Abstract
Background
Nonalcoholic fatty liver disease (NAFLD), the most common cause of liver disease, is frequently diagnosed incidentally on imaging. The goal of the present study was to characterize rates of documentation and evaluation of incidentally identified steatosis.
Methods
Adults who underwent abdominal computed tomography (CT) with incidentally reported steatosis from January 2008 to October 2011 were identified. Individuals with ≥1 primary care appointments within 14 months following imaging were included.
Results
One hundred twenty-seven individuals with newly identified steatosis on imaging were included. Medical record documentation of newly identified steatosis occurred in only 29 individuals (22.8%). Documentation of steatosis within the “impression” section of radiology reports in addition to the report body was associated with significantly higher likelihood of primary care documentation (p=0.007).
Documentation of steatosis was associated higher rates of evaluation for the etiology of steatosis include testing of aminotransferase levels (96.5% vs. 77.5%, p=0.025), alcohol use screening (89.6% vs. 66.3%, p=0.02), and hepatitis C screening (20.6% vs. 2.0%, p=0.002). No patient had documentation of the NAFLD fibrosis score and none were referred for specialist evaluation or for liver biopsy. However, when calculated, the NAFLD fibrosis score identified 14 patients (11%) as high risk for advanced hepatic fibrosis.
Conclusion
Documentation of incidentally identified steatosis is infrequent. Documentation of steatosis was associated with increased rates of aminotransferase testing and alcohol use and hepatitis C screening. An important proportion of individuals (14%) with incidentally identified steatosis are at high risk of fibrosis and may benefit from additional evaluation.
Keywords: fatty liver, primary care, nonalcoholic steatohepatitis, CT scan, imaging, steatosis
With the high prevalence of nonalcoholic fatty liver disease (NAFLD), hepatic steatosis is a frequent incidental finding on imaging studies. Hepatic steatosis, defined histologically as greater than 5% of hepatocytes with macrovesicular fat accumulation, correlates with decreased hepatic attenuation seen on computed tomography (CT).[1] Unenhanced abdominal CT has a sensitivity of 91% and specificity of 99% for the diagnosis of hepatic steatosis.[2] While CT scan can identify the presence of steatosis, it cannot distinguish between the various etiologies of steatosis, including alcoholic liver disease, NAFLD, nonalcoholic steatohepatitis (NASH) and hepatitis C virus (HCV) infection, nor can it evaluate for the presence of hepatic fibrosis or cirrhosis.[3]
NAFLD represents the most frequently etiology of hepatic steatosis.[4] The diagnosis of NAFLD requires evidence of hepatic fat accumulation by imaging or histology and the exclusion of secondary causes of steatosis including hepatitis C infection and alcohol use.[5] Once a diagnosis of NAFLD is confirmed, it is important to distinguish between steatosis which is associated with low risk of liver disease progression and NASH which confers an increased risk of liver-related mortality, cirrhosis, and hepatocellular carcinoma.[6] Scoring systems such as the NAFLD fibrosis score can help determine an individual's risk of NASH and advanced fibrosis and when combined with imaging can identify patients at risk for advanced liver disease and in whom further evaluation is needed.[7] The identification of fatty liver on imaging is often an important first step in bringing patients to clinical attention.
There are limited published data concerning primary care physician (PCP) evaluation and management of patients with NAFLD. A recent survey of PCPs in Wisconsin revealed that 88% of surveyed physicians reported encountering at least one patient with NAFLD in the preceding year and 58% indicated a lack of confidence in their knowledge of NAFLD was a significant barrier to management. [8] Given these findings and the critical role of PCPs in the initial management of this prevalent condition, we sought to characterize the primary care physician-initiated evaluation of individuals with incidentally identified hepatic steatosis on CT scan. We hypothesized that hepatic steatosis identified on imaging would be infrequently documented in the medical record. Further, we hypothesized that evaluation for secondary causes of NAFLD and referral for further evaluation would be infrequent, even among individuals at high risk for advanced fibrosis and cirrhosis.
SUBJECTS AND METHODS
Subjects and Study Design
The radiology database at the Massachusetts General Hospital was queried for renal stone protocol CTs (unenhanced CT) and hematuria protocol CTs (enhanced and unenhanced CT) performed from January 2008 to October 2011. CT scans performed for other indications including abdominal pain were excluded to eliminate indication bias. Imaging reports that included terms “steatosis”, “fatty infiltration”, and “fatty liver” were selected. Patients less than 18 years old and greater than 85 years old at the time of image acquisition were excluded. Patients' electronic medical records were searched using a comprehensive search tool, Queriable Patient Inference Dossier (QPID), for terms “steatosis”, “NAFLD”, “NASH”, “fatty liver”, “hepatic steatosis”, “cirrhosis”, “hepatitis C”, “HCV”, “hepatocellular carcinoma (HCC)”, “HCC”, “Wilson's disease”, “celiac disease”, “autoimmune hepatitis”, “hepatitis B (HCV)”, “HBV”, and “hemochromatosis”. The QPID search tool searches all text present within the electronic medical record including all patient care notes, radiology, and laboratory reports. Patients with previously identified steatosis, chronic HCV infection, chronic HBV infection, autoimmune hepatitis, hemochromatosis, cirrhosis, hepatocellular carcinoma, Wilson's disease, celiac disease, pregnancy or metastatic cancer at time of imaging study were excluded. In order to ensure longitudinal follow-up in our healthcare system, patients without PCPs in our health system or without at least one PCP follow-up appointment within 14 months of imaging were excluded from the study.
Measurements
Patients' medical records were searched to assess baseline data spanning up to 36 months prior to the date of the imaging study demonstrating hepatic steatosis. Baseline data included gender, liver function tests (LFTs), markers of synthetic function including prothrombin time, INR and albumin, complete blood cell count, glycosylated hemoglobin (HGBA1C), fasting glucose, lipid panel, ferritin, weight, height, body mass index (BMI), HCV antibody, HBV surface antigen, diagnosis of hypertension, hyperlipidemia, and diabetes mellitus. For alcohol use screening, search terms “ETOH”, “alcohol”, and “drinks” were used. If multiple sets of labs or other data were obtained during the 36 months prior to imaging study (for example, BMI recorded 3 times in the 36 month period prior to imaging study) the most recent values prior to the imaging study were recorded. A 36 month period was chosen to capture results from infrequently ordered tests (HCV antibody, HBV surface antibody, HBV surface antigen, HGBA1C etc.). Medical records were searched for use of medications known to induce steatosis including amiodarone, tamoxifen, corticosteroids, or methotrexate at any point during the preceding 36 months prior to imaging.
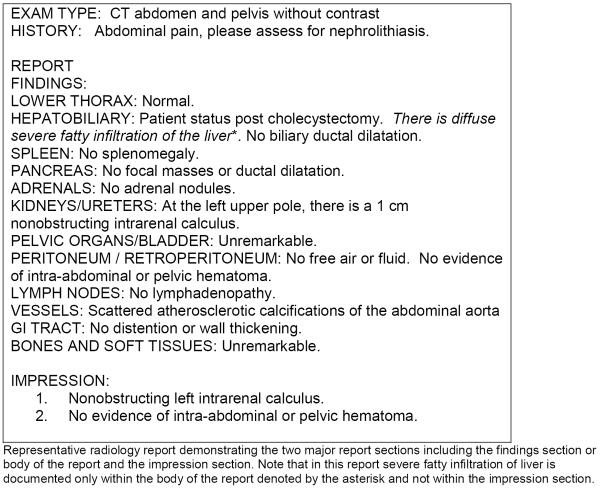
CT scan reports were reviewed to assess location of documentation of hepatic steatosis and categorized as reporting either in body of report alone or within both the body and the impression section of report. The impression portion of radiology reports at our institution consists of numbered statements summarizing key findings in the report (Figure 1).
Figure 1.
Representative radiology report including report body and impression
All PCP documentation was individually reviewed in the 14 months after performance of imaging study for documentation of hepatic steatosis, alcohol use screening, gastroenterology/hepatology referral, laboratory testing including aminotransferase, glucose and insulin levels and use of clinical scoring system to assess for risk of fibrosis such as NAFLD fibrosis score or BARD score. Pathology reports within 14 months after imaging date were reviewed to assess for liver biopsy results. If sufficient data was available, the investigators calculated the NAFLD fibrosis score using an online calculator (http://nafldscore.com/).[7]
Statistical Analysis
Characteristics of patients' whose physicians documented hepatic steatosis after imaging study were compared to patients' whose physicians did not document hepatic steatosis using two-tailed Fisher's exact test for categorical variables or two-sample t-test for continuous variables. The percentage of patients with PCP documented steatosis among patients where steatosis was reported within body versus impression of radiology reports were compared using two-tailed Fisher's exact test. Logistic regression was used to assess predictors of PCP documentation. Two tailed P values of less than 0.05 were considered significant. STATA (StataCorp) was used for analyses.
Results
Baseline characteristics
Two hundred and ninety-three patients with incidentally identified hepatic steatosis on CT scans were identified. Ultimately, 127 patients (43%) were included in the study (96 patients excluded for no primary care follow-up, 56 patients excluded for prior liver disease, and 14 patients excluded for metastatic cancer). Most patients were obese (68%) with 33.8% of patients with BMI >35 and 13.4% with BMI >40. (Table 1) There were high baseline rates of hypertension (60%), hyperlipidemia (60%), and diabetes (27%). Alcohol use was minimal with 47% reporting no regular consumption and only 4 patients (3%) with significant consumption (>14 drinks per week).
Table 1.
Baseline characteristics of study population.
| Characteristic | N=127 | % of total |
|---|---|---|
| Age | ||
| Mean age in years (SD) | 55.4 (12.4) | |
| Gender | ||
| Male | 91 | 72% |
| Female | 36 | 28% |
| Ethnicity | ||
| Caucasian | 100 | 79% |
| Hispanic | 14 | 11% |
| Black | 5 | 4% |
| Asian | 4 | 3% |
| Unknown | 4 | 3% |
| Baseline Characteristic: | 5 | |
| Hypertension* | 76 | 60% |
| Hyperlipidemia* | 76 | 60% |
| Diabetes mellitus* | 34 | 27% |
| Elevated Aminotransferase levels+ | 28 | 22% |
| BMI | ||
| <24.9 | 3 | 2% |
| 25–29 | 27 | 21% |
| >30 | 86 | 68% |
| Missing | 11 | 8% |
| Alcohol Consumption | ||
| Screening Performed | 120 | 94% |
| Quantity of Consumption | ||
| None | 60 | 47% |
| “Social/Occasional” | 37 | 29% |
| “Moderate” | 4 | 3% |
| 1–7/week | 11 | 9% |
| 8–14/week | 4 | 3% |
Baseline hypertension, hyperlipidemia, and diabetes refers to primary care physician documentation of these conditions as problems in the patient's medical record.
Abnormal aminotransferase levels refers to either ALT or AST greater than the upper limit of normal at our institution's central laboratory (ALT >55, AST<40)
Primary care physician initiated evaluation of hepatic steatosis
In the 14-month period following an initial radiology report of hepatic steatosis on CT only 29 patients (22.8%) had PCP documentation of steatosis in the medical record. This included direct communications with patients, adding steatosis to a patient's problem list, or documenting steatosis in a progress note. No patients were referred to a gastroenterologist or hepatologist for further evaluation and no patients had a liver biopsy performed in the follow-up period. In addition, no PCPs documented the application of clinical scores such as the NAFLD fibrosis score or BARD score to assess patients' risk for advanced hepatic fibrosis.
We evaluated patient characteristics that might be associated with PCP documentation of incidentally identified hepatic steatosis (Table 2). BMI, gender, age, diabetes, hypertension, hyperlipidemia, and heavy alcohol consumption (>14 drinks per week) were not significantly associated with PCP documentation of hepatic steatosis. There was a trend towards an association between baseline elevated aminotransferase levels and PCP documentation of steatosis, though this did not reach statistical significance (p=0.08). However, the placement of the findings of steatosis in the radiology report did have a significant impact on PCP documentation. When reporting of hepatic steatosis was included in both the body of radiographic reports and in the “impression” section, PCPs were significantly more likely to document presence of hepatic steatosis in patients' medical records than if the finding was included only in the body of the report (30.1% vs. 9.1%, p=0.007) (Table 2).
Table 2.
Baseline patient characteristics stratified according to primary care physician documentation of steatosis
| Patient characteristic | Patients with PCP documented steatosis | Patients without PCP documented steatosis | P value |
|---|---|---|---|
| Age (SD) (yr) | 54.4 (12.6) | 55.7 (12.4) | 0.63 |
| Male sex (%) | 73.4 | 65.6 | 0.48 |
| BMI (SD) (kg/m2) | 32.8 (5.8) | 34.3 (6.3) | 0.31 |
| Diabetes (%) | 20.6 | 28.5 | 0.48 |
| Hyperlipidemia (%) | 51.2 | 59.1 | 0.52 |
| Hypertension (%) | 51.7 | 62.2 | 0.38 |
| Elevated aminotransferase levels (%) | 34.5 | 18.4 | 0.08 |
| Documentation of steatosis in impression of radiology report (%) | 30.1 | 9.1 | 0.0075 |
| Alcohol consumption >14 drinks/week (%) | 3.4 | 3.2 | 1.0 |
This table refers to clinical and laboratory data present within the medical record at date of radiographic identification of hepatic steatosis. PCP documented steatosis included documentation of steatosis within medical problem list, any progress note, or direct patient communication (letter or phone call) within the medical record. Elevated transaminase refers to either elevated ALT (>55) or AST (>40) present at time of image acquisition.
We sought to characterize the impact of PCP documentation of hepatic steatosis on their subsequent evaluation of the etiology of the steatosis. Patients' whose physicians documented steatosis in the medical record were more likely to have aminotransferase levels checked (96.5% vs. 77.5%, p=0.025), and undergo alcohol use (89.6% vs. 66.3%, p=0.018), and hepatitis C screening (20.6% vs. 2.0%, p=0.0018) (Table 3). There were no differences between groups in rates of screening for dyslipidemia, insulin resistance, or ferritin levels.
Table 3.
Primary care physician initiated screening evaluation after radiographic identification of hepatic steatosis
| Screening test | Patients with PCP documented steatosis (percent screened) | Patients without PCP documented steatosis (percent screened) | P value |
|---|---|---|---|
| Aminotransferase levels | 96.5% | 77.5% | 0.025 |
| Alcohol screening | 89.6% | 66.3% | 0.018 |
| Hepatitis C screening | 20.6% | 2.0% | 0.0018 |
| Insulin resistance/Diabetes mellitus screening | 51.7% | 62.2% | 0.38 |
| Lipid panel | 89.6% | 84.6% | 0.76 |
This table refers to clinical and laboratory screening initiated by primary care physicians within 14 months of date of radiographic identification of hepatic steatosis. Insulin resistance screening included either hemoglobin A1C or fasting glucose measurements. Application of fibrosis score refers to documentation of NAFLD fibrosis score of BARD score within the medical record.
There was sufficient data within the medical record at time of CT scan to calculate the NAFLD fibrosis score in 105 patients (82.6%). Forty-nine patients (38.5%) had NAFLD fibrosis scores less than −1.455 corresponding to a low risk for advanced fibrosis, 42 patients (32.8%) had indeterminate scores between −1.455 and 0.676, and 14 patients (11%) had scores greater than 0.676 corresponding to a high risk of advanced fibrosis. Only 2 of 14 patients with a high-risk NAFLD fibrosis score had hepatic steatosis documented within the medical record and none were referred for further evaluation or liver biopsy.
Discussion
Our study demonstrates that hepatic steatosis identified incidentally on imaging studies was infrequently documented by PCPs. In addition, the present study found that when steatosis was documented higher rates of screening for abnormal aminotransferase levels as well as higher levels of screening for hepatitis C and alcohol use disorders occurred. This finding suggests that when incidentally identified steatosis is recognized it results in a change in practice and a more comprehensive evaluation for possible liver disease.
In addition, we found that prominent placement of the finding of hepatic steatosis in radiographic reports, specifically in the “impression” section that serves to summarize important findings, was associated with a significant increase in documentation. This finding suggests that many PCPs may be aware of the importance of steatosis as a clinical problem but that a lack of emphasis on this finding in radiographic reports may limit their awareness of the new diagnosis. The addition of hepatic steatosis to the impression section or conclusions of radiographic reports may improve the recognition and evaluation of steatosis.
To address the impact of PCP recognition of incidentally identified steatosis on clinical outcomes we assessed NAFLD fibrosis scores for all individuals were data was available. The NAFLD fibrosis score incorporates age, BMI, presence of hyperglycemia or diabetes, aminotransferase levels, platelet count, and albumin level and can be easily utilized in the primary care setting. Further, the NAFLD fibrosis score is validated to identify NAFLD patients at risk for advanced fibrosis.[9] Recently, Angulo et al. demonstrated that the NAFLD fibrosis score was a strong predictor of liver-related complications and death in a large retrospective study of patients with NAFLD and a mean follow-up of 104.8 months.[10] By applying the NAFLD fibrosis score to the study patient population we identified 14 patients (11%) at high risk for advanced fibrosis and cirrhosis who may also be an increased risk for hepatocellular carcinoma. These high risk patients warrant additional evaluation and more intensive follow-up including referral to a specialist, evaluation for liver biopsy and consideration of HCC screening. Surprisingly, none of the patients in our study were referred for specialist evaluation or for liver biopsy during our study time period.
This low rate poses several potential problems in the care of these patients. First, with high population mobility and transitions in care of providers there is potential for patients with this condition to receive inadequate follow-up. Second, patients in whom steatosis is not documented may undergo unnecessary imaging studies to evaluate liver function test abnormalities that occur later in time. If steatosis is documented and appropriate work-up for secondary causes is performed, then the diagnosis of NAFLD can be made and may obviate need for additional workup for abnormal aminotransferase levels noted at a later point in time. Most importantly, by not documenting the presence of this condition and communicating with patients, clinically significant cases of NASH and advanced fibrosis may be missed and may progress without treatment or appropriate cancer screening.
Our study had several limitations. First, it is possible that providers did recommend additional testing for patients found to have steatosis and this was performed at other facilities or not completed by the patient. However, the lack of any mention of the incidentally discovered steatosis in the medical record argues against this. Additionally, this was a single center study and may reflect unique institutional practices and biases. In addition, the limited duration of our study does not allow for the assessment of important clinical outcomes such as progression to cirrhosis or liver-related complications. To estimate this, we calculated the NAFLD fibrosis score which is a predictor of the presence of advanced fibrosis and liver-related mortality. However, longer term studies would be needed to determine who PCP recognition and evaluationof incidentally identified steatosis would change clinical outcomes.
In conclusion, documentation of incidentally indentified radiographic steatosis by PCPs at our institution was limited. Inclusion of steatosis in the impression section of radiographic reports was associated with higher rates of PCP documentation. Further, documentation of steatosis was associated with higher rates of aminotransferase testing, HCV and alcohol screening. Clinical scoring systems, specialist referrals, and liver biopsy were infrequently utilized in evaluation of these patients at this study institution. Future educational efforts for PCPs should focus on use of clinical scoring systems, timing of specialist referral, and strategies for screening for secondary causes of steatosis. Furthermore, radiology departments should include hepatic steatosis within the impression of reports when identified to improve PCP recognition of this common condition.
Acknowledgments
Grant Support: KEC receives funding from NIK K23DK099422-0.
Footnotes
Disclosures: The authors have nothing to disclose.
REFERENCES
- 1.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 2.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 3.Kneeman JM, Misdraji J, Corey KE. Secondary causes of nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2012;5(3):199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–530. e1. doi: 10.1016/j.cgh.2011.03.020. quiz e60. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2011;142(7):1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 8.Said A, Gagovic V, Malecki K, et al. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758–65. [PubMed] [Google Scholar]
- 9.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–9. e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]