100 | Similar to autolysin E | 0.394 | |
| N315-SA1458 | lytH | N-Acetylmuramoyl-l-alanine amidase | 0.393 |
| N315-SA0109 | sirC | Lipoprotein | 0.378 |
| N315-SA0106 | lctP | l-Lactate permease homolog | 0.376 |
| N315-SA0682 | Similar to ditripeptide ABC transporter | 0.370 | |
| N315-SA2053 | Glucose uptake protein homolog | 0.331 | |
| N315-SA0479 | nupC | Pyrimidine nucleoside transport protein | 0.315 |
| N315-SA0111 | sirA | Lipoprotein | 0.312 |
| N315-SA2339 | Similar to antibiotic transport-associated protein | 0.291 | |
| N315-SA0566 | Similar to iron-binding protein | 0.287 | |
| N315-SA2233 | Similar to integral membrane efflux protein | 0.281 | |
| N315-SA0325 | glpT | Glycerol-3-phosphate transporter | 0.281 |
| N315-SA2112 | Similar to sodium-dependent transporter | 0.278 | |
| N315-SA1025 | mraY | Phospho-N-muramic acid-pentapeptide translocase | 0.265 |
| N315-SA0600 | Similar to pyrimidine nucleoside transporter | 0.255 | |
| N315-SA1978 | Similar to ferrichrome ABC transporter (permease) | 0.203 | |
| N315-SA0010 | azlC | Similar to amino acid permease | 0.161 |
| N315-SA2300 | Similar to glucarate transporter | 0.141 | |
| N315-SA0691 | sstD | Lipoprotein, similar to ferrichrome ABC transporter | 0.126 |
| N315-SA0374 | pbuX | Xanthine permease | 0.089 |
| N315-SA0579 | Similar to Na+/H+ antiporter | 0.080 | |
| N315-SA0411 | ndhF | NADH dehydrogenase subunit 5 | 0.065 |
| N315-SA2302 | stpC | Similar to ABC transporter | 0.046 |
| N315-SA2303 | smpC | Similar to membrane-spanning protein | 0.041 |
| Information pathways | |||
| N315-SA1883 | kdpE | KDP operon transcriptional regulatory protein | 5.42 |
| N315-SA2429 | ArgR | Similar to arginine repressor | 3.92 |
| N315-SA2296 | Similar to transcriptional regulator, MerR family | 3.72 | |
| N315-SA2418 | Similar to two-component response regulator | 2.13 | |
| N315-SA0460 | pth | Peptidyl-tRNA hydrolase | 0.490 |
| N315-SA0652 | Similar to transcription regulation protein | 0.452 | |
| N315-SA1853 | Similar to DNA mismatch repair protein MutS | 0.445 | |
| N315-SA1287 | asnS | Asparaginyl-tRNA synthetase | 0.441 |
| N315-SA0348 | Similar to transcription terminator | 0.440 | |
| N315-SA2358 | Similar to transcriptional regulator (TetR/AcrR family) | 0.382 | |
| N315-SA1697 | Similar to protein-tyrosine phosphatase | 0.364 | |
| N315-SA1120 | Similar to transcription regulator GntR family | 0.354 | |
| N315-SA0298 | pfoR | Similar to regulatory protein PfoR | 0.333 |
| N315-SA1550 | tyrS | Tyrosyl-tRNA synthetase | 0.325 |
| N315-SA2482 | pcp | Pyrrolidone-carboxylate peptidase | 0.297 |
| N315-SA1583 | rot | Repressor of toxins (Rot) | 0.295 |
| N315-SA0653 | fruR | Similar to transcription repressor of fructose operon | 0.229 |
| N315-SA0904 | Probable ATL autolysin transcription regulator | 0.191 | |
| N315-SA1725 | sspB | Staphopain, cysteine proteinase | 0.074 |
| Intermediary metabolism | |||
| N315-SA0328 | Similar to NADH-dependent FMN reductase | 7.25 | |
| N315-SA0122 | butA | Acetoin (diacetyl)reductase | 5.04 |
| N315-SA2297 | Similar to GTP-pyrophosphokinase | 3.25 | |
| N315-SA1142 | glpD | Aerobic glycerol-3-phosphate dehydrogenase | 2.97 |
| N315-SA0016 | purA | Adenylosuccinate synthase | 2.37 |
| N315-SA2397 | Similar to pyridoxal-phosphate-dependent aminotransferase | 2.04 | |
| N315-SA2001 | Similar to oxidoreductase, aldo/keto reductase family | 2.01 | |
| N315-SA1201 | trpD | Anthranilate phosphoribosyltransferase | 0.495 |
| N315-SA1685 | mutY | Similar to A/G-specific adenine glycosylase | 0.481 |
| N315-SA2111 | Similar to phosphoglycolate phosphatase | 0.475 | |
| N315-SA1052 | gmk | Guanylate kinase homolog | 0.462 |
| N315-SA2279 | Similar to phosphomannomutase | 0.458 | |
| N315-SA0902 | HisC homolog | 0.448 | |
| N315-SA0177 | argJ | Arginine biosynthesis bifunctional protein homolog | 0.436 |
| N315-SA1310 | ansA | Probable l-asparaginase, gene-ansA | 0.429 |
| N315-SA1309 | cmk | Cytidylate kinase | 0.427 |
| N315-SA1749 | Similar to aspartate transaminase protein | 0.423 | |
| N315-SA2140 | Similar to esterase | 0.412 | |
| N315-SA0507 | Similar to N-acyl-l-amino acid amidohydrolase | 0.407 | |
| N315-SA0568 | Similar to l-2-haloalkanoic acid dehalogenase | 0.374 | |
| N315-SA2213 | bioB | Biotin synthase | 0.373 |
| N315-SA0514 | Similar to deoxypurine kinase | 0.356 | |
| N315-SA1121 | Similar to processing proteinase homolog | 0.340 | |
| N315-SA1203 | trpF | Phosphoriborylanthranilate isomerase | 0.323 |
| N315-SA2342 | thgA | Similar to O-acetyltransferase | 0.280 |
| N315-SA0511 | Similar to UDP-glucose 4-epimerase-related protein | 0.256 | |
| N315-SA1202 | trpC | Indole-3-glycerol phosphate synthase | 0.246 |
| N315-SA2395 | ldh | l-Lactate dehydrogenase | 0.213 |
| N315-SA0180 | bmQ | Similar to branched-chain amino acid transport system carrier protein | 0.192 |
| N315-SA1200 | trpG | Anthranilate synthase component II | 0.120 |
| N315-SA1199 | trpE | Similar to anthranilate synthase component I | 0.101 |
| N315-SA0373 | xprT | Xanthine phosphoribosyltransferase | 0.077 |
| Other functions | |||
| N315-SA0899 | sspC | Cysteine protease | 7.24 |
| N315-SA0900 | sspB | Cysteine protease precursor | 7.03 |
| N315-SA0150 | cap5G | Capsular polysaccharide synthesis enzyme | 4.80 |
| N315-SA2006 | Similar to MHC class II analog | 4.69 | |
| N315-SA0149 | cap5F | Capsular polysaccharide synthesis enzyme | 4.58 |
| N315-SA0148 | cap5E | Capsular polysaccharide synthesis enzyme | 4.05 |
| N315-SA0146 | cap5C | Capsular polysaccharide synthesis enzyme | 3.31 |
| N315-SA0147 | cap5D | Capsular polysaccharide synthesis enzyme | 3.30 |
| N315-SA0145 | cap5B | Capsular polysaccharide synthesis enzyme Cap5B | 2.43 |
| N315-SA0144 | cap5A | Capsular polysaccharide synthesis enzyme | 2.34 |
| N315-SA0841 | Similar to cell surface protein Map-w | 2.22 | |
| N315-SA1709 | Similar to ferritin | 2.18 | |
| N315-SA0754 | Similar to lactococcal prophage ps3 protein 05 | 0.498 | |
| N315-SA1835 | int | Similar to integrase (pathogenicity island SaPln1), gene = int | 0.431 |
| N315-SA1559 | Similar to smooth muscle caldesmon | 0.426 | |
| N315-SA0797 | nifU-3 | Similar to nitrogen fixation protein NifU | 0.407 |
| N315-SA0780 | Similar to hemolysin | 0.331 | |
| N315-SA0746 | nuc | Staphylococcal nuclease | 0.170 |
| N315-SA1766 | HP (bacteriophage φN315) | 0.069 | |
| N315-SA1775 | Similar to scaffolding protein (bacteriophage φN315) | 0.048 | |
| N315-SA1765 | HP (bacteriophage φN315) | 0.042 | |
| N315-SA1777 | HP (bacteriophage φN315) | 0.038 | |
| N315-SA1771 | HP (bacteriophage φN315) | 0.036 | |
| N315-SA1762 | HP (bacteriophage φN315) | 0.036 | |
| Similar to unknown proteins | |||
| N315-SA0326 | CHP (lactoylglutathione lyase and related lyases) | 7.94 | |
| N315-SA0327 | CHP (flavin-dependent oxidoreductases) | 7.50 | |
| N315-SA2479 | CHP | 5.04 | |
| N315-SA0007 | Predicted sugar kinase | 4.57 | |
| N315-SA0380 | CHP (pathogenicity island SaPln2) | 3.07 | |
| N315-SA0381 | CHP (pathogenicity island SaPln2) | 2.80 | |
| N315-SA1235 | CHP | 2.04 | |
| N315-SA1890 | CHP | 2.03 | |
| N315-SA0230 | CHP | 2.02 | |
| N315-SA0941 | CHP | 0.497 | |
| N315-SA0467 | CHP (predicted ATPase of the PP-loop superfamily) | 0.489 | |
| N315-SA1838 | CHP (predicted metal-dependent membrane protease) | 0.481 | |
| N315-SA2487 | rarD | Similar to RarD protein | 0.462 |
| N315-SA1696 | CHP | 0.453 | |
| N315-SA1448 | CHP (TPR repeat-containing proteins) | 0.452 | |
| N315-SA0329 | CHP | 0.445 | |
| N315-SA2328 | CHP (putative effector of murein hydrolase, LrgB) | 0.434 | |
| N315-SA0979 | CHP | 0.433 | |
| N315-SA1928 | HP | 0.421 | |
| N315-SA1601 | crcB | CHP (integral membrane protein) | 0.404 |
| N315-SA2377 | CHP | 0.398 | |
| N315-SAS081 | CHP (ATPase involved in DNA repair) | 0.391 | |
| N315-SA0334 | CHP (Sec-independent protein secretion pathway) | 0.378 | |
| N315-SA2305 | CHP | 0.372 | |
| N315-SA0840 | CHP (phospholipid-binding protein) | 0.370 | |
| N315-SA1903 | CHP | 0.366 | |
| N315-SA0543 | CHP (uncharacterized BCR) | 0.353 | |
| N315-SA0413 | CHP | 0.332 | |
| N315-SA0341 | Similar to low-temperature requirement A protein | 0.329 | |
| N315-SA0345 | CHP (methionine synthase I) | 0.324 | |
| N315-SA0257 | CHP (SAM-dependent methyltransferases) | 0.323 | |
| N315-SA2096 | CHP | 0.320 | |
| N315-SA0773 | CHP (predicted membrane protein) | 0.312 | |
| N315-SA2212 | Similar to 8-amino-7-oxononanoate synthase | 0.303 | |
| N315-SA1252 | CHP (histone acetyltransferase) | 0.298 | |
| N315-SA2452 | CHP (domain typically associated with flavoprotein oxygenases) | 0.282 | |
| N315-SA0870 | CHP (predicted permease) | 0.267 | |
| N315-SA0335 | CHP (Sec-independent protein secretion pathway) | 0.265 | |
| N315-SA0556 | CHP | 0.203 | |
| N315-SA0753 | CHP (lysine efflux permease) | 0.179 | |
| N315-SA2219 | CHP (uncharacterized membrane protein) | 0.133 | |
| N315-SA0739 | CHP | 0.129 | |
| N315-SAS001 | CHP | 0.128 | |
| N315-SA0412 | CHP | 0.078 | |
| No similarity | |||
| N315-SAS016 | HP | 12.74 | |
| N315-SA0883 | HP | 5.97 | |
| N315-SA1233 | HP | 2.25 | |
| N315-SA0414 | HP | 0.496 | |
| N315-SA2126 | HP | 0.495 | |
| N315-tRNA12 | tRNA-Pro | 0.491 | |
| N315-SA1943 | HP | 0.491 | |
| N315-SA1215 | HP | 0.486 | |
| N315-tRNA11 | tRNA-Arg | 0.479 | |
| N315-SA0613 | HP | 0.470 | |
| N315-SA2485 | HP | 0.457 | |
| N315-SA0088 | HP | 0.443 | |
| N315-tRNA57 | tRNA-Lys | 0.416 | |
| N315-tRNA47 | tRNA-Leu | 0.409 | |
| N315-SA1607 | HP | 0.402 | |
| N315-SA0955 | HP | 0.372 | |
| N315-tRNA07 | tRNA-Thr | 0.355 | |
| N315-SA0105 | HP | 0.344 | |
| N315-SA2118 | HP | 0.335 | |
| N315-SA0336 | HP | 0.326 | |
| N315-SA0363 | HP | 0.321 | |
| N315-SA2055 | HP | 0.321 | |
| N315-SA2224 | HP | 0.309 | |
| N315-SA2249 | HP | 0.259 | |
| N315-SA0749 | HP | 0.233 | |
| N315-tRNA06 | tRNA-Val | 0.219 | |
| N315-SA0748 | HP | 0.214 | |
| N315-SA0889 | HP | 0.179 | |
| N315-SA2488 | HP | 0.157 | |
| N315-SA1778 | HP (bacteriophage φN315) | 0.089 | |
| N315-SA1768 | HP (bacteriophage φN315) | 0.050 | |
| N315-SA1770 | HP (bacteriophage φN315) | 0.050 | |
| N315-SA1774 | HP (bacteriophage φN315) | 0.046 | |
| N315-SA1776 | HP (bacteriophage φN315) | 0.044 | |
| N315-SAS060 | HP (bacteriophage φN315) | 0.041 | |
| N315-SA1769 | HP (bacteriophage φN315) | 0.040 | |
| N315-SA1773 | HP (bacteriophage φN315) | 0.032 | |
| N315-SA1772 | HP (bacteriophage φN315) | 0.031 | |
| N315-SA1767 | HP (bacteriophage φN315) | 0.027 | |
| No N315 ORF | |||
| set5 | 100% protein ID Set5, exotoxin 5, and HsdM-like protein gene | 40.43 | |
| sspA | 99.4% protein ID S. aureus glutamic acid-specific protease | 7.19 | |
| cap8H | 100% protein ID to capsular polysaccharide synthase enzyme Cap8H | 5.11 | |
| cap8J | 100% protein ID to capsular polysaccharide synthesis enzyme Cap8J | 4.52 | |
| 58.1% protein ID malofactic enzyme, Oenococcus oeni bacteria | 3.05 | ||
| 68.1% protein ID SA0329 CHP | 2.39 | ||
| 92.8% protein ID MW1748 HP | 2.25 | ||
| 84.4% protein ID MW0360 HP | 2.16 | ||
| 96.7% protein ID to MW2134 HP | 0.500 | ||
| 95.6% protein ID SA2230 | 0.473 | ||
| 58.4% protein ID BH3950 transposase (10), Bacillus halodurans | 0.467 | ||
| 49.1% protein ID MW2618 | 0.463 | ||
| COL-SA1788 | HP | 0.434 | |
| 87.6% protein ID MW0584 | 0.423 | ||
| COL-SA2299 | HP | 0.421 | |
| Similar to splE | 62.6% protein ID serine protease SplE | 0.420 | |
| 97.7% protein ID to SA1559 | 0.419 | ||
| 26.9% protein ID MW2498 | 0.418 | ||
| COL-SA0866 | HP | 0.411 | |
| Serine protease | 55.4% protein ID serine protease SplB | 0.409 | |
| 96% protein ID MW2325 | 0.387 | ||
| 95.5% protein ID MW0355 HP | 0.371 | ||
| 22.2% protein ID SA0283 HP | 0.345 | ||
| 89.4% protein ID SA0553 CHP | 0.338 | ||
| 78.2% protein ID MW1720 HP | 0.315 | ||
| COL-SA1556 | HP | 0.311 | |
| 99.4% protein ID to MW0053 CHP | 0.308 | ||
| 45.9% protein ID BH3950 transposase (10), Bacillus halodurans | 0.290 | ||
| 97.7% protein ID MW0355 HP | 0.289 | ||
| splB | 97.7% protein ID to serine protease SplB | 0.280 | |
| 79.2% protein ID MW1043 HP | 0.270 | ||
| COL-SA2728 | HP | 0.237 | |
| 99.5% id to SAV1992 HP | 0.209 | ||
| 37.3% protein ID MW1769 HP | 0.202 | ||
| COL-SA1140 | sai-1 | 29-kDa cell surface protein | 0.164 |
| 85.2% protein ID MW1042 HP | 0.141 | ||
| 99.5% protein ID MWP018 | 0.138 | ||
| 99.8% protein ID MWP016 S. aureus plasmid pMW2 | 0.096 | ||
| 93.4% protein ID SAV1953 φPVL ORF 20 and 21 homolog | 0.079 | ||
| Mu50-SAV1953 | φPVL ORF 20 and 21 homolog | 0.071 | |
| 100% protein ID to MWP017 HP | 0.070 | ||
| 100% protein ID MW1894 HP | 0.060 | ||
| 78% protein ID MW1892 HP | 0.055 | ||
| 83.2% protein ID SA1763 HP | 0.032 | ||
| 100% protein ID SAP019 HP, S. aureus N315 plasmid N315B | 0.031 |
Based on the published sequence of S. aureus strain N315. For genes not present in N315, the gene name and description given are from the S. aureus strain COL genome, available from The Institute for Genomic Research website (http://www.tigr.org) or by the putative function.
Abbreviations: PTS, phosphotransferase; HP, hypothetical protein; CHP, conserved hypothetical protein; SAM, S-adenosylmethionine; ID, identity.
Normalized values based on the expression ratio (ER), which is defined as the expression level in exponential-phase cells/expression level in stationary-phase cells.
TABLE 4.
Genes differentially expressed in a biofilm versus stationary growth phase
| N315 ORFa | Common namea | Producta,b | ERc |
|---|---|---|---|
| Cell envelope and cellular processes | |||
| N315-SA0655 | fruA | Fructose-specific permease | 14.43 |
| N315-SA0263 | Similar to proton antiporter efflux pump | 9.55 | |
| N315-SA2142 | semB | Similar to multidrug resistance protein | 4.96 |
| N315-SA0293 | Similar to formate transporter NirC | 4.64 | |
| N315-SA1140 | glpF | Glycerol uptake facilitator | 4.40 |
| N315-SA2185 | narG | Respiratory nitrate reductase alpha chain | 4.08 |
| N315-SA2183 | narJ | Similar to nitrate reductase delta chain | 3.87 |
| N315-SA2184 | narH | Nitrate reductase beta chain NarH | 3.56 |
| N315-SA2053 | Glucose uptake protein homolog | 3.55 | |
| N315-SA0166 | Similar to nitrate transporter | 3.39 | |
| N315-SA0167 | Similar to membrane lipoprotein SrpL | 3.29 | |
| N315-SA0702 | llm | Lipophilic protein affecting bacterial lysis rate and methicillin resistance level | 2.83 |
| N315-SA2222 | Similar to bicyclomycin resistance protein TcaB | 2.75 | |
| N315-SA0411 | ndhF | NADH dehydrogenase subunit 5 | 2.41 |
| N315-SA2176 | narK | Nitrite extrusion protein | 2.16 |
| N315-SA1960 | mtlF | PTS system, mannitol-specific IIBC component | 0.486 |
| N315-SA1381 | pbp3 | Penicillin-binding protein 3 | 0.482 |
| N315-SA1219 | Similar to phosphate ABC transporter | 0.463 | |
| N315-SA2311 | Similar to NAD(P)H-flavin oxidoreductase | 0.460 | |
| N315-SA0367 | Similar to nitroflavin reductase | 0.410 | |
| N315-SA1982 | Similar to transporter | 0.397 | |
| N315-SA0260 | Similar to ribose transporter RbsU | 0.385 | |
| N315-SA2074 | modA | Probable molybdate-binding protein | 0.384 |
| N315-SA1848 | amt | Probable ammonium transporter | 0.343 |
| N315-SA0138 | Similar to alkylphosphonate ABC transporter | 0.323 | |
| N315-SA2203 | EmrB/QacA subfamily | Similar to multidrug resistance protein | 0.304 |
| N315-SA0420 | Similar to ABC transporter ATP-binding protein | 0.286 | |
| N315-SA0422 | Similar to lactococcal lipoprotein | 0.272 | |
| N315-SA0421 | Similar to ABC transporter permease protein | 0.256 | |
| N315-SA0589 | Similar to ABC transporter ATP-binding protein | 0.163 | |
| N315-SA0849 | Similar to peptide-binding protein OppA | 0.148 | |
| Information pathways | |||
| N315-SA0653 | fruR | Similar to transcription repressor of fructose operon | 14.57 |
| N315-SA0476 | Similar to transcription regulator GntR family | 4.98 | |
| N315-SA1058 | def | Similar to polypeptide deformylase | 2.35 |
| N315-SA0460 | pth | Peptidyl-tRNA hydrolase | 2.07 |
| N315-SA1516 | phoP | Alkaline phosphatase synthesis transcriptional regulatory protein | 0.479 |
| N315-SA0130 | Similar to trehalose operon transcriptional repressor | 0.477 | |
| N315-SA1805 | Repressor homolog (bacteriophage φN315) | 0.390 | |
| N315-SAS042 | rpmG | 50S ribosomal protein L33 | 0.362 |
| N315-SA1394 | glyS | Glycyl-tRNA synthetase | 0.351 |
| N315-SA1149 | glnR | Glutamine synthetase repressor | 0.319 |
| N315-SA1360 | Xaa-Pro dipeptidase | 0.294 | |
| Intermediary metabolism | |||
| N315-SA0654 | fruB | Fructose-1-phosphate kinase | 17.08 |
| N315-SA1959 | glmS | Glucosamine-fructose-6-phosphate aminotransferase | 9.27 |
| N315-SA0143 | adhE | Alcohol-acetaldehyde dehydrogenase | 5.79 |
| N315-SA2186 | nasF | Uroporphyrin III C-methyl transferase | 4.56 |
| N315-SA2187 | nasE | Assimilatory nitrite reductase | 4.15 |
| N315-SA1929 | pyrG | CTP synthase | 3.97 |
| N315-SA2188 | nirB | Nitrite reductase | 3.38 |
| N315-SA0973 | kdtB | Phosphopantetheine adenyltransferase homolog | 2.29 |
| N315-SA0572 | Similar to esterase or lipase | 0.493 | |
| N315-SA0528 | Similar to hexulose-6-phosphate synthase | 0.484 | |
| N315-SA1231 | dal | Similar to alanine racemase | 0.471 |
| N315-SA2120 | Similar to amino acid amidohydrolase | 0.464 | |
| N315-SA0008 | hutH | Histidine ammonia-lyase | 0.461 |
| N315-SA1584 | Lysophospholipase homolog | 0.456 | |
| N315-SA1230 | hipO | Hippurate hydrolase | 0.435 |
| N315-SAS044 | dmpI | 4-Oxalocrotonate tautomerase | 0.432 |
| N315-SA1225 | lysC | Aspartokinase II | 0.429 |
| N315-SA1229 | dapD | Tetrahydrodipicolinate acetyltransferase | 0.423 |
| N315-SA0258 | rbsK | Probable ribokinase | 0.422 |
| N315-SA0820 | glpQ | Glycerophosphoryl diester phosphodiesterase | 0.419 |
| N315-SA0181 | entB | Similar to isochorismatase | 0.416 |
| N315-SA0512 | ilvE | Branched-chain amino acid aminotroansferase homolog | 0.415 |
| N315-SA2204 | gpm | Phosphoglycerate mutase | 0.408 |
| N315-SA1227 | dapA | Dihydrodipicolinate synthase | 0.406 |
| N315-SA2155 | mqo | Similar to malate:quinone oxidoreductase | 0.404 |
| N315-SA1724 | purB | Adenylosuccinate lyase | 0.394 |
| N315-SA0304 | nanA | N-Acetylneuraminate lyase subunit | 0.384 |
| N315-SA1150 | glnA | Glutamine-ammonia ligase | 0.362 |
| N315-SA1531 | ald | Alanine dehydrogenase | 0.357 |
| N315-SA1228 | dapB | Dihydrodipicolinate reductase | 0.354 |
| N315-SA2125 | hutG | Similar to formiminoglutamase | 0.353 |
| N315-SA0098 | Similar to aminoacylase | 0.346 | |
| N315-SA2127 | rpiA | Similar to ribose 5-phosphate isomerase | 0.341 |
| N315-SA1226 | asd | Aspartate semialdehyde dehydrogenase | 0.341 |
| N315-SA1545 | serA | d-3-Phosphoglycerate dehydrogenase | 0.341 |
| N315-SA0679 | hisC | Similar to histidinol-phosphate aminotransferase | 0.337 |
| N315-SA0658 | Similar to plant metabolite dehydrogenases | 0.335 | |
| N315-SA0656 | nagA | Probable N-acetylglucosamine-6-phosphate deacetylase | 0.333 |
| N315-SA1184 | citB | Aconitate hydratase | 0.329 |
| N315-SA0915 | folD | FolD bifunctional protein | 0.326 |
| N315-SA1858 | ilvD | Dihydroxy-acid dehydratase | 0.280 |
| N315-SA0430 | gltB | Glutamate synthase large subunit | 0.263 |
| N315-SA1614 | menC | o-Succinylbenzoic acid synthetase | 0.234 |
| N315-SA0431 | gltD | NADH-glutamate synthase small subunit | 0.199 |
| N315-SA1553 | fhs | Formyltetrahydrofolate synthetase | 0.168 |
| N315-SA0016 | purA | Adenylosuccinate synthase | 0.117 |
| N315-SA0926 | purD | Phosphoribosylamine-glycine ligase | 0.049 |
| N315-SA0917 | purK | Phosphoribosylaminoimidazole carboxylase carbon dioxide fixation chain | 0.034 |
| N315-SA0916 | purE | Similar to phosphoribosylaminoimidazole carboxylase | 0.030 |
| N315-SA0918 | purC | Phosphoribosylaminoimidazolesuccinocarboxamide synthetase homolog | 0.023 |
| Other functions | |||
| N315-SA2460 | icaD | Intercellular adhesion protein D | 34.06 |
| N315-SA1898 | Similar to SceD precursor | 26.36 | |
| N315-SA2206 | sbi | IgG-binding protein | 26.16 |
| N315-SA1000 | Similar to fibrinogen-binding protein | 11.51 | |
| N315-SA2097 | Similar to SsaA | Similar to secretory antigen precursor | 4.74 |
| N315-SA2164 | Similar to phage infection protein precursor | 2.80 | |
| N315-SA1382 | sodA | Superoxide dismutase SodA | 0.497 |
| N316-SA1606 | Plant metabolite dehydrogenase homolog | 0.480 | |
| N315-SA0841 | Similar to cell surface protein Map-w | 0.448 | |
| N315-SA1549 | htrA | Similar to serine proteinase Do, heat shock protein | 0.434 |
| N315-SA2406 | gbsA | Glycine betaine aldehyde dehydrogenase | 0.399 |
| N315-SA0659 | Similar to CsbB stress response protein | 0.390 | |
| N315-SA1312 | ebpS | Elastin-binding protein | 0.363 |
| N315-SA0755 | Similar to general stress protein 170 | 0.340 | |
| N315-SA1170 | katA | Catalase | 0.309 |
| N315-SA0091 | plc | 1-Phosphatidylinositol phosphodiesterase precursor | 0.300 |
| N315-SA2405 | betA | Choline dehydrogenase | 0.252 |
| Similar to unknown proteins | |||
| N315-SA0213 | CHP | 17.26 | |
| N315-SA2256 | CHP | 4.76 | |
| N315-SA0341 | HP similar to low-temperature requirement A protein | 3.95 | |
| N315-SA1176 | CHP | 3.05 | |
| N315-SA0929 | CHP | 3.04 | |
| N315-SA1431 | CHP | 3.00 | |
| N315-SA1340 | CHP (lactoylglutathione lyase) | 2.53 | |
| N315-SAS027 | CHP | 2.50 | |
| N315-SA1932 | Similar to HP T13D8.31 Arabidopsis thaliana | 2.35 | |
| N315-SA1464 | yajC | CHP (preprotein translocase subunit YajC) | 2.30 |
| N315-SA1540 | CHP (GAF domain-containing protein) | 2.24 | |
| N315-SA0165 | Similar to α-helical coiled-coil protein SrpF | 2.16 | |
| N315-SA0114 | CHP | 2.10 | |
| N315-SA0529 | CHP (predicted sugar phosphate isomerase involved in capsule formation, GutQ) | 0.486 | |
| N315-SA1019 | CHP | 0.467 | |
| N315-SA1737 | CHP (3-carboxymuconate cylase) | 0.466 | |
| N315-SA0801 | CHP (IscA) | 0.463 | |
| N315-SA1543 | CHP (predicted redox protein, regulator of disulfide bond formation) | 0.462 | |
| N315-SA1380 | CHP (5-formyltetrahydrofolate cyclo-ligase) | 0.454 | |
| N315-SA1129 | CHP (predicted HD superfamily hydrolase) | 0.447 | |
| N315-SA0861 | CHP (hemoglobin-like proteins) | 0.443 | |
| N315-SA0230 | CHP | 0.438 | |
| N315-SA1280 | CHP | 0.430 | |
| N315-SA0957 | CHP | 0.429 | |
| N315-SA1331 | CHP (predicted oxidoreductases) | 0.393 | |
| N315-SA1689 | CHP | 0.384 | |
| N315-SA0513 | CHP (predicted phosphatases, Gph) | 0.379 | |
| N315-SA2367 | CHP (predicted hydrolases or acyltransferases) | 0.356 | |
| N315-SA1167 | CHP (predicted hydrolases of the HAD superfamily) | 0.337 | |
| N315-SA1690 | CHP | 0.324 | |
| N315-SA0089 | Similar to DNA helicase | 0.315 | |
| N315-SA0873 | CHP | 0.315 | |
| N315-SA1544 | Similar to soluble hydrogenase 42-kDa subunit | 0.314 | |
| N315-SA0741 | CHP (predicted acetyltransferase) | 0.310 | |
| N315-SA0362 | CHP | 0.310 | |
| N315-SA1281 | CHP | 0.310 | |
| N315-SA0649 | CHP (predicted DNA-binding proteins with PD1-like DNA-binding motif) | 0.286 | |
| N315-SA0407 | CHP (chromosome segregation ATPases) | 0.254 | |
| N315-SA1611 | CHP (dipeptidyl aminopeptidases/acylaminoacyl-peptidases) | 0.227 | |
| N315-SA0919 | CHP (phosphoribosylformylglycinamidine [FGAM] synthase) | 0.027 | |
| No similarity | |||
| N315-SA0663 | HP | 7.97 | |
| N315-SA2281 | HP | 5.10 | |
| N315-SA0779 | HP | 3.44 | |
| N315-SA2376 | HP | 3.22 | |
| N315-SA0885 | HP | 3.16 | |
| N315-SA1670 | HP | 2.80 | |
| N315-SA2126 | HP | 2.62 | |
| N315-SA0336 | HP | 2.59 | |
| N315-SA0571 | HP | 2.37 | |
| N315-SA2058 | HP | 2.17 | |
| N315-SA0397 | lpl2 | HP (pathogenicity island SaPln2) | 0.488 |
| N315-SAS031 | HP | 0.480 | |
| N315-SA1168 | HP | 0.472 | |
| N315-SA0372 | HP | 0.468 | |
| N315-SA0404 | lpl8 | HP (pathogenicity island SaPln2) | 0.447 |
| N315-SA1319 | HP | 0.421 | |
| N315-SA0090 | HP | 0.366 | |
| N315-SA1546 | HP | 0.293 | |
| N315-SA0406 | HP | 0.258 | |
| N315-SA2497 | HP | 0.208 | |
| N315-SA0408 | HP | 0.198 | |
| N315-SA2496 | HP | 0.188 | |
| No N315 ORF | 72.4% protein ID to MW1041 | 25.20 | |
| COL-SA0674 | HP | 6.12 | |
| COL-SA1165 | HP | 5.57 | |
| 98.1% protein ID to MW2274 CHP | 5.33 | ||
| 98.1% protein ID to NasE assimilatory nitrite reductase | 4.25 | ||
| 57% protein ID to spyM18_1050 HP, S. pyogenes MGAS8232 | 3.55 | ||
| 88.2% protein ID to MW2323 | 3.11 | ||
| 100% protein ID to SAP023 S. aureus N315 plasmid pN315B | 2.32 | ||
| 100% protein ID to MW2396 | 0.497 | ||
| COL-SA1345 | HP | 0.491 | |
| 100% protein ID to SA1320 HP | 0.482 | ||
| 100% protein ID to lpl11 HP, S. aureus MW2 | 0.465 | ||
| COL-SA2676 | LPXTG | LPXTG-motif cell wall anchor domain protein | 0.456 |
| 91.5% protein ID to lpl2 HP, S. aureus N315 | 0.447 | ||
| COL-SA0293 | CHP | 0.444 | |
| 39.1% protein ID to lin05-11 Listeria innocua | 0.436 | ||
| 26.6% protein ID to LigW 5-carboxyvanillate decarboxylase, Sphingomonas paucimobilis | 0.416 | ||
| 92.7% protein ID to Lpl7 HP, S. aureus N315 | 0.405 | ||
| binL | 99.5% protein ID to BinL DNA-invertase, S. aureus plasmid pMW2 | 0.394 | |
| 94.8% protein ID to BinL DNA invertase, S. aureus plasmid pMW2 | 0.392 | ||
| COL-SA0601 | HP | 0.372 | |
| 89.8% protein ID to Lpl10 HP, S. aureus MW2 | 0.364 | ||
| 25.8% protein ID to MA2121 CHP, Methanosarcina acetivorans C2A | 0.363 | ||
| 88% protein ID to MW1374 CHP | 0.363 | ||
| 74.8% protein ID to lpl5 HP, S. aureus N315 | 0.331 | ||
| COL-SA1343 | HP | 0.328 | |
| 48.6% protein ID to ycnB homolog of multidrug resistance protein B, B. subtilis | 0.309 | ||
| 71% protein ID to MW1201 HP | 0.282 | ||
| 93.5% protein ID to MW0402 HP | 0.276 | ||
| 84% protein ID to lpl2 HP, S. aureus N315 | 0.261 | ||
| 35.9% protein ID to Cgl0945 putative multicopper oxidases, Corynebacterium glutamicum | 0.259 | ||
| 57.8% protein ID to CopB ATPase, Enterococcus hirae | 0.214 | ||
| 64.2% protein ID to SA0753 CHP | 0.191 | ||
| 33.6% protein ID to RtxC, Bradyrhizobium elkanii | 0.158 |
Based on the published sequence of S. aureus strain N315. For genes not present in N315, the gene name and description given are from the S. aureus strain COL genome, available from The Institute for Genomic Research website (www.tigr.org or by the putative function.
Abbreviations: PTS, phosphotransferase; IgG, immunoglobulin G; CHP, conserved hypothetical protein; HP, hypothetical protein; HAD, haloacid dehalogenase-family protein; ID, identity.
Normalized values based on the expression ratio (ER), which is defined as the expression level in exponential-phase cells/expression level in stationary-phase cells.
TABLE 5.
Genes differentially expressed in a biofilm versus exponential and stationary phase
| N315 ORFa | Common namea | Producta,b | ER vs EPc | ER vs SPc |
|---|---|---|---|---|
| Cell envelope and cellular processes | ||||
| N315-SA2426 | arcD | Arginine/ornithine antiporter | 59.91 | 5.49 |
| N315-SA1881 | kdpA | Probable potassium-transporting ATPase A chain | 51.58 | 11.30 |
| N315-SA1880 | kdpB | Probable potassium-transporting ATPase B chain | 30.99 | 9.53 |
| N315-SA1042 | pyrP | Uracil permease | 25.54 | 7.97 |
| N315-SA1879 | kdpC | Probable potassium-transporting ATPase C chain | 20.82 | 8.40 |
| N315-SA0417 | Similar to sodium-dependent transporter | 7.33 | 16.49 | |
| N315-SA2081 | Similar to urea transporter | 6.49 | 4.65 | |
| N315-SA1688 | Similar to teichoic acid translocation ATP-binding protein TagH | 0.47 | 0.42 | |
| N315-SA0233 | PTS enzyme, maltose and glucose specific, factor II homolog | 0.44 | 0.15 | |
| N315-SA0848 | oppF | Oligopeptide transport system ATP-binding protein homolog | 0.42 | 0.16 |
| N315-SA0847 | oppD | Oligopeptide transport system ATP-binding protein homolog | 0.39 | 0.15 |
| N315-SA0845 | oppB | Oligopeptide transport system permease protein | 0.38 | 0.16 |
| N315-SA2242 | CHP (predicted permease) | 0.37 | 0.35 | |
| N315-SA0846 | oppC | Similar to oligopeptide transport system permease protein | 0.37 | 0.17 |
| N315-SA0758 | Similar to thioredoxin | 0.28 | 0.34 | |
| N315-SA2261 | Similar to efflux pump | 0.25 | 0.45 | |
| N315-SA2132 | Similar to ABC transporter (ATP-binding protein) | 0.24 | 0.34 | |
| N315-SA0217 | Similar to periplasmic iron-binding protein BitC | 0.23 | 0.24 | |
| N315-SA1699 | Similar to transporter | 0.20 | 0.34 | |
| N315-SA1987 | opuD | Glycine betaine transporter OpuD homolog | 0.11 | 0.21 |
| Information pathways | ||||
| N315-SA2424 | acrR | Similar to transcription regulator Crp/Fnr family protein | 48.16 | 9.87 |
| N315-SA1041 | pyrR | Pyrimidine operon repressor chain A | 16.05 | 6.82 |
| N315-SA2320 | Similar to regulatory protein PfoR | 9.87 | 4.66 | |
| N315-SA2502 | rnpA | RNase P protein component | 3.82 | 5.26 |
| N315-SA2134 | Similar to DNA 3-methyladenine glycosidase | 0.48 | 0.40 | |
| N315-SA0815 | Peptidyl-prolyl cis-trans isomerase homolog | 0.44 | 0.49 | |
| N315-SA2278 | Similar to mutator protein MutT | 0.41 | 0.41 | |
| N315-SA1626 | hsdM | Type I restriction enzyme homolog (SaPln3) | 0.41 | 0.48 |
| N315-SA0097 | Similar to transcription regulator AraC/XylS family | 0.40 | 0.49 | |
| N315-SA2144 | Similar to transcriptional regulator (TetR/AcrR family) | 0.39 | 0.37 | |
| N315-SA0189 | hsdR | Probable type I restriction enzyme restriction chain | 0.30 | 0.36 |
| N315-SA1806 | Probable ATP-dependent helicase (bacteriophage φN315) | 0.29 | 0.25 | |
| Intermediary metabolism | ||||
| N315-SA2427 | arcB | Ornithine transcarbamoylase | 124.22 | 5.83 |
| N315-SA2428 | arcA | Arginine deiminase | 114.59 | 5.45 |
| N315-SA2425 | arcC | Carbamate kinase | 37.87 | 5.76 |
| N315-SA1044 | pyrC | Dihydroorotase | 17.84 | 6.11 |
| N315-SA1045 | carA | Carbamoyl-phosphate synthase small chain | 13.13 | 6.03 |
| N315-SA1047 | pyrF | Orotidine-5-phosphate decarboxylase | 10.65 | 4.93 |
| N315-SA1046 | carB | Carbamoyl-phosphate synthase large chain | 10.50 | 4.97 |
| N315-SA2082 | ureA | Urease gamma subunit | 9.68 | 3.12 |
| N315-SA2083 | ureAB | Urease beta subunit | 9.29 | 2.97 |
| N315-SA1048 | pyrE | Orotate phosphoribosyltransferase | 8.94 | 4.87 |
| N315-SA2319 | sdhB | Similar to beta-subunit of l-serine dehydratase | 8.90 | 4.84 |
| N315-SA2084 | ureC | Urease alpha subunit | 8.40 | 3.50 |
| N315-SA2086 | ureF | Urease accessory protein | 8.34 | 3.33 |
| N315-SA2088 | ureD | Urease accessory protein | 7.72 | 3.67 |
| N315-SA2085 | ureE | Urease accessory protein | 7.26 | 2.82 |
| N315-SA2087 | ureG | Urease accessory protein | 6.78 | 3.16 |
| N315-SA2318 | sdhA | Similar to l-serine dehydratase | 6.49 | 4.24 |
| N315-SA1043 | pyrB | Aspartate transcarbamoylase chain A | 4.69 | 6.34 |
| N315-SA2007 | Similar to α-acetolactate decarboxylase | 4.40 | 3.09 | |
| N315-SA0821 | argH | Argininosuccinate lyase | 3.93 | 14.65 |
| N315-SA0822 | argG | Argininosuccinate synthase | 3.52 | 13.48 |
| N315-SA2008 | budB | α-Acetolactate synthase | 3.29 | 2.53 |
| N315-SA1155 | cls | Cardiolipin synthetase homolog | 2.73 | 2.28 |
| N315-SA1160 | nuc | Thermonuclease | 2.30 | 2.47 |
| N315-SA2258 | Similar to diaminopimelate epimerase | 2.03 | 2.60 | |
| N315-SA1940 | deoD | Purine nucleoside phosphorylase | 0.46 | 0.49 |
| N315-SA1615 | menE | O-Succinylbenzoic acid-CoA ligase | 0.44 | 0.21 |
| N315-SA0925 | purH | Bifunctional purine biosynthesis protein | 0.43 | 0.04 |
| N315-SA0241 | Similar to 4-diphosphocytidyl-2C-methyl-d-erythritol synthase | 0.42 | 0.46 | |
| N315-SA0963 | pyc | Pyruvate carboxylase | 0.41 | 0.26 |
| N315-SA0011 | Similar to homoserine-o-acetyltransferase | 0.39 | 0.44 | |
| N315-SA0534 | atoB | Acetyl-CoA c-acetyltransferase | 0.38 | 0.43 |
| N315-SA0920 | purQ | Phosphoribosylformylglycinamidine synthase I | 0.37 | 0.02 |
| N315-SA0923 | purM | Phosphoribosylformylglycinamidine cyclo-ligase | 0.34 | 0.03 |
| N315-SA0924 | purN | Phosphoribosylglycinamide formyltransferase | 0.34 | 0.03 |
| N315-SA0242 | Similar to xylitol dehydrogenase | 0.34 | 0.44 | |
| N315-SA0921 | purL | Phosphoribosylformylglycinamidine synthetase | 0.33 | 0.03 |
| N315-SA0922 | purF | Phosphoribosylpyrophosphate amidotransferase | 0.33 | 0.03 |
| N315-SA0344 | metE | 5-Methyltetrahydropteroyltriglutamate-homocysteine | 0.28 | 0.43 |
| Methyltransferase | ||||
| N315-SA0022 | Similar to 5′-nucleotidase | 0.26 | 0.29 | |
| N315-SA1814 | Similar to succinyl-diaminopimelate desuccinylase | 0.23 | 0.20 | |
| N315-SA0266 | CHP (ABC-type multidrug transport system, ATPase component) | 0.15 | 0.23 | |
| Other functions | ||||
| N315-SA2353 | ssaA | Similar to secretory antigen precursor | 3.64 | 5.70 |
| N315-SA0270 | ssaA | Similar to secretory antigen precursor | 0.42 | 0.39 |
| N315-SA1629 | splC | Serine protease | 0.39 | 0.49 |
| N315-SA0107 | spa | Immunoglobulin G-binding protein A precursor | 0.01 | 0.04 |
| Similar to unknown proteins | ||||
| N315-SA0023 | CHP | 0.50 | 0.35 | |
| N315-SA0814 | kapB | CHP | 0.48 | 0.44 |
| N315-SA1692 | CHP (putative intracellular protease/amidase) | 0.46 | 0.37 | |
| N315-SA0518 | CHP (predicted flavoprotein) | 0.46 | 0.43 | |
| N315-SA1612 | CHP (NTP pyrophosphohydrolases) | 0.34 | 0.17 | |
| N315-SA1133 | CHP | 0.32 | 0.44 | |
| N315-SA2371 | CHP | 0.30 | 0.26 | |
| N315-SA0559 | CHP (histone acetyltransferase HPA2 and related acetyltransferases) | 0.29 | 0.36 | |
| N315-SA0872 | CHP (enterochelin esterase and related enzymes) | 0.29 | 0.44 | |
| N315-SA2131 | CHP (ABC-type Na+ efflux pump, permease component) | 0.27 | 0.38 | |
| N315-SA1733 | CHP | 0.26 | 0.29 | |
| N315-SA2322 | CHP (permeases of the drug/metabolite transporter superfamily) | 0.22 | 0.45 | |
| N315-SA0269 | HP | 0.13 | 0.10 | |
| N315-SA0359 | CHP (uncharacterized membrane protein) | 0.13 | 0.37 | |
| No similarity | ||||
| N315-SA1049 | HP | 7.42 | 4.41 | |
| N315-SA0575 | HP | 2.14 | 2.10 | |
| N315-SA1152 | HP | 0.46 | 0.46 | |
| N315-SA0752 | HP | 0.41 | 0.49 | |
| N315-SAS025 | HP | 0.39 | 0.47 | |
| N315-SA2372 | HP | 0.38 | 0.28 | |
| N315-SA0364 | HP | 0.36 | 0.41 | |
| N315-SA1332 | HP | 0.34 | 0.26 | |
| N315-SA1015 | HP | 0.34 | 0.23 | |
| N315-SA2373 | HP | 0.32 | 0.20 | |
| N315-SA0740 | HP | 0.29 | 0.24 | |
| N315-SA0268 | HP | 0.17 | 0.17 | |
| N315-SA0267 | HP | 0.17 | 0.19 | |
| N315-SA1726 | HP | 0.08 | 0.17 | |
| No N315 ORF | ||||
| COL-SA2069 | HP | 42.86 | 13.21 | |
| 87.1% protein ID to Ssp extracellular ECM and plasma-binding protein | 33.06 | 135.82 | ||
| No hit in GenPept | 16.42 | 28.08 | ||
| mapN | 99.7% protein ID to MapN protein | 14.76 | 35.81 | |
| 86.9% protein ID to SA1813 (possibly hemolysin) | 6.80 | 12.73 | ||
| 46.1% protein ID to lin0925 putative membrane protein, Listeria innocua | 5.73 | 23.61 | ||
| 50.7% protein ID to lin0924, Listeria innocua | 5.52 | 14.61 | ||
| 63.2% protein ID to MW0768 | 4.58 | 2.71 | ||
| COL-SA1559 | HP | 3.68 | 2.70 | |
| 91% protein ID to SA0093 HP | 0.37 | 0.28 | ||
| 41.4% protein ID to HsdS probable restriction modification system | 0.36 | 0.35 | ||
| 45.5% protein ID to SA2490 | 0.33 | 0.46 | ||
| COL-SA1043 | Glycosyl transferase, group 1 | 0.33 | 0.15 | |
| 100% ID to SA1814 | 0.31 | 0.25 | ||
| 97.9% protein ID to structure of cassette chromosome (SCC)-like element, stra | 0.30 | 0.21 | ||
| 61.3% protein ID to SA0553 CHP | 0.28 | 0.50 | ||
| COL-SA0653 | CHP | 0.23 | 0.46 | |
| 24.1% protein ID to BdrC3, Borrelia hermsii | 0.23 | 0.34 | ||
| 26.5% protein ID to RSc1168 CHP, Ralstonia solanacearum | 0.22 | 0.43 | ||
| 29.4% protein ID to PF1843 chromosome segregation protein Smc, Pyrococcus furiosus | 0.22 | 0.26 | ||
| COL-SA0654 | CHP | 0.21 | 0.48 | |
| 50% protein ID to NMB0372 HP, Neisseria meningitidis | 0.19 | 0.48 | ||
| 82.5% protein ID to SA0276 | 0.17 | 0.26 | ||
| 32.9% protein ID to ParA, B. subtilis | 0.14 | 0.30 | ||
| COL-SA0095 | spa | Immunoglobulin G-binding protein A precursor | 0.02 | 0.08 |
Based on the published sequence of S. aureus strain N315. For genes not present in N315, the gene name and description given are from the S. aureus strain COL genome, available from The Institute for Genomic Research website (www.tigr.org or by the putative function.
Abbreviations: PTS, phosphotransferase; CHP, conserved hypothetical protein; CoA, coenzyme A; NTP, nucleoside triphosphate; HP, hypothetical protein; ID, identity; ECM, extracellular matrix.
Normalized values based on the expression ratio (ER), which is defined as the expression level in biofilms/expression level in exponential-phase (EP) or stationary-phase (SP) cells.
We also identified 84 genes whose expression was reduced by a least a factor of at least 2 by comparison with both planktonic growth conditions (Table 5). Included were 25 genes in eight possible operons including an oligopeptide transport system (opp; N315-SA0845-SA0848) and the genes responsible for purine biosynthesis (pur; N315-SA0920-SA0925). Most genes in the other six putative operons encode hypothetical or conserved hypothetical proteins with no known function. However, one well-defined gene that was drastically downregulated in biofilms (60 to 139 times higher in the exponential-phase cultures and 12 to 27 times higher in the stationary-phase cultures) was spa, the gene that encodes protein A.
Confirmation of transcriptional profiling by real-time PCR.
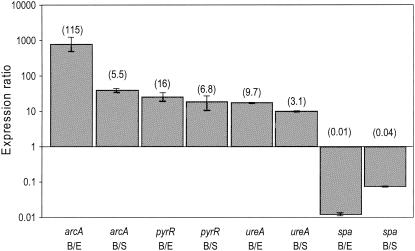
To verify the results of our microarray experiments, we used real-time PCR to examine the relative expression levels of selective target genes. These comparisons were done using RNA isolated from two independent cultures representing each of three growth conditions (biofilm and exponential and stationary growth phases). As observed in our profiling experiments, the arcA, pyrR, and ureA transcripts were present in greater quantities in the biofilm samples than in both exponential- and stationary-phase planktonic cultures (Fig. 5). Indeed, while the patterns of gene expression observed with real-time PCR were consistent with our profiling experiments, the results from the real-time PCR experiments suggest that our profiling experiments may underestimate the actual differences. As with our profiling experiments, we also found that spa was significantly downregulated in biofilms on the basis of real-time PCR comparisons (Fig. 5). Collectively, the real-time PCR results provide independent verification of our DNA microarray results.
FIG. 5.
Relative expression levels as determined by real-time PCR. Expression levels of the arcA, pyrR, ureA, and spa genes was determined by real-time PCR. Relative expression levels are illustrated as the ratio of the expression level observed in biofilms (B) versus exponential-phase (E) or stationary-phase (S) planktonic cultures. The numbers in parentheses above the bars indicate the relative expression levels determined by transcriptional profiling.
Roles of genes regulated by sarA in biofilm formation.
For the reasons discussed above, we are particularly interested in genes that are differentially expressed in biofilms and are part of the sarA regulon. Therefore, we compiled a list of genes that were reported by Dunman et al. (19) to be regulated by sarA and were either induced or repressed in a biofilm compared to either planktonic condition. This analysis revealed 27 genes that were part of the sarA regulon and were differentially expressed in biofilms (Table 6). Because these genes may be genes that are required for biofilm formation, genes that are induced in biofilms and positively regulated by sarA would be of particular interest; however, we identified only four genes (sdhB, carA, an unidentified ORF with similarity to a major histocompatibility complex [MHC] class II analog, and a hypothetical protein) that fell into this category. At the same time, it may be equally important that specific genes be turned off to facilitate biofilm formation, and we identified eight genes (arc, phoP, pbp3, nuc, ndhG, spa, and two hypothetical proteins) that were repressed in biofilms and negatively regulated by sarA. The remaining genes were divergently regulated by sarA and in biofilms; however, the possibility that the impact of sarA is indirect in these cases cannot be ruled out.
TABLE 6.
SarA-regulated genes differentially expressed in a biofilm
| N315 ORFa | Common namea | Producta,b | B/Ec | B/Sc | SarAd |
|---|---|---|---|---|---|
| N315-SAS016 | HP | 12.74 | Up | ||
| N315-SA2425 | arcC | Carbamate kinase | 37.87 | 5.76 | Down |
| N315-SA2424 | arcR | Transcription regulator Crp/Fnr family protein | 48.16 | 9.87 | Down |
| N315-SA2321 | HP | 0.33 | Up | ||
| N315-SA2319 | sdhB | Similar to beta: subunit of l-serine dehydratase | 8.90 | 4.84 | Up |
| N315-SA2125 | hutG | Similar to formiminoglutamase | 0.35 | Up | |
| N315-SA2006 | Similar to MHC class II analog | 4.69 | Up | ||
| N315-SA1928 | HP | 0.42 | Up | ||
| N315-SA1611 | CHP | 0.23 | Up | ||
| N315-SA1553 | fhs | Formyltetrahydrofolate synthetase | 0.17 | Down | |
| N315-SA1516 | phoP | Alkaline phosphatase synthesis | 0.48 | Up | |
| N315-SA1381 | pbp3 | Penicillin-binding protein 3 | 0.48 | Down | |
| N315-SA1319 | HP | 0.42 | Up | ||
| N315-SA1140 | glpF | Glycerol uptake facilitator | 4.40 | Down | |
| N315-SA1120 | Similar to transcription regulator GntR family | 0.35 | Up | ||
| N315-SA1045 | carA | Carbamoyl-phosphate synthase small chain | 13.13 | 6.03 | Up |
| N315-SA0923 | purM | Phosphoribosylformylglycinamidine cyclo-ligase | 0.34 | 0.03 | Down |
| N315-SA0900 | sspB | Cysteine protease precursor | 7.03 | Down | |
| N315-SA0899 | sspC | Cysteine protease | 7.24 | Down | |
| N315-SA0754 | Similar to lactococcal prophage ps3 protein 05 | 0.50 | Up | ||
| N315-SA0746 | nuc | Staphylococcal nuclease | 0.17 | Down | |
| N315-SA0412 | CHP | 0.08 | Down | ||
| N315-SA0411 | ndhF | NADH dehydrogenase subunit 5 | 0.07 | 2.41 | Down |
| N315-SA0363 | HP | 0.32 | Down | ||
| N315-SA0107 | spa | Immunoglobulin G-binding protein A precursor | 0.01 | 0.04 | Down |
| N315-SA0016 | purA | Adenylosuccinate synthase | 2.37 | 0.12 | Up |
| fnbB | Fibronectin-binding protein homolog | 0.46 | Up |
Based on the published sequence of S. aureus strain N315. For genes not present in N315, the gene name and description given are from the S. aureus strain COL genome, available from The Institute for Genomic Research website (www.tigr.org or by the putative function.
Abbreviations: HP, hypothetical protein; CHP, conserved hypothetical protein.
Normalized values based on expression levels in biofilms versus exponential-phase cells (B/E) or biofilms versus stationary-phase cells (B/S).
Regulation by SarA as reported by Dunman et al. (19).
DISCUSSION
Valle et al. (66) was the first to demonstrate that mutation of sarA results in a reduced capacity to form a biofilm. They concluded that this was due, at least in part, to reduced expression of the icaADBC operon. At the same time, Valle et al. (66) also suggested that SarA enhances biofilm formation by suppressing production of a second, unidentified protein that was either a repressor of PNAG synthesis or was involved in the turnover of PNAG. On this basis, they proposed a model in which the impact of sarA on biofilm formation was dependent on two pathways, both of which functioned by moderating the production of PNAG.
In this report, we also demonstrated that mutation of sarA results in reduced production of PNAG. To further investigate the impact of this on biofilm formation in our clinical isolate, we generated an ica mutant and examined its capacity to form a biofilm both in vitro and in vivo. Surprisingly, our ica mutant formed a biofilm comparable to that of the parental strain under both static and flow conditions. As in our previous experiments (5), the capacity of our UAMS-1 sarA mutant to form a biofilm was reduced in comparison to both the wild-type strain and its corresponding ica mutant. This clearly indicates that the impact of sarA on biofilm formation, at least as defined under in vitro growth conditions, involves a pathway that is independent of the icaADBC operon.
The role of ica in vivo has been addressed in S. epidermidis with contradictory results (10, 23, 41, 47, 49, 55, 56, 57, 62). However, few studies have addressed this issue in S. aureus. Cramton et al. (14) demonstrated that ica is present in S. aureus and that it is required for PIA production and biofilm formation in that species. However, the mutagenesis experiments were limited to a single strain of S. aureus (SA113) that was derived from NCTC 8325 by chemical mutagenesis, so these results may not be representative of the situation observed in clinical isolates. More recently, Vandecasteele et al. (67) analyzed expression of biofilm-associated genes, including icaA and icaC, both in vitro and in vivo. While expression of both genes was induced upon initial exposure to foreign bodies, this induction peaked shortly after the introduction of bacteria and was followed by a slow decrease over time. These results suggest that the ica operon is mainly associated with the initial colonization phase of biofilm formation, rather than maturation and persistence. This is consistent with our results and the fact that our analysis was limited to mature biofilms. Additionally, Francois et al. (23) compared an S. aureus strain and its corresponding ica mutant using a tissue cage model and found that the ica mutant retained the capacity to colonize at a level comparable to that of the wild-type strain. They concluded on this basis that biofilms were not an important factor in their model. However, we believe there are two possible alternative explanations. The first is that their model does not accurately reflect the need to form a biofilm. The second is that their ica mutant retained the capacity to form a biofilm under in vivo conditions. Our results would support the latter hypothesis. Specifically, we found that while the UAMS-1 sarA mutant demonstrated a reduced capacity to colonize catheters in vivo, the UAMS-1 ica mutant colonized the same substrates as well as the wild-type strain did. These results also suggest that PNAG is not required for in vivo colonization by S. aureus and that sarA regulates genes required for biofilm formation independent of its ability to modulate ica expression and/or the production of PNAG. We would note, however, that our results are also limited to a single strain, and it is certainly possible that UAMS-1 has an alternative means of promoting intercellular accumulation that attenuates the need for PIA. Whether this is true of other clinical isolates of S. aureus remains to be determined, but previous work in our laboratory has confirmed that all other sarA-mediated phenotypes are conserved among clinical isolates like UAMS-1 (5, 7).
Dunman et al. (19) recently reported the results of microarray-based transcriptional profiling experiments with S. aureus sarA and agr mutants. These studies confirmed that sarA has global regulatory effects that are mediated through both agr-dependent and agr-independent pathways. In an effort to determine which of these might be involved in biofilm formation and/or maintenance of the sessile lifestyle, we performed comprehensive transcriptional profiling with RNA isolated from mature S. aureus biofilms. Although it would be preferable to do these experiments using RNA derived from biofilms grown in vivo, our attempts to isolate a sufficient quantity of high-quality RNA from in vivo samples have thus far been unsuccessful. However, our studies indicating that mutation of sarA results in a reduced capacity to form a biofilm both in vitro and in vivo suggest that biofilms grown in flow cells may also provide a relevant source of RNA for transcriptional profiling. On this basis, we isolated RNA from UAMS-1 grown in flow cells and compared the pattern of gene expression to RNA from the same strain grown in planktonic culture. The results of our comparisons to both exponential- and stationary-phase planktonic cultures clearly indicate that our flow cell biofilm model represents a unique growth environment. Indeed, we identified a total of 580 genes that are differentially expressed in biofilms by comparison to either or both planktonic conditions.
Several of the operons that were induced in biofilms have been found to be important in acid tolerance in other bacterial species. Indeed, maintenance of pH homeostasis within the bacterial cell and buffering of the surrounding microenvironment have been associated with biofilm formation in the oral bacteria Streptococcus salivarius (34). One way bacteria combat acidic environments is to produce alkaline compounds, such as ammonia, that can neutralize the acids. Two ways in which bacteria generate ammonia are through the urease and arginine deiminase (ADI) pathways. Interestingly, we found that multiple genes from both of these pathways were induced in S. aureus biofilms by comparison to both planktonic conditions.
Under anaerobic conditions, some bacteria are also able to generate ATP as an energy source through catabolism of arginine via the ADI pathway, which is widely distributed in bacteria (16), archaea (54), and eucarya (60). The ADI pathway is comprised of three enzymatic reactions, catalyzed by arginine deiminase (arcA), ornithine transcarbamoylase (arcB), and carbamate kinase (arcC). Collectively, these enzymes convert arginine to ornithine, ammonia, and CO2, yielding 1 mol of ATP per mol of arginine consumed. ArcD is an arginine-ornithine transporter that catalyzes the uptake of arginine and concomitant export of ornithine, while arcR encodes an activator of the ADI operon that is a member of the Crp-Fnr family of regulators. We found that all five of these genes were significantly induced in S. aureus biofilms (Table 5). One additional gene that was upregulated 3.9-fold in a biofilm compared to exponential-phase cells is the arginine repressor encoded by argR (Table 3). Under anaerobic conditions in the presence of arginine, ArgR represses anabolic ornithine carbamoyltransferase and induces the ADI pathway. In addition to its role in generating ATP anaerobically, the ADI pathway is one of two major ammonia-generating pathways utilized by oral bacteria to maintain pH homeostasis when growing in a biofilm. Ammonia generated by the deimination of arginine can neutralize acids generated by bacterial glycolysis.
The ADI system and its role in acid resistance have also been correlated with virulence in Streptococcus pyogenes. ADI in this species was originally called streptococcal acid glycoprotein (SAGP) and was characterized as being an inhibitor of stimulated human peripheral blood mononuclear cell proliferation (17, 18). In addition, it is thought that the acid sensitivity of a SAGP-negative mutant is responsible for its reduced ability to enter and survive in epithelial cells (18).
Also included among the genes induced in biofilms (Table 5) were seven genes that comprise the urease operon (ureABCEFGD). Urease (urea amidohydrolase) is a nickel-containing enzyme that catalyzes the hydrolysis of urea to yield two molecules of ammonia and one molecule of CO2. Ureases of most bacteria are composed of three distinct subunits encoded by three contiguous genes, ureA, ureB, and ureC. Urease gene clusters also encode accessory genes, in addition to these structural genes, that are required for the de novo synthesis of active urease. Urease activity is essential for the colonization of the gastric mucosa by Helicobacter pylori and colonization of the urinary tract by both Proteus mirabilis and Staphylococcus saprophyticus (20, 25, 30). In addition, urease is thought to play a central role in the pathogenesis of Ureaplasma urealyticum urinary and respiratory tract infections (27, 35).
Recently, Saïd-Salim et al. (58) found that genes of the urease operon in S. aureus are negatively regulated by the SarA homolog Rot (repressor of toxin). Interestingly, expression of rot was repressed in biofilms, although this was limited to the comparison with exponential-phase planktonic cultures. The trigger for induced transcription of the urease operon in S. aureus has not yet been studied. However, urease synthesis by Klebsiella aerogenes is stimulated under conditions of nitrogen starvation, such as when the bacteria are cultured in minimal medium containing a poor nitrogen source, such as proline, arginine, or histidine (24). In H. pylori, expression of the urease operon is upregulated by a pH-dependent, posttranscriptional regulatory mechanism. More specifically, Akada et al. (1) showed that a shift to an acidic pH resulted in a significant increase in the level of ure operon mRNA, even in the presence of inhibitors of transcription. Until recently, it was believed that urease was associated with the cell surface and that it directly neutralized the microenvironment surrounding the cell (50). However, it is now thought to play a more important role as an intracellular enzyme required for acid resistance (61).
In addition to the production of ammonia, cation transport ATPases, such as the high-affinity K+-specific transport system encoded by the kdp operon, can also contribute to pH homeostasis through the exchange of K+ for H+ (13). In this study, we found that three genes of the kdp operon (kdpABC) were induced in biofilms by comparison to both planktonic growth conditions (Table 5) and two other genes (kdpDE) were induced by comparison to the exponential growth phase (Table 3). In E. coli, the Kdp system is composed of the ion motive P-type ATPase encoded by the kdpFABC operon, and expression of the kdpFABC operon is regulated by an adjacent operon, kdpDE (2). KdpD is a membrane-spanning sensor kinase, and KdpE is a cytosolic transcriptional activator. In response to an appropriate signal(s) (membrane stretch, alteration in turgor pressure, and external and internal potassium levels), KdpD transphosphorylates KdpE, which in turn upregulates transcription of the kdpFABC operon (2, 43). Interestingly, van der Laan et al. (68) recently reported that NH4+ ions strongly stimulate the ATPase activity of the KdpFABC complex in E. coli.
Several operons of the pyrimidine nucleotide biosynthetic (pyr) pathway (pyrRPBC, carAB, and pyrFE) were also induced in biofilms (Table 5). The pathway for the de novo synthesis of pyrimidines consists of six enzymatic steps leading to the formation of UMP. The first step in the pyrimidine biosynthetic pathway is the formation of carbomyl-phosphate (CP) from bicarbonate, glutamine (or ammonia), and ATP by CP synthase, which is encoded by the carAB genes. Interestingly, CP is also required for the biosynthesis of arginine. In B. subtilis, PyrR regulates transcription of the pyr operon by binding in a uridine nucleotide-dependent fashion to pyr mRNA and altering the secondary structure of the downstream mRNA (37, 38). Binding of PyrR to the downstream mRNA stabilizes a binding loop and prevents the formation of the antiterminator (39). In the absence of PyrR or when levels of the nucleotides UMP and UTP are low, the antiterminator is a stable secondary structure, and transcription of the downstream genes continues (39). While we do not know if the function of B. subtilis and S. aureus PyrR is conserved, our results showing that all of the genes in the pyr operon were induced in biofilms suggests that the level of UMP in cells growing in a biofilm is severely limited. In addition, upregulation of the pyr operon and subsequent CP production may be required for synthesis of sufficient levels of arginine to be used by the ADI pathway during anaerobic growth.
Taken together, the results of our array experiments suggest that mature biofilms are growing anaerobically and that genes of the acid tolerance response are upregulated in response to an acidic environment. While the regulatory pathway for the acid tolerance response in S. aureus has not been well characterized, there have been studies suggesting that the global regulators SigB and SarA are involved. Specifically, mutation of sigB results in strains with impaired abilities to respond to acid and to induce a stationary-phase acid tolerance response (8, 32). In addition, in the absence of RsbU, expression from the SigB-dependent sarA promoter was significantly reduced at pH 5.5 (46).
Our analysis revealed 27 genes that were differentially expressed in biofilms and were part of the sarA regulon as defined by Dunman et al. (19) (Table 6). Given the different mechanistic possibilities of how SarA modulates biofilm production, it is difficult to determine which of these genes might be the most relevant candidates for further consideration. For example, because SarA mutants have a reduced capacity to form a biofilm, SarA would presumably be required for production of an activator of biofilm formation or a required effector molecule, in which case transcription of the relevant gene(s) would be upregulated by SarA and in a biofilm. However, it is also possible that SarA represses production of an effector that is deleterious to biofilm formation, in which case the SarA target would be downregulated within a biofilm. One possibility in that regard is that the increased production of proteases observed in sarA mutants (7) results in degradation of a required surface protein. However, Valle et al. (66) concluded on the basis of both mutagenesis experiments and experiments employing specific inhibitors that protease production was not responsible for the decreased capacity of a sarA mutant to form a biofilm. Nevertheless, it remains possible that a similar scenario in which SarA represses transcription of some factor that has a negative impact on biofilm formation is involved. Moreover, both of these scenarios are based on the assumption that the SarA-dependent regulation of the relevant effector(s) is direct, and it is also possible that sarA modulates biofilm formation in an indirect fashion, perhaps via one of the increasing number of SarA homologs (4, 9, 45). It is also possible that sarA is required for production of an adhesin or some other effector protein that is required only for initial stages of biofilm formation. In this case, expression of the relevant gene may be transient in a fashion that would not have been apparent in our profiling experiments focusing on mature biofilms.
Finally, it should be noted that biofilms are not homogenous populations of cells and because the experiments we performed did not address this issue, it is certainly possible that we failed to detect genes that are within the sarA regulon but are differentially expressed only within certain regions of the biofilm. At the same time, this would imply that the genes we did identify are either differentially expressed throughout the biofilm or that our results actually underestimate the degree of differential gene expression observed within more limited regions. It should also be noted that the profiling experiments of Dunman et al. (19) were done using a first-generation GeneChip that was limited to only 85% of the genes identified in a single strain (COL) of S. aureus, and it is certainly possible that this limited the identification of relevant genes that are conserved among clinical isolates like UAMS-1. Moreover, the profiling experiments of Dunman et al. (19) were done using RNA isolated from derivatives of the S. aureus strain RN6390, and we have previously demonstrated that the regulatory circuits observed in RN6390 and its corresponding sarA and agr mutants are different from those of other strains, including UAMS-1 (7). To address these issues, we are currently performing transcriptional profiling experiments using RNA isolated from UAMS-1 sarA and agr mutants and the more comprehensive chips used for the biofilm profiling experiments described here.
REFERENCES
- 1.Akada, J. K., M. Shirai, H. Takeuchi, M. Tsuda, and T. Nakazawa. 2000. Identification of the urease operon in Helicobacter pylori and its control by mRNA decay in response to pH. Mol. Microbiol. 36:1071-1084. [DOI] [PubMed] [Google Scholar]
- 2.Altendorf, K., P. Voelkner, and W. Puppe. 1994. The sensor kinase KdpD and the response regulator KdpE control expression of the kdpFABC operon in Escherichia coli. Res. Microbiol. 145:374-381. [DOI] [PubMed] [Google Scholar]
- 3.Arciola, C. R., L. Baldassarri, and L. Montanaro. 2001. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 39:2151-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvidson, S., and K. Tegmark. 2001. Regulation of virulence determinants in Staphylococcus aureus. Int. J. Med. Microbiol. 291:159-170. [DOI] [PubMed] [Google Scholar]
- 5.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins, J. S., A. F. Gillaspy, T. M. Rechtin, B. K. Hurlburt, and M. S. Smeltzer. 1999. The staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesin gene (cna) in an agr-independent manner. Mol. Microbiol. 33:317-326. [DOI] [PubMed] [Google Scholar]
- 7.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, P. F., S. J. Foster, E. Ingram, and M. O. Clements. 1998. Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung, A. L., and G. Zhang. 2002. Global regulation of virulence determinants in Staphylococcus aureus by the SarA protein family. Front. Biosci. 1:1825-1842. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, G. D., L. Baldassarri, and W. A. Simpson. 1994. Colonization of medical devices by coagulase-negative staphylococci, p. 45-78. In A. L. Bisno and F. A. Waldvogel (ed.)., Infections associated with indwelling medical devices, 2nd ed. ASM Press, Washington, D.C.
- 11.Clements, M. O., and S. J. Foster. 1999. Stress resistance in Staphylococcus aureus. Trends Microbiol. 7:458-462. [DOI] [PubMed] [Google Scholar]
- 12.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter, P. D., and H. Colin. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramton, S. E., M. Ulrich, F. Götz, and G. Döring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degnan, B. A., M. A. Kehoe, and J. A. Goodacre. 1997. Analysis of human T cell responses to group A streptococci using fractionated Streptococcus pyogenes proteins. FEMS Immunol. Med. Microbiol. 17:161-170. [DOI] [PubMed] [Google Scholar]
- 18.Degnan, B. A., M. C. Fontaine, A. H. Doebereiner, J. J. Lee, P. Mastroeni, G. Dougan, J. A. Goodacre, and M. A. Kehoe. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton, K. A., J. V. Gilbert, E. A. Joyce, A. E. Wanken, T. Thevenot, P. Baker, A. Plaut, and A. Wright. 2002. In vivo complementation of ureB restores the ability of Helicobacter pylori to colonize. Infect. Immun. 70:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans, R. P., C. L. Nelson, W. R. Bowen, M. G. Kleve, and S. G. Hickmon. 1998. Visualization of bacterial glycocalyx with a scanning electron microscope. Clin. Orthop. Relat. Res. 347:243-249. [PubMed] [Google Scholar]
- 22.Fowler, V. G., Jr., P. D. Fey, L. B. Reller, A. L. Chamis, G. R. Corey, and M. E. Rupp. 2001. The intercellular adhesin locus ica is present in clinical isolates of Staphylococcus aureus from bacteremic patients with infected and uninfected prosthetic joints. Med. Microbiol. Immunol. 189:127-131. [DOI] [PubMed] [Google Scholar]
- 23.Francois, P., P. H. Tu Quoc, C. Bisognano, W. L. Kelly, D. P. Lew, J. Schrenzel, S. E. Cramton, F. Götz, and P. Vaudaux. 2003. Lack of biofilm contribution to bacterial colonisation in an experimental model of foreign body infection by Staphylococcus aureus and Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 35:135-140. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich, B., and B. Magasanik. 1977. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J. Bacteriol. 131:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatermann, S., and R. Marre. 1989. Cloning and expression of Staphylococcus saprophyticus urease gene sequences in Staphylococcus carnosus and contribution of the enzyme to virulence. Infect. Immun. 57:2998-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 27.Hedelin, H., J. E. Brorson, L. Grenabo, and S. Pettersson. 1984. Ureplasma urealyticum and upper urinary tract stones. Br. J. Urol. 56:244-249. [DOI] [PubMed] [Google Scholar]
- 28.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 29.Heinrichs, J. H., M. G. Bayer, and A. L. Cheung. 1996. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J. Bacteriol. 178:418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, B. D., C. V. Lockatell, D. E. Johnson, J. W. Warren, and H. L. Mobley. 1990. Construction of urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 58:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadurugamuwa, J. L., L. Sin, E. Albert, J. Yu, K. Francis, M. DeBoer, M. Rubin, C. Bellinger-Kawahara, T. R. Parr, Jr., and P. R. Contag. 2003. Direct continuous method for monitoring biofilm infection in a mouse model. Infect. Immun. 71:882-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, Y. H., Y. Y. Chen, and R. A. Burne. 2000. Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ. Microbiol. 2:169-177. [DOI] [PubMed] [Google Scholar]
- 35.Ligon, J. V., and G. E. Kenny. 1991. Virulence of ureaplasmal urease for mice. Infect. Immun. 59:1170-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowen, P., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 37.Lu, Y., and R. L. Switzer. 1996. Transcriptional attenuation of the Bacillus subtilis pyr operon by the PyrR regulatory protein and uridine nucleotides in vitro. J. Bacteriol. 178:7206-7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, Y., R. J. Turner, and R. L. Switzer. 1995. Roles of the three transcriptional attenuators of the Bacillus subtilis pyrimidine biosynthetic operon in the regulation of its expression. J. Bacteriol. 177:1315-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu, Y., R. J. Turner, and R. L. Switzer. 1996. Function of RNA secondary structures in transcriptional attenuation of the Bacillus subtilis pyr operon. Proc. Natl. Acad. Sci. USA 93:14462-14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermid, K. P., D. W. Morck, M. E. Olson, N. D. Boyd, A. E. Khoury, M. K. Dasgupta, and J. W. Costerton. 1993. A porcine model of Staphylococcus epidermidis catheter-associated infection. J. Infect. Dis. 168:897-903. [DOI] [PubMed] [Google Scholar]
- 42.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 43.Nakashima, K., A. Sugiura, K. Kanamaru, and T. Mizuno. 1993. Signal transduction between the two regulatory components involved in the regulation of the kdpABC operon in Escherichia coli: phosphorylation-dependent functioning of the positive regulator, KdpE. Mol. Microbiol. 7:109-116. [DOI] [PubMed] [Google Scholar]
- 44.Ni Edhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 45.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 46.Palma, M., and A. L. Cheung. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 69:7858-7865. [DOI] [PMC free article] [PubMed]
- 47.Patrick, C. C., S. V. Hetherington, P. K. Roberson, S. Henwick, and M. M. Sloas. 1995. Comparative virulence of Staphylococcus epidermidis isolates in a murine catheter model. Ped. Res. 37:70-74. [DOI] [PubMed] [Google Scholar]
- 48.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 49.Perdreau-Remington, F., M. A. Sande, G. Peters, and H. F. Chambers. 1998. The abilities of Staphylococcus epidermidis wild-type strain and its slime-negative mutant to induce endocarditis in rabbits are comparable. Infect. Immun. 66:2778-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phadnis, S. H., M. H. Parlow, M. Levy, D. Ilver, C. M. Caulkins, J. B. Connors, and B. E. Dunn. 1996. Surface localization of Helicobacter pylori urease and heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prigent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohde, H., J. K. Knobloch, M. A. Horstkotte, and D. Mack. 2001. Correlation of biofilm expression types of Staphylococcus epidermidis with polysaccharide intercellular adhesin synthesis: evidence for involvement of icaADBC genotype-independent factors. Med. Microbiol. Immun. 190:105-112. [DOI] [PubMed] [Google Scholar]
- 54.Ruepp, A., and J. Soppa. 1996. Fermentative arginine degradation in Halobacterium salinarium (formerly Halobacterium halobium): genes, gene products, and transcripts of the arcRACB gene cluster. J. Bacteriol. 178:4942-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed]
- 58.Saïd-Salim, B., P. M. Dunman, F. M. McAleese, D. Macapagal, E. Murphy, P. J. McNamara, S. Arvidson, T. J. Foster, S. J. Projan, and B. N. Kreiswirth. 2003. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 185:610-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schembri, M. A., K. Kjærgaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-267. [DOI] [PubMed] [Google Scholar]
- 60.Schofield, P. J., M. R. Edwards, J. Matthews, and J. R. Wilson. 1992. The pathway of arginine catabolism in Giardia intestinalis. Mol. Biochem. Parasitol. 51:29-36. [DOI] [PubMed] [Google Scholar]
- 61.Scott, D. R., D. Weeks, C. Hong, S. Postius, K. Melchers, and G. Sachs. 1998. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology 114:58-70. [DOI] [PubMed] [Google Scholar]
- 62.Shiro, H., E. Muller, N. Gutierrez, S. Boisot, M. Grout, T. D. Tosteson, D. Goldmann, and G. B. Pier. 1994. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are virulent in a rabbit model of endocarditis. J. Infect. Dis. 169:1042-1049. [DOI] [PubMed] [Google Scholar]
- 63.Stanley, N. R., R. A. Britton, A. D. Grossman, and B. A. Lazazzera. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 65.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 66.Valle, J., A. Toledo-Arana, C. Berasain, J. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 67.Vandecasteele, S. J., W. E. Peetermans, R. Merckx, B. J. Rijnders, and J. Van Eldere. 2003. Reliability of the ica, aap, and atlE genes in the discrimination between invasive, colonizing and contaminant Staphylococcus epidermidis isolates in the diagnosis of catheter-related infections. Clin. Microbiol. Infect. 9:114-119. [DOI] [PubMed] [Google Scholar]
- 68.Van der Laan, M., M. Gassel, and K. Altendorf. 2002. Characterization of amino acid substitutions in KdpA, the K+-binding and -translocating subunit of the KdpFABC complex to Escherichia coli. J. Bacteriol. 184:5491-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vuong, C., C. Gerke, G. A. Somerville, E. R. Fischer, and M. Otto. 2003. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J. Infect. Dis. 188:706-718. [DOI] [PubMed] [Google Scholar]
- 70.Vuong, C., H. L. Saenz, F. Götz, and M. Otto. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182:1688-1693. [DOI] [PubMed] [Google Scholar]
- 71.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 72.Wolz, C., D. McDevitt, T. J. Foster, and A. L. Cheung. 1996. Influence of agr on fibrinogen binding in Staphylococcus aureus Newman. Infect. Immun. 64:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolz, C., C. Goerke, R. Landmann, W. Zimmerli, and U. Fluckiger. 2002. Transcription of clumping factor A in attached and unattached Staphylococcus aureus in vitro and during device-related infection. Infect. Immun. 70:2758-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolz, C., P. Pöhlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding proteins by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36:230-243. [DOI] [PubMed] [Google Scholar]
- 75.Ziebuhr, W., V. Krimmer, S. Rachid, I. Lössner, F. Götz, and J. Hacker. 1999. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol. Microbiol. 32:345-356. [DOI] [PubMed] [Google Scholar]