Abstract
Integrative, multilevel approaches investigating neurobiological systems relevant to threat detection promise to advance understanding of the pathophysiology of major depressive disorder (MDD). In this study we considered key neuronal and hormonal systems in adolescents with MDD and healthy controls (HC). The goals of this study were to identify group differences and to examine the association of neuronal and hormonal systems. MDD and HC adolescents (N = 79) aged 12–19 years were enrolled. Key brain measures included amygdala volume and amygdala activation to an emotion face-viewing task. Key hormone measures included cortisol levels during a social stress task and during the brain scan. MDD and HC adolescents showed group differences on amygdala functioning and patterns of cortisol levels. Amygdala activation in response to emotional stimuli was positively associated with cortisol responses. In addition, amygdala volume was correlated with cortisol responses, but the pattern differed in depressed versus healthy adolescents, most notably for unmedicated MDD adolescents. The findings highlight the value of using multilevel assessment strategies to enhance understanding of pathophysiology of adolescent MDD, particularly regarding how closely related biological threat systems function together while undergoing significant developmental shifts.
The public health impact of depression may be substantially mitigated if adequate attention is directed to effectively understand and treat depression early in development. Depressive disorders are associated with impairment, chronic suffering, and early death, and impact about 16% of the population (Kessler, Avenevoli, & Merikangas, 2001). Historical trends suggest that depression is on the rise and is the third leading cause of global burden of disease worldwide (Berndt et al., 2000; World Health Organization, 2008). Depression in adolescence is of particular importance (Zalsman, Brent, & Weersing, 2006). Not only is depression commonly first evident during adolescence, but an early onset of depression is associated with a poor prognosis (Lewinsohn, Clarke, Seeley, & Rohde, 1994; Weissman et al., 1999; Zisook et al., 2007).
Major depressive disorder (MDD) has been characterized as a multisystemic disorder affecting brain and body (Insel & Charney, 2003). Inclusion of multiple levels of analysis provides an opportunity to examine the interplay across relevant systems. The focus on depression early in development is a priority because adolescents are more sensitive to stress (Compas & Wagner, 1991), and the neurobiological systems relevant to threat detection and stress regulation are continuing to undergo maturational refinement (e.g., Lenroot & Giedd, 2006; Luciana & Collins, 2012; Romeo & McEwen, 2006). Neuroscience research on adolescent MDD to date has identified anomalous functioning in systems involved in responding to threats in the environment, including key brain regions (e.g., Cullen et al., 2009, 2010; Thomas et al., 2001; Yang et al., 2010) and the hypothalamic–pituitary–adrenal (HPA) axis (e.g., Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001; Rao, Hammen, Ortiz, Chen, & Poland, 2008). However, these approaches are limited by focusing primarily on either the neural or the hormonal aspects of the biological threat response system. Research with adult depression has begun to examine interplay across systems; while these findings may have limited developmental relevance, consideration of multiple levels of analysis provides a useful framework for advancing our understanding of the complex neurobiology that underlies the pathophysiology of depression (e.g., Pruessner et al., 2010). The current work uses multiple levels of analysis to examine the interplay of systems relevant to threat response.
There are several existing models that highlight the challenges of threat processing for those struggling with depression (Drevets, 1999; Ghashghaei & Barbas, 2002; Mayberg, 1997; Nestler et al., 2002; Phillips, Drevets, Rauch, & Lane 2003; Price & Drevets, 2010). Fronto-limbic circuitry and HPA axis functioning are two key systems important for threat processing, and preclinical studies have demonstrated clear links between these systems (e.g., Diorio, Viau, & Meaney, 1993; McEwen, 1995; Reul & de Kloet, 1985; Sullivan & Gratton, 2002). Presumably in certain pathological conditions, excessive limbic activation may lead to overstimulation of the HPA axis, resulting in the release of stress hormones whose cumulative effects include alterations in receptor functioning as well as deleterious long-term consequences for neuronal health (e.g., McEwen, 1995; Musselman & Nemeroff, 1993). There is preliminary evidence that HPA axis normalization can be achieved when treatment is effective (Fisher, Gunnar, Chamberlain, & Reid, 2000; Pariante, Kim, Makoff, & Kerwin, 2003). Adolescence may represent a critical window of development in which interventions for depression could be most successful in terms of alternating these stress response patterns (Levine 1957), reducing the likelihood of neurodegeneration and gene expression alterations (de Kloet, 2003; Kaufman & Charney, 2001; Meaney & Szyf, 2005).
Highly complex neural networks make up the threat response system of the brain and the associated hormonal cascade. Given that this field is still in a preliminary phase of inquiry, we chose to focus on the amygdala, a central node of the threat response network circuitry that is posited to affect hormonal systems. There is evidence of excitatory modulation of the amygdala on the HPA axis (Van de Kar & Blair, 1999). The amygdala and the HPA system are both key components in a response system that detects and orchestrates a regulatory response from distributed networks. The amygdala also affects the glucocorticoid feedback processing that is disrupted in depression (Herman, Flak, & Jankord, 2008).
Several studies to date have considered the interplay between amygdala functioning and HPA axis functioning in healthy adults and adults suffering from depression. Drevets et al. (2002) found a positive association between glucose metabolism in the amygdala, as assessed by positron emission tomography scans, and cortisol levels during the scan in depressed adults. Cunningham-Bussel et al. (2009) reported that the right amygdala blood oxygen level dependent response to visual images of the World Trade Center attack was positively correlated with cortisol values during a scan in healthy adults, and right amygdala activation was correlated with the cortisol preversus postscan change scores. Another study showed that exogenous cortisol administration led to increased noradrenergic activation in the amygdala (van Stegeren et al., 2007). Exogenous cortisol has also been found to “decouple” the amygdala from executive control brain regions (Henckens, van Wingen, Joels, & Fernandez, 2012). While there is some conflicting evidence (e.g., Holsen et al., 2013; Lovallo, Robinson, Glahn, & Fox, 2010), overall these studies suggest that elevated amygdala activation is associated with cortisol activation in both healthy and MDD populations.
In addition to amygdala functioning, amygdala volume may also be linked with HPA axis functioning. Some work has suggested links between the volumes of other limbic structures, including the pituitary gland and the hippocampus, to HPA axis functioning in depressed adults (Axelson et al., 1992, 1993; Dedovic et al., 2010; Treadway et al., 2009), while other studies have failed to document such an association (e.g., Colla et al., 2007; Vythilingam et al., 2004). Among the few studies that have examined amygdala volume more specifically, there has been limited evidence of across-system associations (Kronenberg et al., 2009; Pruessner et al., 2010; Schuhmacher et al., 2012). These results may be due to variance in methods used to assess HPA axis functioning, which range from considering cortisol under basal conditions to highly variable stress reactive paradigms (Pressner et al., 2010). These results may also be because depression is sometimes associated with larger amygdala volumes and in other cases smaller amygdala volumes (Hamilton, Siemer, & Gotlib, 2008; Leuner & Shors, 2013). In a meta-analysis, Hamilton et al. (2008) noted that depressed adults undergoing antidepressant pharmacotherapy tend to have larger amygdala volumes. Finally, because the adolescent neurobiological stress system is undergoing development, applications of the adult literature may be limited when considering adolescent depression.
Research is needed to begin parsing out the contributions of these important factors in adolescence. Performing analyses geared toward elucidating structural and hormonal interplay may be able to demonstrate new links between stress and later vulnerability for depressive and other disorders. To date, there have been only a handful of studies that have examined the interplay between systems in typically developing adolescents or in adolescents at risk for depression. Thomason, Hamilton, and Gotlib (2011) found that in a sample of healthy adolescents who completed functional magnetic resonance imaging (fMRI) after a social stress paradigm, higher cortisol stress response was correlated with increased functional connectivity between the salience network and the subgenual anterior cingulate cortex. When examining potential correspondence between early life stress and HPA axis function, Burghy et al. (2012) found that increased cortisol due to higher early life stress in young girls predicted lower functional connectivity between the amygdala and the ventromedial prefrontal cortex 14 years later. Another study, by Liu et al. (2012), showed that in a group of adolescents experiencing stressful life conditions, who completed the Trier Social Stress Test (TSST) followed by an fMRI, a greater cortisol response correlated with less activity in the left hippocampus while viewing fearful faces. Critically important questions considering links between brain and HPA functioning in clinically depressed adolescents have yet to be addressed.
The purpose of this study was to conduct multilevel assessments of threat systems in adolescents with and without depression using multiple levels of analysis, and to evaluate the correspondence across systems (neuronal structure and responses, hormonal responses, and behavior). Assessments addressed amygdala volume and functioning. Evaluation of the hormonal stress system included two paradigms to measure stress reactivity and recovery. The TSST was used to evaluate HPA axis functioning in response to a social stressor on a different day than the brain scan. To obtain an HPA measure that was more temporally linked to our neurocircuitry measure, we also assessed cortisol levels before and after the brain scan. The first study aim was to evaluate differences between depressed and well adolescents for each of these stress indices. Based on previous work (e.g., Rosso et al., 2005; Yang et al., 2010), we predicted that adolescents with MDD would exhibit smaller amygdala size, greater amygdala functioning, and a different pattern of hormonal response to stress. The second study aim was to examine the interplay between amygdala and HPA axis indices. We predicted that associations between the neuronal and hormonal systems would be found and to some extent these associations would differ between MDD and healthy adolescents; however, no specific predictions were made with regard to the directions of the associations. We also explored possible differences between medicated and unmedicated depressed adolescents (Aihara et al., 2007).
Methods
Participants
Participants were 79 adolescents between the ages of 12 and 19 years old (M age = 15.90, SD = 1.87): 52 adolescents with MDD and 27 healthy controls (HC). Participants were primarily females (75.95%) and most identified themselves as Caucasian (65.8%), followed by African American (8.9%), Hispanic (8.9%), Asian (3.8%), and Native American (1.3%), with the remaining participants self-identifying as “other” (20.3%). Participants were able to select more than one option for race/ethnicity. MDD and HC participants were matched at recruitment on sex, age, and race.
Participants were recruited using a variety of different strategies, primarily through community postings, and from inpatient and outpatient clinical services at the University of Minnesota and the surrounding area. On the first visit, diagnostic interviews were conducted. The TSST and the brain scan were conducted on two separate subsequent visits. The study was approved by the University of Minnesota’s Institutional Review Board. All participants completed signed informed consent and/or assent (if under 18), and all participants received monetary compensation for their participation after completing each of the three visits.
Measures
Diagnosis and symptom assessment. The presence or absence of a DSM-VI-TR Axis I disorder(s) was confirmed by a semistructured diagnostic interview. Participants under 18 years of age and a legal guardian completed independent interviews using the Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997). The KSADS-PL interviews were conducted by highly trained clinical psychologists, child psychiatrists, or advance trainees enrolled in graduate clinical psychology doctoral programs under the direct supervision of a clinician. For participants 18 or 19 years of age, a parent interview was not conducted. Many MDD participants (67%) suffered from at least one comorbid mental illness, which consisted most commonly of an anxiety disorder (67%) and/or attention-deficit/hyperactivity disorder (ADHD; 13%). In addition to diagnostic status, clinicians obtained information about the duration of illness (MDD). Upon having conducted the KSADS-PL, clinicians compete a Global Assessment of Functioning score rating (M = 54.35, SD = 8.35) and the Children’s Depression Rating Scale (mean T score = 77.29, SD = 6.13; Poznanski & Mokros, 1996) on participants in the MDD group. The Children’s Depression Rating Scale is a semistructured interview that assesses 17 symptom areas related to depression, including those that serve as criteria in the DSM-IV. All participants completed the Beck Depression Inventory II (Beck, Steer, & Brown, 1996) at each study visit, which documented a significant difference for the average Beck Depression Inventory II score across visits between the HC (M = 2.33, SD = 3.40) and the MDD groups (M = 25.54, SD 12.28).
Within the context of the KSAD-PL, information about medication status was obtained. MDD participants were classified based on medication status. One subgroup of 16 participants was receiving medication for depression and another subgroup of 36 participants was not receiving medication for depression. Participants diagnosed with ADHD who were only receiving stimulants for the treatment of ADHD were included in the unmedicated sample provided that they abstained from taking the medication on the day of the brain scan. Two participants did not abstain; both had deviated from the recommended protocol and taken psychostimulants earlier in the day, but by the time of the scan the effects of the medication were likely partially or totally worn off given the typical half-life of approximately 6 hr for these medications.
DSM-IV diagnoses were established through a consensus meeting that incorporated information from the independent parent and child interviews, rating scales, and, if available, medical records. Participants were eligible for the HC group if they had no evidence of an Axis I diagnosis. Participants were eligible for the patient group if they had a primary diagnosis of MDD. Exclusionary diagnoses for both the MDD and HC groups included pervasive developmental disorder, bipolar disorder, schizophrenia, a neurological disorder, or a chronic or serious medical condition. An additional exclusion criteria was evidence of a below average IQ. Participants were excluded if their IQ, as assessed by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), was lower than 80. Although the average IQ scores for both groups fell in the normal range, as shown on Table 1, the HC group had a significantly higher IQ than the MDD group (with the average IQs of the unmedicated MDD group appearing the lowest). In the eight cases where IQ data was missing that were retained in this sample, all participants were achieving at an average or above rate at school and had not been identified for special services, suggesting that their IQ was likely to be at or above the average range of functioning.
Table 1.
Demographic and clinical characteristics of adolescents with MDD and HC participants
| MDD All (n = 52) |
MDD Med (n = 16) |
MDD No Med (n = 36) |
HC (n = 27) |
|
|---|---|---|---|---|
| Demographic Characteristics | ||||
|
| ||||
| Age (years) mean ± SD | 15.69 ± 1.71 | 16.10 ± 1.13 | 15.50 ± 1.90 | 16.32 ± 2.10 |
| Gender (male/female) | 11/41 | 3/13 | 8/28 | 8/19 |
| IQ mean ± SD | 104.85 ± 14.99*
(n = 48) |
110.60 ± 13.72 (n = 15) |
102.24 ± 15.01*
(n = 33) |
111.38 ± 11.05 (n = 24) |
| Right handed, n (%) | 44 (91.67%; n = 48) | 15 (93.75%) | 29 (90.63%; n = 32) | 24 (92.31%; n = 26) |
|
| ||||
| Ethnicity, n (%) | ||||
|
| ||||
| Caucasian | 36 (69.23%) | 11 (68.75%) | 25 (69.44%) | 16 (59.26%) |
| African American | 6 (11.54%) | 1 (6.25%) | 5 (13.89%) | 1 (3.70%) |
| Hispanic | 5 (9.62%) | 2 (12.50%) | 3 (8.33%) | 2 (7.41%) |
| Asian | 1 (1.92%) | 0 | 1 (2.78%) | 2 (7.41%) |
| Native American | 1 (1.92%) | 1 (6.25%) | 0 | 0 |
| Other | 8 (15.38%) | 3 (18.75%) | 5 (13.89%) | 8 (29.63%) |
|
| ||||
| Medication Class, n (%) | ||||
|
| ||||
| Selective serotonin reuptake inhibitors | 13 (25%) | 13 (81.25%) | 0 | |
| Atypical antidepressants | 8 (15%) | 8 (50%) | 0 | |
| Mood stabilizers | 1 (2%) | 1 (6%) | 0 | |
| Atypical antipsychotics | 1 (2%) | 1 (6%) | 0 | |
| Stimulants | 7 (13%) | 5 (31%) | 2 (6%) | |
| Selective norepinephrine reuptake inhibitors |
1 (2%) | 1 (6%) | 0 | |
| Tricyclic antidepressants | 1 (2%) | 1 (6%) | 0 | |
|
| ||||
| Illness History Description, and Details | ||||
|
| ||||
| Duration of current illness (months) mean ± SD |
9.76 ± 11.56 (n = 51) | 10.38 ± 11.46 | 9.49 ± 11.76 (n = 35) | NA |
| Global Assessment of Functioning mean ± SD |
54.35 ± 8.35 | 53.94 ± 8.31 | 54.53 ± 8.47 | NA |
| CDRS T scores mean ± SD | 77.29 ± 6.13(n = 46) | 78.16 ± 7.40 (n = 14) | 76.91 ± 5.57 | NA |
| BDI average mean ± SD | 25.54 ± 12.28*** | 24.50 ± 11.03*** | 26.00 ± 12.92*** | 2.33 ± 12.28 |
| Current comorbidity n (%) | 35 (67.31%) | 11 (68.75%) | 24 (66.67%) | NA |
| Comorbid ADHD n (%) | 7 (13%) | 3 (19%) | 4 (11%) | |
| Comorbid anxiety disorder n (%) | 35 (67%) | 10 (63%) | 25 (69%) | |
| Time of TSST | 14:54 (n = 49) | 15:15 | 14:43 (n = 33) | 15:12 (n = 26) |
| Time of MRI | 14:52 (n = 50) | 16:36 | 14:02 (n = 34) | 15:25 (n = 23) |
| Experience with MRI n (%) | 12 (23.08%) | 4 (25%) | 8 (22.22%) | 10 (37.04%) |
| Intracranial volume cm3 (mean ± SD) | 1528 ± 148 | 1540 ± 169 | 1523 ± 140 | 1581 ± 201 |
Note: MDD, Major depressive disorder; HC, healthy control; Med, medicated; No Med, not medicated; CDRS, Children’s Depression Rating Scale; BDI, Beck Depression Inventory; ADHD, attention-deficit/hyperactivity disorder; TSST, Trier Social Stress Test; MRI, magnetic resonance imaging.
p ≤ .05 when compared to healthy controls.
**p ≤ .01 when compared to healthy controls.
p ≤ .001 when compared to healthy controls.
Assessment of brain structure and function. Participants completed a MRI scan using a Siemens 3 Tesla TIM Trio scanner (Erlangen, Germany) that is housed at the Center for Magnetic Resonance Research at the University of Minnesota. A 5-min scan was administered to acquired to obtain a structural image using a T1-weighted high-resolution magnetization prepared gradient echo sequence (repetition time = 2530 ms, echo time = 3.65 ms, inversion time = 1100 ms, flip angle = 78, 224 coronal slices, field of view = 256 mm, voxel size 1 × 1 × 1 mm, matrix size = 256 × 256, GRAPPA = 2). In addition to several other scans (e.g., resting), an fMRI scan was administered during which participants completed the subsequently described emotion face-matching task. The fMRI scan consisted of an echo planar imaging sequence, which was used to collect 197 T2-weighted whole-brain functional volumes in the context of the task (34 3.0 mm contiguous interleaved axial slices; aligned to anterior and posterior commissures with –308 tilt, repetition time = 2000 ms, echo time = 28 ms, flip angle = 808, field of view = 200 mm, voxel size 3.1×3.1×3.0 mm, matrix = 64 × 64).
Amygdala volume analyses. Volumetric data was processed using the FreeSurfer 5.3.0 software (http://surfer.nmr.mgh. harvard.edu/), including brain extraction and parcellation of data into a standard set of anatomically based regions of white and gray matter. FreeSurfer output was visually inspected; when any errors were identified, they were manually corrected, and the pipeline’s remaining steps were repeated. Although many regions of interest were identified using this software, for the purposes of this study, we focused on the volumetric data that was produced for the right and left amygdala.
Emotion face-matching task and data analysis. The emotion face-matching task (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002) used E-Prime software and was projected on a screen inside the bore of the MRI scanner that the participant could see using a mirror attached to the head coil. The task entailed both affective and control stimuli. For the affective stimuli, Ekman faces (Ekman & Friesen, 1976) were used to portray anger and fear, which included six images of each gender and emotion. The control stimuli consisted of circles, horizontal ellipses, and vertical ellipses. Participants were instructed to match the stimuli presented on the top row with one of the two stimuli presented in the bottom row using a button box. Specifically, participants were instructed to match the shape for the control stimuli to faces with emotional expression (fear or anger) for the affective stimuli. The task was presented in 13 24-s counterbalanced blocks (3 fixation, 5 shape, and 5 emotion). This task took approximately 6.5 min to complete in the scanner.
Analysis of fMRI data was conducted using software tools from the FMRIB software library (http://fsl.fmrib.ox.ac.uk) version 4.1.8. Preprocessing steps included motion correction, brain extraction, high-pass temporal filtering, prewhitening, regression of motion parameters, and registration to MNI standard space. A first-level analysis for each data set was conducted to regress the task model onto the fMRI data at each voxel of the brain. We included a covariate of no interest, which allowed us to regress out those volumes in which motion (relative to the preceding volume) exceeded our threshold of 1.5 mm in any direction (half the size of one voxel). We considered two explanatory variables from the block-design task (matching emotion faces and matching neutral shapes) and two contrasts (matching emotion faces minus fixation and matching emotion faces minus matching shapes). Left and right amygdala masks from the Harvard Oxford Subcortical Structural Atlas were then used to extract the average z score for all voxels within these regions from the emotion minus fixation and the emotion minus shape contrasts of the regression results for each participant. These average z score values were then used for group comparison and correlation analyses. The primary index of focus here was on amygdala (right and left) activation for the emotion face minus fixation contrast. Follow-up analyses were also conducted with the index that assesses amygdala functioning for emotion minus shapes.
HPA axis assessments. Participants completed a slightly modified version of the TSST, a task that has been found to reliably elicit a stress response (Kirschbaum, Pirke, & Hellhammer, 1993). Participants were asked to spend 5 min preparing a speech to introduce themselves to a job committee and were informed that another task would follow the speech task. After the preparation period, participants were escorted to another room in front of two unfamiliar evaluators wearing white lab coats, who were trained to remain neutral and to avoid giving reassurance or feedback. Participants were first asked to deliver their speech (5 min) and then asked to do a serial subtraction task with corrective feedback provided by experimenters (5 min). Participants were debriefed immediately following the completion of the TSST.
A total of five salivary samples were collected throughout this visit: the first before speech preparation (0 min), the second immediately following the TSST (15 min), and the final three at approximately 30, 45, and 60 min. Instead of lengthening the visit, researchers eased subject burden by having them complete tasks that don’t typically activate stress responding (e.g., Gunnar, Talge, & Herrera, 2009), including rating scales and computerized neuropsychological tests, during the time the two final samples were collected.
Experiences and expressions of stress were recorded within the context of the TSST. After completion of the TSST, participants were asked to rate a series of questions on a scale of 1 (calm) to 5 (high stress), including, “How stressful was giving the speech ( job interview)?” and “How stressful was the subtraction task?” A mean score across these two items was used as the summary score for experienced stress. The two examiners for the TSST independently rated participant’s behavior on a scale of 1 (not stressed at all) to 6 (discontinued the procedure because the participant was so stressed) for the following items, “How stressed did the participant appear during the story telling task ( job interview)?” and “How stressed did the participant appear during the arithmetic task?” These scores were averaged for the participant. The two examiner ratings were positively correlated r (74) = .41, p = .01. A mean score across examiner ratings was used as the summary score for expressed stress.
Cortisol was also assessed within the context of the MRI scan: there is accumulating evidence that the MRI scan procedure may be considered a moderate stressor, likely due to the movement restrictions, loud noises, and novel task features required, particularly for scan-naive participants (Eatough, Shirtcliff, Hansen, & Pollak, 2009; Peters, Cleare, Papadopoulos, & Fu, 2011; Tessner, Walker, Hochman, & Hamann, 2006). Researchers collected a saliva sample upon arrival to the MRI facility and again immediately after completing the 75-min scan.
For each saliva sample, participants facilitated salivary excretion by chewing Trident Original gum for 20–30 s before spitting out the saliva and gum. Participants then pushed their saliva through a straw and into a 1.5 ml vial. Samples were labeled and stored in a –25 8C freezer until they were shipped to Universität Trier in Trier, Germany, for analysis. Researchers used assay methods consistent with Dressendörfer, Kirschbaum, Rohde, Stahl, and Strasburger (1992). Summary indices of cortisol values: across the TSST, cortisol was represented by the area under the curve ground (AUCg) and the AUC from the initial pretest sample (AUCi; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Difference scores (MRI CORT) were the primary summary index for cortisol levels linked to the brain scan.
Statistical analysis
Preliminary analyses were conducted using Kruskal–Wallis (continuous characteristics) and Fisher exact tests (categorical characteristics) to determine whether there were any demographic or other characteristic differences across the MDD and HC groups (two group analysis). We also considered how the behavioral and biological indices were correlated. To address the first study aim, general linear models were used to assess whether MDD and HC differed on amygdala structure, amygdala functioning, HPA axis functioning on the TSST, and HPA axis functioning during the MRI. To address the second study aim, a series of general linear models were conducted with each of AUCi, AUCg, and MRI CORT as separate dependent variables, to consider if there were associations with the amygdala (structure or function) either overall or differentially by risk status of the adolescent (a total of 12 analyses with three HPA outcomes and four possible predictors: right and left amygdala volume and right and left amygdala functioning). Differential associations with HPA axis functioning, modeled as the interaction between amygdala (structure or function) and risk status, would represent differences between MDD and HC participants in the interplay between the components of the biological stress system.
For the previously described analyses, we carefully considered appropriate statistical controls. With few exceptions, demographic characteristics were comparable across the groups; however, IQ was significantly different across groups. Although IQ was initially considered as an adjusting variable in all models, it was not retained (any changes in the results were most likely to be due to changes in degrees of freedom in the models and are noted subsequently). Because MDD and HC showed robust group differences on experiences and expressions of stress during the TSST (described below), we included this summary index as an adjusting variable in all models of HPA axis functioning on the TSST (e.g., AUCg). In addition, for all analyses that considered amygdala volume as either dependent or independent variable, intracranial volume was included as an adjusting variable. For all analyses that considered MRI CORT as either dependent or independent variable, time of day of the scan’s onset, cortisol levels pre-MRI, and previous experience with a brain scan (yes/no) were included as adjusting variables.
Finally, as exploratory analyses, this same modeling process was repeated using three groups: unmedicated MDD, medicated MDD, and HC participants. Results were reported below when the three group comparisons were significant ( p < .05).
Results
Participant characteristics by group are shown in Table 1. Preliminary analyses considered the correlations among behavioral, neuronal, and HPA axis variables of relevance to threat processing (Table 2). Right and left amygdala volumes were positively correlated, as were right and left amygdala response to the emotion face-matching task. There was no evidence that the structure and the function of the amygdala were correlated with each other in this sample. With regard to HPA axis functioning, cortisol levels were correlated between the TSST and the MRI CORT. In addition, right amygdala functioning within the context of the emotion face-matching task was positively correlated with AUCg cortisol functioning during the TSST.
Table 2.
Correlations for primary study variables
| Test | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|
| 1. TSST ratings | −.22 | −.15 | .20 | .18 | .16 | .01 | −.20 |
| 2. Right amygdala volume | .80** | −.00 | .05 | .00 | .12 | −.11 | |
| 3. Left amygdala volume | −.06 | −.04 | −.06 | −.04 | .02 | ||
| 4. Right amygdala functioning | .81** | .30* | .05 | −.05 | |||
| 5. Left amygdala functioning | .23 | −.04 | −.04 | ||||
| 6. TSST AUCg | .27* | −.16 | |||||
| 7. TSST AUCi | −.49** | ||||||
| 8. MRI CORT difference |
Note: TSST, Trier Social Stress Test; TSST ratings, z score means of self-reports and experimenter observations; AUCg, area under the curve ground levels of salivary cortisol; AUCi, area under the curve initial levels of salivary cortisol; MRI CORT difference, pre magnetic resonance imaging – post magnetic resonance imaging levels of salivary cortisol.
p = .05 (two tailed).
p = .01 (two tailed).
Our first study aim was to examine whether MDD and HC adolescents differed on indexes of behavioral and neurobiological functioning (descriptive information is provided on Table 3). Consideration of experiences and expression of stress within the context of the TSST was assessed across participant groups. The summary score of self-reported experiences of stress and experimenter ratings of expressed stress during the TSST were significantly higher for the MDD group than the HC group, F (72) = 20.47, p < .001). In addition, there was a significant difference across the three groups, F (72) = 20.47, p < .001). Post hoc comparisons showed that medicated MDD participants were significantly higher on behavioral stress than were HC ( p < .001) and that the unmedicated MDD participants were significantly higher on behavioral stress than were HC ( p = .004).
Table 3.
Descriptive information about adolescents with MDD and HC participants stress functioning
| Characteristics | Total Sample M (SD) |
MDD All M (SD) |
MDD Med M (SD) |
MDD No Med M (SD) |
HC M(SD) |
|---|---|---|---|---|---|
| Behavioral index of stress (N = 74) | |||||
| TSST ratings (z score) | 0.00 ± 0.85 | 0.29 ± 0.77 | 0.27 ± 0.90 | 0.30 ± 0.72 | −0.54 ± 0.72 |
| Amygdala structure (N = 79) | |||||
| Right amygdala volume | 1693.65 ± 221.12 | 1675.05 ± 203.03 | 1717.78 ± 169.97 | 1656.06 ± 215.60 | 1729.49 ± 252.57 |
| Left amygdala volume | 1583.84 ± 191.76 | 1583.05 ± 177.32 | 1604.10 ± 169.37 | 1573.69 ± 182.28 | 1584.38 ± 220.54 |
| Amygdala function, emotion matching task (N = 66) |
|||||
| Right amygdala | 0.77 ± 0.84 | 0.93 ± 0.81 | 0.84 ± 0.82 | 0.98 ± 0.82 | 0.43 ± 0.80 |
| Left amygdala | 1.52 ± 0.56 | 1.62 ± 0.60 | 1.60 ± 0.55 | 1.62 ± 0.62 | 1.31 ± 0.49 |
| HPA axis functioning | |||||
| TSST AUCg (N = 74) | 19.39 ± 13.89 | 19.97 ± 14.74 | 17.75 ± 10.42 | 21.08 ± 16.53 | 18.32 ± 12.36 |
| TSST AUCi (N = 74) | 1.28 ± 11.51 | 0.08 ± 12.09 | −1.64 ± 14.06 | 0.94 ± 11.12 | 3.49 ± 10.21 |
| MRI CORT difference (N = 69) | −0.02 ± 0.20 | 0.00 ± 0.20 | 0.03 ± 0.18 | −0.01 ± 0.21 | −0.07 ± 0.19 |
Note: MDD, Major depressive disorder; HC, healthy control; Med, medicated; No Med, not medicated; HPA, hypothalamic–pituitary–adrenal axis; TSST, Trier Social Stress Test; AUCg, area under the curve ground levels of salivary cortisol; AUCi, area under the curve initial levels of salivary cortisol; MRI, magnetic resonance imaging; MRI CORT difference, pre-MRI – post-MRI levels of salivary cortisol.
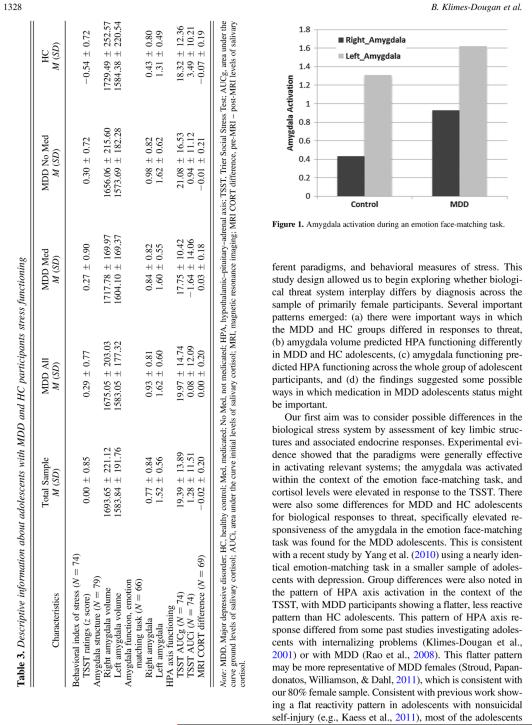
There were no significant MDD and HC differences for mean volumes for each of left or right amygdalae, nor were there any significant three-group differences. For example, the MDD and HC group results for amygdala volume were F (1, 77) = 0.00, p = .96 for the left amygdala and F (1, 77) = 1.08, p = .30 for the right amygdala. As predicted, mean right amygdala activation, F (1, 64) = 5.48, p = .02, and left amygdala activation, F (1, 64) = 4.10, p = .05, was significantly different between MDD and HC (Figure 1). This significant difference for right amygdala functioning was still evident when IQ was entered as a control variable. There were no significant three-group differences for right or left amygdala functioning.
Figure 1.
Amygdala activation during an emotion face-matching task.
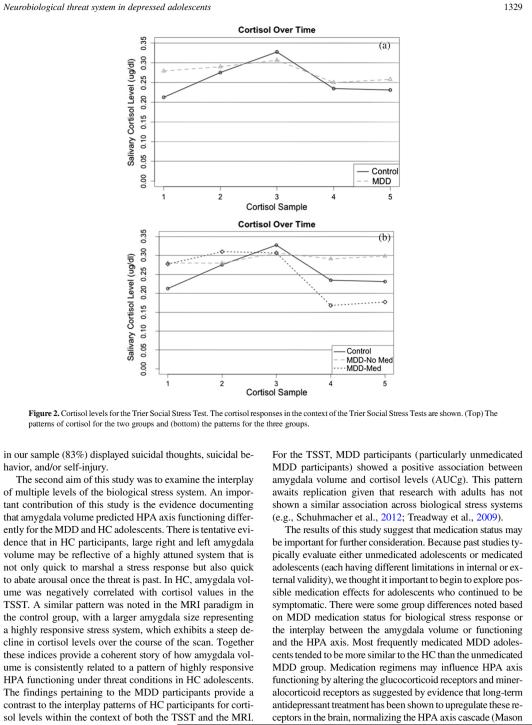
We evaluated unadjusted MDD and HC differences for various indices of HPA axis functioning. There were no significant MDD and HC differences for the TSST for AUCg, F (1, 72) = 0.24, p = .63, or AUCi, F (1, 72) = 1.49, p = .23. Results were largely the same after controlling for important confounders. However, as shown on Figure 2, there were differences in patterns of linear slope the shape of the cortisol response versus time for the patterns of responses between unmedicated MDD and HC, with a high and flat-peaked time trajectory for the HC group for which the cortisol measurements started low and ended low ( p value for negative second-order effect in the first four time measurements was .0093), a low and flatter cortisol versus time pattern for the unmedicated MDD group ( p = .05 for positive second-order effect, relative to HC), and a combination of those two patterns for the medicated MDD group. MRI CORT did not differ across groups, MDD versus HC: F (1, 67) = 1.90, p = .17. Nor did MRI CORT significantly differ across groups after controlling for time of MRI onset, cortisol at MRI onset, and prior scan experience. No significant three-group differences were found for AUCg, AUCi, or MRI CORT.
Figure 2.
Cortisol levels for the Trier Social Stress Test. The cortisol responses in the context of the Trier Social Stress Tests are shown. (Top) The patterns of cortisol for the two groups and (bottom) the patterns for the three groups.
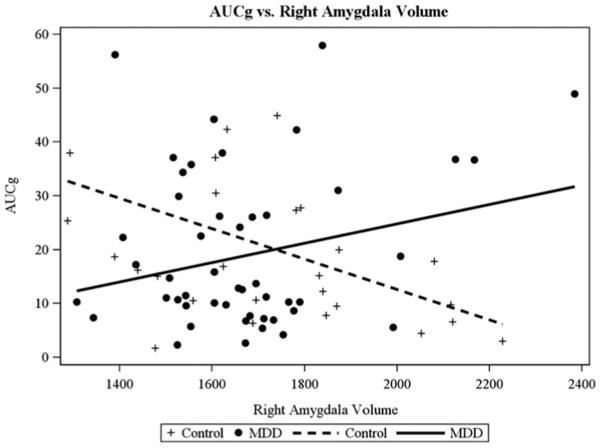
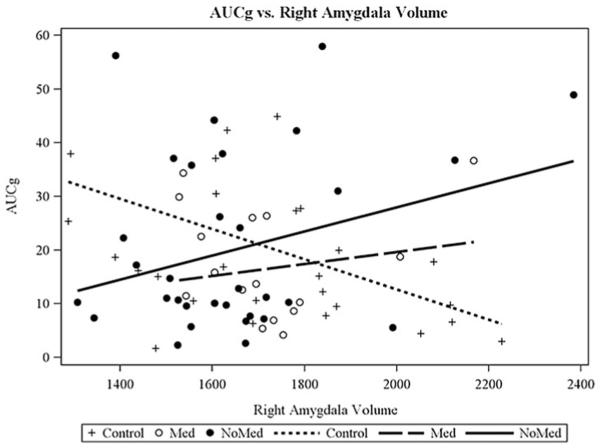
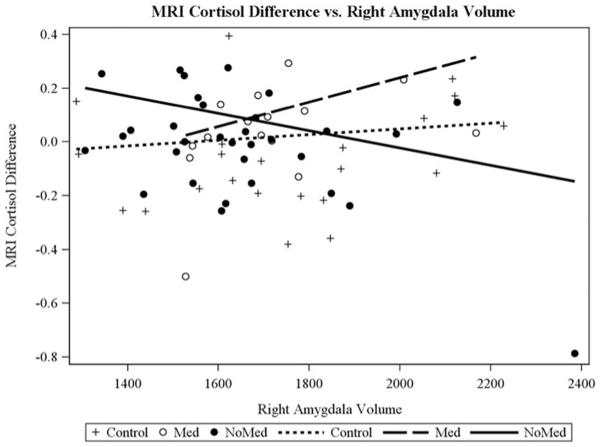
Our second study aim was to examine whether MDD and HC adolescents differed in the interplay between amygdala structure and function with HPA axis functioning, after controlling for the confounders identified in the Statistical Analysis section. There was a significant interaction between right amygdala volume and participant group when predicting TSST AUCg for two-group comparison, F (1, 67) = 9.55, p = .003 (Figure 3), and three-group comparison, F (2, 65) = 5.24, p = .008 (Figure 4). There was also a significant interaction between right amygdala volume and participant group when predicting MRI CORT for the three-group comparison, F (2, 60) = 4.51, p = .01 (Figure 5), but not for the two-group comparison, F (1, 62) = 1.56, p = .21. Group-specific slopes (or pooled-group slopes, as appropriate) of volume with cortisol response are shown in Table 4. There was a significant interaction between left amygdala volume and participant group when predicting TSST AUCg, two group: F (1, 67) = 5.33, p = .02 (this pattern was similar to that found for right amygdala); three group: F (2, 65) = 2.81, p = .06 (this pattern was similar to that found for the right amygdala), but not when predicting MRI CORT AUCg, two group: F (1, 62) = 0.92, p = .34; three group: F (2, 60) = 2.69, p = .08. There were no significant findings for AUCi for any of these analyses.
Figure 3.
Different patterns of correspondence between salivary cortisol levels during the Trier Social Stress Test (area under the curve with respect to ground) and the right amygdala volume were found for the depressed (major depressive disorder) and healthy control adolescents.
Figure 4.
Different patterns of correspondence between salivary cortisol levels during the Trier Social Stress Test (area under the curve with respect to ground) and the right amygdala volume were found for the unmedicated depressed, the medicated depressed, and the healthy control adolescents.
Figure 5.
Different patterns of correspondence between salivary cortisol levels during the MRI and the right amygdala volume were found for the unmedicated depressed, the medicated depressed, and the healthy control adolescents.
Table 4.
Interplay regressions adjusted for reported/ observed stress during the Trier Social Stress Test and intracranial volume
| Slope | AUCg SE (Slope) |
pa | |
|---|---|---|---|
|
| |||
| Left | |||
| Volume effect in | |||
| No med MDD (vs. HC vol. effect) | 0.04 | 0.02 | .02 |
| Med MDD (vs HC vol. effect) | 0.03 | 0.02 | .19 |
| All MDD (vs. HC vol. effect) | 0.04 | 0.02 | .02 |
| HC | −0.03 | 0.02 | .03 |
| Activation effect (all groups) | 5.96 | 3.42 | .09 |
|
| |||
| Right | |||
|
| |||
| Volume effect in | |||
| No med MDD (vs. HC vol. effect) | 0.05 | 0.02 | .0021 |
| Med MDD (vs. HC vol. effect) | 0.04 | 0.02 | .11 |
| All MDD (vs. HC vol. effect) | 0.05 | 0.01 | .003 |
| HC | −0.03 | 0.01 | .03 |
| Activation effect (all groups) | 5.19 | 2.32 | .03 |
Note: AUCg, Area under the curve ground levels of salivary cortisol; No med MDD, unmedicated adolescents major depressive disorder; HC, healthy controls; Med MDD, medicated adolescents MDD. Subjects diagnosed with MDD with med MDD and no med MDD did not experience a significant left amygdala volume or right amygdala volume effects, unless it was relative to HC.
The value for the difference from HC or slope = 0.
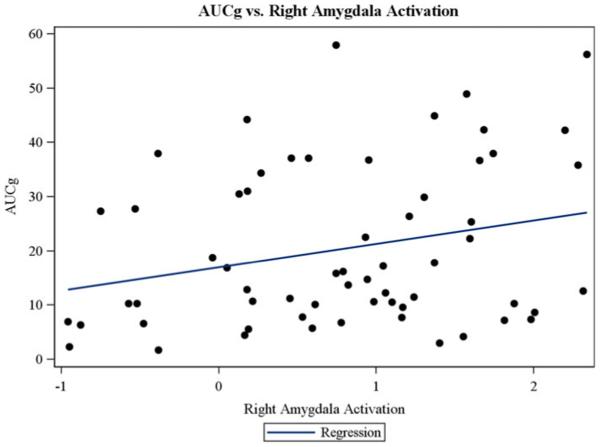
Next we examined whether MDD and HC adolescents differed in the interplay between amygdala function and HPA axis functioning after controlling for the confounders listed in Statistical Analysis. There was a significant association of right ( p = .03; Figure 6), but not left ( p = .09) amygdala functioning with AUCg cortisol response to the TSST for all groups combined. Pooled-group slopes of activation with cortisol response are shown in Table 4 and depicted on Figure 6. However, there were no significant interactions between (either right or left) amygdala functioning and participant group when predicting AUCg, AUCi, and MRI CORT, whether examining the two-or three-group comparisons.
Figure 6.
(Color online) Correspondence between salivary cortisol levels during the Trier Social Stress Test (area under the curve with respect to ground) and the right amygdala functioning were found for the whole sample.
Discussion
To our knowledge, this is the first study to consider the interplay of the neural and hormonal systems of key relevance to stress regulation in depressed and well adolescents. Multilevel assessment included structural and functional measurement of the amygdala, hormonal response to stress in two different paradigms, and behavioral measures of stress. This study design allowed us to begin exploring whether biological threat system interplay differs by diagnosis across the sample of primarily female participants. Several important patterns emerged: (a) there were important ways in which the MDD and HC groups differed in responses to threat, (b) amygdala volume predicted HPA functioning differently in MDD and HC adolescents, (c) amygdala functioning predicted HPA functioning across the whole group of adolescent participants, and (d) the findings suggested some possible ways in which medication in MDD adolescents status might be important.
Our first aim was to consider possible differences in the biological stress system by assessment of key limbic structures and associated endocrine responses. Experimental evidence showed that the paradigms were generally effective in activating relevant systems; the amygdala was activated within the context of the emotion face-matching task, and cortisol levels were elevated in response to the TSST. There were also some differences for MDD and HC adolescents for biological responses to threat, specifically elevated responsiveness of the amygdala in the emotion face-matching task was found for the MDD adolescents. This is consistent with a recent study by Yang et al. (2010) using a nearly identical emotion-matching task in a smaller sample of adolescents with depression. Group differences were also noted in the pattern of HPA axis activation in the context of the TSST, with MDD participants showing a flatter, less reactive pattern than HC adolescents. This pattern of HPA axis response differed from some past studies investigating adolescents with internalizing problems (Klimes-Dougan et al., 2001) or with MDD (Rao et al., 2008). This flatter pattern may be more representative of MDD females (Stroud, Papandonatos, Williamson, & Dahl, 2011), which is consistent with our 80% female sample. Consistent with previous work showing a flat reactivity pattern in adolescents with nonsuicidal self-injury (e.g., Kaess et al., 2011), most of the adolescents in our sample (83%) displayed suicidal thoughts, suicidal behavior, and/or self-injury.
The second aim of this study was to examine the interplay of multiple levels of the biological stress system. An important contribution of this study is the evidence documenting that amygdala volume predicted HPA axis functioning differently for the MDD and HC adolescents. There is tentative evidence that in HC participants, large right and left amygdala volume may be reflective of a highly attuned system that is not only quick to marshal a stress response but also quick to abate arousal once the threat is past. In HC, amygdala volume was negatively correlated with cortisol values in the TSST. A similar pattern was noted in the MRI paradigm in the control group, with a larger amygdala size representing a highly responsive stress system, which exhibits a steep decline in cortisol levels over the course of the scan. Together these indices provide a coherent story of how amygdala volume is consistently related to a pattern of highly responsive HPA functioning under threat conditions in HC adolescents. The findings pertaining to the MDD participants provide a contrast to the interplay patterns of HC participants for cortisol levels within the context of both the TSST and the MRI. For the TSST, MDD participants (particularly unmedicated MDD participants) showed a positive association between amygdala volume and cortisol levels (AUCg). This pattern awaits replication given that research with adults has not shown a similar association across biological stress systems (e.g., Schuhmacher et al., 2012; Treadway et al., 2009).
The results of this study suggest that medication status may be important for further consideration. Because past studies typically evaluate either unmedicated adolescents or medicated adolescents (each having different limitations in internal or external validity), we thought it important to begin to explore possible medication effects for adolescents who continued to be symptomatic. There were some group differences noted based on MDD medication status for biological stress response or the interplay between the amygdala volume or functioning and the HPA axis. Most frequently medicated MDD adolescents tended to be more similar to the HC than the unmedicated MDD group. Medication regimens may influence HPA axis functioning by altering the glucocorticoid receptors and miner-alocorticoid receptors as suggested by evidence that long-term antidepressant treatment has been shown to upregulate these receptors in the brain, normalizing the HPA axis cascade (Mason & Pariante, 2006). However, a recent meta-analysis of the treatment response literature with adults has brought into question the evidence that HPA axis functioning is altered with pharmacotherapy (McKay & Zakzanis, 2010). This remains a question for further inquiry in adolescent samples undergoing randomized control medication trials.
There was no evidence of group differences for the associations between amygdala activation and cortisol responses during threat. Across groups, high amygdala activation during matching of negative emotion faces was predictive of higher overall HPA axis functioning. A similar pattern was found (data not shown), even considering a more pure index of amygdala activation that was based on amygdala activation minus activation of the shape-matching task. Given that high activation of the amygdala and a flat cortisol pattern in response to stress was more characteristic of MDD adolescents, regardless of overall patterns it is possible that the positive association between amygdala and HPA axis activation may have different implications for the MDD and HC groups. It was somewhat surprising that, in this study, amygdala activation was related to cortisol levels that were measured on a different day (the TSST) but not to the more time-linked cortisol levels that were collected before and after the scan (MRI CORT). It is likely that engaging in the emotion face-matching task while undergoing a scan (even for those who had not been previously scanned) was not sufficiently potent to activate the HPA axis. By contrast, others have found that amygdala activation in healthy adults viewing traumatic imagery (pictures of the World Trade Center attack) was positively associated with cortisol reactivity that was collected prior to and following the brain scan (Cunningham-Bussel et al., 2009).
The approach taken in this study represents an important first step in trying to address the extraordinarily complex task of considering multiple and interacting levels of biological stress systems. We measured threat detection systems in the brain and the body. One strength of this study was that it considered both brain volume and function of the amygdala, a pivotal structure to threat detection. While brain structure is thought to underlie brain functioning (Hebb, 1949), rarely are strong associations between structure and function noted. Future multilevel research is also needed to expand the current work to investigate other key regions implicated in threat processing that have high concentrations of glucocorticoid receptors such as the hippocampus, anterior cingulate cortex, and prefrontal cortex (PFC). Future multilevel work should also expand upon the current finding to include assessment of a broader array of regulatory regions implicated in threat response. Models of emotion processing suggest reciprocal ventral and dorsal systems (Phillips et al., 2003). Some have suggested a reciprocal function of the PFC and limbic/hormonal response, although others have suggested increased recruitment of these regions in response to threat. For example, one study found an inverse correlation between rostral anterior cingulate cortex volume and average diurnal basal cortisol levels in depressed adults (Treadway et al., 2009). Another study found that individuals with the greatest increases in glucose metabolism in the medial PFC (BA 9 and BA 10) in response to stress were likely to have the lowest cortisol AUCg scores for the TSST (Kern et al., 2008). Wang et al. (2005) assessed stress responses using a modified TSST within the scan and salivary cortisol prior to and following the scan. They noted a positive association between right PFC and cortisol levels in healthy adults. Similarly, Jahn et al. (2010) reported a positive association between brain metabolism in a number of ventral medial and limbic regions, including the subgenual PFC and pregenual PFC (BA 25/24), and cortisol under threat conditions in adolescent rhesus monkeys. These latter studies go against the reciprocal theory and suggest that as limbic systems respond, regulatory regions also respond to threat. This area of research is yet in its infancy and the conflicting finding highlight that these complex issues of interplay between systems require further investigation.
Another strength of this study was that two assessments of potential threat were assessed. Even though assessments were conducted on different days, in different settings, and with different experiments, the results indicated moderate correlations between the cortisol levels within the context of the MRI and the TSST paradigm. However, it was somewhat surprising to find that HPA axis activation in the MRI scanner was not related to amygdala functioning. Instead, cortisol levels during the TSST were positively correlated with amygdala functioning within the context of the emotion face-viewing task. In an ideal situation, proceeding with methodologies that allow researchers to temporally map stress responses of the neuroendocrine system onto neuronal activation would be desirable (see Dedovic et al., 2010). However, the same stressor may be sufficiently potent to activate key limbic regions, but may need to be more intense and protracted to activate the peripheral stress response. In addition, a better understanding of HPA functioning under basal and stress conditions is warranted, for the results may differ considerably across these estimates of HPA axis functioning (e.g., Cunningham-Bussel et al., 2009; Root et al., 2009). Additional refinements may include (a) assessing more saliva samples over a longer period of time to better evaluate recovery of the HPA axis, (b) using psychological stressors that elicit a stronger stress response within ethical constraints, (c) including a broader array of hormonal assessment (e.g., ACTH, DHEA, and DHEA-sulfate) given the cross-regulation of a broader hormonal network in adolescence (e.g., Marceau et al., 2014), and (d) including fewer task demands (e.g., limiting tasks demands before and after the TSST).
In the future it will be important to assess experiences and expressions of stress more comprehensively, particularly in the context of the MRI. Correspondence between experiences, expressions, or physiological responses to strong emotions/stress is often limited (e.g., Bauer, Quas, & Boyce, 2002; Gross, 1998). In this study, the findings show moderate correspondence between experiences and expression of emotion, r (74) = .44, p < .0001. Experience and expression of stress in the TSST was entered as a summary control variable in the primary study analyses because the results of this study are consistent with past research (Holsen et al., 2013) showing that MDD adolescents experienced and expressed higher levels of stress than did HC adolescents. However, there was minimal evidence that experienced or expressed stress was related to neuronal or hormonal indexes. One exception was based on the question “Did the participant appear to be relieved to have completed the TSST?” (a high score represents “extremely relieved”). Experimenter ratings were significantly correlated with right, r (62) = .34, p = .008, and left, r (62) = .28, p = .02, amygdala activation and may reflect an important aspect threat recovery. It will be fruitful to continue to examine further how observed emotions maps onto brain activity.
In conclusion, the results of this study suggest differential links between amygdala structure and volume and neuroendocrine responses to stress in adolescents with MDD in comparison to HC. Further investigation is warranted to disentangle the processes more pertinent to normative maturation (e.g., functional connectivity across affective and cognitive control regions), the timing of onset and intensity of depression, and change over time with treatment. This is especially important in regard to how these clinical characteristics may be reflected in the adolescent’s developing brain and extended physiological networks. This line of work would presumably serve to bolster current conceptualizations of the pathophysiology of depression early in development and may have implications for intervention efforts that take advantage of the plasticity that is still evident in neurobiological systems important during adolescence. Preventive intervention efforts may capitalize on this line of research, for it would be important to know in what ways altering the functioning of one system may influence stress functioning more broadly. Schuhmacher et al. (2012) recently showed that larger amygdala volume prior to treatment was associated with a “normalization” of the hormonal stress response as measured by the dexamethasone /corticotrophin releasing hormone tests. Our team has identified that anomalous HPA axis functioning predicts treatment responses in young children with internalizing problems (Klimes-Dougan, Klingbeil, & August, 2009) and adolescents with depression (Gunlicks-Stoessel, Mufson, Cullen, & Klimes-Dougan, 2013). Considering these broader systems and their interplay is likely to provide a richer description of neurobiological functioning that may further enhance prediction of treatment response. Application of these results may also consider how intervention alters biological threat systems’ functioning (Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Longitudinal research by Frodl et al. (2008) has found brain morphology changes in gray matter density in brain regions implicated in the threat system for depressed adults, and patients that remitted during the follow-up assessment showed less volume decline than those who failed to remit. There are certainly exciting possibilities for the field, and translation of this neurobiological research will be critical to advance current clinical practice and aid in the development of more effective neurobiologically informed interventions.
Acknowledgments
The authors first and foremost thank the adolescents who contributed to this study. We also deeply appreciate the staff, the trainees, and the many volunteers who contributed to this project. The study was funded by a Deborah E. Powell Center for Women’s Health Seed Grant (to B.K.-D.) as well as by the National Institute of Mental Health (K23MH090421), the National Alliance for Research on Schizophrenia and Depression, the University of Minnesota Graduate School, and the Minnesota Medical Foundation (to K.R.C.).
References
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Research. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Doraiswamy PM, Boyko OB, Rodrigo Escalona P, McDonald WM, Ritchie JC, et al. In vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: Relationship to dexamethasone suppression test results in patients. Psychiatry Research. 1992;44:63–70. doi: 10.1016/0165-1781(92)90070-j. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, et al. Hypercortisolemia and hippocampal changes in depression. Psychiatry Research. 1993;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown KB. Beck Depression Inventory II. Harcourt Brace; San Antonio, TX: 1996. [Google Scholar]
- Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, et al. Lost human capital from early-onset chronic depression. American Journal of Psychiatry. 2000;157:940–947. doi: 10.1176/appi.ajp.157.6.940. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala–prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla M, Kronenberg G, Deuschle M, Meichel K, Hagen T, Bohrer M, et al. Hippocampal volume reduction and HPA-system activity in major depression. Journal of Psychiatric Research. 2007;42:587–595. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Compas BE, Wagner BM. Psychosocial stress during adolescence: Intrapersonal and interpersonal processes. In: Gore S, Colton ME, editors. Adolescence, stress and coping. Aldine de Gruyter; New York: 1991. [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L. Mueller, B. A., et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, et al. Altered white matter microstructure in adolescents with major depression: A preliminary study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:173–183. doi: 10.1097/00004583-201002000-00011. doi:10.1016/j.jaac.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, et al. Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoeuroendocrinology. 2009;34:694–704. doi: 10.1016/j.psyneuen.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, et al. Cortisol awakening response and hippocampal volume: Vulnerability for major depressive disorder? Biological Psychiatry. 2010;68:847–853. doi: 10.1016/j.biopsych.2010.07.025. doi:10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones, brain and stress. Endocrine Regulations. 2003;37:51–68. [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic–pituitary–adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. doi:10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical–amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. doi:10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME, et al. Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharmacology Biochemistry and Behavior. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children’s behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. doi:http://dx.doi.org/10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, et al. Depression-related variation in brain morphology over 3 years: Effects of stress? Archives of General Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. doi:10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Integration of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. doi:10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gunlicks-Stoessel M, Mufson L, Cullen KR, Klimes-Dougan B. Depressed adolescents’ cortisol reactivity during parent–adolescent conflict and response to interpersonal psychotherapy (IPT-A) Journal of Affective Disorders. 2013;150:1125–1128. doi: 10.1016/j.jad.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. doi.org/10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. Wiley; New York: 1949. [Google Scholar]
- Henckens MJAG, van Wingen AG, Joels M, Fernadez G. Cortiscosteriod induced decouping of the amygdala in men. Cerbral Cortex. 2012;22:2336–2345. doi: 10.1093/cercor/bhr313. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Progress in Brain Research. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. doi:10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen L. M, Lancaster, K., Klibanski A, Whitfield-Babrieli S, Cherkerzian S, Ubka S, et al. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:733–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Charney DS. Research on major depression. Journal of the American Medical Association. 2003;289:3167–3168. doi: 10.1001/jama.289.23.3167. doi:10.1001/jama.289.23.3167. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, et al. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic–pituitary–adrenal activity across different contexts. Biological Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess M, Hille M, Parzer P, Maser-Gluth C, Resch F, Brunner R. Alterations in the neuroendocrinological stress response to acute psychosocial stress in adolescents engaging in nonsuicidal self-injury. Psychoneuroendocrinology. 2011;37:157–161. doi: 10.1016/j.psyneuen.2011.05.009. doi:10.1016/j.psyneuen. 2011.05.009. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. doi:10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. doi:10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangus KR. Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The “Trier Social Stress Test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropshcobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual difference in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Klingbeil DK, August G. HPA axis functioning of children enrolled in Early Risers Prevention Program; Paper presented at the Society for Research in Child Development; Denver, CO. Apr, 2009. [Google Scholar]
- Kronenberg G, Tebartz van Elst L, Regaen F, Deuschle M, Heuser I, Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. Journal of Psychiatric Research. 2009;43:1112–1117. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. doi:10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde D. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Liu J, Chaplin TM, Wang F, Sinha R, Mayes LC, Blumberg HP. Stress reactivity and corticolimbic response to emotional faces in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:304–312. doi: 10.1016/j.jaac.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT. Acute effects of hydrocortisone on the human brain: An fMRI study. Psychoneuroendocrinology. 2010;35:15–20. doi: 10.1016/j.psyneuen.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Collins PF. Incentive motivation, cognitive control, and the adolescent brain: Is it time for a paradigm shift? Child Development Perspectives. 2012;6:392–399. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C, Dorn LD, et al. Within-adolescent coupled changes in cortisol with DHEA and testosterone in response to three stressors during adolescence. Psychoneuroendocrinology. 2014;41:33–45. doi: 10.1016/j.psyneuen.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BL, Pariante CM. The effects of antidepressants on the hypothalamic–pituitary–adrenal axis. Drug News Perspect. 2006;19:603. doi: 10.1358/dnp.2006.19.10.1068007. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. Journal of Psychiatry and Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stressful experience, brain, and emotions. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT Press; Cambridge, MA: 1995. pp. 1117–1136. [Google Scholar]
- McKay MS, Zakzanis KK. The impact of treatment on HPA axis activity in unipolar major depression. Journal of Psychiatric Research. 2010;44:183–192. doi: 10.1016/j.jpsychires.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Musselman D, Nemeroff CB. The role of cortisotropin-releasing factor in the pathophysiology of psychiatric disorders. Psychiatry Annals. 1993;23:676–681. [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. doi:10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Kim RB, Makoff A, Kerwin RW. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. British Journal of Pharmacology. 2003;139:1111–1118. doi: 10.1038/sj.bjp.0705357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Cleare AJ, Papadopoulos A, Fu CH. Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoeuroendocrinology. 2011;36:737–741. doi: 10.1016/j.psyneuen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception: II. Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. The Children’s Depression Rating Scale—Revised (CDRS-R) Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, et al. Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations. Psychoeuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoeuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. doi:10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen H, Ortiz LR, Chen L, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. doi:10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J, de Kloet E. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Root JC, Tuescher O, Cunningham-Bussel A, Pan H, Epstein J, Silbersweig D, et al. Frontolimbic function and cortisol reactivity in response to emotional stimuli. NeuroReport. 2009;20:429–434. doi: 10.1097/WNR.0b013e328326a031. doi:10.1097/WNR.0b013e328326a031. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Schuhmacher A, Mössner R, Jessen F, Scheef L, Block W, Belloche AC, et al. Association of amygdala volumes with cortisol secretion in unipolar depressed patients. Psychiatry Research. 2012;202:96–103. doi: 10.1016/j.pscychresns.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh pediatric neurobehavioral studies. Psychoneuroendocrinology. 2011;36:1226–1238. doi: 10.1016/j.psyneuen.2011.02.017. doi:10.1016/j.psy neuen.2011.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic–pituitary–adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. doi:10.1016/S0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Human Brain Mapping. 2006;27:889–895. doi: 10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Hamilton JP, Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. Journal of Child Psychology and Psychiatry. 2011;52:1026–1034. doi: 10.1111/j.1469-7610.2011.02422.x. doi:10.1111/j.1469-7610.2011.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLOS One. 2009;4:e4887. doi: 10.1371/journal.pone.0004887. doi:10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Frontiers in Neuroendocrinology. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiological Learning and Memory. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biological Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) American Psychological Association; San Antonio, TX: 1999. [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, et al. Depressed adolescents grown up. Journal of the American Medical Association. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- World Health Organization The Global Burden of Disease 2004 update. 2008 Retrieved June 16, 2012, from http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, et al. Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Brent DA, Weersing VR. Depressive disorders in childhood and adolescence: An overview: Epidemiology, clinical manifestation and risk factors. Child and Adolescent Psychiatric Clinics of North America. 2006;15:827–841. doi: 10.1016/j.chc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, et al. Effect of age at onset on the course of major depressive disorder. American Journal of Psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. doi:10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]