Abstract
The olfactory system of Drosophila melanogaster provides a powerful model to study molecular and cellular mechanisms underlying function of a sensory system. In the 1970s Siddiqi and colleagues pioneered the application of genetics to olfactory research and isolated several mutant Drosophila with odorant-specific defects in olfactory behaviour, suggesting that odorants are detected differentially by the olfactory system. Since then basic principles of olfactory system function and development have emerged using Drosophila as a model. Nearly four decades later we can add computational methods to further our understanding of how specific odorants are detected by receptors. Using a comparative approach we identify two categories of short amino acid sequence motifs: ones that are conserved family-wide predominantly in the C-terminal half of most receptors, and ones that are present in receptors that detect a specific odorant, 4-methylphenol, found predominantly in the N-terminal half. The odorant-specific sequence motifs are predictors of phenol detection in Anopheles gambiae and other insects, suggesting they are likely to participate in odorant binding. Conversely, the family-wide motifs are expected to participate in shared functions across all receptors and a mutation in the most conserved motif leads to a reduction in odor response. These findings lay a foundation for investigating functional domains within odorant receptors that can lead to a molecular understanding of odor detection.
Keywords: Drosophila, motifs, odor receptor, olfaction
1. Introduction
The Odorant receptor gene family in insects encodes seven-transmembrane-domain proteins that detect a wide variety of volatile chemicals. Individual receptors detect a subset of odorants with a high degree of specificity and sensitivity (Hallem and Carlson 2006; Kreher et al. 2008; Mathew et al. 2013). Insect Ors have a novel inside-out seven-transmembrane topology and the functional receptor is composed of a heteromeric complex comprising a variable canonical Or and an obligate non-canonical subunit Orco that together can act as a ligand-dependent ion channel (Benton et al. 2006; Sato et al. 2008). Structure-function analyses of these proteins have revealed some functionally important parts of the protein (Nakagawa et al. 2012); yet, little is known about the odor-binding regions.
Here we identify portions of insect odor receptors that are likely to participate in binding to specific odorants and in signalling-related functions. We combine bioinformatic analysis and functional analysis using electrophysiology to identify short motifs of conserved amino acid sequences in the N-terminal half of the canonical Ors that are involved in detection of a specific structural class of phenolic odors in Drosophila melanogaster. Using these predicted phenol-binding motifs, we screen insect odor receptor sequence space to identify candidate phenol-binding Ors from the genomes of several species of moths, bees and beetles, as well as two mosquito species that may utilize phenol receptors to detect their human hosts. We also identify C-terminal motifs that are shared among most members of the Or family, and are likely to play conserved roles in odor receptor function.
2. Materials and methods
2.1 Bioinformatics
Sequence analysis was performed using the MEME and MAST tools available at the MEME Suite of Motif-based sequence analysis tools (http://meme.nbcr.net/meme/).
2.2 Drosophila stocks
Transgenic constructs were injected into w1118; Δhalo/CyO, and third chromosome insertions were retained. The Δhalo deficiency (also called Δab3A) and the `empty neuron' have been described previously (Dobritsa et al. 2003; Hallem et al. 2004a, b). Stocks carrying UAS-Or46aA and UASAgOR1 were described previously.
2.3 Construction of transgenes
To generate UAS-Or46aB, a cDNA with the coding region of isoform Or46aB was obtained by RT-PCR from an antennal preparation of mRNA and cloned in frame with an N-terminal myc-tag into pUAST. For UAS-AgOrX, point mutations in the AgOr1 ORF were introduced using a PCR cloning strategy using the existing UAS-AgOr1 construct as a template.
2.4 Electrophysiology
Action potential responses from ORNs to odor stimuli were recorded and analysed as described previously (Dobritsa et al. 2003). Briefly, an electrode was placed into a sensillum in contact with the lymph surrounding the dendrites of the ORNs, and the responses were quantified from counts of action potentials generated by the neurons during the 0.5 s stimulus period. Non-stimulated impulse rate was subtracted from the responses. Chemicals for odor stimuli were obtained from Sigma-Aldrich and were >99% pure. They were dissolved in paraffin oil at 1% v/v for liquid chemicals or at 10 mg/mL for chemicals solid at RT, yielding a 10−2 solution, and diluted further in decadic steps. Odor cartridges were prepared by dropping 50 μL of a solution on a half-inch filter roundel placed in a Pasteur pipette. Stimuli were presented by placing the tip the Pasteur pipette into the air stream (37.5 mL/s) over the fly, and diverting air (3.75 mL/s) through the pipette for 0.5 s. A cartridge was used for a maximum of three presentations. Up to three sensilla were recorded on each fly.
3. Results and discussion
The Odorant receptor gene family in Drosophila melanogaster consists of ~60 members that have limited amino acid sequence conservation (~20%). The crystal structures of these receptors are not known, thus posing a challenge for identification of structural features involved in odor detection. However, the odor response properties have been determined for most of these receptors (Hallem and Carlson 2006; Kreher et al. 2008), and orthologs of the Or gene family have been identified from 11 other Drosophila species. Electro-physiological analysis of the antennal large basiconic sensilla and the sensilla of the maxillary palp in different Drosophila species suggest that odor responses are widely conserved across tens of millions of years of evolution.
D. melanogaster receptors can be loosely classified according to chemical features of the odorants that they respond to: aromatic rings, sulphur compounds, terpenes and terpenoids, lactones, esters, alcohols, aldehydes, ketones, acids, and long-chain hydrocarbons. Receptors with low levels of sequence identity can respond to the same odors, and thus the identification of functional odorant-binding domains within the proteins by simple sequence comparisons would be a difficult task. In order to identify functional domains within odor receptor proteins, we turned to a bioinformatic approach. In order to test whether odor receptors with divergent sequences that respond to the same odors may share smaller domains of common odor-binding sequences, we looked for short amino acid motifs. We selected receptors that are known to detect the structurally related compounds, 2-methylphenol and 4-methylphenol. Initial analysis using Clustal-W sequence alignments did not reveal clearly defined conserved regions, probably due to the low level of amino acid identity across the protein sequences.
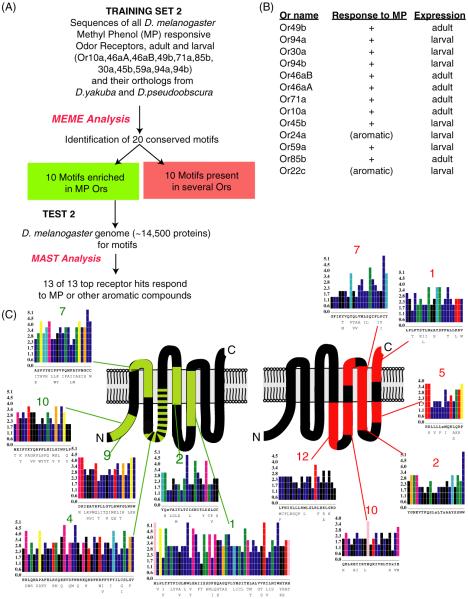
We therefore turned to a more powerful computational approach to identify shared amino acid motifs called MEME (Multiple Em for Motif Elicitation) (Bailey et al. 2009) that performs local, gapless multiple sequence alignments. The initial training set included 5 odorant receptors (Or10a, Or46aA, Or49b, Or71a and Or85b) that are expressed in the olfactory organs of the adult D. melanogaster and have been shown either to respond to the two phenols or map to neurons that respond to these compounds (Goldman et al. 2005; Hallem and Carlson 2006). Additionally, to incorporate information from evolutionary conservation in the identification of motifs, we also included the orthologs for each of these 5 Ors from a closely related species (D. yakuba) and a distantly related species (D. pseudoobscura). MEME identified at least 20 amino acid motifs that are shared across multiple receptor sequences in the training set (figure 1A). These motifs ranged between 10 to 40 amino acids in length and were present across the lengths of the proteins.
Figure 1.
A computational method to predict phenol-responsive odor receptors. (A) Schematic of a computational approach to identify amino acid motifs shared among odor receptors that respond to phenols. (B) Odor receptors that are identified from scanning the Drosophila genome using conserved amino acid motifs found in phenol odor receptors. (C) Amino acid sequence alignment of Or46aA and Or46aB. (D) Odorant response spectra of the ΔHalo ab3A ORNs ectopically expressing Or46aB at odor doses of 10−2 (center panel) and 10− (right panel) (means and SEMs). Error bars = S.E.M.; n=10–12.
We next tested the efficacy of each of these motifs in predicting phenol detection with the expectation that if these motifs could predict phenol responsiveness of receptors, they likely participated in odor-binding. We computationally screened the ~14,000 proteins in the D. melanogaster genome using MAST (Motif Alignment and Search Tool) (Bailey et al. 2009) using these 20 motifs, and as expected, the top 5 matches correspond to the 5 Or proteins that were used in the training set (figure 1B). Moreover the following 10 highest scoring hits contained 4 of the 5 larval Ors (Or94a, Or45b, Or94b, and Or30a) that are known to respond to 2-methylphenol or 4-methylphenol, and that were not part of the training set. Of the remaining 6 receptors, 2 are larval-specific Ors that do not respond to 2-methylphenol/4-methylphenol but respond to structurally related aromatic odors, 2 others that do not respond to 2-methylphenol/4-methylphenol, and Or46aB whose odor responses have not yet been reported.
Or46aB has low-level amino acid identity (~35%) to the alternatively spliced Or46aA protein (figure 1C). In order to test the computational prediction that Or46aB is a 4-methylphenol receptor, we functionally expressed it in the D. melanogaster in vivo `empty neuron' system (Dobritsa et al. 2003; Hallem and Carlson 2004). As predicted, Or46aB responded strongly to 4-methylphenol, from among a panel of diverse odorants that were tested using single-sensillum electrophysiology (figure 1D, left panel). To further characterize the response spectrum of this receptor, we tested a number of structurally-related phenols at a lower concentration and discovered that it is most sensitive to 2-methylphenol and phenol (figure 1D, right panel). Taken together, these experiments suggest that among the 20 amino acid motifs identified using MEME there are predictors of responses to phenol odors and some of them may constitute phenol-binding regions within the receptor proteins.
In order to refine the motifs, we expanded the training set by adding the sequences of the 5 larval Ors that respond to 2-methylphenol/4-methylphenol to the 5 adult receptors and including Or46aB. As done previously, we added the orthologs from two additional species for each of the receptors and used MEME to identify the top 20 amino acid motifs that were shared between members of training set 2 (figure 2A). We assumed that these 20 motifs would fall into two categories, ones specific to phenol receptors that may be involved in binding phenols, and others shared across many odor receptors that may play other shared structural roles required for all Ors. In order to distinguish between these two types of motifs, we performed a similar MEME analysis on all 60 D. melanogaster odor receptors. By manual comparison of the motif sequences and positions across the two sets we identified 10 that are specific to the phenol receptors in training set 2.
Figure 2.
Computational identification of motifs that are phenol-specific and family-wide. (A) Schematic of a computational approach to identify amino acid motifs shared among odor receptors that respond to phenols 2-methylphenol or 4-methylphenol indicated as MP. (B) Odor receptors that are identified from scanning the Drosophila genome using conserved amino acid motifs enriched in phenol odor receptors. (C) Schematic representing the locations of the phenol-specific motifs and the family-wide motifs on a secondary structure model of odor receptors. The most common amino acid compositions are indicted below each position on the X-axis and their MEME-determined conservation score indicated on Y-axis for the best-conserved sequence motifs.
We next tested whether these motifs predicted 2-methylphenol/4-methylphenol responsive odorant receptors in the D. melanogaster genome using MAST analysis (figure 2A–B). As expected the top scoring hits from the >14,000 proteins encoded by the genome are the 11 phenol receptors from training set 2, along with two others. The other two (Or24a and Or22c) are receptors that respond to structurally related aromatic odorants, and the hits beyond show a substantially lower score (not shown). The simplest interpretation of these results is that the 10 amino acid motifs we have identified are predictors of 2-methylphenol/4-methylphenol-responsive receptors. These results suggest that the sequences of these motifs represent regions of the receptors that are involved in the specific binding to phenol odors.
Interestingly the 10 phenol-receptor motifs were present mainly in the N-terminal half of the odor receptors, while the other 10 motifs that are present family-wide are predominantly in the C-terminal half of the odor receptors (figure 2C; supplementary figure 1). The C-terminal region of Or proteins is generally better conserved, perhaps supporting common functions family-wide, while the N-terminal half is more divergent, perhaps to bind to different types of odors. The positional map of the 10 phenol-specific motifs represents features that may interact directly with the odor molecules. Phenol-specific motifs are widespread in the N-terminal half of the proteins (figure 2C; supplementary figure 1). It is not unusual for multiple regions of a receptor to form part of a ligand-binding pocket. The composition of the phenol-specific motifs varies and several different classes of amino acids are represented in the well-conserved residues within the motifs (figure 2C).
4-methylphenol is a component of human sweat and is thought to be a part of host odor blends that are detected by insect vectors like the malaria mosquito Anopheles gambiae and other mosquitoes. Identification of 4-methylphenol and other host-odor responsive receptors from vector insect species would improve our understanding of the molecular basis of host-seeking behavior. A large family of 79 AgOr genes has been identified from the A. gambiae genome and we hypothesized that the phenol-specific motifs may be conserved and we could use their occurrence to predict phenol detectors. We used MAST to map the D. melanogaster phenol motifs in the genome of the distantly related A. gambiae. We identified 6 AgOrs, which had better scores (E-value<0.000073) than other hits (figure 3). Of these 6 AgOrs, 5 have been functionally characterized either in the D. melanogaster empty neuron system or in Xenopus oocytes (Hallem et al. 2004a; Carey et al. 2010; Wang et al. 2010). Four of the 5 receptors predicted (AgOr1, AgOr2, AgOr10, and AgOr34) respond strongly to phenol odorants such as 4-methylphenol. The fifth receptor does not respond to 2-methylphenol and 4-methylphenol, but to structurally related aromatic ring compounds such as benzaldehyde and acetophenone. Taken together, these results suggest that the motifs identified from Drosophila may be useful in identifying phenol-responsive Ors from mosquitoes. The phenol-specific motifs are likely to be partially conserved for hundreds of millions of years of insect evolution.
Figure 3.
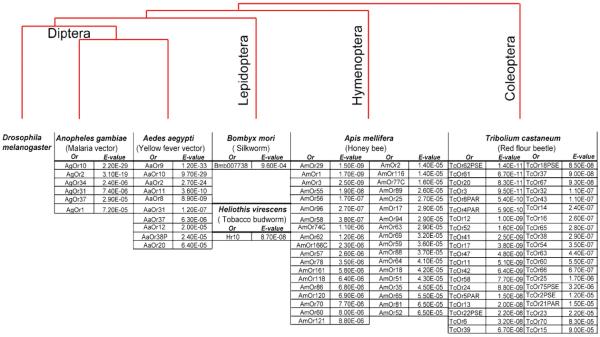
Identification of putative phenol odor receptors from other insects. Lists of odor receptors identified by the Drosophila phenol-specific motifs. E-values for each receptor are indicated in a separate cell. Species are represented in a phylogenetic tree.
Encouraged by these results we decided to test whether the Drosophila phenol-specific motifs could be used to predict phenol odor receptors from other mosquito species (figure 3). As the genome sequences of a number of insect species are becoming available, large families of odor receptor genes are continually being identified on the basis of their sequence similarity to the D. melanogaster Or genes (Hill et al. 2002; Robertson and Wanner 2006; Bohbot et al. 2007; Wanner et al. 2007; Engsontia et al. 2008). We scanned the genome of Aedes aegypti, the dengue and yellow fever mosquito, and identified 10 receptors that have scores comparable to those of the candidates from A. gambiae. D. melanogaster and the mosquitoes A. gambiae and A. aegypti are ~250 million years apart evolutionarily and belong to the same Order, Diptera.
To test whether Drosophila motifs may be useful with more distantly related insect species of economic importance from other Orders such as Lepidopterans, Hymenopterans and Coleopterans, we used MAST to screen the sequences of predicted odor receptor proteins in these species. A very small number of candidates were identified as hits among Lepidopteran species: a single candidate in Heliothis virescens (tobacco budworm) with a score comparable to the Anopheles receptors; and one in Bombyx mori (silk-worm) with a lower score. In contrast, a large number of candidates were predicted from Apis mellifera (honey bee), 37 with scores comparable to the Anopheles phenol odor receptors. The A. mellifera odor receptor family is much larger than Drosophila and shows species-specific expansions. Our results suggest that a large number of the A. mellifera receptors may be involved in detecting phenols or other structurally related aromatic ring compounds. Perhaps a foraging worker honeybee encounters a wide array of flowers and may need to detect and discriminate a variety of aromatic ring compounds that are released in floral blends. The red flour beetle Tribolium castaneum has a large known odor receptor family (n~250) and we predict that ~40 candidate T. castaneum receptors may respond to phenols or closely related aromatic ring compounds. Taken together, these results show that we are able to identify putative phenol-specific odor receptors from a wide variety of species, simply by using a motif-based bioinformatic screening.
The second class of conserved motifs that we identified comprise 10 that are concentrated in the C-terminal half of the proteins and are shared across a large number of odor receptors, notwithstanding their odor sensitivities. These motifs may reside in regions of the receptors that play a role in a function common to all odor receptors. Techniques such as FingerPRINT scans and Conserved Domain predictions suggested that the motifs we identified are unique to the odor receptors; we did not find any similarity between them and domains in other proteins, including ion channels, GPCRs and transporters. Studies have demonstrated that canonical Or proteins can heterodimerize with an obligate partner Orco (Larsson et al. 2004; Benton et al. 2006; Neuhaus et al. 2005). Interestingly we find that 4 of the 10 shared C-terminal motifs are present in Orco (Motifs 1, 2, 7 and 10) (supplementary figure 2). Of these the ones with the highest conservation are Motif 1 spanning TM7 and the extracellular C-terminus, and Motif 2 spanning part of TM6 and intracellular region 3.
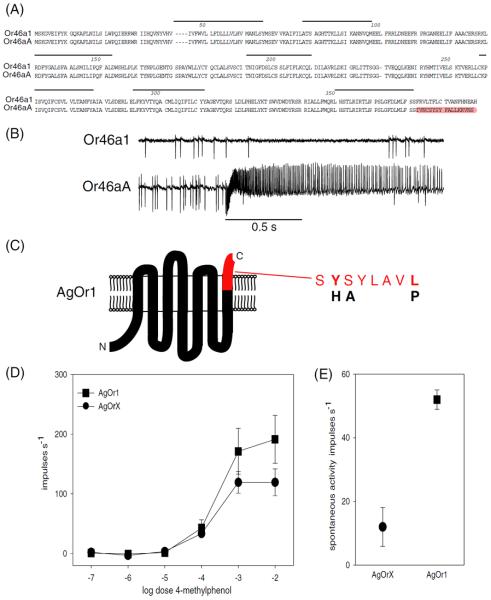
We tested the function of the well-conserved Motif 1 in vivo. The Or46a gene locus produces two alternative spliced mRNAs, which are predicted to encode two nearly identical proteins, Or46a1 and Or46aA, that differ only in the 18 amino acids at the C-terminal end. The Or46aA protein has the highly conserved Motif 1 within its terminal amino acids, whereas the Or46a1 does not (figure 4A). We wanted to examine whether there are any functional differences between these two receptors, that are identical but for the presence or absence of Motif 1. We functionally expressed the cDNA of each isoform individually in the `empty neuron' system (Dobritsa et al. 2003). While Or46aA conferred a strong excitatory response to 4-methylphenol, and other related odorants (figure 1D and 4B), Or46a1 conferred no responses indicating that it may not be a functional receptor protein (figure 4B). Since Or46a1 is identical to Or46aA except for the 18 residues in the C-terminus, these data suggest that Motif 1 is essential for Or46a function.
Figure 4.
(A) Amino acid sequence alignment of the alternatively spliced gene products Or46aA and Or46a1. (B) Representative traces showing the electrophysiological responses of ORNs. The top trace shows that there is no response to 4-methylphenol from the Δab3A neuron that ectopically expresses Or46a1. The bottom trace shows responses from the Δab3A neuron that is ectopically expressing Or46aA, upon 0.5 s stimulation (bar) with odorant 4-methylphenol. (C) Schematic of AgOr1 with location of Motif 1 indicated in red. The black letters indicate the three point mutations generated in the Motif 1 region to produce AgOrX. (D) Dose response curves showing the responses of AgOr1 and AgOrX to 4-methylphenol when expressed ectopically in the Δab3A ORNs of Drosophila. The values on the Y-axis represent the average spikes/second response to the odor stimulus minus the average spontaneous spiking frequency for each genotype. (E) Spontaneous firing rates of Δab3A neurons ectopically expressing AgOr1 and AgOrX.
The Motif 1 sequence is well conserved in the C-terminus region of insect odor receptors, including in A. gambiae. In order to identify a functional role for this conserved motif in odorant receptors, we tested the consequences of a mutation in this motif in the Anopheles AgOr1 protein (Fox et al. 2001; Hallem et al. 2004a). We generated transgenic flies containing a mutated version of UAS-AgOr1, called UASAgOrX, which encodes a protein with a 3 amino acid substitution within Motif 1 (SHAYLAVP) as compared to wild type (SYSYLAVL) (figure 4C). We ectopically expressed the mutant AgOrX protein in the empty neuron, and recorded the responses to the AgOr1 ligand 4-methylphenol. Neurons expressing the mutant AgOrX protein showed a reduced response to 4-methylphenol across concentrations when compared to the neurons expressing AgOr1 (figure 4D). Moreover, the dose response curve plateaued at a lower maximum spike frequency. The simplest interpretation of this result is that C-terminal conserved Motif 1 is required for robust Or protein function. However, whether this effect is due to reduced efficiency in coupling to Orco or an effect on some other aspect of odor receptor function remains to be elucidated.
In the absence of an odor stimulus, the spontaneous activity of a neuron has been shown to be dependent on the odor receptor that it expresses (Hallem et al. 2004a, b). The spontaneous activity of the AgOrX protein is substantially reduced when compared to AgOr1, further supporting the interpretation that the function of AgOrX is impaired (figure 4E).
The number of identified insect odor receptors is growing at a fast pace as additional genome sequencing projects for insects of medical or economical importance are being completed. Methods to decode insect odor receptors have been extremely valuable so far; however, current methods are not economical in terms of use of time and resources required to identify receptors that detect specific odor cues, such as those that mediate host-seeking behaviour in insect disease vectors and pests. A computational screening method to identify candidate receptors that may respond to specific odors as outlined here is advantageous for selecting receptors for further in-depth experimental studies. Moreover, the size of the initial training set for the algorithm may be increased to accommodate the growing number of insect odor receptors that are being functionally characterized. Thus, we may be able to use similar odor-specific motif-based prediction methods with ever-increasing sizes of training sets for a large number of specific odors.
We note in closing that this endeavour can be seen as a logical extension of the pioneering research of Obaid Siddiqi. In 1978, in a remarkable article published in the Proceedings of the Indian Academy of Science, Obaid and his student Veronica Rodrigues published the isolation of a collection of olfactory mutants of Drosophila. Some mutants were defective in response to ethyl acetate but normal in response to benzaldehyde, whereas others showed a reciprocal phenotype. The Conclusions section of that landmark article began with a forthright acknowledgment, `The experiments described here are still at an early stage', and proceeded immediately to a prophetic assertion, `It is, nevertheless, evident that the olfactory and gustatory mutants of Drosophila provide a convenient way of exploring the organization of the chemosensory pathway'. Thus began a quest that has lasted more than 35 years: the use of genetics to understand insect olfaction.
The odor-specific phenotypes of Siddiqi's early mutants raised intriguing questions about the molecules, cells and circuits by which odors are distinguished. Following his precedent, others began to use genetics, and subsequently molecular biology, to explore the underlying mechanisms of odor discrimination in the fly. Eventually these efforts led to the identification of receptors that confer responses to subsets of odors, and a rapidly expanding field has acquired an ever-increasing understanding of how signals from these receptors are transmitted and processed via olfactory circuits (Vosshall and Stocker 2007; Su et al. 2009; Wilson 2013). The current study extends the exploration of odor discrimination in new dimensions by exploiting the power of computational analysis. The technology is modern, but the underlying problems are classic. They inspired the creativity of Obaid Siddiqi, and his lasting legacy has inspired and continues to inspire many of us ever since.
Supplementary Material
Footnotes
Supplementary materials pertaining to this article are available on the Journal of Biosciences Website at http://www.ias.ac.in/jbiosci/sep2014/supp/Ray.pdf
References
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot J, Pitts RJ, Kwon HW, Rutzler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. Biol. 2007;16:525–537. doi: 10.1111/j.1365-2583.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Engsontia P, Sanderson AP, Cobb M, Walden KK, Robertson HM, Brown S. The red flour beetle's large nose: an expanded odorant receptor gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2008;38:387–397. doi: 10.1016/j.ibmb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Fox A, Pitts R, Robertson H, Carlson JR, Zwiebel L. Candidate odor receptors from the malaria vector mosquito, Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. The odor coding system of Drosophila. Trends Genet. 2004;20:453–459. doi: 10.1016/j.tig.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Fox AN, Zwiebel LJ, Carlson JR. Mosquito receptor for human-sweat odorant. Nature. 2004a;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004b;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Mathew D, Martelli C, Kelley-Swift E, Brusalis C, Gershow M, Samuel AD, Emonet T, Carlson JR. Functional diversity among sensory receptors in a Drosophila olfactory circuit. Proc. Natl. Acad. Sci. USA. 2013;110:E2134–2143. doi: 10.1073/pnas.1306976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Pellegrino M, Sato K, Vosshall LB, Touhara K. Amino acid residues contributing to function of the heteromeric insect olfactory receptor complex. PLoS ONE. 2012;7:e32372. doi: 10.1371/journal.pone.0032372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Stortkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Wanner KW. The chemoreceptor super-family in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006;16:1395–1403. doi: 10.1101/gr.5057506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner KW, Anderson AR, Trowell SC, Theilmann DA, Robertson HM, Newcomb RD. Female-biased expression of odourant receptor genes in the adult antennae of the silkworm, Bombyx mori. Insect Mol. Biol. 2007;16:107–119. doi: 10.1111/j.1365-2583.2007.00708.x. [DOI] [PubMed] [Google Scholar]
- Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu. Rev. Neurosci. 2013;36:217–241. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.