Abstract
Background:
The present study was directed to evaluate the forms of oropharyngeal candidiasis (OPC) and their correlation with CD4+ cell counts in human immunodeficiency virus (HIV) patients.
Materials and Methods:
This was a descriptive and analytical cross-sectional study carried out for a 2-year period, in which quantitative data collection methods were used. 50 patients with HIV infection were evaluated. Relationship between OPC and CD4+ was investigated.
Results:
Five different clinical forms were noticed on examination: pseudomembranous candidiasis 20/38 (P) was the most common one (52.6%) followed by erythematous 5/38 (13.15%), angular cheilitis 5/38 (13.15%) (AC), a combination of AC and E 4/38 (10.52%) or AC, E and P 4/38 (10.52%). Candida albicans was the most frequent specie isolated in 35 cases of OPC (92%). Candida tropicalis was isolated in 2 cases (5.26%) and Candida glabrata in 1 case (2.64%). The majority of patients with OPC had cell counts 28/38 (73%) <200 cells/mm3, followed by 9/38 (23%) at CD4+ cell counts of 201-499 cells/mm3.
Conclusion:
Oral Candida colonization and invasive infection occur more frequently in HIV-positive patient and is significantly more common in patients with CD4+ cell counts <200 cell/mm3.
Keywords: Acquired immune deficiency syndrome, candidiasis, CD4+, human immunodeficiency virus, oral lesions
Introduction
Oral cavity is colonized by Candida albicans or other Candida species in 40-60% of healthy persons. In immune-compromised patients, Candida species can trigger a variety of disease manifestations ranging from localized mild oral lesion to a disseminated candidiasis.1
Many factors contribute to the development of oropharyngeal candidiasis (OPC) including malnutrition, poor oral hygiene, dental malocclusion, and immunosuppression.2
Diagnosis and treatment of oral lesions caused by Candida species are of utmost importance in human immunodeficiency virus (HIV)-positive patients who, despite the initiation of triple antiretroviral therapy (ART), continue to suffer from significant Candida associated morbidity.3-5
According to the Joint United Nations Program on HIV/acquired immune deficiency syndrome (AIDS) as of 2013, approximately 35.3 millions people have HIV worldwide with the number of new infections that year being about 2.1 millions.6
OPC has been described as the most frequent opportunistic fungal infection among HIV-positive patients, and it has been estimated that more than 90% of HIV-positive patients develop this infection at some time during the progression of their disease.7-9 OPC is an opportunistic infection of soft buccal mucosa. OPC can appear as erythematous patches or white, scrapable lesions and is often one of the first clinical signs of HIV infection.9,10 OPC is observed with a higher prevalence in patients with CD4+ counts below 200/mm3 or a high viral load (>10,000 copies/mL).4,5,7-9 OPC caused by C. albicans is generally managed by judicious use of fluconazole.2,4,7-9 A rise in resistant organisms may be due to prolonged or frequent treatment with azoles.10 An epidemiologic shift of Candida species could significantly impact the utility of fluconazole as empiric treatment for candidiasis in patients with HIV/AIDS.11
The present study was directed to evaluate the forms of OPC and their correlation with CD4+ cell counts in HIV patients. Counts in HIV patients.
Materials and Methods
This was a descriptive and analytical cross-sectional study carried out for a 2-year period, in which quantitative data collection methods were used. 50 patients with HIV infection were evaluated. The relationship between OPC and CD4+ was investigated. Ethical clearance was obtained, and every participant signed informed consent. Patient records, available at the Odontology unit of Saint-Antoine Hospital, were initially studied and then the patients were asked to visit the clinic for a further evaluation. A complete medical history was taken and a physical examination of the oral cavity, head, and neck area was performed on each patient. The variables studied, including medical history, physical examination, socio-demographic characteristics, socio-behavioral factors, experience with oral lesions, and laboratory tests, were reviewed. Based on the findings of a physical examination and laboratory tests, patients were prescribed essential medication and repeated examination, and follow-up visits were considered.
Clinical and microbiological assessment of subjects
One dental surgeon, who was blinded to the clinical staging, carried out all oral examinations. Patients were examined while seated in the dental chair and a well-illuminated room. Extra oral and perioral areas were examined first, followed by intraoral tissues for any abnormalities. Diagnosis of oral lesions was implemented using European Community clearinghouse guidelines for presumptive diagnosis of OPC.12
Blood samples were obtained on the same day as the oral examinations, and their results were recorded onto each participant’s questionnaire.
Candida colonization was defined as isolation of Candida species from the oral cavity. A single oral swab was collected from each study participant by passing a sterile swab firmly across buccal mucosa, floor of mouth, dorsal tongue in cases of asymptomatic patients, and from the base of the oral lesion in cases of symptomatic patients. Swabs were cultured on Sabouraud’s dextrose agar with chloramphenicol 0.5g/l, then incubated at 37°C and observed daily for 7 days. Pure growth of Candida species was considered for analysis. Candida was identified by conventional tests and species identification was performed using the germ tube test, growth on CHROM agar Candida Medium (DRG International Inc. Dehydrated media, Springfield, U.S.A), and sugar assimilation tests.12,13
All patients who had oral lesions and from whom Candida species were isolated were considered to have OPC. Recent CD4+ levels were analyzed by the flow cytometer method, and a history of intake of ART was noted. Based on the WHO classification14 the CD4+ cell counts ≥500 cells/mm3 was classified as “type 1,” CD4+ cell count of >201 to <499 cells/mm3 as “type 2” and CD4+ cell count of ≤200 cells/mm3 as “type 3.”
Statistical analysis
Patient data, microbiological results, and CD4+ counts were collected and protected with a password. Data included age, gender, socio-behavioral factors, OPC, antifungal use, ART, CD4+ count, were entered in Excel data sheet (Microsoft Corporation Seattle, USA). All statistical calculations were performed using SPSS software version 20 (IBM SPSS, Chicago, USA).
Results
Of the 50 consenting participants, 5 (10%) were females and 45 (90%) males.
The median age in this study group was 39 years (28-57). Of the participants, 38 (76%) had a history of sexual contamination, 11 (22%) had a history of sharing intravenous needles, and one (2%) had a history of homosexuality and sharing intravenous needles.
Most participants had CD4+ count (cells/mm3) was <200, 201-499, and >500 in 32 cases (64%), 16 cases (32%) and 2 cases (4%), respectively, and the mean CD4+ count (cells/mm3) was 167.12. Median duration of ART was 2 years (range, 1.5 months to 6 years). Overall, Candida species was isolated from the oral cavity in 38/50 (76%). HIV-associated oral lesions were observed in all patients (periodontal disease, herpetic lesions, hairy leukoplakia, gingivitis, oral ulceration, Kaposi’s sarcoma, and Non-Hodgkin lymphoma). Five different clinical presentations of OPC were noticed on examination. Pseudomembranous candidiasis 20/38 (P) was the most common clinical presentation of OPC (52.6%) followed by erythematous 5/38 (13.15%), angular cheilitis 5/38 (13.15%) (AC) and a combination of AC and E 4/38 (10.52%) or AC, E and P 4/38 (10.52%) (Figure 1).
Figure 1.
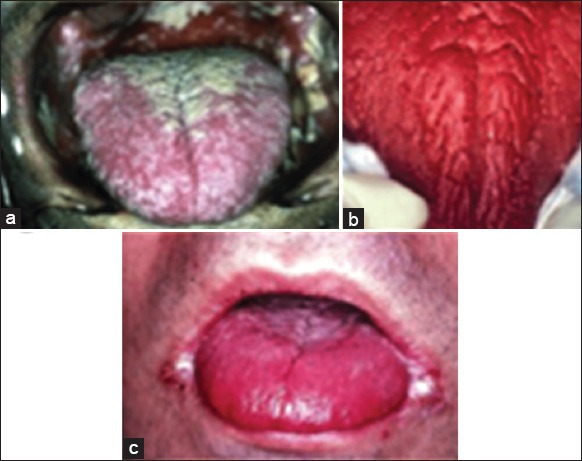
Clinical forms of oropharyngeal candidiasis: (a) pseudomembranous, (b) erythematous (c) angular cheilitis.
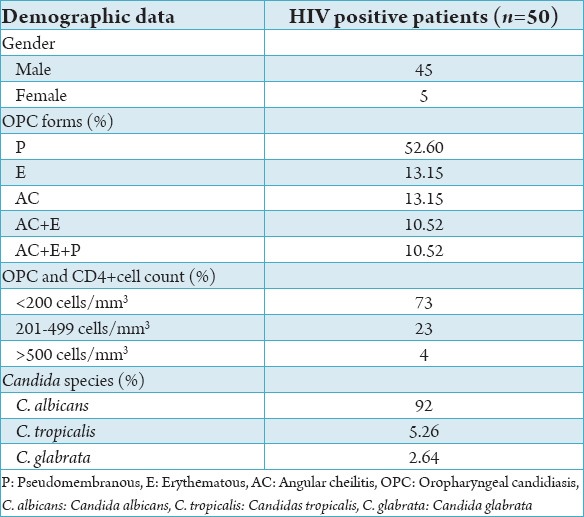
C. albicans was the most frequent species isolated in 35 cases of OPC (92%). Candida tropicalis was isolated in 2 cases (5.26%) and Candida glabrata in 1 case (2.64%). The distribution of oral lesions based on the CD4+ count of HIV-infected patients showed that 62% of the oral lesions occurred at CD4+ count <200 cells/mm3 (mean CD4+), about 26% oral lesions were seen at CD4+ count of 201-499 cells/mm3 (mean CD4+) whereas 12% cases of oral lesions were seen at CD4+ count >500 cells/mm3 (mean CD4). The majority of patients with OPC had cell counts 28/38 (73%) <200 cells/mm3, followed by 9/38 (23%) at CD4+ cell counts of 201-499 cells/mm3 (Table 1).
Table 1.
Profile of HIV patients with OPC form distribution, CD4+ count and Candida species
Discussion
OPC is the most common fungal infection in HIV patients and fluconazole efficiency in treating this infection has been proven. However, certain non-albican species (C. glabrata, Candida Krusei) proved to be less susceptible to flunconazole than C. albicans. Since they were isolated in HIV patients.7,13
Impact of ART on opportunistic infections in HIV patients is continuously evolving: Patel and co-workers (2012) studied the changing aspect of OPC epidemiology in 215 HIV/AIDS patients by assessing yeast colonization from oral rinse samples. C. albicans was the most prevalent and C. glabrata and Candida dubliniensis were identified in 29% of cultures. Decrease susceptibility to flunonazole was studied and detected in 10% of isolates.9
In a prospective cross-sectional study of predisposing factors for oropharyngeal colonization of yeasts in HIV-infected patients, Lin et al. (2013) reviewed 105 patients, among whom, 54 (51.4%) were colonized with yeasts and among the 68 isolates, C. albicans accounted for 73.5 %, C. tropicalis for 5.95%, C. glabrata for 5.9%, and C. dubliniensi for 4.4%, and there 7.5% Candida isolates resistant to flunonazole and a higher prevalence of yeast colonization was observed in patients with CD4+ count <200 cells/mm3.15
Thompson et al. (2013) studied OPC in 122 HIV-infected patients in whom infection was observed in one-third: C. albicans was the most implicated pathogen and oral yeast colonization in 81.1%, despite the availability of ART, and resistant yeasts occurred in 25.3% of patients.4
In an observational cohort, Arribas et al. (2000) determined the relationship between ART and changes in prevalence and amount of OPC. They found that the majority of 99 observed patient presented OPC with a CD4+ count <200 cells/mm3.16
In our study, the mean age was 39 years, and male gender was 90%, and female was 10%.
About 76% of our patients were contaminated with HIV by sexual transmission and 22% by sharing intravenous needles and 2% by a combination of sexual and intravenous sharing needles.
Candida species was isolated from the oral cavity in 76%. Similar figures were recorded by Thompson et al.,4 Castro et al.13 and Lin et al.15
Earlier studies2,5,15,16 showed that pseudomembranous candidiasis was the most common in clinical appearance of OPC. These findings shared our clinical observation.
C. albicans was isolated in 92% of our cases followed by C. tropicalis and C. glabrata. Some of the recent report1,4,8 have also reported the same species while others12,13 described other species as C. dubliniensis beside the mentioned species.
These variations could be related to the differences in the socio-demographic characteristics and socio-behavioral factors or may be due to increased identification of the species, which can be mistaken phenotypically as C. albicans.17
Distribution of OPC lesions and the CD4+ cell counts, 73% associated with <200 cells/mm3 is a common observation with other studies.4,5,18 The only given explication may be the decrease of the immunodeficiency of the immune system.
Fluconazole therapy shows efficacy in the treatment of OPC. Current guidelines note that for AIDS patients, 200 mg/day of fluconazole is acceptable and does not lead to resistance.19
Conclusion
Oral Candida colonization and invasive infection occur more frequently in HIV-positive patient and are significantly more common in patients with CD4+ cell counts <200 cell/mm3. ART reduces OPC. C. albicans continues to be the most frequently isolated species in OPC. Fluconazole therapies still the treatment of choice in OPC. Our results emphasize the need of continued awareness of OPC as a major clinical sign in HIV population.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Sánchez-Vargas LO, Ortiz-López NG, Villar M, Moragues MD, Aguirre JM, Cashat-Cruz M, et al. Point prevalence, microbiology and antifungal susceptibility patterns of oral Candida isolates colonizing or infecting Mexican HIV/AIDS patients and healthy persons. Rev Iberoam Micol. 2005;22(2):83–92. doi: 10.1016/s1130-1406(05)70014-0. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Lei L, Tan D, Jiang L, Zeng X, Dan H, et al. Oropharyngeal Candida colonization in human immunodeficiency virus infected patients. APMIS. 2013;121(5):375–402. doi: 10.1111/apm.12006. [DOI] [PubMed] [Google Scholar]
- 3.Delgado AC, de Jesus Pedro R, Aoki FH, Resende MR, Trabasso P, Colombo AL, et al. Clinical and microbiological assessment of patients with a long-term diagnosis of human immunodeficiency virus infection and Candida oral colonization. Clin Microbiol Infect. 2009;15(4):364–71. doi: 10.1111/j.1469-0691.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson GR, 3rd, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, et al. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:488–95. doi: 10.1016/j.tripleo.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berberi A, Noujeim Z. Epidemiology and relationships between CD4+counts and oral lesions amomg 50 patients infected with human immunodeficiency virus. J Int Oral Health. 2015;7:1–4. [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and childrens. Geneva: World Health Organization; 2013. pp. 6–16. [Google Scholar]
- 7.Maurya V, Srivastava A, Mishra J, Gaind R, Marak RS, Tripathi AK, et al. Oropharyngeal candidiasis and Candida colonization in HIV positive patients in northern India. J Infect Dev Ctries. 2013;7(8):608–13. doi: 10.3855/jidc.2801. [DOI] [PubMed] [Google Scholar]
- 8.Sharifzadeh A, Khosravi AR, Shokri H, Asadi Jamnani F, Hajiabdolbaghi M, Ashrafi Tamami I. Oral microflora and their relation to risk factors in HIV+patients with oropharyngeal candidiasis. J Mycol Med. 2013;23(2):105–12. doi: 10.1016/j.mycmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Patel PK, Erlandsen JE, Kirkpatrick WR, Berg DK, Westbrook SD, Louden C, et al. The changing epidemiology of oropharyngeal candidiasis in patients with HIV/AIDS in the era of antiretroviral therapy. AIDS Res Treat. 2012;2012:262471. doi: 10.1155/2012/262471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dore GJ, Cooper DA. HAART's first decade: success brings further challenges. Lancet. 2006;368(9534):427–8. doi: 10.1016/S0140-6736(06)69128-9. [DOI] [PubMed] [Google Scholar]
- 11.Mulu A, Kassu A, Anagaw B, Moges B, Gelaw A, Alemayehu M, et al. Frequent detection of ‘azole’ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis. 2013;13:82. doi: 10.1186/1471-2334-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Classification and diagnostic criteria for oral lesions in HIV infection. EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. J Oral Pathol Med. 1993;22:289–91. [PubMed] [Google Scholar]
- 13.Castro LÁ, Álvarez MI, Martínez E. Pseudomembranous candidiasis in HIV/AIDS patients in Cali, Colombia. Mycopathologia. 2013;175(1-2):91–8. doi: 10.1007/s11046-012-9593-0. [DOI] [PubMed] [Google Scholar]
- 14.Chunchanur SK, Nadgir SD, Halesh LH, Patil BS, Kausar Y, Chandrasekhar MR. Detection and antifungal susceptibility testing of oral Candida dubliniensis from human immunodeficiency virus-infected patients. Indian J Pathol Microbiol. 2009;52(4):501–4. doi: 10.4103/0377-4929.56138. [DOI] [PubMed] [Google Scholar]
- 15.Lin JN, Lin CC, Lai CH, Yang YL, Chen HT, Weng HC, et al. Predisposing factors for oropharyngeal colonization of yeasts in human immunodeficiency virus-infected patients: a prospective cross-sectional study. J Microbiol Immunol Infect. 2013;46(2):129–35. doi: 10.1016/j.jmii.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Arribas JR, Hernández-Albujar S, González-García JJ, Peña JM, Gonzalez A, Cañedo T, et al. Impact of protease inhibitor therapy on HIV-related oropharyngeal candidiasis. AIDS. 2000;14(8):979–85. doi: 10.1097/00002030-200005260-00009. [DOI] [PubMed] [Google Scholar]
- 17.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: A cautionary note. J Clin Microbiol. 2010;48(3):741–52. doi: 10.1128/JCM.01948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 19.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]


