Abstract
Objective:
To assess whether concurrent blood flow restriction (BFR) during low-load resistance training is an efficacious and tolerable means of improving quadriceps strength and volume in women with risk factors for symptomatic knee osteoarthritis (OA).
Design:
Randomized, double-blinded, controlled trial
Setting:
Exercise training clinical research laboratory
Participants:
Women over age 45 years with risk factors for symptomatic knee OA.
Methods:
Participants were randomized to either low-load resistance training (30% 1RM) alone (control) or with concurrent BFR and completed 4 weeks of 3 times per week leg-press resistance training. Those randomized to BFR wore a cuff that progressively restricted femoral blood flow over the weeks of training. Inter-group differences in outcome measures were compared using regression methods, while adjusting for BMI.
Main Outcome Measures:
Isotonic bilateral leg press strength, isokinetic knee extensor strength, and quadriceps volume by MRI were assessed before and after participation. Secondary measures included lower limb muscle power (leg press and stair climb). Knee pain was assessed to determine tolerance.
Results:
Forty women completed the program out of 45 who consented. There were no significant inter-group differences in baseline characteristics except that BMI was lower in the BFR group (p=.0223). Isotonic 1RM improved significantly more in the BFR group (28.3±4.8 kg) than in the control group (15.6±4.5 kg) (p=.0385). Isokinetic knee extensor strength scaled to body mass increased significantly more in the BFR group (0.07±0.03 Nm/kg) than in the control group (-0.05±0.03 Nm/kg) (p=.0048). Changes in quadriceps volume, leg press power, and knee-related pain did not significantly differ between groups.
Conclusions:
Addition of BFR to a 30% 1RM resistance training program was effective in increasing leg press and knee extensor strength in women at risk for knee OA, in comparison with the same program without BFR.
Keywords: blood flow restriction, quadriceps strength, knee osteoarthritis, resistance training
Introduction
An estimated 45% of Americans will develop symptomatic knee osteoarthritis (OA) [1], characterized by radiographic findings and persistent knee pain or stiffness. Recent observational evidence indicates that higher knee extensor strength in women without the disease reduces risk for developing incident symptomatic knee OA (the combination of knee OA with daily symptoms) [2]. Furthermore, in adults who have knee OA, higher knee extensor strength reduces risk for progression of joint space narrowing [3]. Together, these results suggest that knee extensor strength may be a modifiable risk factor for knee OA.
In order to translate these observational findings to an interventional study, a well-tolerated and effective strengthening program is needed. Organizations such as the American College of Sports Medicine have indicated that a minimum resistance training load of 70-85% of the one-repetition maximum (1RM) is necessary to achieve muscle hypertrophy and 60-70% is necessary to gain strength [4]. High loads can be problematic for those who have knee pain or a history of a knee injury or surgery, although these factors confer risk for development or worsening of symptomatic knee OA[1]. Loading an already painful or damaged joint at this percentage of 1RM can be poorly tolerated and possibly injurious for an individual already suffering knee pain and stiffness during activities of daily living. Due to poor tolerance or excess risk conferred by the exercise loads prescribed to elicit strength gains, there is a need for alternative means of increasing strength while avoiding exacerbation of symptoms or disease risk.
Augmentation of resistance training with blood flow restriction (BFR) to the exercising muscle may offer older adults, and particularly those with or at elevated risk for knee OA, the ability to develop the strength necessary to attenuate disease incidence and progression while avoiding deleterious joint loading. There is a growing base of evidence in people without knee OA that supports the ability of BFR combined with low load resistance training to stimulate significant muscle hypertrophy and strength gains even when performed at a resistance of only 15-40% of 1RM [5-7]. The gains in muscle strength and hypertrophy achieved with low-load resistance training and BFR have been similar to those of more traditional strength training programs [7]. Evidence regarding BFR training supports safety equivalent to traditional exercises [8]. The use of BFR with low-load resistance training on women at risk for knee OA may effectively stimulate muscle strength and hypertrophy without exacerbating knee pain.
The aim of this study was to assess the efficacy of a four-week, low-load resistance-training program with concurrent application of BFR to the exercising limbs to improve knee extensor strength (primary), quadriceps muscle volume, and lower limb muscle power in women with risk factors for symptomatic knee OA. This study focused on women because observational studies demonstrated a stronger association between strength and knee OA development in women [2, 9], and strengthening interventions may have different effects on women than men [10]. The secondary aim was to assess whether the training program adversely affected knee-related pain, activities of daily living or quality of life. A similar BFR program has been shown to increase muscle strength and bulk in older adults without knee problems [6], but has not been tested in adults at risk for knee OA. These aims were achieved utilizing a double-blinded, randomized, controlled experimental design, comparing low-load training with and without BFR.
Methods
Participants
A total of 45 women between the ages of 45–65 years volunteered to participate in the study. The participants were recruited through e-mail, postal letters, and study notices in area clinics, newspapers and businesses. All participants had at least one of the following risk factors for symptomatic knee OA: Body Mass Index (BMI) greater than or equal to 25 kg/m2, a history of a knee joint injury or surgery, knee symptoms (pain, aching, or stiffness) on most of the last 30 days, or reported being told that they had radiographic knee OA. No participants had been involved in resistance training in the three months prior to the study. Furthermore, no participants had any conditions that would prevent them from safely taking part in this exercise intervention (e.g. bilateral knee replacements; lower limb surgery in the last six months; back, hip or knee problems that affect walking; diagnosis of inflammatory joint or muscle disease, such as rheumatoid or psoriatic arthritis or polymyalgia rheumatica; neurologic diagnoses, such as multiple sclerosis or peripheral neuropathy; history of cancer, peripheral vascular disease or deep venous thrombosis; history of myocardial infarction or stroke in the last year; chest pain during exercise or at rest; or need for supplemental oxygen).
The study was approved by the Institutional Review Board (IRB) of The University of Iowa and registered at clinicaltrials.gov (study ID NCT01311206). The study was conducted between 9/23/2011 and 11/8/2011. All participants provided written informed consent following completion of an IRB-approved consent process. Participants' vital signs were assessed to confirm blood pressure less than 180/100 and resting heart rate >40 and <110 beats per minute while seated in a chair.
Research staff members, who were not involved in outcome assessments, 1:1 randomized participants to low-load exercise with or without BFR. A random number generator (randomization.com) was used for group allocation and sealed opaque envelopes, containing group assignments, were opened sequentially. Participants were unaware of which exercise intervention was considered therapeutic and were instructed not to discuss their intervention experience with other study participants if they met them incidentally. Appointments were conducted individually and staggered to avoid interaction between participants.
Prior to initiation of the exercise protocol and following completion, a single assessor completed assessments of each outcome measure. The assessor was uninvolved with the training and blinded to the group to which participants had been randomized [11]. To enhance the quality of the measurements, the staff member who assessed outcome measures was trained and certified in the strength testing protocols and the equipment was calibrated before initiation of the study.
Bilateral Leg-Press Isotonic Strength and Power
In order to determine the appropriate training load, the isotonic leg press strength of each participant was measured on an instrumented pneumatic leg press (Keiser A420, Keiser, Fresno, CA). Participants were seated with both feet on the foot pedals and their knees and hips flexed at 90-degree angles. Participants completed 2 submaximal (lower resistance) sets of 10 repetitions as a warm-up and then began the strength testing by performing bilateral leg presses with increasing weight and standardized verbal encouragement until they could no longer press their legs through their full range of motion. Participants’ 1RM was estimated by finding a challenging weight that each participant could bilateral leg press at least once but no more than 5 times. After each test, participants rated their perceived exertion level on a visual rating of perceived exertion (RPE) scale to determine the difficulty of the resistance and were also asked about whether they experienced joint pain. If the load was considered to be too light (i.e. rated as “somewhat hard” on the RPE scale or participants perceived that she could complete greater than 5 repetitions), then they were given a 3-minute break before attempting with a higher resistance. Participants did not report pain that interfered with pushing during the leg press 1RM testing.
The 1RM estimation method of Lombardi, et al., as cited in McNair, was used to estimate each participant's 1RM based on the number of repetitions completed at the highest resistance the participant could leg press, using the equation: 1RM = (Number of Repetitions).01 × Resistance [12]. The derived 1RM was used to determine the 30% 1RM load used for low-load training.
Following strength testing, bilateral leg press muscle power was assessed at 40% of the estimated 1RM, as is commonly tested in older adults[13]. Participants were again positioned as described above. Again, a standardized script was used to promote maximum effort. After a practice repetition to accommodate to the resistance, participants were instructed to extend their legs as quickly as possible to a point of almost full knee extension and then to slowly return the pedals to the start position. A 30–60 second rest period was provided between attempts. Five attempts at maximal speed were made, and the highest value (Watts), measured and digitally output by the Keiser leg press, was considered to be the peak power. At the follow-up visit, a median of three days following completion of the four weeks of training, the same assessor who remained blinded to treatment allocation repeated these procedures for assessment of bilateral leg press strength and power.
Quadriceps Volume
Magnetic Resonance Imaging (MRI) was used to assess quadriceps volume in 12 participants, six randomly selected from the control group, and six randomly selected from the intervention group (randomization.com). Assessment of trophic changes in the quadriceps muscle were accomplished by acquiring bilateral T1-weighted gradient echo imaging from the iliac crest to the tibial tuberosity using a 3.0T Siemens TIM Trio MRI scanner (Siemens Corporation, Washington, DC). Two volumes were acquired before and after the intervention, one with fat and water in phase (TE = 4.5 ms) and one with opposed phase (TE = 2.2 ms). Images were acquired in the axial plane with 512 × 336 matrix, 8 mm slices, 2 mm gap, and 24 slices per volume. Three volumes were acquired to cover the entire quadriceps muscle volume with automatic table repositioning between each set of scans. The 3 volumes were concatenated to a single volume in post-acquisition processing. The entire set of volumes was acquired over approximately 20 minutes.
Manual segmentation of the quadriceps was completed by tracing the outer borders of the muscle group in each axial slice using a Cintiq 21UX interactive display (Wacom Technology Corp., Vancouver, WA) and the Medical Image Processing, Analysis, and Visualization (MIPAV) software (Center for Information Technology, National Institutes of Health, Bethesda, MD)[14]. Image analysis tools within MIPAV were used to calculate muscle volume, as this has been shown to have high reproducibility and inter-rater reliability (ICC(2,1) = 0.98, p < .001). All MRI analyses were performed by a single analyzer who was blinded to not only the conditions of the participants and time points, but also to the specific aims and methods of the study to ensure unbiased data.
Maximum Isokinetic Strength
While isotonic leg press strength was most specific to the intervention, isokinetic knee extensor strength was measured as well due to the association of this measure with development of incident symptomatic and progressive knee OA [2, 9]. At baseline and final visits, peak isokinetic knee extensor torque for each lower limb was measured using a Biodex System 3 Dynamometer (Biodex Medical Systems, Inc., Shirley, NY). Biodex medical system software version 3.30, System 3 PRO, Rev N was used for data acquisition. Participants were seated in a chair with a hip joint angle of 85°. The center of the lateral femoral condyle was visually aligned with the axis of rotation of the dynamometer. The seat back was positioned such that the participants were comfortably sitting with their back flush against the seat back, and the popliteal fossa overhanging the front edge of the seat by approximately 2 fingerbreadths, leaving the knee free to move. The dynamometer arm length was adjusted to each participant and the shin pad was secured proximal to the medial malleoli with straps. Participants were stabilized with bilateral shoulder straps, a lap belt, and strap over the thigh to be tested, such that participants could not lift their thigh or body off the chair upon extension. Settings for the chair and dynamometer arm positions were recorded upon initial testing and were again used for follow-up testing.
Testing was completed first on the right and then on the left. Participants were familiarized with the strength-testing equipment and counseled on proper lifting technique before performing four practice repetitions, at a low degree of effort (approximately 50% effort) prior to the strength test. After correction for gravitational torque and the warm-up with sub-maximal contractions, participants were instructed to perform 4 maximal isokinetic knee extensions and flexions at 60°/sec through their maximal range of motion between 90° of knee flexion and complete extension. This measure has been found to have excellent test-retest reliability in women with or at risk for knee OA [15, 16]. A research assistant, certified in the research protocol, used a standardized script to verbally encourage participants’ maximal effort. Peak isokinetic knee extensor strength for each lower limb was recorded (Nm).
Stair Climb Muscle Power
As a more functional measure of lower limb power, participants were instructed to ascend eight stairs (vertical height of 1.441 m) as quickly as possible. Participants were instructed to use handrails only if necessary for balance or safety. The stopwatch was started when the participant initiated foot movement and was stopped when both feet arrived on the top step. Times for the two trials, attempted on the same day, were recorded to the nearest 0.01 second and averaged. The reliability for this test has been reported to be excellent (ICC = 0.97) [17]. Stair climb power (Watts) was calculated as the product of gravitational force (Newtons) and vertical distance (meters) divided by time (seconds) [13]. This measure allows comparison of muscle power, scaled to body mass.
Assessment of Knee Pain (Tolerance of the Intervention)
In order to determine whether the intervention led to worsening of knee pain, knee pain was assessed with the Knee Osteoarthritis Outcome Score (KOOS) [18]. The KOOS is a 42-item self-administered questionnaire that covers 5 patient-relevant dimensions: Pain, Other Disease-Specific Symptoms, activities of daily living (ADL), Sport and Recreation, and Knee-related Quality of Life (QOL). This instrument is reliable and responsive in people with knee OA, and sensitive to changes[19].
Intervention
Low-Load Resistance Training Protocol: All participants completed the exercise protocol as outlined in Figure 1, performing 4 sets of bilateral leg presses at 30% of their 1RM, 3 times a week for 4 weeks, using the instrumented leg press [20]. The participants’ training load was not adjusted during the training period. Participants randomized to the control group completed this without using the BFR device and those randomized to the BFR group completed the training while wearing the device. The total time that the cuff was applied was 6.5 minutes—5 minutes of exercise and 1.5 minutes of rest between sets. Individual contraction duration lasted 4 seconds with a 2 second shortening: 2 second lengthening contraction duty cycle, as this has been shown previously to increase the fatigue index [21, 22].
Figure 1.
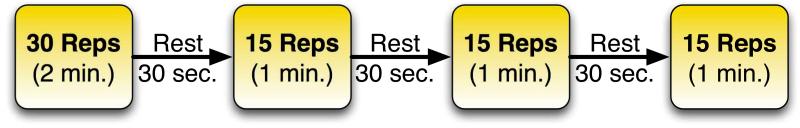
Protocol for BFR Cuff Inflation
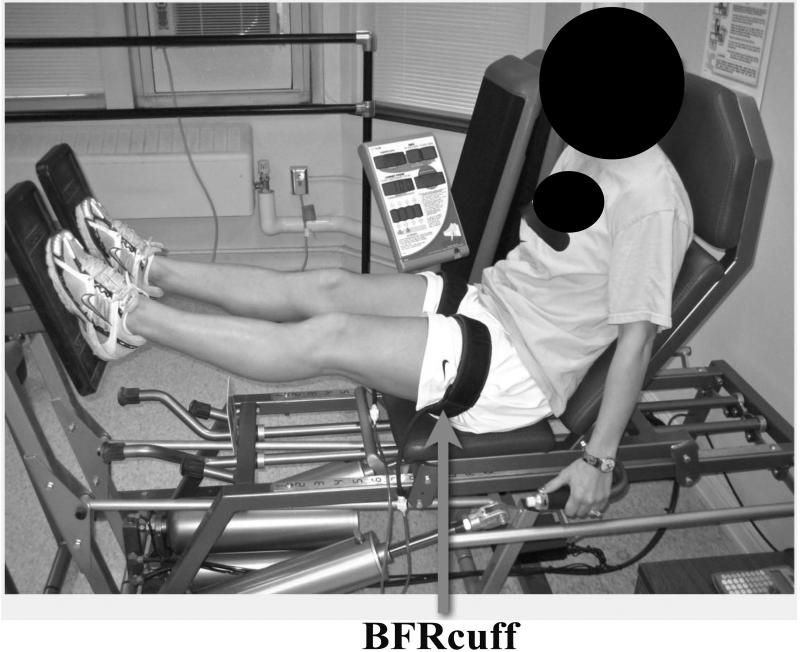
Blood flow restriction (BFR): Femoral blood flow was partially restricted using the Kaatsu Master BFR device (Sato Sports Plaza, Tokyo, Japan), that consists of a control unit, pneumatic pump and two pneumatic cuffs (65 mm in width and 650 mm in length). This BFR device restricts arterial and occludes venous blood flow and causes pooling of blood in capacitance vessels distal to the cuffs (Figure 2) [7, 8, 23]. Before training each leg, participants sat in a chair and the cuff was applied to the proximal thigh as near to the hip joint as was comfortable. For the first training session, the cuffs were inflated to an initial pressure of 30 mmHg (for participant comfort). For all subsequent training sessions, the cuffs were tightened to an initial pressure of 40 mmHg and inflated per the manufacturer’s instructions (Table 1) [24]. The vertical position of the cuff on the thigh was measured using a soft tape measure and the distance from proximal pole of the patella to the distal edge of the cuff was recorded to ensure repeatability at all visits. Once positioned and tightened to the initial cuff pressure, the pneumatic cuffs were then inflated until the desired exercise pressure was reached (Table 1). During Week 1, the beginning incremental inflation pressure was 100 mmHg. For Weeks 2, 3 and 4, the beginning inflation pressure was 120 mmHg[22]. The belt was repeatedly pressurized for 1 minute and then depressurized for 10 seconds in increments of 20 mmHg from 100, 120, or 140 mmHg to the final exercise pressure based on the week of the training, as detailed in Table 1.[24] Participants then performed the 4 sets of leg presses as described above at the final exercise pressure. During exercise and rest periods illustrated in Figure 1, the cuff pressure was continuously maintained and monitored by the BFR apparatus as previously described [23].
Figure 2.
BFR Low-Load Resistance Training
*Model demonstrating research methods is not an actual research subject
Table 1.
Partial Blood Flow Restriction Cuff Pressures
| Week and Session # (week.session) |
Initial Cuff Pressure upon Application (mmHg) |
One Minute Incremental Inflation Pressures (mmHg)* |
Final Exercise Pressure (mmHg) |
|||
|---|---|---|---|---|---|---|
| 1.1 | 30 | 100 | 120 | 140 | 160 | |
| 1.2 | 40 | 100 | 120 | 140 | 160 | |
| 1.3 | 40 | 100 | 120 | 140 | 160 | |
| 2.1-2.3 | 40 | 120 | 140 | 160 | 180 | |
| 3.1-3.3 | 40 | 120 | 140 | 160 | 180 | 200 |
| 4.1-4.3 | 40 | 120 | 140 | 160 | 180 | 200 |
The cuff was repeatedly inflated for 1 minute at each indicated pressure and then deflated for 10 seconds before continuing to the next pressure level.
Statistical Methods
An a priori sample size was estimated based upon prior data collected for the clinically significant difference in isokinetic knee extensor torque [9], and appropriate standard deviations within and between groups. At a two-sided significance level of 0.025, a standard deviation in the knee extensor strength response variable of 12.2 Nm, and a power of 0.80 to detect an inter-group difference in means of 11.4 Nm, a minimum of 19 participants would be necessary in each arm of this two-treatment parallel-design study. To retain sufficient statistical power even with a dropout rate of up to 20%, we aimed to recruit a total of 46 participants. For the secondary outcome, change in muscle volume, a sample size calculation indicated that 6 participants in each of the two groups would provide approximately 80% power to detect an inter-group difference of 3% ± 2% at an alpha level of 0.05. Therefore, 12 participants were randomly selected for muscle volume measurements.
Participant demographics that met normality assumption were summarized using the means±SD. Baseline characteristics of each intervention group were compared using 2-sample t-tests. For each participant, the difference between the pre-intervention and post-intervention measurement values was calculated. The number of visits attended was not normally distributed and was analyzed with non-parametric methods. For within-group analyses, paired t-tests were used for each person-based variable of interest (e.g. 1RM, muscle power, stair climb test, KOOS). For the main study analyses, the inter-group differences in person-based outcome variables were compared using linear regression, adjusting for BMI. For limb-based outcome variables (i.e. knee extensor muscle strength and volume), mixed models were constructed to control for limb as a repeated factor within participants. As significant inter-group differences existed in BMI, analyses were adjusted for BMI, using regression methods appropriate to the data.
Results
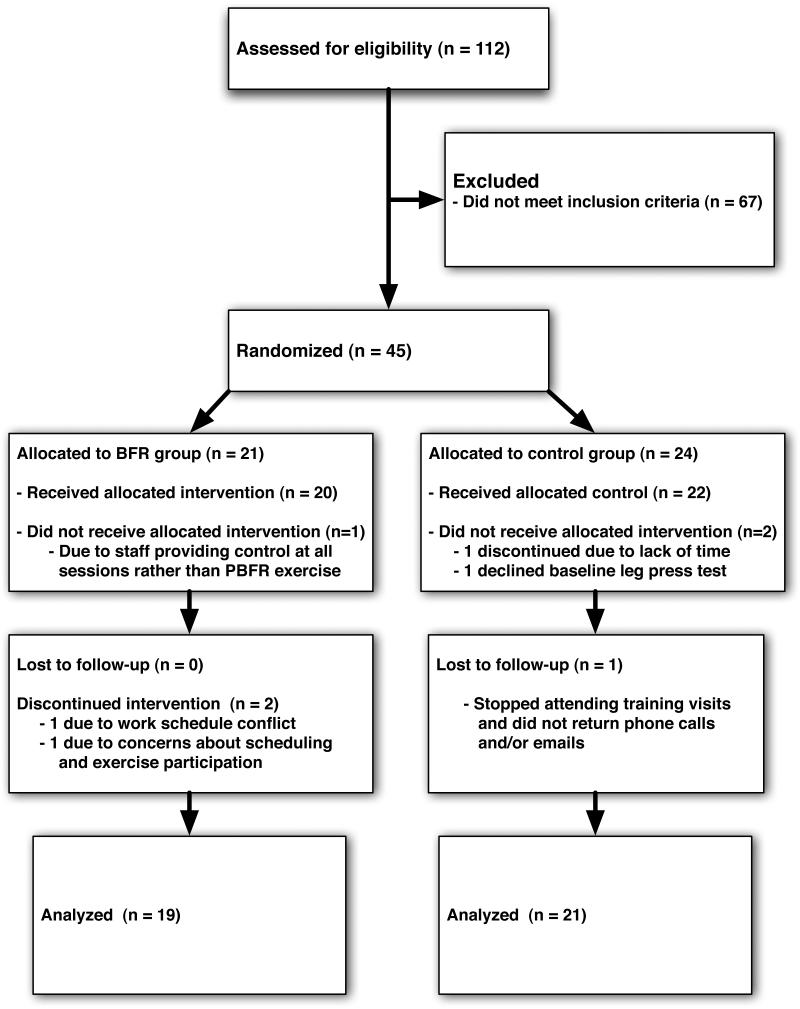
A total of 45 women met eligibility criteria and were enrolled in the study (Figure 3). Following enrollment, five participants discontinued the study due to lack of time (N=3), inability to tolerate the intervention (N=1), and lost to follow-up (N=1). At baseline, there was a statistically significant difference in body size, smaller in the BFR group compared with the control group (Table 2). However, outcomes data was not significantly different when adjusted for BMI. No other baseline characteristics differed between groups. Eight of 24 control participants and five of 21 BFR participants had radiographic knee OA (p=.4819).
Figure 3.
CONSORT Diagram
Table 2.
Baseline Subject Characteristics (means±SD)
| Measurement | Control Group | BFR Group | p-value |
|---|---|---|---|
| Age (years) | 54.6±6.9 | 56.1±5.9 | .4339 |
| Height (cm) | 165.5±6.0 | 161.9±6.0 | .0504 |
| Mass (kg) | 88.0±16.3 | 75.0±11.5 | .0040 |
| BMI (kg/m2) | 32.1±5.2 | 28.7±4.4 | .0223 |
| Scaled Leg Press 1RM (kg per kg body mass) |
2.1±.5 | 2.3±.6 | .2195 |
| Scaled 40% 1RM Leg Press Power (Watts) |
11.3±2.9 | 12.6±2.5 | .1188 |
| Scaled Isokinetic Knee Extensor Torque (Nm/kg) |
1.3±.4 | 1.3±.3 | .8461 |
| Stair Climb Power (Watts) | 404.3±118.4 | 364.3±71.2 | .1721 |
| Knee Pain (KOOS) | 76.0±20.0 | 80.5±16.9 | .4293 |
| Quadriceps Volume (cm3) | 1,030.8±65.2 | 948.0±71.4 | .4139 |
1RM=1 repetition maximum
KOOS= Knee Osteoarthritis Outcome Score
Data expressed as standard deviation.
There were no significant differences in the number of training sessions attended between groups, with median (interquartile range) of 12 (12, 12) sessions attended by control participants and 12 (11, 12) by BFR participants (p=.1681).
Isokinetic knee extensor strength was unchanged in the control group, but improved in the BFR group with a significant inter-group difference (Table 3). In addition, there was a significantly greater increase in isotonic leg press 1RM in the BFR group in comparison with the control group. There were no other significant inter-group differences detected in outcome measures. However, there were significant increases in stair climb power in both groups. In addition, there was no worsening of knee-specific pain in either group, and no statistically significant differences between the groups (Table 3).
Table 3.
Change in Measurements Between Baseline and Follow up {p-values for Pre-Post Within Group Comparisons} and p-Values for Between-Group Comparisons
| Measurement | Control Group | BFR Group | p-values Comparing Groups |
|---|---|---|---|
| Scaled Leg Press 1RM (kg per kg body mass) |
+0.2±.3 {p=.0046} |
+0.4±.3 {p<.0001} |
.0385 |
| Scaled 40% 1RM Leg Press Power (Watts) |
+0.42±.26 {p=.1102} |
+0.62±.27 {p=.0288} |
.6173 |
| Scaled Isokinetic Knee Extensor Torque (Nm/kg)* |
−0.05±.03 {p=.0537} |
+0.07±.03 {p=.0243} |
.0048 |
| Stair Climb Power (Watts) | +53.4±11.0 {p<.0001} |
+29.3±11.6 {p=.0163} |
.1520 |
| Knee Pain (KOOS) | +1.8±2.7 {p=.5209} |
+2.0±2.8 {p=.4834} |
.9574 |
| Quadriceps Volume (%Change)* | +0.01±.73 {p=.9912} |
+1.3±.80 {p=.1362} |
.2604 |
All inter-group comparisons are adjusted for BMI.
Data expressed as standard deviation.
Least squares means±SE for limb-based parameters.
Discussion
The long-term aim of this line of research is to assess whether there is a well-tolerated and efficacious means of modifying risk for incident symptomatic and progressive knee OA. Previously, knee extensor strength was found to be protective against incident symptomatic knee OA as well as knee OA progression [2, 3, 9]. However, well-tolerated means of strengthening of the quadriceps while avoiding excessive loading the knee joint have been elusive.
In this study, knee extensor strength and leg-press 1RM improved with addition of BFR to low-load exercise. Importantly, this resistance-training program was effective without exacerbating knee pain, indicating that adding BFR to a low-load resistance-training program does not have a negative impact with regard to knee pain.
The improvement in strength with BFR in comparison with the control group in this study is consistent with the results of numerous studies of BFR with low-load resistance training in people without knee OA. In those studies, combining BFR to the exercising muscle with low-load (~20% of 1RM) resistance training elicited significant increases in muscle strength, of a similar magnitude to those completing high-load resistance training [6, 7, 25-27]. Therefore, our study adds additional evidence that this training modality is effective for stimulating muscle strength, even when performed at relatively low loads.
There were clear intergroup differences between the change in isokinetic knee extensor strength and isotonic leg-press 1RM in the BFR group in comparison with the control group. Whereas knee extensor strength decreased in the control group and increased in the BFR group, the finding of an increase in leg press strength in both groups may relate to a learning effect for this task. As the learning effect would not be expected to differ between participants randomized to the individual groups (BFR group 28.3±4.8 kg and control group 15.6±4.5 kg), this suggests that even if a learning effect explained the increase in the low-load control group, the addition of BFR appears to have induced an effect that exceeded the learning effect.
Despite the increase in strength, an increase in muscle volume was not detected in this study. Based on the means and standard deviations for change in quadriceps volume, presented in Table 3, the effect size (d) was 1.68. At an alpha level of 0.05, a total of 6 participants in each of the groups would have provided 86% power to detect a statistically significant difference of this magnitude between groups. One of the 12 participants who underwent MRI at baseline discontinued participation, leaving only 11 participants for analyses and limiting power to detect a difference of this size. Although not statistically significant with data for 11 participants, the relatively greater change in muscle volume in the BFR than in the control participants in our study could indicate that the study was under-powered to detect this small a difference in muscle hypertrophy (1.3%). However, numerous prior studies have reported greater increases in muscle cross-sectional area (CSA) following BFR interventions [28-34], and most recently this also was seen in older adults [6].
There are several additional potential reasons why an increase in quadriceps volume may not have been detected. While leg-press exercise is not selective for the quadriceps as it also involves gluteal musculature, Yasuda, et al. did report muscle hypertrophy in a similar, but unblinded leg-press/knee extension study in older adults, published after the completion of this study[6]. The study by Yasuda, et al. detected an 8% increase in quadriceps muscle CSA on MRI. The results of that study may have differed from those of our study due to the greater frequency and duration of the training—11 minutes × 2 times per week × 12 weeks in that study and 7 minutes × 3 times per week × 4 weeks in our study. That study also progressed the training loads every three weeks based on 1RM tests, while in our 4-week study, the training load was not progressed, given the shorter duration and our aim of quantifying the effect of progression of the BFR pressure. In addition to progressing the load, in the study by Yasuda et al, training pressure was initiated at 120 mmHg the first day of training and increased by 10-20 mmHg each subsequent session until reaching 270 mmHg [6] the final week. In contrast, in our study, the initial training session used a cuff pressure of 160 mm Hg and adjusted the cuff pressure in weeks 2 and 3 to a training pressure of 200 mmHg during the final 2 weeks. Although it is possible that the dose of BFR in our study may have been insufficient to induce muscle hypertrophy, higher pressures have not been found to induce greater muscle fatigue than lower pressures [21]. Thus, it is more likely that, in addition to the lower statistical power due to one MRI-participant dropping out, the shorter duration without progression of load may have contributed to an increase in quadriceps volume not being detected in our study.
Strengths of the current study included the strict inclusion criteria that allow the results to be generalizable to women with identified risk factors for developing incident symptomatic or progressive knee OA. The randomized, controlled trial (RCT) design also strengthened internal validity, allowing assessment of the efficacy and potential adverse affects of the BFR resistance training intervention. The blinding of participants and assessors to the treatment allocation (both groups completed low-load resistance training unaware of the other training program and evaluators were unaware of the intervention to which participants had been allocated) reduced both selection bias and confounding in the study results.
Although the strict inclusion criteria and RCT design strengthened internal validity, it also could limit generalizability to populations with different characteristics, such as those with pre-existing symptomatic knee OA. In addition, the study results are based on an intensive, supervised exercise program that may not reflect results in a different environment. Lastly, the design of the study built on protocols from prior studies, but was limited by gaps in knowledge regarding the optimal use of BFR during exercise training. Since the completion of this study in 2011, there have been advances in knowledge supporting the need to determine cuff size and pressure on a participant-specific basis, based on thigh circumference [35]. Therefore, future use of BFR exercise could potentially induce a greater effect through more optimal dosing of the BFR. Lastly, while evidence suggests that continuous BFR at 20% 1RM may be the most effective protocol during training to induce muscle hypertrophy [21], the exact dose and most effective duration of training still varies across studies and remains unclear, especially in older adults.
Current BFR training methods have evolved from 40 years of research and development [36]. Recent studies have demonstrated efficacy and some mechanisms for the effect of BFR training eliciting significant strength gains as well as increasing the ability of older adults to perform functional tasks. The results of our study suggest that BFR methods may also be an effective means of strengthening older women with risk factors for incident symptomatic knee OA. This potential solution to the challenge of strengthening people with at-risk knees, may enable older adults to increase strength to the extent necessary to confer protection against knee joint worsening, while minimizing deleterious joint loading through low-load training.
Continuing this line of research could have a significant positive impact on public health, by using an inexpensive means of well-tolerated and safe strengthening exercise that can be completed in community environments. Initial investigation of this exercise strategy suggests that it is well tolerated and has the potential to increase muscle strength in older women at risk for symptomatic knee OA. Additional research is needed to determine whether low-load resistance training with concurrent BFR is effective for increasing strength in men at risk for knee OA, determine optimal dosing for men and women, as well as determine whether this type of training may reduce incidence and progression of symptomatic knee OA. Reduction of this primary cause of disability [37], through cost-effective preventive exercise, has high potential to reduce the burden of disease, thereby improving quality of life for older adults.
Conclusions
When augmented with BFR, an average load of 30% 1RM appears to be sufficient to increase knee extensor and leg press strength in women at risk for symptomatic knee OA, in comparison with the same resistance-training program without BFR. Future studies of low-load resistance training using BFR should assess the dosing parameters that most effectively increase muscle strength and volume.
Acknowledgements
The authors appreciate the participants’ time in making this study possible, Cam Koch for his work in generating the quadriceps volume measurements and Natalie Glass for coordinating this study. This research was supported by a Dennis W. Jahnigen Career Development Scholars Award, a Beeson Career Development Award in Aging (NIH/NIA 1K23AG030945) and an American College of Sports Medicine Research Foundation Kaatsu Training Research Grant. The investigators retained full independence in the conduct of this research. No authors report conflicts of interest with regard to this research. None of the authors received funds from KAATSU International, the manufacturer and patent holder of the KAATSU Master equipment used in this study. The results of the present study do not constitute endorsement by the ACSM.
Source of Funding and Conflict of Interest Statement:
Funding for this project was received from the American College of Sports Medicine Research Foundation Kaatsu Training Research Grant, a Dennis W. Jahnigen Career Development Scholars Award and a Beeson Career Development Award in Aging (NIH/NIA 1K23AG030945). None of the authors received funds from KAATSU International, the manufacturer and patent holder of the KAATSU Master equipment used in this study. The equipment used in this study was borrowed from Dr. Takashi Abe, who also provided the first author with training in the use of the equipment.
Footnotes
No authors report conflicts of interest with regard to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Neil A. Segal, Departments of Orthopaedics & Rehabilitation, Radiology, and Epidemiology; The University of Iowa.
Glenn N. Williams, Department of Physical Therapy & Rehabilitation Science; The University of Iowa.
Maria Davis, Department of Orthopaedics & Rehabilitation; The University of Iowa.
Robert B. Wallace, Department of Epidemiology; The University of Iowa.
Alan Mikesky, Department of Kinesiology; Indiana University-Purdue University Indianapolis.
References
- 1.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal NA. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18:769–775. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segal NA, Glass NA, Felson DT, et al. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc. 2010;42:2081–2088. doi: 10.1249/MSS.0b013e3181dd902e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Sports M. American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 5.Loenneke JP, Wilson JM, Marin PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112:1849–1859. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda T, Fukumura K, Fukuda T, et al. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand J Med Sci Sports. 2013 doi: 10.1111/sms.12087. Epub date (2013/06/05) [DOI] [PubMed] [Google Scholar]
- 7.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol (1985) 2000;88:2097–2106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 8.Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011;21:510–518. doi: 10.1111/j.1600-0838.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 9.Segal NA, Torner JC, Felson D, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–1217. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labarbera KE, Murphy BG, Laroche DP, Cook SB. Sex differences in blood flow restricted isotonic knee extensions to fatigue. J Sports Med Phys Fitness. 2013;53:444–452. [PubMed] [Google Scholar]
- 11.Liu CJ, LaValley M, Latham NK. Do unblinded assessors bias muscle strength outcomes in randomized controlled trials of progressive resistance strength training in older adults? Am J Phys Med Rehabil. 2011;90:190–196. doi: 10.1097/PHM.0b013e31820174b3. [DOI] [PubMed] [Google Scholar]
- 12.McNair PJ, Colvin M, Reid D. Predicting maximal strength of quadriceps from submaximal performance in individuals with knee joint osteoarthritis. Arthritis care & research. 2011;63:216–222. doi: 10.1002/acr.20368. [DOI] [PubMed] [Google Scholar]
- 13.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Archives of physical medicine and rehabilitation. 2007;88:604–609. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical Image Processing, Analysis & Visualization In Clinical Research. IEEE Computer-Based Medical Systems (CBMS) 2001 [Google Scholar]
- 15.Segal NA, Torner JC, Felson DT, et al. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PM R. 2009;1:459–465. doi: 10.1016/j.pmrj.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessel J. Isometric strength measurements of knee extensors in women with osteoarthritis of the knee. J Rheumatol. 1996;23:328–331. [PubMed] [Google Scholar]
- 17.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 18.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita S, Abe T, Drummond MJ, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–910. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 21.Cook SB, Clark BC, Ploutz-Snyder LL. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc. 2007;39:1708–1713. doi: 10.1249/mss.0b013e31812383d6. [DOI] [PubMed] [Google Scholar]
- 22.Fujita S, Mikesky AE, Sato Y, Abe T. Fatigue characteristics during maximal concentric leg extension exercise with blood flow restriction. Int. J. KAATSU Training Res. 2007;3:27–31. [Google Scholar]
- 23.Iida H, Kurano M, Takano H, et al. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol. 2007;100:275–285. doi: 10.1007/s00421-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda T, Fukumura K, Fukuda T, et al. Effects of low-intensity, elastic band resistance exercise combined with blood flow restriction on muscle activation. Scand J Med Sci Sports. 2014;24:55–61. doi: 10.1111/j.1600-0838.2012.01489.x. [DOI] [PubMed] [Google Scholar]
- 25.Laurentino GC, Ugrinowitsch C, Roschel H, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012;44:406–412. doi: 10.1249/MSS.0b013e318233b4bc. [DOI] [PubMed] [Google Scholar]
- 26.Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86:308–314. doi: 10.1007/s00421-001-0561-5. [DOI] [PubMed] [Google Scholar]
- 27.Yasuda T, Loenneke JP, Thiebaud RS, Abe T. Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS One. 2012;7:e52843. doi: 10.1371/journal.pone.0052843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100:1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 29.Abe T, Sakamaki M, Fujita S, et al. Effects of low-intensity walk training with restricted leg blood flow on muscle strength and aerobic capacity in older adults. J Geriatr Phys Ther. 2010;33:34–40. [PubMed] [Google Scholar]
- 30.Abe T, Yasuda T, Midorikawa T, et al. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Training. 2005;1:6–12. [Google Scholar]
- 31.Fujita T, Brechue WF, Kurita Y, Sato Y, Abe T. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int. J. KAATSU Training Research. 2008;4:1–8. [Google Scholar]
- 32.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. 2011;21:e231–241. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki H, Sakamaki M, Yasuda T, et al. Increases in thigh muscle volume and strength by walk training with leg blood flow reduction in older participants. J Gerontol A Biol Sci Med Sci. 2011;66:257–263. doi: 10.1093/gerona/glq182. [DOI] [PubMed] [Google Scholar]
- 34.Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32:2035–2039. doi: 10.1097/00005768-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses. 2012;78:151–154. doi: 10.1016/j.mehy.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y. The history and future of KAATSU Training. Int. J. KAATSU Training Research. 2005;1:1–5. [Google Scholar]
- 37.Hootman J, Brault M, Helmick C, Theis K, Armour B. Prevalence and Most Common Causes of Disability Among Adults - United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]