Abstract
Background:
Toothpastes are considered as one of the most common and usable cosmetic and hygienic materials. Such materials contain chemicals which may have an adverse effect on oral tissue in humans. The present study aimed to compare the toxic effect of current commercial toothpastes including Iranian products and imported types which are consumed globally on oral epithelial- and HeLa cells as well as to evaluate their antibacterial effect on Streptococcus mutans in Shiraz, Iran.
Materials and Methods:
In this experimental study, 16 types of commercial toothpastes were prepared, and their effect was determined on primer epithelial cells of the oral cavity and HeLa cells. Toothpastes anti streptococcal property and toxicity were examined in vitro in different intervals of 1, 2, and 5 min. Data collection and analysis were done using one-way analysis of variance.
Results:
All experimented toothpastes revealed variable toxic effects on cultured cells. Through an increase in the time of exposure with toothpastes, the toxicity of these materials substantially increased (P = 0.005). On the other hand, all tested toothpastes showed varying degrees of anti-streptococcal effect in the laboratory (P = 0.005).
Conclusions:
The most cytotoxic effect on primer epithelial cells of oral mucosa and HeLa cells, respectively, belongs to Bath, Daroogar2, Latifeh2, Crend, Sehat, Nasim and Aqua fresh toothpastes; however, the least cytotoxic effect on primer epithelial cells of oral mucosa and HeLa cells, respectively, belongs to Pronamel followed by Crest (sensitive), Close-up, Oral-B, Signal, Colgate, Paradent, and AME.
Keywords: Antibacterial effect, cytotoxicity, HeLa cell, oral epithelial cell, toothpastes
Introduction
The most common bacterial infections which make the involved patients refer to dental clinics are periodontal diseases and dental caries.1,2
As an infectious disease, dental caries is resulted from the accumulation of plaque on the surface of the teeth and leads to the destruction of dental tissue.2 Streptococcus mutans is recognized as the main opportunistic pathogen of dental caries which can demineralize the enamel. Poor oral hygiene is considered as a major reason for the accumulation and emergence of this harmful effect.2
Periodontal disease is another bacterial disorder that may results in tooth mobility and tooth loss by affecting the supporting structures. The traditional periodontal pathogens include Streptococci, Spirochetes, and Bacteroides.2
Good oral health has a major influence on an individual’s quality of life. There exists an increasing global demand for the development of new preventive and treatment methods and products that are also economical, safe, and effective.
The most widely practiced oral hygiene method is tooth brushing that could not occasionally be sufficient to provide these requirements. Review of the related literature shows that many dental products such as toothpastes with antimicrobial activity have a crucial effect on the elimination of both dental biofilm and gingivitis. These agents decrease the plaque-induced disease and are used as an alternative product in plaque control.3,4
Several clinical researchers have demonstrated the antimicrobial effects of toothpastes on oral bacteria.1,5 A study in Iran, compared the plaque control activity of two Iranian products (Pooneh and Nasim) with Crest Regular and showed that there was no significant difference between the two products in terms of their plaque control activity.5 In contrast, another study showed the minimal antibacterial effect of Bath and Pooneh III toothpastes (Iranian-products) than crest cavity protection toothpaste.1
In Brazil, Carvalho et al. had evaluated the antimicrobial activity of six children toothpastes. They selected three fluoride-free experimental toothpastes, including cashew-based, mango-based and fluoride-free, and extracts. They compared these experimental toothpastes with two commercially fluoride-free and fluoridated toothpastes. Their investigation showed that cashew fluoride-free toothpaste had inhibitory activity against S. mutans and lactobacillus acidophilus.6
Since materials added to toothpastes may have a cytotoxic effect, a bulk of studies has so far been conducted. Cytotoxicity of two toothpastes was investigated by Torrado et al. They examined the toxicity of crest extra whitening and NMTD toothpaste. NMTD is experimental toothpaste based on a mixture of ion-exchange resin. None of the toothpastes resulted in marked increases in cytotoxicity with the time of incubation.7
With respect to the antibacterial and cytotoxicity effects of toothpaste little information is available in Iran. On the other hand, there is a dearth of research on comparing Iranian products with commercial types. Given this limited information, the present study aimed to compare toothpastes toxicity on oral epithelial and HeLa cells as well as to assess their antibacterial effect on S. mutans.
Materials and Methods
Determination of antimicrobial activities of microorganisms
In this study, the antibacterial activities of the Iranian commercial types of toothpastes were evaluated against standard types of S. mutans (ATCC000).
Determination of minimum inhibitory concentration (MIC)
Using the broth microdilution method recommended by the clinical and laboratory standards institute, MICs were determined with some modifications.8 To determine the antibacterial activities, serial dilutions of the toothpastes (2,4,8,16,64,128) were prepared in Muller-Hinton media (Merck; Darmstadt, Germany). S. mutans strains were suspended in Muller Hinton media and using a spectrophotometer method (this yields stock suspension of 1-1.5 × 108 cells/ml of bacteria), the cell densities were adjusted to 0.5 McFarland standards at 530 nm wavelengths. 100 µl of the working inoculums was added to the100 µl of various concentration of toothpaste in the microtiter plates incubated in a humid atmosphere at 37°C for 24 h 200 µl of uninoculated medium was included as a sterility control (blank). In addition, growth controls (medium with inoculums but without) were also included. The growth in each well was compared to that of the growth control well. MICs were visually determined and defined as the lowest concentration of each toothpaste which inhibited ≥95% growth reduction to compare with the growth control well. Each experiment was performed in triplicate.
Moreover, media from wells with S. mutans showed no visible growth on Muller-Hinton agar (Merck; Darmstadt, Germany) to determine minimum bactericidal concentration (MBC). MBCs were determined as the lowest concentration yielding no more than 4 colonies, which corresponds to a mortality of 98% of the microbes in the initial inoculums.8
Cell culture
Vero and primary human epithelial cells were grown in DMEM medium supplemented with 10% fetal bovine serum (GIBCO) containing 100 u/ml penicillin and 100 ug/ml streptomycin cells incubated at 37°C under 5% CO2 in a 96-well plate and was left overnight. Various concentrations of each toothpaste was prepared in cell culture medium (2, 4, 8, 16, and 64, 128) then added to the cells followed by 24 h incubation at 37°C. The test control contained untreated cells. Each treatment was performed in triplicate. Using MTT methods, the cells were recovered for cytotoxicity.
The control includes normal epithelial and HeLa cells in proper culture media.
Cytotoxicity assay
100 µl of MTT (0.5 mg/ml) was added to each well upon 24 h exposure to different concentrations of toothpaste, and then it was incubated in the dark for 4 h. To solubilize the formazone crystal sodium dodecyl sulfate (10%) in HCl (×1) was added, and a plate was incubated overnight. Optical density reading was measured at 540 nm in a microplate reader.9
Results
Antimicrobial effect of toothpastes
The results obtained from the present study revealed that all tested toothpastes demonstrated a significant antimicrobial activity against S. mutans (P = 0.005).
None of the toothpastes showed any inhibitory effect on the growth of S. mutans in the concentration of 1.256.
Minimal inhibitory concentration (MIC) for all toothpastes was 1.128.
Cytotoxic effect of the toothpastes
Comparison of cytotoxicity effect of toothpastes on epithelial and HeLa cells in 1 min
There existed a statistically significant difference between the toxicity of all tested toothpaste on the epithelial and HeLa cells in 1 min (P = 0.005).
Among all toothpastes, pronamel had no significant toxicity in comparison with that in the control group (P > 0.05).
Comparison of cytotoxicity effect of toothpastes on epithelial and HeLa cells in 2 min
There was a statistically significant difference between the toxicity of all tested toothpaste on epithelial and HeLa cells in 2 min (P = 0.005).
Compared with the control group, all toothpastes had also significant toxicity (P = 0.005); however, only pronamel had no notable effect on HeLa cell in comparison with the control group (P = 0.005).
Comparison of cytotoxicity effect of toothpastes on epithelial and HeLa cells in 5 min
A statistical significant difference was observed among the toxicity of all tested toothpastes on epithelial and HeLa cells after 5 min (P = 0.005).
Compared to the control group, a significant toxicity was found in tested toothpastes (P = 0.005).
The most cytotoxic effect on primer epithelial cells of the oral mucosa and HeLa cells belonged to Bath, Daroogar2, Latifeh2, Crend, Sehat, Nasim, and Aqua fresh toothpastes, respectively.
On the other hand, the least cytotoxic effect on primer epithelial cells of oral mucosa and HeLa cells belonged to Pronamel followed by Crest (sensitive), Close-up, Oral-B, Signal, Col gate Paradent, and AME, respectively (Table 1).
Table 1.
Cytotoxicity and anti-streptococcal activity of commercial toothpastes.
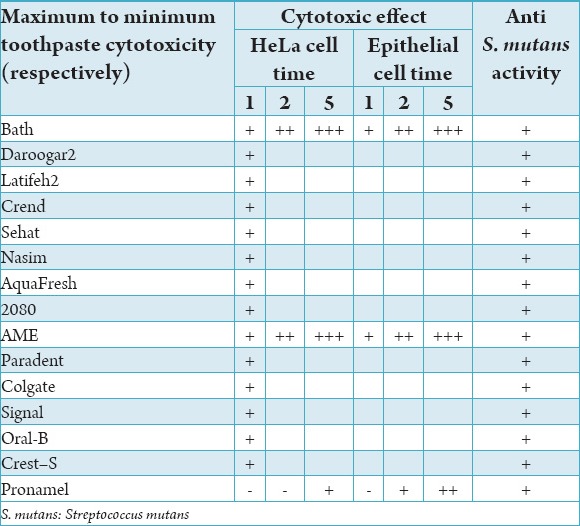
The cytotoxicity of toothpastes was significantly increased through an increase in the time of exposure from 1 to 5 min (Table 1).
Discussion
All chemical agents employed for dental use are to be evaluated in terms of the presence of any cytotoxicity. Different experiments established by ADA and FDI are required for a complete assessment of dental materials. One of the primary tests would be the use of cell cultures to determine the toxic effects of such materials.5
Toothpastes have increasingly been used by patients for daily dental care; however, the effects of these agents on oral mucosal cells have not so far been precisely evaluated. Since this material is in constant contact with the mucosa, all adverse effects of such agents should be verified. The main objective of the current study was to evaluate the cytotoxicity of 16 Iranian commercial toothpastes on oral epithelium and HeLa cells.
The present study revealed that after exposure to cultured cells, all tested toothpastes had some degrees of toxicity. Moreover, compared to the control group, the cytotoxicity of toothpastes was significantly increased and caused cell death via an increase in the time of exposure. This finding suggests that the cytotoxic effects on viable cells are time-dependent.
Sodium loril sulfate (SLS) is known as one of the most toxic agent in toothpastes used as detergent and cosmetics–health agent. The research conducted by Gerckens and Herlofson showed that SLS is the most toxic agent on mucosal cells and cause epithelial desquamation.10,11 It should be noted that all 16 tested toothpastes in this study contained SLS.
Other ingredients including sodium monofluorophosphate, silicone dioxide, hydrated silica, sodium benzoate, preservatives, colors, flavors, and essences may also have a toxic effect.12
Torrado et al. have compared the toxic effect of crest whitening with NMTD toothpastes and reported that they had no significant effect on cell viability and that there was no evidence of cell toxicity.7 This result is not consistent with that of our study which may partly be attributed to the cell type tested in the two studies.
In the current study, epithelial and HeLa cells were used while in Torrado study mouse fibroblast cells were cultured. These cells are probably more resistant to the toxic effects of toothpastes.
Rantanen et al. showed that SLS could irritate the oral mucosa while betaine had not such effect.12
Betaine is a kind of detergent applied in some toothpaste for patients with xerostomia. In our study, none of the tested toothpastes contained betaine.
Both Dumas et al. in 2007 and Phan et al. in 2006 reported inhibitory effect of triclosan on S. mutans.13,14
Magnusson in 2007 demonstrated that the amount of S. mutans decreased significantly after 6 months of using toothpastes containing triclosan, amino fluoride, and stannous fluoride. However, this effect was not detected within one to three months of usage.15
Prasanat in 2011 revealed the inhibitory effect of triclosan on S. mutans, Escherichia coli and Candida albicans.2
In the present study, all these toothpastes contained either fluoride, paraben, triclosan or all of them.
In this study, we examined the inhibitory effect of toothpastes on S. mutans growth; however, the intensity of this effect (the maximum or minimum antimicrobial effect) was not taken into account.
Kowitz et al. addressed the effect of anti-tartar toothpastes on the oral mucosa. They evaluated four toothpastes and concluded that tartar control toothpastes may cause redness, desquamation, and ulceration of the oral tissue.16
Delattre et al. demonstrated that pyrophosphate (anti-tartar agent) may directly or indirectly irritate the oral mucosa.17
In our study, we examined newly anti- tartar toothpaste called 2080. The active ingredient of this dentifrice is tocopheryl acetate. We found that its toxic effects on epithelial cells did not differ significantly from other toothpastes except for the pronamel. Detergents were other components of these types of toothpastes which may result in gingival desquamation.
Sudha Patil et al. demonstrated that toothpaste containing neem and fluoride showed desirable effect in reducing the oral microorganisms, particularly S. mutans.18
All studied toothpastes in the current research had an antibacterial effect, and all of them contained fluoride, so fluoride may be partly attributed to their antimicrobial effect.
It should be noted that this study aimed not to determine the substances which cause toxicity in toothpaste since in several articles these ingredients have implicitly been considered.
Fortunately, the cytotoxicity showed by most of these toothpastes in the laboratory did not contribute to any major problems for public health. Nevertheless, future research as well as a long time follow-up is required to determine any side effect of dentifrices.
Given the limitations of this study, oral cavity condition differs from in vitro status and many factors such as saliva, mucus layer, creatine levels, blood flow, and normal flora can influence the oral cavity protection from harmful materials.
More accurate attention of all pharmaceutical factories and laboratories is highly recommended toward the side effects of these products and to use better compounds that have minimal toxicity in their products.
Conclusion
The results obtained in this study include:
All toothpastes tested in this study had a toxic effect on HeLa cells as well as on the epithelial cell, and their toxic effects were increasing with the time interval of consumption.
The highest rate of toxicity on oral mucosal primer epithelial cells was related to Bath, Daroogar 2, Latifeh 2, Crend, Sehat, Nasim, and Aqua fresh toothpastes, respectively.
The lowest rate of toxicity on oral mucosal primer epithelial cells were related to Pronamel followed by Crest (sensitive), Close-up, Oral-B, Signal, Col gate Paradent, and AME, respectively.
All toothpastes tested in this study had antimicrobial effects against S. mutans.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Sadeghi M, Assar S. An in vitro antimicrobial activity of ten Iranian-made toothpastes. Dent Res J (Isfahan) 2009;6(2):87–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Prasanth M. Antimicrobial efficacy of different toothpastes and mouthrinses: an in vitro study. Dent Res J (Isfahan) 2011;8(2):85–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Fine DH, Furgang D, Markowitz K, Sreenivasan PK, Klimpel K, De Vizio W. The antimicrobial effect of a triclosan/copolymer dentifrice on oral microorganisms in vivo. J Am Dent Assoc. 2006;137(10):1406–13. doi: 10.14219/jada.archive.2006.0053. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki F, Pannuti CM, Imbronito AV, Pessotti W, Saraiva L, de Freitas NM, et al. Efficacy of a herbal toothpaste on patients with established gingivitis – a randomized controlled trial. Braz Oral Res. 2006;20(2):172–7. doi: 10.1590/s1806-83242006000200015. [DOI] [PubMed] [Google Scholar]
- 5.Sentila R, Gandhimathi A, Karthika S, Suryalakshmi R, Michael A. In-vitro evaluation and comparison of the anti-microbial potency of commercially available oral hygiene products against Streptococcus mutans. Indian J Med Sci. 2011;65(6):250–9. [PubMed] [Google Scholar]
- 6.Carvalho FG, Negrini Tde C, Sacramento LV, Hebling J, Spolidorio DM, Duque C. The in vitro antimicrobial activity of natural infant fluoride-free toothpastes on oral micro-organisms. J Dent Child (Chic) 2011;78(1):3–8. [PubMed] [Google Scholar]
- 7.Torrado A, Valiente M, Zhang W, Li Y, Muñoz CA. Cytotoxicity of a new toothpaste based on an ion exchange resin mixture. Am J Dent. 2005;18(4):267–9. [PubMed] [Google Scholar]
- 8.Khan F, Khan A, Kazmi SU. Prevalence and Susceptibility Pattern of Multi Drug Resistant Clinical Isolates of Pseudomonas aeruginosa in Karachi. Pak J Med Sci. 2014;30(5):951–4. doi: 10.12669/pjms.305.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeow WS, Ziauddin MF, Maxhimer JB, Shamimi-Noori S, Baras A, Chua A, et al. Potentiation of the anticancer effect of valproic acid, an antiepileptic agent with histone deacetylase inhibitory activity, by the kinase inhibitor Staurosporine or its clinically relevant analogue UCN-01. Br J Cancer. 2006;94(10):1436–45. doi: 10.1038/sj.bjc.6603132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerckens B, Eisinger G, Kaden P, Krüger W. Comparative studies of toothpastes and toothpaste ingredients in biological systems: 1. Can various toothpastes be differentiated by relative biological effectiveness in cell culture studies? Oralprophylaxe. 1991;13(2):55–60. [PubMed] [Google Scholar]
- 11.Herlofson BB, Barkvoll P. Oral mucosal desquamation caused by two toothpaste detergents in an experimental model. Eur J Oral Sci. 1996;104(1):21–6. doi: 10.1111/j.1600-0722.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 12.Rantanen I, Jutila K, Nicander I, Tenovuo J, Söderling E. The effects of two sodium lauryl sulphate-containing toothpastes with and without betaine on human oral mucosa in vivo. Swed Dent J. 2003;27(1):31–4. [PubMed] [Google Scholar]
- 13.Dumas ER, Engelman EE, Venell JA. In vitro antibacterial comparison of dentifrices claiming antigingivitis and gum-healing properties. Oral Heath Prev Dent. 2007;12(2):243–6. [Google Scholar]
- 14.Phan TN, Marquis RE. Triclosan inhibition of membrane enzymes and glycolysis of Streptococcus mutans in suspensions and biofilms. Can J Microbiol. 2006;52(10):977–83. doi: 10.1139/w06-055. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson K, Petersson LG, Birkhed D. Effect of dentifrices with antimicrobial agents on mutans streptococci in saliva and approximal dental plaque in orthodontic patients. Oral Health Prev Dent. 2007;5(3):223–7. [PubMed] [Google Scholar]
- 16.Kowitz G, Jacobson J, Meng Z, Lucatorto F. The effects of tartar-control toothpaste on the oral soft tissues. Oral Surg Oral Med Oral Pathol. 1990;70(4):529–36. doi: 10.1016/0030-4220(90)90226-i. [DOI] [PubMed] [Google Scholar]
- 17.DeLattre VF. Factors contributing to adverse soft tissue reactions due to the use of tartar control toothpastes: report of a case and literature review. J Periodontol. 1999;70(7):803–7. doi: 10.1902/jop.1999.70.7.803. [DOI] [PubMed] [Google Scholar]
- 18.Patil S, Venkataraghavan K, Anantharaj A, Patil Sh. Comparison of two commercially available toothpastes on the salivary streptococcus mutans count in urban preschool children: An in vivo study. Int Dent SA. 2010;12(4):72–82. [Google Scholar]


