Abstract
In end-stage heart failure, left ventricular assist devices (LVADs) represent an exciting new frontier in which post-device-implantation survival approaches that of heart transplant. However, expansion of this technology is still limited by complications that impact morbidity and mortality. Thus, it is essential to identify and optimize modifiable predictors of poor outcomes. One such predictor may be hypertension (HTN). Not only may chronic HTN as a traditional cardiovascular risk factor be present during long-term LVAD support, but HTN may also contribute to device malfunction or device-associated complications. Although current guidelines identify blood pressure (BP) control as important to outpatient continuous flow (CF) LVAD management, there is no evidence base to support these guidelines. Indeed, our comprehensive literature search did not identify any studies that evaluated post-device-implantation HTN as a potential predictor of adverse CF-LVAD outcomes. Hypertension among CF-LVAD patients is likely a relatively unstudied factor because of difficulties using standard non-invasive techniques to measure BP in the setting of reduced pulsatile flow. Fortunately, recent research has elucidated the meaning of Doppler BP measurements and validated a slow-deflation cuff system for BP measurements in the setting of CF-LVAD support. Therefore, CF-LVAD researchers and clinicians may i) consider potential mechanisms relating HTN to poor outcomes, ii) realize that HTN management is a stated goal despite scarce evidence, and iii) utilize the new reliable and valid methods for outpatient BP measurement that make research and management possible. It is critical and now feasible that research on HTN in the CF-LVAD patient population move forward.
Keywords: hypertension, blood pressure, left ventricular assist devices, outcomes
Introduction
The survival rate with current LVAD technology is approaching that of heart transplant. 1 Further improvement in LVAD survival stemming from evolving technology and management approaches may justify a future shift in triaging patients from transplant to destination therapy LVAD. In the meantime, LVAD complications negatively impact morbidity and mortality to an extent that limits such expansion of this technology. Pending technological advances, clinicians and researchers must continue to elucidate modifiable predictors of poor outcomes in order to optimize contemporary, continuous-flow (CF) LVAD management and thereby reduce morbidity and mortality.
Hypertension (HTN) is an established long-term risk factor for cardiovascular disease and may represent a risk factor for poor outcomes among CF-LVAD patients. Not only may HTN cause CF-LVAD dysfunction or contribute to CF-LVAD-associated complications but, additionally, chronic HTN, as a traditional cardiovascular risk factor, may negatively impact outcomes during long-term CF-LVAD support.2, 3 However, there is little literature to provide an evidence base for HTN management among LVAD patients, mainly because of methodological challenges in its measurement due to reduced pulse pressure (PP).
The goal of this review is to describe the state of current knowledge regarding HTN as a prognostic factor among patients on CF-LVAD support.
Hypertension as a Potential Risk Factor among LVAD Patients
Hypertension may be a risk factor for poor outcomes among CF-LVAD patients by contributing to device dysfunction and device-related complications (Figure). The mechanisms involved reflect important hemodynamic and pathophysiologic consequences of CF-LVAD’s unique physiology. Unlike in the setting of a pulsatile LVAD, afterload or systemic vascular resistance may greatly affect the amount of cardiac output support provided by a CF-LVAD.3 Thus, poorly controlled BP may have detrimental consequences in LVAD patients for many different reasons. First, by decreasing CF-LVAD flow it may contribute to device thrombosis. Thrombosis itself may in turn lead both to device dysfunction resulting in worsening heart failure and to thromboembolism resulting in stroke as clots can propel through the device, embolizing systemically.4 Second, by reducing CF-LVAD flow and ventricular unloading, poorly controlled BP may increase left ventricular filling pressures leading to worsening heart failure symptoms and eventually to hospitalization for hemodynamic optimization using intravenous diuretic and inotropic agents. Third, elevated right and left ventricular filling pressure may lead to stretch- and subendocardial ischemia-induced ventricular arrhythmias,5 particularly among CF-LVAD patients with a history of pre-operative ventricular arrhythmias.6 Finally, HTN may also promote de-novo aortic insufficiency (AI) among CF-LVAD patients, with increased afterload possibly contributing to decreased frequency of aortic valve opening, commissural fusion, and ultimately AI.7, 8 Our recent CF-LVAD cohort analysis does not necessarily support this hypothesis that HTN might induce AI but our analysis of BP as a potential risk factor for AI was limited by the study’s cross-sectional nature and few BP measurements.9
Figure.
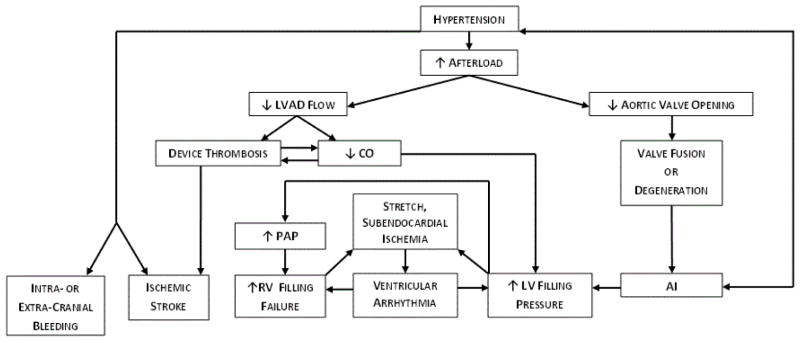
Pathophysiological relationships between HTN and device-related complications in CF-LVADs.
AI = aortic insufficiency; CO = cardiac output; LV = left ventricular; PAP = pulmonary artery pressure; RV = right ventricular
With longer duration of LVAD support, patients can be also exposed to HTN as a traditional risk factor, as it exists among non-LVAD patients. The duration of LVAD support has extended considerably: 44% of adult, first-time mechanical circulatory support recipients are undergoing destination therapy device implantation and 10% undergo bridge-to-transplant implantation with only a “moderate” or “unlikely” chance of ever receiving a heart transplant.10 Even patients with a higher likelihood of transplantation have progressively longer device duration because transplant wait list times are extending in several UNOS Regions and for blood type O patients in particular.11 Therefore, with chronic support, exposure to traditional risk factors such as HTN is an important consideration.
Chronic, poorly controlled HTN can affect CF-LVAD patients in several ways. Sustained HTN can be linked with systemic complications such as vascular disease and bleeding.2, 12 Hypertension can perpetuate and contribute to the deterioration of AI independently of aortic valve opening.13 Additionally, HTN is a well-established risk factor for both ischemic and non-traumatic hemorrhagic stroke in non-LVAD patients.14–16 Hemorrhagic stroke among CF-LVAD patients is of particular concern because the incidence rate is high, ranging from 0.05 (HeartMate, Thoratec)17 to 0.09 (HVAD, HeartWare)18 events per patient year, and it represents one of leading causes of CF-LVAD associated death.19 Finally, poorly controlled HTN in the setting of anticoagulation – a standard of LVAD management – poses an even greater bleeding risk. Two scores – HAS-BLED and HEMORR2HAGES – designed and utilized to assess bleeding risk among patients on anticoagulation (albeit with the clinical indication being atrial fibrillation rather than LVAD) both include uncontrolled HTN as an independent risk factor.20, 21
Hypertension as a Target in CF-LVAD Management
Manuscripts as well as current International Society for Heart and Lung Transplantation guidelines regarding the clinical management of LVADs identify HTN management as a specific component of post-implantation patient care, although specific BP goals for CF-LVAD patients vary slightly according to the publication.2, 3, 22 Wilson et al. suggest a MAP goal of 70–90 mmHg2 while Slaughter et al. suggest 70–80 mmHg.3 International Society for Heart and Lung Transplantation guidelines advise a goal MAP <80 mmHg, but this recommendation is a Class IIb recommendation based on level of evidence C.22 This guideline is based on expert panel consensus due to a lack of randomized studies regarding CF-LVAD patient care.22
An assessment of the extent of this evidence gap was warranted and, as such, we undertook a comprehensive literature search on HTN and outcomes among CF-LVAD patients. Indeed, our methodical search yielded minimal data, providing an important confirmation of the dearth of evidence on this topic. Our search is described below.
Methods and Results of a Literature Search on HTN and CF-LVAD Outcomes
A comprehensive search of the literature was conducted to identify studies relating HTN to adverse outcomes among CF-LVAD patients. Medline was searched through January 17, 2014 with a search strategy based on a combination of three categories of keyword variations: 1) assist devices (heart/(left) ventricular/continuous flow assist device; 2) mechanical circulatory support, HeartMate); and 3) BP (BP, MAP, pulse pressure, systolic/systole), and outcomes (morbidity, mortality, survival/survival rate/survival analysis, post-operative complications, complications, adverse events, patient readmission/re-hospitalization). The search focused on English language studies of human subjects.
The search yielded 397 studies, of which one was relevant to the original search question. However, closer reading showed that it discussed only a single patient case.23 The case study described a patient with poor device output in the setting of HTN, leading the authors to suggest that managing afterload in LVAD patients is an important clinical consideration.23
Other articles identified in the search were reviewed in detail, but they did not address the search question. Seven articles evaluated clinical outcomes among CF-VAD patients but were found to include only pre-LVAD implantation BP measurements24, 25 or HTN diagnosis.8, 26–29 Another four studies sought to predict which patients are most likely to be readmitted or survive post-CF-LVAD;30–33 however, these analyses and risk scores focus nearly exclusively on pre-operative variables and none addressed post-operative BP or HTN or any other medically modifiable, post-operative predictors of outcomes.
Therefore, our comprehensive search did not identify any literature to inform an evidence-based summary on the potential contributions of HTN to CF-LVAD complications. One possible reason for the dearth of data on this important topic is the difficulty in measuring and interpreting BP in CF-LVAD patients.
Measuring BP in the Setting of LVAD Support for HTN Research and Management
Non-invasive BP measurement has been problematic with reduced pulsatile flow. Early generation LVADs incorporated pumps producing pulsatile flow, mimicking physiologic conditions for end-organ perfusion and producing readily measurable systolic blood pressure (SBP) and diastolic blood pressure (DBP). However, pulsatile technology has disadvantages, warranting newer generation CF LVAD designs. Contemporary CF-LVADs produce low-pulsatile flow that translates clinically into lower SBP, PP34 and MAP35 compared to those pressures produced by pulsatile devices.
Currently, the gold-standard for BP measurement among CF-LVAD patients is an invasive arterial line, which is not feasible in the outpatient setting.36 Unfortunately, despite being accurate, traditional automated BP monitors only successfully obtain a BP measurement approximately 50% of the time due to reduced PP with CF-LVAD.37 Alternatives include Doppler ultrasound with sphygmomanometer as well as newer cuff-based technologies.
Doppler is used by many LVAD centers, though it requires specific technical expertise and it produces a single measurement that has had unclear meaning.36 To address this limitation, we recently compared arterial-line and Doppler BP measurements among 30 CF-LVAD in-patients to better understand what Doppler BP measurements represent.38 We found that Doppler BP measurements most closely reflect arterial-line SBP but may also closely reflect MAP in cases of low PP (i.e., below the sample median).38 Therefore, Doppler BP consistently represents SBP and only in situations of low PP should Doppler BP be used as a surrogate for MAP.
Despite this better understanding of Doppler BP measurements, the issue of technical expertise persists, rendering it impractical for use among inexperienced clinic staff or for home BP monitoring. Therefore, newer technologies such as the Terumo Elemano BP Monitor – a novel slow cuff deflation device – have been developed to provide an alternative, valid method of CF-LVAD BP monitoring that requires less technical expertise for obtaining traditional SBP and DBP measurements. Terumo Elemano measurements may closely reflect gold-standard arterial-line measurements: we found that the correlation between Terumo Elemano and arterial line was as high as 0.83 for SBP and as high as 0.75 for MAP.38 Terumo Elemano underestimated SBP by 0.3 mmHg and MAP by 1.7 mmHg.
Thus, our data support the practice of first using Terumo Elemano to measure BP among CF-LVAD patients. We also suggest that if Terumo Elemano measurements are unsuccessful or inconsistent across multiple readings, Doppler should be used to measure SBP in this population.
Conclusion
Research on HTN among CF-LVAD patients is largely absent despite a reasonable and logical expectation that it is likely a risk factor for poor outcomes in this patient population. Expert-consensus guidelines do identify BP control as an important component of outpatient CF-LVAD management,22 but these guidelines do not draw from an evidence base because there are no data available from original research studies on this topic, as demonstrated by our comprehensive literature search. The lack of research regarding HTN among CF-LVAD patients is likely driven by measurement difficulties using standard techniques in the setting of non-pulsatile flow.
Fortunately, recent advances in BP measurement methods for CF-LVAD patients have helped to elucidate the meaning of a Doppler BP measurement and to validate a novel slow-cuff deflation device for BP measurements,38 raising the potential for future studies on HTN among CF-LVAD patients. From this perspective, important evidence may be added from the recently commenced and ongoing supplemental cohort of the ENDURANCE protocol, in which HVAD destination therapy CF-LVAD patients undergo BP management according to a predefined algorithm using Doppler and Terumo and are followed for clinical outcomes, with particular attention paid to neurological events. Other critical next research steps are to: 1) identify the best methods and strategies by which to measure, monitor, and manage BP in hospital, clinic and home settings; 2) establish an evidence-based normal and goal BP range for CF-LVAD patients – perhaps eventually different goals based on the type of CF device pump implanted (i.e., axial or centrifugal); and 3) determine whether HTN is in fact a predictor of CF-LVAD outcomes. Supported by such information, BP control could be an important element of impactful medical management among the growing population of end-stage heart failure patients with long-term CF-LVAD support.
Acknowledgments
Grants/Contracts/Financial Support:
NIH T32 Training Grant HL007343 (Dr. Wasson)
The A. L. Mailman Family Foundation White Plains, NY (Dr. Colombo)
The Schwartz Scholarship New York, NY (Dr. Jorde)
Thoratec (Drs. Jorde and Naka have each received consulting fees of less than $5000 annually)
HeartWare (Drs. Jorde and Uriel have each received consulting fees of less than $5000 annually)
Jarvik (Dr. Jorde has received consulting fees of less than $5000 annually)
Dr. Paolo Colombo would like to acknowledge the A. L. Mailman Family Foundation for its research support. Dr. Ulrich Jorde would like to acknowledge the Schwartz Scholarship.
Abbreviations
- AI
aortic insufficiency
- BP
blood pressure
- CF
continuous flow
- DBP
diastolic blood pressure
- HTN
hypertension
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LVAD
left ventricular assist device
- MAP
mean arterial pressure
- PP
pulse pressure
- SBP
systolic blood pressure
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson L, Miller M, Young JB. Long-term mechanical circulatory support (destination therapy): On track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584–603. doi: 10.1016/j.jtcvs.2012.05.044. discussion 597–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson SR, Givertz MM, Stewart GC, Mudge GH., Jr Ventricular assist devices the challenges of outpatient management. J Am Coll Cardiol. 2009;54:1647–1659. doi: 10.1016/j.jacc.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S, Adamson RM, Blood MS, Camacho MT, Idrissi KA, Petty M, Sobieski M, Wright S, Myers TJ, Farrar DJ. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation. 2012;125:1304–1315. doi: 10.1161/CIRCULATIONAHA.111.060830. [DOI] [PubMed] [Google Scholar]
- 5.Hansen DE, Craig CS, Hondeghem LM. Stretch-induced arrhythmias in the isolated canine ventricle. Evidence for the importance of mechanoelectrical feedback. Circulation. 1990;81:1094–1105. doi: 10.1161/01.cir.81.3.1094. [DOI] [PubMed] [Google Scholar]
- 6.Garan AR, Yuzefpolskaya M, Colombo PC, Morrow JP, Te-Frey R, Dano D, Takayama H, Naka Y, Garan H, Jorde UP, Uriel N. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: Need for primary prevention? J Am Coll Cardiol. 2013;61:2542–2550. doi: 10.1016/j.jacc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Mudd JO, Cuda JD, Halushka M, Soderlund KA, Conte JV, Russell SD. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. The Journal of Heart and Lung Transplantation. 2008;27:1269–1274. doi: 10.1016/j.healun.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circulation: Heart Failure. 2010;3:668–674. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorde UP, Uriel N, Nahumi N, Bejar D, Gonzalez-Costello J, Thomas SS, Han J, Morrison KA, Jones S, Kodali S, Hahn RT, Shames S, Yuzefpolskaya M, Colombo P, Takayama H, Naka Y. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circulation: Heart Failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.113.000878. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Timothy Baldwin J, Young JB. Fifth intermacs annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of Heart and Lung Transplantation. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 11.United states organ transplantation: Optn & srtr annual data report 2011. 2012 [Google Scholar]
- 12.del Conde I, Halperin JL. Ineligibility for anticoagulation in patients with atrial fibrillation. The American journal of medicine. 2013;126:105–111. doi: 10.1016/j.amjmed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Bekeredjian R, Grayburn PA. Valvular heart disease: Aortic regurgitation. Circulation. 2005;112:125–134. doi: 10.1161/CIRCULATIONAHA.104.488825. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 16.Marsh JD, Keyrouz SG. Stroke prevention and treatment. J Am Coll Cardiol. 2010;56:683–691. doi: 10.1016/j.jacc.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 17.Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: An analysis of more than 900 heartmate ii outpatients. Journal of the American College of Cardiology. 2014;63:880–888. doi: 10.1016/j.jacc.2013.08.1656. [DOI] [PubMed] [Google Scholar]
- 18.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier OH, Jeevanandam V, Anderson AS, Kormos RL, Teuteberg JJ, Levy WC, Naftel DC, Bittman RM, Pagani FD, Hathaway DR, Boyce SW. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 19.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. Journal of the American College of Cardiology. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. CHEST Journal. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 21.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: Results from the national registry of atrial fibrillation (nraf) American heart journal. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J. The 2013 international society for heart and lung transplantation guidelines for mechanical circulatory support: Executive summary. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Kouatli Y, Neill S, Pagani F. Considerations on afterload management for patients with centrifugal ventricular assist devices. International Journal of Artificial Organs. 2013;36:69–72. doi: 10.5301/ijao.5000173. [DOI] [PubMed] [Google Scholar]
- 24.Bogaev RC, Pamboukian SV, Moore SA, Chen L, John R, Boyle AJ, Sundareswaran KS, Farrar DJ, Frazier OH, HeartMate IICI. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. Journal of Heart & Lung Transplantation. 2011;30:515–522. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the intermacs scale to predict outcomes after mechanical assist device implantation. Journal of Heart & Lung Transplantation. 2009;28:827–833. doi: 10.1016/j.healun.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO Journal. 2012;58:499–502. doi: 10.1097/MAT.0b013e318268ea80. [DOI] [PubMed] [Google Scholar]
- 27.Kato TS, Schulze PC, Yang J, Chan E, Shahzad K, Takayama H, Uriel N, Jorde U, Farr M, Naka Y, Mancini D. Pre-operative and post-operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:1–8. doi: 10.1016/j.healun.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW. An analysis of pump thrombus events in patients in the heartware advance bridge to transplant and continued access protocol trial. The Journal of Heart and Lung Transplantation. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Holman WL, Kormos RL, Naftel DC, Miller MA, Pagani FD, Blume E, Cleeton T, Koenig SC, Edwards L, Kirklin JK. Predictors of death and transplant in patients with a mechanical circulatory support device: A multi-institutional study. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:44–50. doi: 10.1016/j.healun.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Imamura T, Kinugawa K, Shiga T, Endo M, Kato N, Inaba T, Maki H, Hatano M, Yao A, Nishimura T, Hirata Y, Kyo S, Ono M, Nagai R. Novel risk scoring system with preoperative objective parameters gives a good prediction of 1-year mortality in patients with a left ventricular assist device. Circulation journal : official journal of the Japanese Circulation Society. 2012;76:1895–1903. doi: 10.1253/circj.cj-12-0182. [DOI] [PubMed] [Google Scholar]
- 32.Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: The heartmate ii risk score. J Am Coll Cardiol. 2013;61:313–321. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 33.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Stulak JM, Joyce L, Daly R, Park SJ, Kushwaha SS. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 34.Kamdar F, Boyle A, Liao K, Colvin-adams M, Joyce L, John R. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. The Journal of Heart and Lung Transplantation. 2009;28:352–359. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Klotz S, Deng MC, Stypmann J, Roetker J, Wilhelm MJ, Hammel D, Scheld HH, Schmid C. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. The Annals of thoracic surgery. 2004;77:143–149. doi: 10.1016/s0003-4975(03)01336-5. discussion 149–150. [DOI] [PubMed] [Google Scholar]
- 36.Markham DW, Fu Q, Palmer MD, Drazner MH, Meyer DM, Bethea BT, Hastings JL, Fujimoto N, Shibata S, Levine BD. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circulation: Heart Failure. 2013;6:293–299. doi: 10.1161/CIRCHEARTFAILURE.112.969873. [DOI] [PubMed] [Google Scholar]
- 37.Bennett MK, Roberts CA, Dordunoo D, Shah A, Russell SD. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. Journal of Heart & Lung Transplantation. 2010;29:593–594. doi: 10.1016/j.healun.2009.11.604. [DOI] [PubMed] [Google Scholar]
- 38.Lanier GM, Orlanes K, Hayashi Y, Murphy J, Flannery M, Te-Frey R, Uriel N, Yuzefpolskaya M, Mancini DM, Naka Y, Takayama H, Jorde UP, Demmer RT, Colombo PC. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circulation: Heart Failure. 2013;6:1005–1012. doi: 10.1161/CIRCHEARTFAILURE.112.000186. [DOI] [PubMed] [Google Scholar]


