Abstract
Myofibroblasts after its discovery in 1971 as the principal cell for wound healing has come a long way as far as research is concerned. The primary focus of research has been regarding preventing certain unwanted effects of this cell such as wound contraction and scarring. As far as the oral and maxillofacial region is concerned, the primary concern of this untoward effect is during repair of cleft palate surgically which results impaired development of palate and the dentoalveolar structures. This review focuses on the basic aspects of myofibroblasts such as its origin, formation, function in wound healing, role in wound contraction and ways by which its unwanted effects can be overcome to improve the quality of the post surgical complications of cleft palate surgery.
Keywords: Cleft palate, complications of wound healing, factors affecting myofibroblasts, myofibroblasts, wound healing
Introduction
Myofibroblasts were first described by Gabbiani et al., in 1971 in the granulation tissue and was identified to have a role in wound contraction. They described the structure of these cells ultramicroscopically as a modified fibroblast with smooth muscle like features, as they showed bundles of microfilaments, with dense bodies scattered in between them and showed gap junctions.1 Since then various authors have described this cell in histological and immunohistochemical aspects. The simplest definition that has been proposed till date was quoted by Powell et al., in an invited review in 1999. They described myofibroblast as a fibroblast with smooth-muscle cell-like features.2 In a review article by Eyden in 2008 a more complex definition based on the previous findings by various authors was given. The cell was described as one with stellate or spindle morphology which had a palely eosinophillic but prominent cytoplasm. The pericellular matrix contains inter alia collagen and glycosaminoglycans. Immunohistochemically these cells were positive for vimentin, alpha-smooth muscle actin (α-SMA), non-muscle myosin and extra domain A (EDA) cellular fibronectin (EDA-FN) and ultrastructurally these cells contain prominent rough endoplasmic reticulum, a golgi apparatus, myofilaments with focal densities and possess gap junctions. It has been clearly mentioned that this definition applies only to the fully differentiated myofibroblasts and not to the neoplastic myofibroblasts.3
The chief physiological function of myofibroblasts in mammalian tissues has been in wound healing and specifically in contraction of the wound. Typically there are four overlapping phases of wound healing: Haemostasis, inflammation, proliferation, and remodelling. Each of these phases form a continuum which heavily relies upon a fine balance of molecular signs that involve an intricate series of ordered and inter-related events that are: Chemotaxis, mitosis, neovascular synthesis of extracellular matrix (ECM) and the formation of a scar.4 Myofibroblasts play an important role in the contraction of the wound that occurs during the proliferation phase. The contraction is considered one of the important events in wound healing because it results in the closure of the wound.5 However, this action of the myofibroblasts is considered to create a scenario like a double edged sword. A fine balance has to be maintained as on one side, it is beneficial because it helps to narrow down the defect but on the other side an excessive action can result in undesirable contracture and scarring.6 An undesirable complication of these cells with regard to the head and neck region is during the surgical repair of cleft palate where an excessive contraction and scar formation impairs the growth of maxilla and also affects the dento-alveloar development.7 With this basic understanding let us review the role of myofibroblasts in wound healing and contraction and also the clinical implications with regard to orofacial clefts especially cleft lip and palate.
Myofibroblasts
Origin
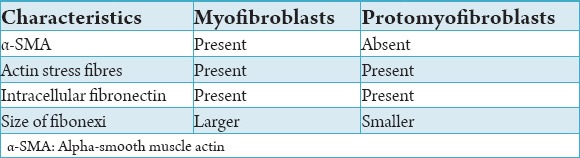
Although a lot is known about the structure, ultrastructure, immunophenotype of the myofibroblasts, there is lot of debate regarding their origin. Some of the authors in the late 1970’s believed that the myofibroblasts developed from smooth muscle cells.8 Other concepts state that myofibroblasts develop directly from mesenchymal cells,9 epithelial cells10 pericytes11 or circulating fibrocytes.12 The most widely accepted concept is that myofibroblasts originate from fibroblasts.1 In a recent review by Hinz et al., in 2007, it was proposed that myofibroblasts may differentiate from any of the above cell types, although they concluded that the main myofibroblast progenitor after injury of different tissues seemed to be the locally residing fibroblast.13 Now it is believed that there is a transient cell involved in the development of myofibroblasts from the fibroblasts, termed the “protomyofibroblast.”14 The main difference between the promyofiroblasts and the myofibroblasts is the absence of α-SMA in the protomyofibroblasts. Both these cells contain actin stress fibers and intracellular fibronectin. One more difference is in the size of the fibronexi. The protomyofibroblasts have small fibronexi whereas the fibronexi in the myofibroblasts are large.5 (Table 1)
Table 1.
Comparision between myofibroblasts and protomyofibroblasts.
Structure
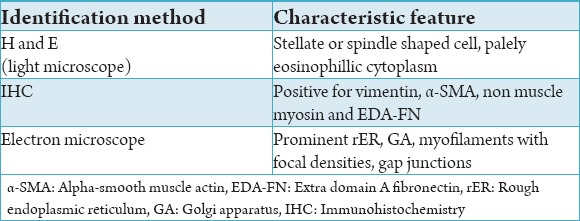
To understand the functional significance of the myofibroblasts it is very much important to understand the structure. Thus we emphasize first on the structure to give a better understanding of this cell. As mentioned earlier myofibroblasts are modified fibroblasts that have the ability to contract. This contractile property is given to this cell by its cytoskeleton which contains stress fibres mainly α-SMA. The presence of α-SMA in myofibroblasts was identified by Darby et al. in the year 1990.15 Another important component of the cytoskeleton is the non-muscle myosin. The presence of this element in the myofibroblasts was first reported by Eddy et al., who concluded that the myofibroblasts were specialised non muscle like cell and not a smooth muscle cell based upon their finding of non muscle myosin in the myofibroblasts.16 The contractile force that has been produced intracellularly has to be transferred outside the cell to the matrix proteins to bring about wound contaction or closure. The structure that assists in transmission of force from the actin microfilaments within the cell to the extracellular matrix protein fibronectin has been termed the fibronexus. In a review by Eyden, it was described as a device for providing contact between myofibroblasts and matrix that mediates continuity between intracellular contractile filaments and extracellular matrix proteins.17 The extracellular matrix proteins involved with the myofibroblasts are the fibronectin which are attached via the integrins.18,19 At this point of time it should be understood that there are two types of fibronectin in the human body. One is the plasma fibronectin (pFN) and the other is the cellular fibronectin (cFN). It has been found that there are structural differences between cFN and pFN. This difference occurs due to alternate splicing of the fibronectin mRNA which produces three variants namely EDA-FN, extra domain-B (EDB-FN) and IIICS. Our interest is the cFN that contains EDA-FN and EDB-FN and is seen in the surface of the fibroblasts. It has been established that EDA-FN is present on the surface of the myofibroblasts and is important for its differentiation from fibroblasts.20-22 Thus the important structures to be remembered to understand the physiology of the myofibroblasts are the α-SMA, non muscle myosin, fibronexus, EDA-FN and the integrins. The summary of the characteristics of myofibroblasts are given in Table 2.
Table 2.
Characteristics of a myofibroblast.
Factors affecting differentiation
As mentioned earlier the fibroblasts first differentiate into protomyofibroblasts and further into myofibroblasts. There numerous factors governing this differentiation.
-
(i)
Fibroblast to protomyofibroblast differentiation
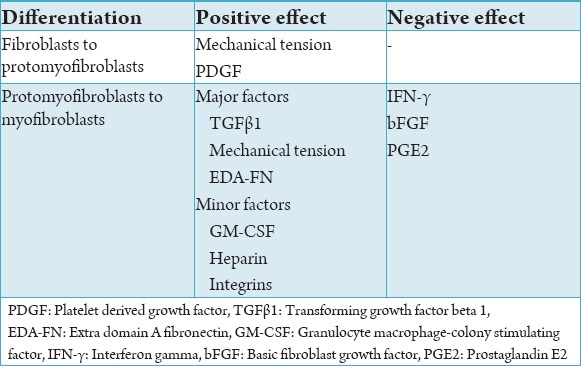
Basically there are two factors that initiate the differentiation of fibroblasts to protomyofibroblasts. First is the mechanical tension and second is the platelet derived growth factor (PDGF). The migrating fibroblasts promote the assembly of the stress fibres which is characteristic of the protomyofibroblast. There is an increase in the number of fibroblasts in the site and concurrently secretion of more ECM and fibronectin. The fibroblasts exert small tractional forces on the newly formed matrix, reinforce cell-matrix contacts, develop intracellular contractile stress fibers and hence become protomyofibroblasts.23 The role of PDGF was studied in 1996 by Boström et al., who concluded that the role of PDGF was limited to the differentiation of fibroblasts in protomyfibroblasts as they do not induce the formation of α-SMA.24 Now, it is generally agreed that the mechanical tension produced by the fibroblasts plays a major role in differentiation into protomyfibroblasts and also PDGF might have some role in it.25
-
(ii)
Protomyofibroblast to myofibroblast differentiation
Further differentiation of the protomyofibroblasts to mature myofibroblasts includes numerous factors. They can be broadly classified as major and minor factors that contribute to the differentiation. The major factors involved are the mechanical tension, transforming growth factor beta 1 (TGFβ1), and EDA-FN.5
Major Factors
TGFβ1
Various studies have been performed to study the role of TGFβ1 in the differentiation of myofibroblasts. Desmoulière et al., in 1993 published a study in which they studied the expression of α-SMA, which is an important component of the myofibroblasts. Subcutaneous injection of TGFβ1 in rats induced the expression of α-SMA in the cultured fibroblasts thus concluding that TGFβ1 played an important role in differentiation of mature myofibroblasts.26 In the year 2000 Vaughan et al., made an attempt to study the functional characteristics of myofibroblasts under the influence of TGFβ1. The results of their study showed that TGFβ1 not only increases the expression of α-SMA but also enhances the assembly of the stress fibres that is required to generate the mechanical tension and also increases the expression of fibronectin and the focal adhesion complexes which are important characteristics of the myofibroblasts.27
EDA-FN
The role EDA-FN also has been extensively studied by various authors in both fetal and post natal wound healing. It has been well established that EDA-FN is expressed in embryogenesis and fetal wound healing. Among the various functions of EDA-FN in embryogenesis, the chief function as far as the ECM and wound healing is concerned, is to help in the assembly of the various matrix constituents.28 But EDA-FN does not induce the differentiation of fibroblasts into mature myofibroblasts in this stage. This can be explained by the fact that TGFβ1 is absent in fetal wounds as proved by the results by Whitby and Ferguson29 Another important finding as observed by Hinz et al., is that presence of TGFβ1 and EDA-FN alone does not induce differentiation of mature myofibroblasts. Mechanical tension is also an important factor to induce this differentiation.30 Thus in a nut shell it is to be understood that TGFβ1, EDA-FN and mechanical tension are required for differentiation into myofibroblasts and a synergistic action is required for such an event and each of these factors individually cannot induce the formation of mature myofibroblasts.
Minor Factors
The minor factors that have found to have role in the differentiation of protomyofibroblasts to myofibroblasts are as follows:
-
(i)
Granulocyte macrophage-colony stimulating factor (GM-CSF)
-
(ii)
Heparin
-
(iii)
Integrins
-
(iv)
Cytokines - interferon gamma (IFN-γ), basic fibroblast growth factor (bFGF), prostaglandin E2 (PGE2).
GM-CSF
Numerous studies have been conducted to study the role of GM-CSF in the formation of granulation tissue and differentiation of myofibrolasts. In a study by Vyalov et al., by immunohistichemical methods they found that GM-CSF induced the formation of α-SMA in the granulation tissue.31 In a study by Shephard et al., who studied the differentiation of myofibroblasts from keratinocytes, observed that GM-CSF does not induce this differentiation directly but rather increases the expression of TGFβ1 and thus indirectly plays a role in differentiation of myofibroblasts.32
Heparin
Studies to understand the role of heparin in the differentiation of myofibroblasts goes back to 1992 when Desmoulière et al., investigated the effect of heparin on granulation tissue. Their results showed that it was able to stimulate the expression of α-SMA in vitro but later found that tumour necrosis factor alpha was required for this differentiation in vivo and hence is considered to play a minor role in formation of mature myofibroblasts.33,34
Integrins
The interest in the role of integrins for the differentiation of myofibroblasts came through when investigators tried to understand the pathway of TGFβ1 and formation of myofibroblasts. In 2004 a study by Lygoe et al., showed that αvβ1, αvβ3 and αvβ5 integrins played a crucial role in that pathway.35 This later was confirmed by Liu et al., in 2010 who observed similar results in vivo and concluded that integrins play a role in differentiation of myofibroblasts through TGFβ1.36 It has also been established that integrins play a role in functioning of myofibroblasts. They help mediate the contractilie forces generated within these cells via the cell junctions to the extracellular matrix.24
Cytokines
Certain cytokines have been found to be involved in differentiation of myofibroblasts especially in their inhibition such as IFN-γ, bFGF and PGE2. IFN-γ has been found to downregulate the formation of myofibroblasts by inhibiting the TGFβ1 induced formation of myofibroblasts.37 The other important functions that has been attribute to this cytokine is that it reduces the expression of α-SMA, prevents collagen formation and reduces collagen lattice contraction during wound healing.38,39
The role bFGF has also been extensively studied by numerous authors. In 1999, Khouw et al’s study showed that bFGF inhibited the formation of myofibroblasts.40 The other important aspect of bFGF as identified by Ishiguro et al., was that in addition to the role of bFGF in reduced expression of α-SMA, it could also induce apoptosis of myofibroblasts but not fibroblasts.41 PGE2 has also been found to reduce the expression of α-SMA via the TGFβ1 induced formation of myofibroblasts.42,43 Thus these cytokines have been indicated to reduce the adverse effects of myofibroblasts during wound healing and contraction as described further in this article. All the factors affecting the myofibroblasts have been summarized in Table 3.
Table 3.
Factors affecting differentiation from fibroblasts to protomyofibroblasts to myofibroblasts.
Myofibroblasts, wound contraction and its clinical implications
As mentioned earlier the chief function of myofibroblasts in wound healing is that causes wound contraction thereby reducing the margins of the wound. Physiologically the myofibroblasts disappear by apoptosis after wound healing, thereby preventing excess contraction. Another aspect that has been attributed to myofibroblasts in wound healing is scar formation.3 These adverse effects has been of keen interest to various researchers especially those interested in healing after correction of cleft palate surgically. This excessive wound contraction and scarring leads to impaired dento-alveolar development and other ill effects.6 So the researchers have tried to reduce the action of myofibroblasts after the healing phase is over by various methods. From the above description of myofibroblasts we could achieve this by the following mechanisms,
-
i)
Inhibition of factors that induce myofibroblast differentiation
-
ii)
Stimulation of factors that inhibit myofibroblast differention
-
iii)
Stimulation of apoptosis of myofibroblasts
-
iv)
Impairing the function of myofibroblasts.
Inhibition of factors that induce myofibroblast differentiation
The two important treatment modalities investigated to inhibit the myofibroblast differentiation are blocking of TGFβ1 and EDA-FN. The investigation began by producing introducing neutralising antibodies to TGFβ1 in vitro. Results showed that this procedure reduced the expression of α-SMA in the early stages of wound healing, inhibited collagen lattice contraction and prevented formation of a scar.44,45 However introduction of these antibodies in the later stages caused excessive wound contraction and scarring and hence introduction of these neutralising antibodies have to choreographed to perfection to achieve successful results.46 Blocking of EDA-FN vitro also produced promising results by reducing the expression of α-SMA. Other advantages that have been found that it would reduce the risk of formation of scars.47 Thus targeting EDA-FN has been suggested but studies are further required to efficiently use these with good effect.
Stimulation of factors that inhibit myofibroblast differentiation
The possible role of cytokines mentioned earlier that play a minor role in differentiation of protomyofibroblasts to myofibroblasts has also been investigated thoroughly. Although these results have been promising, studies concerning its role on oral mucosal repairs has been very limited. The role if IFN-γ in palatal wounds was studied in 2000 by Cornelissen et al. They found that IFN-γ significantly reduced the number of myofibroblasts at the site. One more important finding was that they reduced the scar formation and the collagen content and thus concluded it to be a promising pharmaceutical agent to reduce wound contraction and scarring after cleft palate surgery.38 Perhaps the most of promising results have been provided by the cytokine bFGF on palatal wound healing. This has been attributed to the fact that bFGF not only reduces the expression of α-SMA and thereby affecting the differentiation of myofibroblasts but also induces apoptosis of myofibroblasts at the right time thereby reducing the ill effects of myofibroblasts such as excessive contraction and scar formation.41 Studies on the role of PGE2 have not been promising regarding oral mucosal repairs. This is primarily due to the fact that in vivo administration of PGE2 has lot of side effects such a diarrhoea, lethargy and flushing.48 Thus it can be concluded that research in field of targeting IFN-γ and bFGF might produce fruitful results.
Stimulation of apoptosis of myofibroblasts
Induction of apoptosis of myofibroblasts at the right time is also one of the possibilities to reduce untoward contraction on palatal wounds and scarring. As mentioned earlier introduction of bFGF and TGFB1 might help for this particular action and studies have shown success in this aspect in vivo.46 Another technique that has been used but with limited success has been inducing disruption of integrin mediated cell adhesion. This has its ill effects because integrins play a chief role in other cell to cell and cell to matrix interaction and may produce untoward effects.49
Impairing the function of myofibroblasts
The chief function of myofibroblasts producing the untowards effects in platal cleft repair is the excessive contraction. As discussed earlier this contraction is brought about by the transmission of stress generated inside the cell by the stress fibers to the exterior of the cell by the integrins. So blocking this transmemberane protein has been proposed to reduce the ill effects of myofiblasts in wound repair. But as discussed in the previous section this will again affect the normal extracellular matrix functions and hence cannot be used in good effect for reducing the wound contraction or scarring produced by cleft palate repair.50
Conclusion
Myofibroblasts are the most important of the cells involved in wound healing. Although it has a lot of beneficial effects few untoward reactions such as wound contraction and scarring have also been attributed to it. These untoward effects are of special importance in the oral and maxillofacial region in the field of surgeries for cleft palate repair. Understanding the molecular mechanisms of myofibroblast formation, differentiation and applying it to prevent ill effects is very important for normal development of palate and dentoalveolar structures in patients who have undergone surgeries for repair of cleft palate. Various molecules have been targeted to achieve such modifications. Of these alterations in the expression of EDA-FN and bFGF have shown promising results and certain other cytokines such as TGFβ1 and IFN-α have also produce stable results. Inspite of all these positive results, lot of research has to be carried out to practically apply such treatment modalities.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 2.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.Eyden B. The myofibroblast in health and disease. Rev Esp Patol. 2008;41(1):3–10. [Google Scholar]
- 4.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J Tissue Viability. 2011;20(4):108–20. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 6.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. 2nd ed. New York: Oxford University Press; 2004. p. 477. [Google Scholar]
- 7.Millard DR., Jr Reconstructive rhinoplasty for the lower two-thirds of the nose. Plast Reconstr Surg. 1976;57(6):722–8. doi: 10.1097/00006534-197606000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sottiurai VS, Fry WJ, Stanley JC. Ultrastructure of medial smooth muscle and myofibroblasts in human arterial dysplasia. Arch Surg. 1978;113(11):1280–8. doi: 10.1001/archsurg.1978.01370230070008. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Malagon H. Proliferative disorders of myofibroblasts. Ultrastruct Pathol. 1993;17(3-4):211–20. doi: 10.3109/01913129309027767. [DOI] [PubMed] [Google Scholar]
- 10.Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, et al. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant. 2003;18(9):1777–84. doi: 10.1093/ndt/gfg231. [DOI] [PubMed] [Google Scholar]
- 11.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 12.Metz CN. Fibrocytes: A unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60(7):1342–50. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbiani G. The evolution of the myofibroblast concept: A key cell for wound healing and fibrotic diseases. G Gerontol. 2004;52:280–2. [Google Scholar]
- 14.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: One function, multiple origins. Am J Pathol. 2007;170(6):1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–9. [PubMed] [Google Scholar]
- 16.Eddy RJ, Petro JA, Tomasek JJ. Evidence for the nonmuscle nature of the “myofibroblast” of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988;130(2):252–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Eyden BP. Brief review of the fibronexus and its significance for myofibroblastic differentiation and tumor diagnosis. Ultrastruct Pathol. 1993;17(6):611–22. doi: 10.3109/01913129309027797. [DOI] [PubMed] [Google Scholar]
- 18.Singer II, Kawka DW, Kazazis DM, Clark RA. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: Immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984;98(6):2091–106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racine-Samson L, Rockey DC, Bissell DM. The role of alpha1beta1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem. 1997;272(49):30911–7. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 20.Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, et al. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989;109(1):357–66. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White ES, Muro AF. Fibronectin splice variants: Understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life. 2011;63(7):538–46. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- 22.Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010;24(11):4503–12. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14(5):538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85(6):863–73. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 25.van Beurden HE, Von den Hoff JW, Torensma R, Maltha JC, Kuijpers-Jagtman AM. Myofibroblasts in palatal wound healing: Prospects for the reduction of wound contraction after cleft palate repair. J Dent Res. 2005;84(10):871–80. doi: 10.1177/154405910508401002. [DOI] [PubMed] [Google Scholar]
- 26.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257(1):180–9. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 28.ffrench-Constant C. Alternative splicing of fibronectin – many different proteins but few different functions. Exp Cell Res. 1995;221(2):261–71. doi: 10.1006/excr.1995.1374. [DOI] [PubMed] [Google Scholar]
- 29.Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147(1):207–15. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 30.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyalov S, Desmoulière A, Gabbiani G. GM-CSF-induced granulation tissue formation: Relationships between macrophage and myofibroblast accumulation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):231–9. doi: 10.1007/BF02899267. [DOI] [PubMed] [Google Scholar]
- 32.Shephard P, Hinz B, Smola-Hess S, Meister JJ, Krieg T, Smola H. Dissecting the roles of endothelin, TGF-beta and GM-CSF on myofibroblast differentiation by keratinocytes. Thromb Haemost. 2004;92(2):262–74. doi: 10.1160/TH03-11-0669. [DOI] [PubMed] [Google Scholar]
- 33.Desmoulière A, Rubbia-Brandt L, Grau G, Gabbiani G. Heparin induces alpha-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest. 1992;67(6):716–26. [PubMed] [Google Scholar]
- 34.Schmitt-Gräff A, Desmoulière A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: An example of fibroblastic cell plasticity. Virchows Arch. 1994;425(1):3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- 35.Lygoe KA, Norman JT, Marshall JF, Lewis MP. AlphaV integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 2004;12(4):461–70. doi: 10.1111/j.1067-1927.2004.12402.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B, et al. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674–82. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K, Sano K, Yuba K, Katsumura K, Nakano T, Tanaka K, et al. Inhibition of induction of myofibroblasts by interferon gamma in a human fibroblast cell line. Int Immunopharmacol. 2003;3(9):1273–80. doi: 10.1016/S1567-5769(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 38.Cornelissen AM, Maltha JC, Von den Hoff JW, Kuijpers-Jagtman AM. Local injection of IFN-gamma reduces the number of myofibroblasts and the collagen content in palatal wounds. J Dent Res. 2000;79(10):1782–8. doi: 10.1177/00220345000790100901. [DOI] [PubMed] [Google Scholar]
- 39.Jansen RG, van Kuppevelt TH, Daamen WF, Kuijpers-Jagtman AM, Von den Hoff JW. Interferon-γ-loaded collagen scaffolds reduce myofibroblast numbers in rat palatal mucosa. Eur J Orthod. 2011;33(1):1–8. doi: 10.1093/ejo/cjp129. [DOI] [PubMed] [Google Scholar]
- 40.Khouw IM, van Wachem PB, Plantinga JA, Vujaskovic Z, Wissink MJ, de Leij LF, et al. TGF-beta and bFGF affect the differentiation of proliferating porcine fibroblasts into myofibroblasts in vitro. Biomaterials. 1999;20(19):1815–22. doi: 10.1016/s0142-9612(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 41.Ishiguro S, Akasaka Y, Kiguchi H, Suzuki T, Imaizumi R, Ishikawa Y, et al. Basic fibroblast growth factor induces down-regulation of alpha-smooth muscle actin and reduction of myofibroblast areas in open skin wounds. Wound Repair Regen. 2009;17(4):617–25. doi: 10.1111/j.1524-475X.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 42.Garrison G, Huang SK, Okunishi K, Scott JP, Kumar Penke LR, Scruggs AM, et al. Reversal of myofibroblast differentiation by prostaglandin E(2) Am J Respir Cell Mol Biol. 2013;48(5):550–8. doi: 10.1165/rcmb.2012-0262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L417–28. doi: 10.1152/ajplung.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339(8787):213–4. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 45.Yokozeki M, Moriyama K, Shimokawa H, Kuroda T. Transforming growth factor-beta 1 modulates myofibroblastic phenotype of rat palatal fibroblasts in vitro. Exp Cell Res. 1997;231(2):328–36. doi: 10.1006/excr.1997.3473. [DOI] [PubMed] [Google Scholar]
- 46.Funato N, Moriyama K, Baba Y, Kuroda T. Evidence for apoptosis induction in myofibroblasts during palatal mucoperiosteal repair. J Dent Res. 1999;78(9):1511–7. doi: 10.1177/00220345990780090501. [DOI] [PubMed] [Google Scholar]
- 47.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100(11):6736–40. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadden HL, Henke CA. Induction of lung fibroblast apoptosis by soluble fibronectin peptides. Am J Respir Crit Care Med. 2000;162:1553–60. doi: 10.1164/ajrccm.162.4.2001015. [DOI] [PubMed] [Google Scholar]
- 50.Yang CH, Liu CZ, Huang TF, Yang CM, Lui KR, Chen MS, et al. Inhibition of RPE cell-mediated matrix adhesion and collagen gel contraction by crovidisin, a collagen-binding snake venom protein. Curr Eye Res. 1997;16(11):1119–26. doi: 10.1076/ceyr.16.11.1119.5106. [DOI] [PubMed] [Google Scholar]


