Abstract
The torECAD operon encoding the trimethylamine oxide (TMAO) respiratory system of Shewanella oneidensis is positively controlled by the TorS/TorR two-component system when TMAO is available. Activation of the tor operon occurs upon binding of the phosphorylated response regulator TorR to a single operator site containing the direct repeat nucleotide sequence TTCATAN4TTCATA. Here we show that the replacement of any nucleotide of one TTCATA hexamer prevented TorR binding in vitro, meaning that TorR specifically interacts with this DNA target. Identical direct repeat sequences were found in the promoter regions of torR and of the new gene torF (SO4694), and they allowed TorR binding to both promoters. Real-time PCR experiments revealed that torR is negatively autoregulated, whereas torF is strongly induced by TorR in response to TMAO. Transcription start site location and footprinting analysis indicate that the operator site at torR overlaps the promoter −10 box, whereas the operator site at torF is centered at −74 bp from the start site, in agreement with the opposite role of TorR in the regulation of the two genes. Since torF and torECAD are positively coregulated by TorR, we propose that the TorF protein plays a role related to TMAO respiration.
Trimethylamine oxide (TMAO) is a small compound mainly found in aquatic environments (15). In a number of marine animals including fish and crustaceans, it stabilizes proteins against the denaturing effect of stresses such as hydrostatic pressure or high urea or salt concentration (20, 31, 32). This protective role is not yet clearly established for bacteria, but many of them can use TMAO as a terminal electron acceptor for anaerobic respiration (3, 28). For example, Shewanella strains, which are gram-negative bacteria with wide respiratory capacities, can reduce TMAO efficiently to generate energy during fish spoilage (13, 14, 16). The main TMAO respiratory pathway of Shewanella species comprises a periplasmic terminal reductase (TorA) containing a molybdenum cofactor and a pentaheme c-type cytochrome (TorC) anchored to the inner membrane (9, 12). The genes encoding the Tor pathway are clustered in the torECAD operon, and this operon is regulated by the TorS/TorR two-component system (6). When TMAO is available in the medium, the sensor TorS transphosphorylates the response regulator TorR which, in turn, activates the torECAD operon by binding to a single operator site in the operon promoter (12).
A similar Tor respiratory system is present in Escherichia coli, and its torCAD structural operon is also controlled by a TorS/TorR signal transduction system (18, 24). The E. coli TorS sensor detects the presence of not only TMAO but also immature TorC to allow optimal production of the structural components of the Tor respiratory system in inducing conditions (1, 19). The physiological relevance of this subtle negative autoregulation by apocytochrome TorC is probably that TorC maturation is the limiting step of the Tor system biogenesis (11). Overproduction of the c-type cytochrome maturation machinery relieves the negative autoregulation by increasing the extent of TorC maturation (1). In addition to the torCAD operon, TorR-P activates the tnaLAB operon encoding the tryptophanase (TnaA) and a low-affinity tryptophan permease (TnaB). The physiological reason for the coregulation of torCAD and tnaLAB is that the tryptophanase activity protects E. coli against the alkaline stress generated by the production of alkaline TMA during TMAO respiration (7). Indeed, TnaA reverses alkalinization by producing acidic products from l-tryptophan.
In this study, we show that TorR of Shewanella oneidensis activates torECAD and a new gene called torF (SO4694) and represses its own gene by binding to specific operator sites containing a direct repeat of the hexanucleotide sequence TTCATA separated by four nucleotides. torF encodes a protein that belongs to a new family of proteins of unknown function, and its coregulation with torECAD suggests that the TorF protein plays a key role in the TMAO respiratory system.
MATERIALS AND METHODS
Strains, media, and growth conditions.
All strains of S. oneidensis used in this study are derivatives of strain MR1-R (6, 26). Strains SOR-3 and SOS-2 are, respectively, torR and torS insertion mutants. S. oneidensis was grown at 30°C in Luria-Bertani rich medium, complemented with 40 mM l-lactate and 20 mM HEPES as described by Myers and Myers (27). E. coli strains MC4100 and LCB436 [MC4100 but Δ(torSTRCAD)] were grown at 37°C in Luria-Bertani medium (12). To maintain plasmid selection in E. coli, ampicillin was added at a concentration of 50 μg/ml.
DNA manipulations.
DNA preparation, restriction endonuclease digestion, purification, and ligation were carried out according to standard procedures. The transformation of E. coli was performed as described by Chung and Miller (8).
Plasmid constructions.
To create plasmid pPTorRSO, we performed PCR by using S. oneidensis chromosomal DNA as a template and the primer pair pR1-pR2 (Table 1) to generate a DNA fragment extending from −182 to + 19 (nucleotide position relative to the translation start site of torR). The PCR product was cloned into the SmaI site of pGE593 (10), and the resulting plasmid (pPTorRSO) was introduced into strain LCB436. The appropriate cloning orientation was determined by PCR. The absence of mutation in the cloned fragment was checked by DNA sequencing.
TABLE 1.
Synthetic oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| WT | 5′-TTTTAAAATTTTCATAATTTTTCATATATTGTCAAAGCC-3′ |
| M1 | 5′-TTTTAAAATTTTGCTAATTTTTCATATATTGTCAAAGCC-3′ |
| M2 | 5′-TTTTAAAATTTTCATAATTTTTGCTATATTGTCAAAGCCCATTCATCCC-3′ |
| M3 | 5′-TTTTAAAATTGTCATAATTTTTCATATATTGTCAAAGCC-3′ |
| M4 | 5′-TTTTAAAATTTGCATAATTTTTCATATATTGTCAAAGCC-3′ |
| M5 | 5′-TTTTAAAATTTTGATAATTTTTCATATATTGTCAAAGCC-3′ |
| M6 | 5′-TTTTAAAATTTTCGTAATTTTTCATATATTGTCAAAGCC-3′ |
| M7 | 5′-TTTTAAAATTTTCAGAATTTTTCATATATTGTCAAAGCC-3′ |
| M8 | 5′-TTTTAAAATTTTCATGATTTTTCATATATTGTCAAAGCC-3′ |
| M9 | 5′-TTTTAAAATTTTCATAAGTTTTCATATATTGTCAAAGCC-3′ |
| M10 | 5′-TTTTAAAATTTTCATAATTTTGCATATATTGTCAAAGCCCATTCATCCC-3′ |
| Erev | 5′-AAGAGTATGAAAATGATGAATCCCAG-3′ |
| pR1 | 5′-CCAGCACACTATAGGCCATGTTC-3′ |
| pR2 | 5′-CATAGTTTGTTAGCGTCCACC-3′ |
| F1 | 5′-CGTGGAGAATCCGAAACCTTAG-3′ |
| F2 | 5′-CCGCATACCAGCCTTGATTGTG-3′ |
| F3 | 5′-CTACGCCTAGCTATCCATAAGC-3′ |
| F5 | 5′-GAAAGTAACGCCCCAGTTAGC-3′ |
| 949A | 5′-GATTAACGGATAGGTAAACGGG-3′ |
| 949B | 5′-AGTGATTATGTGTCGATTAGCC-3′ |
| C1 | 5′-CCTTAGGCGCTGTCAGCATTAG-3′ |
| C2 | 5′-ATGCAGAAGGCTTCGGTATTGG-3′ |
| 16S1 | 5′-CGGACGGGTGAGTAATGCCTAG-3′ |
| 16S2 | 5′-GTAGGGCGTATGCGGTATTAGC-3′ |
| R1 | 5′-AAGGTTATCGCGTGGTTGAGGC-3′ |
| R2 | 5′-GCGTAACTCGCGAGTTAAGCTC-3′ |
| R5 | 5′-CGCGCACGAATAACCACTTCGTCATC-3′ |
| R7 | 5′-AAGCAAAATAAGAGAAACAGAACATG-3′ |
| R8 | 5′-ATGAAAAAGTATGAATAATTCAGTT-3′ |
| lacZ | 5′-CGCCAGGGTTTTCCCAGTCACGAC-3′ |
RNA preparation.
RNA was prepared by using a High Pure RNA isolation kit from Roche Diagnostics according to the manufacturer's instructions but with the slight modification that the DNase I digestion step was carried out twice in order to diminish the quantity of contaminating DNA. When the RNA was prepared in order to perform real-time PCR experiments, an additional third step of DNase I treatment was carried out in solution with RNase-free DNase I (Amersham) between the two passages through columns.
Primer extension analysis.
The transcription start sites of the torR and torF genes were determined in E. coli strain LCB436 carrying plasmid pPTorRSO and in S. oneidensis strain MR1-R, respectively. The strains were grown anaerobically in the presence of 50 mM TMAO until the culture reached an A600 of 0.5. Total RNA was then extracted. The oligonucleotides used as probes were end labeled with [γ-33P]ATP (2,500 Ci/mmol) by using T4 polynucleotide kinase (Gibco-BRL) and purified with a QIAGEN QIAquick nucleotide removal kit. The primer extension reactions were performed with reverse transcriptase (Superscript II; Gibco-BRL). The sequencing ladders were generated with the same oligonucleotides used for the primer extensions.
RT PCR analysis.
Reverse transcriptase PCR (RT PCR) was performed with the Promega Access system. The oligonucleotides used are indicated in Table 1 (see also Fig. 3). One microgram of purified RNA was denatured at 94°C for 2 min in the presence of the primers. Immediately afterwards, reverse transcription and 35 cycles of PCR amplification were carried out according to the supplier's protocol.
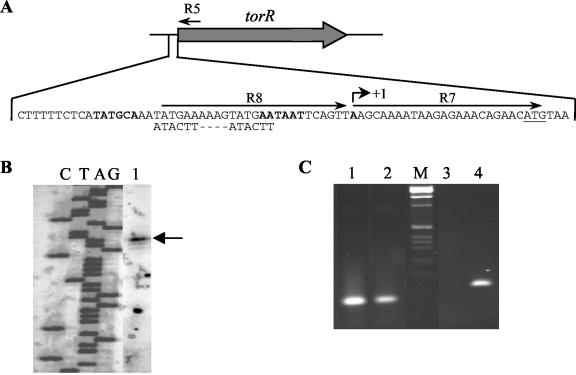
FIG. 3.
(A) Nucleotide sequence of the torR promoter region. The transcription start site (+1) and the positions of oligonucleotides R5, R7, and R8 are indicated. The −10 and −35 regions are indicated in bold, and the ATG initiation codon is underlined. The direct repeat is indicated as a double-strand sequence. (B) Location of the transcription start point of gene torR. The labeled lacZ primer, complementary to the lacZ internal sequence, was annealed to total RNA from E. coli LCB436 carrying plasmid pPTorRSO and extended with RT (lane 1). The sequencing reactions were performed with the same primer as in the primer extension reaction. The samples were loaded on an 8% polyacrylamide-8 M urea electrophoresis gel. The arrow points out the transcription start site. (C) Analysis of the torR gene transcription by RT PCR followed by 2% agarose gel electrophoresis. The RT PCR was carried out with primer R5 and either primer R7 (lane 1 and 2) or R8 (lane 3 and 4). Lanes 2 and 3, RT PCR with 1 μg of total RNA from S. oneidensis MR1-R; lanes 1 and 4, control PCR with genomic DNA; lane M, 1-kb ladder from Gibco BRL.
Real-time PCR.
The relative abundance of the torC, torR, and torF transcripts of various S. oneidensis strains (MR1-R, SOR-3, and SOS-2) grown with or without TMAO (50 mM) was determined by real-time PCR. 16S rRNA was used as a reference standard. Real-time PCR was performed by using a LightCycler instrument and the LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics) according to the manufacturer's instructions. Total RNA, extracted from S. oneidensis strains grown with or without TMAO (50 mM), was reverse transcribed by using random hexamers. cDNA (2 ng) was then mixed with 4 mM MgCl2, a 0.1 μM concentration of each primer, and 2 μl of master mix in a 20-μl final volume. The primer pairs used to quantify the torC, torR, torF, and 16S rRNA gene expression levels were C1-C2, R1-R2, F1-F2, and 16S1-16S2, respectively (Table 1). PCR assay was carried out with one cycle at 95°C for 8 min, followed by up to 45 cycles at 95°C for 15 sec, 60°C for 10 sec, and 72°C for 10 sec. The fluorescence derived from the incorporation of SYBR Green I into the double-stranded PCR products was measured at the end of each cycle to determine the amplification kinetics of each product. The fit points method described by the manufacturer was then applied to the results. Briefly, a horizontal noise band was determined as well as a log line fitting the exponential portion of the amplification curve. The intersections of these log lines with the horizontal noise line identified the crossing points. These crossing points were determined for each gene in both growth conditions. The induction factor was calculated as follows: 2(crossing point in absence of TMAO − crossing point in presence of TMAO). The values were normalized by using values obtained with 16S rRNA. The real-time PCR experiments were performed three times with RNA samples prepared from independent cultures.
Preparation of the TorR protein of S. oneidensis.
Overproduction of the TorR protein of S. oneidensis was achieved by growing 100 ml of strain MC4100 carrying plasmid pRso1 (pBAD24 carrying the torR gene under the control of the arabinose-inducible promoter) (12). When the culture reached an A600 of 1, overproduction of the TorR protein was induced for 1 h with 0.2% arabinose. The cells were then harvested by centrifugation, and the pellet was resuspended in 5 ml of 40 mM Tris-HCl, pH 7.6. The cells were passed through a French press, and the extract was centrifuged at 14,000 rpm in a Sorvall RC5B centrifuge for 10 min. The supernatant was directly loaded on a heparin-Sepharose column (Amersham Pharmacia Biotech). The proteins were eluted with a step gradient of KCl from 100 mM to 1 M. TorR was purified near to homogeneity in the 400 mM KCl fraction.
Gel retardation assays.
The DNA fragments were generated by PCR with the appropriate labeled and unlabeled primers. Labeling was carried out by using [γ-32P]ATP (4,000 Ci/mmol) and T4 polynucleotide kinase (Gibco-BRL), and the labeled fragments were then separated from unincorporated nucleotides (QIAquick nucleotide removal kit; QIAGEN). Binding of TorR to labeled DNA fragments was carried out in a 4-μl reaction mixture containing 50 mM Tris-HCl (pH 8), 1.25 mM EDTA, 0.25 M sucrose, 0.025% bromophenol blue, and 0.25 μg of poly(dI-dC) per μl. After 30 min at room temperature, the samples were loaded and run on a 12.5% polyacrylamide gel (Pharmacia Phast System). The gel was exposed for 3 h at room temperature on a phosphorimager screen.
DNase I footprinting.
The same labeled DNA fragments as those used for the gel retardation assays, encompassing the torR (201 bp) or the torF (386 bp) regulatory regions, were generated by PCR from plasmid pPTorRSO and from MR1-R chromosomal DNA, respectively, with the appropriate labeled and unlabeled primers. The footprinting experiments were performed as follows. About 1 nM of probe was used in 50 μl of binding mix [10 mM Tris HCl (pH 7.5), 50 mM NaCl, 2.5 mM MgCl2, 0.5 mM dithiothreitol, 4% glycerol, and 30 ng of poly(dI-dC) per μl]. Different amounts of the purified TorR protein were then added. After 30 min of incubation at room temperature, DNase I was added (1 U; Promega), and the reaction was conducted for 1 min and then stopped by the addition of 140 μl of DNase stop solution (192 mM sodium acetate, 32 mM EDTA, 0.14% sodium dodecyl sulfate, and 64 μg of yeast RNA per ml). After phenol-chloroform extraction and DNA-ethanol precipitation, the pellets were resuspended in loading solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol) and loaded on an 8% polyacrylamide-8 M urea electrophoresis gel. The location of the protected nucleotides was deduced by running a ladder with the products of the G+A cleavage reaction.
RESULTS AND DISCUSSION
TorR binds to a direct repeat of the hexanucleotide sequence TTCATA in the torECAD promoter.
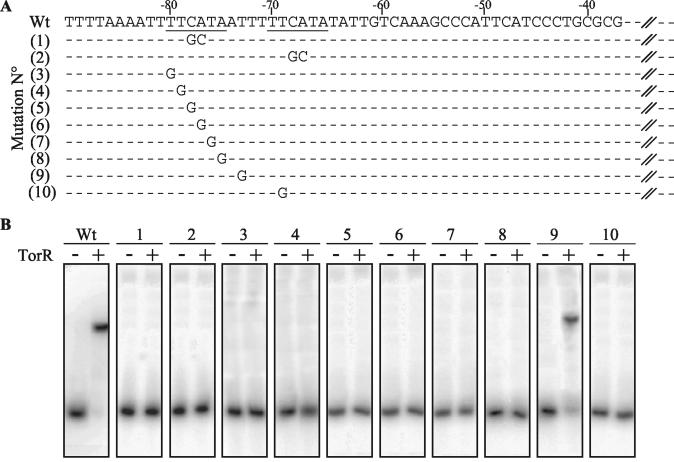
We have previously shown that TorR, the TMAO response regulator of S. oneidensis, induces the torECAD operon by binding to a single operator site located between positions −84 and −60 relative to the transcription start site (12). Inspection of this region revealed the presence of a direct repeat of the hexameric sequence TTCATA (Fig. 1). This tandem direct repeat could be the target of TorR because members of the OmpR family usually interact with direct repeats (5, 21, 22, 25). To test this hypothesis, we first changed the center of each hexamer (italicized) independently by a double mutation (TTCATA→TTGCTA) and carried out a DNA-binding gel shift assay with labeled DNA fragments corresponding to the tor operon region from position −90 to + 119 and purified TorR. As shown in Fig. 1, the DNA fragments containing the double mutation (mutations 1 and 2) were not retarded by a high concentration (1 μM) of TorR, whereas the wild-type fragment was. This preliminary result is consistent with the idea that each hexamer plays a key role in TorR binding. To study further the involvement of the TTCATA hexameric sequence in TorR binding, we replaced each nucleotide of the first hexamer with a guanine residue (Fig. 1). Strikingly, no retardation was observed for any of the six mutated fragments (mutations 3 to 8), meaning that each nucleotide of the first hexamer is essential for TorR binding. To confirm that the two hexamers play a similar role in TorR binding, we replaced one nucleotide of the second hexamer with a guanine residue. As expected, the mutated DNA fragment (mutation 10) was no longer retarded by TorR. In contrast, a point mutation T→G in the four-nucleotide region spacing the tandem repeats did not significantly affect TorR binding (Fig. 1, mutation 9). The same results were obtained when TorR was preincubated with acetyl phosphate (data not shown). Together, these results strongly suggest that the DNA recognition site of TorR comprises at least the sequence TTCATAN4TTCATA, and we propose that like other members of the OmpR family, phosphorylated TorR binds as a dimer to its operator site, with each monomer interacting with one direct repeat (5, 29).
FIG. 1.
Effect of mutations in the torE promoter region on the in vitro binding of TorR. (A) Representation of the wild-type and mutated promoter regions. The DNA fragments (209 bp) were obtained by PCR with Erev as the 3′ primer and WT, M1, M2, M3, M4, M5, M6, M7, M8, M9, or M10 as 5′ primers leading to the wild type and the corresponding mutated (1 to 10) promoter regions. Positions relative to the transcription start site are indicated above the sequences. The direct repeats are underlined. Only bases differing from the wild-type sequence are shown for the mutated fragments. (B) Gel shift analysis. The labeled fragments corresponding to the wild type and mutated (1 to 10) promoter regions were incubated in the absence (−) or presence (+) of a 1 μM concentration of purified TorR protein. Wt, wild type.
Binding of TorR to new promoters.
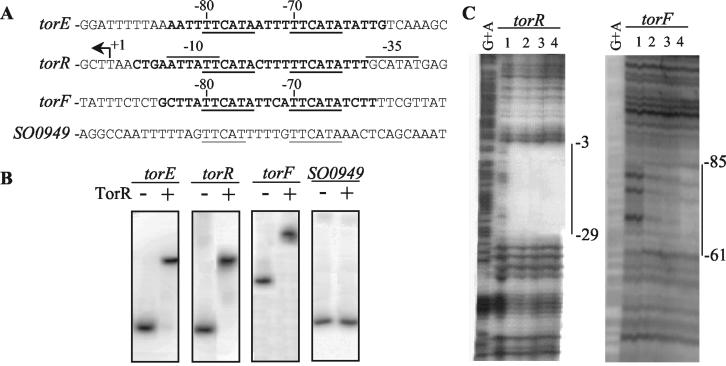
The fact that we knew the specific nucleotide sequence recognized by TorR in the torECAD promoter prompted us to look for homologous nucleotide sequences within the genome of S. oneidensis in order to find new potential targets of TorR. By using the bioMotif utility (http://genetics.mgh.harvard.edu/doc/bioMotif/), we retrieved sequences homologous to the consensus sequence TTCATAN4TTCATA, located in noncoding regions or in the beginning of coding regions. This survey revealed two additional sequences identical to the consensus and located upstream of the coding sequences of torR and of SO4694 (hereafter called torF) and one sequence upstream of SO0949 containing a single change in one hexamer (Fig. 2A). Since these sequences could be TorR binding sites, we checked whether the TorR protein was able to bind to them in vitro. Using a band shift assay, we observed DNA retardation for the promoter DNA of torR and torF but not for that of SO0949 (Fig. 2B). This result shows that TorR binds to the torR and torF promoters, and it confirms that only one base change in one of the TTCATA repeat sequences prevents TorR binding. The same pattern of retardation was observed when TorR was preincubated with 100 mM acetyl phosphate, but the TorR affinity for the torE, torR, and torF promoter DNA was increased two- to threefold, indicating that phosphorylation of TorR increased its affinity for the DNA targets containing the consensus motif (data not shown).
FIG. 2.
(A) Alignment of the torE, torR, torF, and SO0949 promoter regions. The regions protected by TorR are indicated in bold. The direct repeats are underlined. Positions relative to the transcription start sites are indicated above the sequences. For convenience, the complementary sequence of the torR promoter is presented. The direct repeat sequence of SO0949 is centered at −165 bp from the initiation codon. (B) Electrophoretic gel shift analysis of TorR interaction with the torE, torR, torF, and SO0949 promoters. The DNA fragments containing the torE (position −90 to + 119 relative to the transcription start site), torR (position −159 to + 42 relative to the transcription start site), torF (position −306 to + 80 relative to the transcription start site), and SO0949 (position −234 to −54 relative to the initiation codon) promoter regions were obtained by PCR with the primer pairs Wt-Erev, pR2-pR1, F3-F5, and 949A-949B, respectively. The labeled fragments were used in gel shift experiments in the presence (+) or absence (−) of a 1 μM concentration of purified TorR protein. (C) Analysis of TorR binding to the torR and the torF promoter regions by DNase I footprinting experiments. The DNA fragments corresponding to the torR and the torF promoter regions were obtained by PCR by using the primer pairs labeled pR2-unlabeled pR1 and labeled F5-unlabeled F3, respectively. The labeled DNA fragments were digested with DNase I in the presence of the following concentrations of TorR protein: lane 1, no protein; lane 2, 0.25 μM; lane 3, 1 μM; and lane 4, 2.5 μM. The G+A sequencing ladders are shown, and the vertical bars indicate the protected regions.
The same pattern search approach was performed by using a five-nucleotide spacer between the two hexamers (consensus sequence, TTCATAN5TTCATA). Indeed, an additional nucleotide in the spacer modifies the distance between the nucleotide motif of the hexamers from 10 to 11 bp, meaning that the same motifs are still present on the same side of the DNA helix and, thus, might still allow TorR binding. However, no sequence entirely matching the consensus was found within the S. oneidensis genome, and, out of the seven sequences containing a single base change in one hexamer, none allowed TorR binding in vitro (data not shown). These results support the idea that TorR recognizes highly specific sequences present at only a restricted number of sites on the chromosome of S. oneidensis.
To check that TorR binds to the consensus sequence TTCATAN4TTCATA in the torR and torF promoters, we carried out a DNase I footprinting analysis with the DNA fragments used for the retardation experiments. As shown in Fig. 2C, in both cases a single region was protected against DNase I digestion when TorR was present. The protected regions extend over 25 to 27 nucleotides, and they cover the entire direct repeat sequences of the torR and torF promoters. This finding confirms that TorR recognizes operator sites containing a TTCATA repeat and suggests that it controls torR and torF gene expression.
Negative autoregulation of the torR gene.
We tried to define the transcription start site of torR by primer extension experiments with RNA prepared from S. oneidensis MR1-R cells grown anaerobically with or without TMAO. These experiments were unsuccessful, probably because the amount of torR messenger was too low. To solve this problem, we fused the putative promoter region of torR to the promoterless lacZ gene of plasmid pGE593, and we introduced the resulting multicopy plasmid (pPTorRso) into an E. coli strain (LCB436) from which the entire tor locus was deleted to avoid any interference. We then carried out primer extension by using RNA prepared from the plasmid-containing E. coli cells and a primer hybridizing to the 5′ end of lacZ (Fig. 3B). A transcription start site was located 23 bases upstream of the torR start codon. To confirm that the transcription start site of torR was identical in E. coli and S. oneidensis, we performed RT PCR by using RNA extracted from strain MR1-R and appropriate convergent oligonucleotide pairs (Fig. 3). When the upstream primer (R7) that hybridizes to the 5′ end of the potential torR messenger was used, a PCR product of the expected size was observed, but when an upstream primer (R8) complementary to the sequence just upstream of the putative transcription start site was used, no DNA fragment was amplified. The RT PCR experiment thus shows that the position of the torR transcription start site in S. oneidensis is identical or close to that defined by primer extension in E. coli.
A −10 promoter box (AATAAT) close to the E. coli −10 consensus sequence is correctly positioned relative to the start site, but the putative −35 box (TATGCA) is far from the E. coli −35 consensus box (TTGACA), supporting the idea that the torR promoter is weakly expressed in S. oneidensis. Moreover, one hexamer of the TorR operator site overlaps the −10 box, and, as a result, the TorR binding region which extends from position −3 to position −29 covers the −10 box (Fig. 2). Interaction of TorR with the torR promoter might thus hamper the correct binding of the RNA polymerase to this promoter, and, consequently, TorR might repress expression of its own gene. To test a possible negative autoregulation of the torR gene, we performed real-time PCR from total RNA extracted from MR1-R cells grown anaerobically with or without TMAO. The cDNA samples were synthesized by using random hexamers as primers, and the real-time PCR was carried out by using a torR specific primer pair (Table 1, R1-R2). Real-time PCR was also performed with a 16S-specific primer pair (16S1-16S2) to quantify the amount of 16S RNA in each sample, and the relative level of torR transcript was then normalized to that of the 16S RNA. As shown in Table 2, the amount of torR transcript decreased almost threefold when the cells were grown in the presence of TMAO, meaning that the expression of torR is negatively autoregulated, as expected from the in vitro experiments (Fig. 2). Unfortunately, the control experiment with RNA extracted from the torR strain (SOR-3) was not feasible because the mutation in this strain corresponds to an ISSo2 insertion into the torR promoter region, leading to the absence of torR transcription (6). However, the torR transcript levels were similar in the torS strain (SOS-2) grown with or without TMAO (induction increased by a factor of 1.3 ± 0.3 [mean ± standard deviation]), thus confirming that the torR gene is negatively regulated by phosphorylated TorR.
TABLE 2.
Analysis of the expression levels of torC, torF, and torR genes by real-time PCR
| Gene | Induction factora (with TMAO/without TMAO)
|
|
|---|---|---|
| MR1-R | SOR-3 | |
| torC | 21.6 ± 1.9 | 1.2 ± 0.3 |
| torF | 63.1 ± 2.8 | 1.6 ± 0.7 |
| torR | 0.35 ± 0.04 | ND |
Values, normalized to the value of the 16S rRNA, were calculated as indicated in Materials and Methods. Values represent the means ± standard deviations of three independent experiments. ND, not determined.
In E. coli, the torR gene is also negatively autoregulated, but this autoregulation occurs even in a torS strain or in the absence of TMAO (2). In fact, the E. coli torR gene is always repressed because phosphorylated as well as unphosphorylated TorR binds to a high-affinity binding site overlapping the torR transcription start site (30). The situation is quite different in S. oneidensis since torR negative autoregulation occurs in the presence of TMAO and, thus, probably involves only the phosphorylated form of TorR. Consequently, TorR negative autoregulation maintains the TorR concentration at a low level whatever the growth conditions in E. coli, whereas in S. oneidensis, it decreases TorR production when TMAO is present in the medium. The reason for this subtle difference is unknown, but, in general, negative autoregulation has homeostatic properties and allows the production of a precise amount of regulator in the cell. Although more than one-third of the transcriptional factors are negatively autoregulated in E. coli, several response regulators proved to be positively autoregulated (17, 23). One proposal to explain positive autoregulation is that an increased concentration of a given response regulator is required in inducing conditions when the regulator controls many genes and must, therefore, bind to many targets at the same time (4). The restricted number of targets for TorR of E. coli and S. oneidensis might explain why TorR is negatively rather than positively autoregulated in both strains.
Activation of the gene torF (SO4694) by TorR.
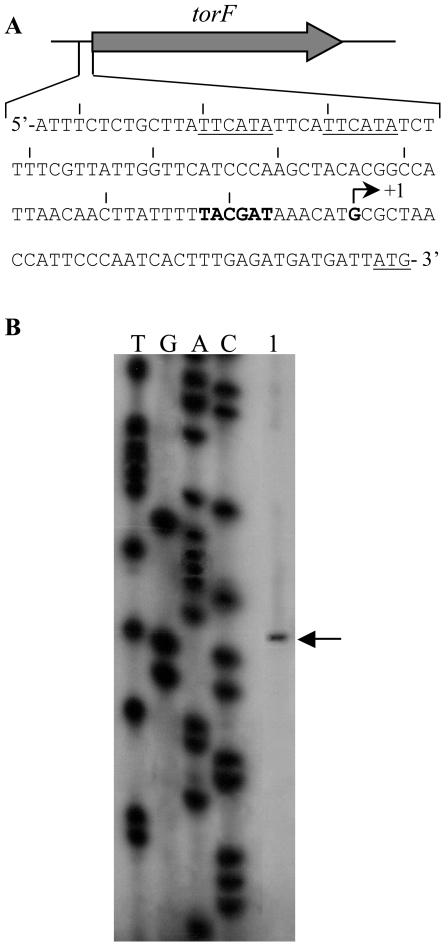
The transcription start site of torF was defined by a primer extension experiment with RNA prepared from MR1-R cells grown anaerobically with TMAO (Fig. 4). A single start site was located 34 bases upstream of the torF initiation codon, and a −10 promoter box (TACGAT) was found correctly positioned relative to the start site. In contrast, no putative −35 box could be found 16 to 18 bp upstream of the −10 box, but the TorR binding site is centered 74 bp upstream of the start site at a position compatible with that of an activator binding site (Fig. 2 and 4). To follow expression of torF, we carried out real-time PCR experiments from total RNA prepared from strains MR1-R and SOR-3 grown with or without TMAO. As shown in Table 2, torF expression was strongly induced by TMAO in strain MR1-R since the amount of torF transcript increased >60-fold when TMAO was added. In contrast, torF was poorly induced in strain SOR-3. These results clearly indicate that TorR is responsible for the strong induction of torF and confirm that TorR mediates TMAO signaling in S. oneidensis.
FIG. 4.
(A) Nucleotide sequence of the torF promoter region. The transcription start site (+1) is indicated. The −10 region is indicated in bold. The ATG initiation codon and the direct repeats are underlined. Vertical bars above the sequence are positioned every 10 bases from the transcription start site. (B) Location of the transcription start point of gene torF. Labeled F5 primer, complementary to a torF internal sequence, was annealed to total RNA from S. oneidensis MR1-R cells grown anaerobically in the presence of TMAO and extended with RT (lane 1). The sequencing reactions were performed with the same primer as in the primer extension reaction. The samples were loaded on an 8% polyacrylamide-8 M urea electrophoresis gel. The arrow points out the transcription start site.
By using a plasmid-borne torE′-lacZ fusion, we have previously shown that the β-galactosidase activities increased almost 40-fold in the presence of TMAO in the wild-type context, whereas no significant increase was observed in the torR strain SOR-3 (6). Although these data are consistent with a strong TMAO induction of the torECAD operon mediated by TorR, they were indirectly assessed from a multicopy plasmid. To confirm tor operon activation and to compare it with that of torF, we carried out real-time PCR with torC-specific primers (C1-C2) and the cDNA samples generated for the torF expression study. As shown in Table 2, the torC induction factor was >20-fold in strain MR1-R, whereas it was close to 1 in strain SOR-3. The real-time PCR experiments thus confirm that the torECAD operon is activated by TorR, but the level of induction is somewhat lower for the torECAD operon than for the torF gene. This result was quite unexpected because the torECAD operon encodes the TMAO reductase respiratory complex and, thus, was supposed to be the main target of the TMAO response regulator TorR. In any case, the fact that torF is strongly induced by TorR suggests that the TorF protein plays a key role either in the TMAO respiratory system or in another TMAO-related pathway. A genome-wide transcriptional analysis has recently revealed that in E. coli the TorS/TorR phosphorelay system positively regulated the tnaLAB operon in addition to torCAD, but the TMAO induction factors, measured either from DNA arrays or from lacZ fusions, were clearly higher for torCAD than for tnaLAB (7).
TorF belongs to a new family of proteins of unknown function.
The torF gene (SO4694) is a monocistronic unit encoding a putative protein of 245 residues with a calculated molecular mass of 26,998 Da. The amino acid sequence was compared with those of the proteins listed in the databases, and significant similarity was detected with several putative proteins encoded by various genomes of proteobacteria including Azotobacter vinelandii (Avin4116), Bordetella pertussis (BP1724), and Caulobacter crescentus (CC2658). However, no protein homologous to TorF is encoded by the related genome of Vibrio cholerae or by that of E. coli, and, in particular, no similarity was found with either TnaA or TnaB. Interestingly, one of the homologous proteins is encoded by a gene of S. oneidensis (SO3502), meaning that the torF gene might have been duplicated in this strain. So far, no biological function has been assigned to any of these homologues. These proteins could thus be classified in a new family of conserved proteins of unknown function.
Concluding remarks.
The analysis of the DNA targets of the TMAO regulator TorR of S. oneidensis revealed that TorR recognizes highly specific operator sites containing a direct repeat of the sequence TTCATA. The TorR binding sites were only found in the promoters of torECAD, torF, and torR, and they allow TMAO induction of the torECAD and torF units and TMAO repression of the torR gene. Since the torF gene which encodes a protein of unknown function is coregulated with the torECAD operon encoding the TMAO respiratory system, we propose that TorF plays a specific role related to TMAO respiration. Future investigation will aim to define the function of TorF and of the other members of the TorF family.
Acknowledgments
We thank A. Manvell and C. Appia-Ayme for reviewing the manuscript, J. C. Patte for helpful suggestions, and L. Théraulaz for expert technical assistance. We also thank the Institute for Genomic Research for genome sequence data.
This work was supported by grants from the Centre National de la Recherche Scientifique and the Université de la Méditerranée. C.B. was supported by grants from the MENRT and from the Fondation pour la Recherche Médicale (FRM).
REFERENCES
- 1.Ansaldi, M., C. Bordi, M. Lepelletier, and V. Méjean. 1999. TorC apocytochrome negatively autoregulates the trimethylamine N-oxide (TMAO) reductase operon in Escherichia coli. Mol. Microbiol. 33:284-295. [DOI] [PubMed] [Google Scholar]
- 2.Ansaldi, M., G. Simon, M. Lepelletier, and V. Méjean. 2000. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J. Bacteriol. 182:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, E. L., and H. S. Kwan. 1985. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 39:131-149. [DOI] [PubMed] [Google Scholar]
- 4.Bijlsma, J. J., and E. A. Groisman. 2003. Making informed decisions: regulatory interactions between two-component systems. Trends Microbiol. 11:359-366. [DOI] [PubMed] [Google Scholar]
- 5.Blanco, A. G., M. Sola, F. X. Gomis-Ruth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure (Cambridge) 10:701-713. [DOI] [PubMed] [Google Scholar]
- 6.Bordi, C., C. Iobbi-Nivol, V. Méjean, and J. C. Patte. 2003. Effects of ISSo2 insertions in structural and regulatory genes of the trimethylamine oxide reductase of Shewanella oneidensis. J. Bacteriol. 185:2042-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bordi, C., L. Théraulaz, V. Méjean, and C. Jourlin-Castelli. 2003. Anticipating an alkaline stress through the Tor phosphorelay system in Escherichia coli. Mol. Microbiol. 48:211-223. [DOI] [PubMed] [Google Scholar]
- 8.Chung, C. T., and R. H. Miller. 1988. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res. 16:3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dos Santos, J. P., C. Iobbi-Nivol, C. Couillault, G. Giordano, and V. Méjean. 1998. Molecular analysis of the trimethylamine N-oxide (TMAO) reductase respiratory system from a Shewanella species. J. Mol. Biol. 284:421-433. [DOI] [PubMed] [Google Scholar]
- 10.Eraso, J. M., and G. M. Weinstock. 1992. Anaerobic control of colicin E1 production. J. Bacteriol. 174:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gon, S., C. Jourlin-Castelli, L. Théraulaz, and V. Méjean. 2001. An unsuspected autoregulatory pathway involving apocytochrome TorC and sensor TorS in Escherichia coli. Proc. Natl. Acad. Sci. USA 98:11615-11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gon, S., J. C. Patte, J. P. Dos Santos, and V. Méjean. 2002. Reconstitution of the trimethylamine oxide reductase regulatory elements of Shewanella oneidensis in Escherichia coli. J. Bacteriol. 184:1262-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gram, L., and P. Dalgaard. 2002. Fish spoilage bacteria-problems and solutions. Curr. Opin. Biotechnol. 13:262-266. [DOI] [PubMed] [Google Scholar]
- 14.Gram, L., and H. H. Huss. 1996. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 33:121-137. [DOI] [PubMed] [Google Scholar]
- 15.Hatton, A. D., and S. W. Gibb. 1999. A technique for the determination of trimethylamine-N-oxide in natural waters and biological media. Anal. Chem. 71:4886-4891. [DOI] [PubMed] [Google Scholar]
- 16.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 17.Hoffer, S. M., H. V. Westerhoff, K. J. Hellingwerf, P. W. Postma, and J. Tommassen. 2001. Autoamplification of a two-component regulatory system results in “learning” behavior. J. Bacteriol. 183:4914-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jourlin, C., M. Ansaldi, and V. Méjean. 1997. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J. Mol. Biol. 267:770-777. [DOI] [PubMed] [Google Scholar]
- 19.Jourlin, C., A. Bengrine, M. Chippaux, and V. Méjean. 1996. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol. Microbiol. 20:1297-1306. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, R. H., and P. H. Yancey. 1999. High contents of trimethylamine oxide correlating with depth in deep-sea taleost fishes, skates, and decapod crustaceans. Biol. Bull. 196:18-25. [DOI] [PubMed] [Google Scholar]
- 21.Kenney, L. J. 2002. Structure/function relationships in OmpR and other winged-helix transcription factors. Curr. Opin. Microbiol. 5:135-141. [DOI] [PubMed] [Google Scholar]
- 22.Lejona, S., A. Aguirre, M. L. Cabeza, V. E. Garcia, and F. C. Soncini. 2003. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J. Bacteriol. 185:6287-6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Antonio, A., and J. Collado-Vides. 2003. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6:482-489. [DOI] [PubMed] [Google Scholar]
- 24.Méjean, V., C. Iobbi-Nivol, M. Lepelletier, G. Giordano, M. Chippaux, and M. C. Pascal. 1994. TMAO anaerobic respiration in Escherichia coli: involvement of the tor operon. Mol. Microbiol. 11:1169-1179. [DOI] [PubMed] [Google Scholar]
- 25.Minagawa, S., H. Ogasawara, A. Kato, K. Yamamoto, Y. Eguchi, T. Oshima, H. Mori, A. Ishihama, and R. Utsumi. 2003. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J. Bacteriol. 185:3696-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 27.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor, L. M., and R. P. Gunsalus. 2000. Anaerobic respiratory growth of Vibrio harveyi, Vibrio fischeri and Photobacterium leiognathi with trimethylamine N-oxide, nitrate and fumarate: ecological implications. Environ. Microbiol. 2:399-406. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, V. L., T. Wu, and A. M. Stock. 2003. Structural analysis of the domain interface in DrrB, a response regulator of the OmpR/PhoB subfamily. J. Bacteriol. 185:4186-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon, G., C. Jourlin, M. Ansaldi, M. C. Pascal, M. Chippaux, and V. Méjean. 1995. Binding of the TorR regulator to cis-acting direct repeats activates tor operon expression. Mol. Microbiol. 17:971-980. [DOI] [PubMed] [Google Scholar]
- 31.Yancey, P. H., W. R. Blake, and J. Conley. 2002. Unusual organic osmolytes in deep-sea animals: adaptations to hydrostatic pressure and other perturbants. Comp. Biochem. Physiol. A 133:667-676. [DOI] [PubMed] [Google Scholar]
- 32.Zou, Q., B. J. Bennion, V. Daggett, and K. P. Murphy. 2002. The molecular mechanism of stabilization of proteins by TMAO and its ability to counteract the effects of urea. J. Am. Chem. Soc. 124:1192-1202. [DOI] [PubMed] [Google Scholar]