Abstract
BACKGROUND
Excision repair cross-complementing gene-1 (ERCC1) and thymidylate synthase (TS) are key regulatory enzymes whose expression patterns are associated with overall survival (OS) in several malignancies. Their expression patterns and prognostic value in resected gastric adenocarcinoma (GAC) are not known.
METHODS
In total, 109 patients who underwent resection for GAC between January 2000 and June 2011 had tissue available for analysis. The primary objective was to assess for the differential expression of ERCC1 and TS using immunohistochemistry. The secondary objective was to assess for the association between OS and the expression of ERCC1 and TS.
RESULTS
The median follow-up was 21.2 months, and the median OS was 28.8 months. Resected GAC exhibited differential expression of ERCC1 (high expression, 23%; n =25) and TS (high expression, 43%; n =47). ERCC1 and TS expression were not associated with OS. In a subset analysis of patients who received chemotherapy (n =73), high ERCC1 expression was associated with decreased OS (16.7 months vs 53.8 months; P =0.03). After controlling for known adverse pathologic features, high ERCC1 expression persisted as a negative prognostic factor in multivariate Cox regression analysis (hazard ratio, 2.5; 95% confidence interval, 1.03–6.0; P =.04). Conversely, in patients who underwent resection only (n =35), high ERCC1 expression demonstrated a trend toward improved OS (40.4 months vs 12.7 months; P =.10); a positive prognostic influence also was present on multivariate analysis (hazard ratio, 0.20; 95% confidence interval, 0.04–0.86; P =.03).
CONCLUSIONS
Resected GAC exhibited differential expression of TS and ERCC1. Among all patients, ERCC1 and TS expression levels were not associated with OS. High ERCC1 tumor expression was associated with decreased OS in the patients who received chemotherapy but was associated with increased OS in those who underwent surgery alone. ERCC1 expression had prognostic value in resected gastric cancer, and further investigation is warranted.
Keywords: gastric adenocarcinoma, ERCC1, thymidylate synthase, biomarkers
INTRODUCTION
Gastric cancer remains the fourth most common cancer diagnosis worldwide and the second leading cause of cancer mortality.1 Surgery remains the mainstay of treatment for resectable gastric adenocarcinoma (GAC), although rates of recurrence remain persistently high, and overall survival (OS) remains poor. Over the past decade, multiple clinical trials have demonstrated an improvement in survival for patients with resected gastric cancer who receive chemotherapy in neoadjuvant or adjuvant settings or both. The Intergroup-0116 study first demonstrated improved survival with the addition of adjuvant 5-fluorouracil (5-FU) and radiation therapy versus surgery alone.2 The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial subsequently demonstrated improved survival with a perioperative chemotherapy regimen of epirubicin, cisplatin, and 5-FU compared with surgery alone.3 The more recent Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) and Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) trials have reinforced the potential survival benefits of platinum-based adjuvant regimens after resection of gastric cancer.4,5 Although a single standardized chemotherapy regimen for gastric cancer has not been uniformly adopted, most regimens typically involve 5-FU and/or a platinum agent.
Clinically significant molecular biomarkers with prognostic value—that is, biomarkers whose expression is independently associated with survival outcomes—have been identified in various malignancies, most notably in breast and lung cancers.6,7 Prognostic biomarkers often prove particularly valuable in early stage disease, in which they can help guide decisions regarding further adjuvant therapy or stratify patients who are enrolled in clinical trials.
Excision repair cross-complementing gene-1 (ERCC1) is a key component of the nucleotide excision repair pathway, which functions to excise bulky intra-strand and interstrand DNA adducts, such as those introduced by platinum drugs, that would otherwise interrupt cellular replication.8 Increased levels of ERCC1 have been associated with resistance to platinum agents.9 We recently demonstrated that ERCC1 expression was an independent prognostic marker for decreased OS after resection of early stage pancreas cancer.10,11 Studies regarding the prognostic role of ERCC1 expression for survival in gastric cancer to date have produced mixed results. High expression of ERCC1 in patients with advanced or metastatic gastric cancer has been associated with decreased survival in several studies,12,13 whereas others have failed to demonstrate any association with ERCC1 expression and survival.14 In 2 recent studies of patients who received adjuvant, cisplatin-based chemotherapy after curative intent resection, high tumor ERCC1 expression again was associated with decreased OS,15,16 whereas a third study reported the opposite; namely, that increased ERCC1 expression was correlated with improved survival.17
Thymidylate synthase (TS) is the rate-limiting enzyme in the pyrimidine nucleotide synthetic pathway for DNA and is a key target of fluoropyrimidine agents, such as 5-FU.18 In patients with advanced colorectal cancer, TS has been identified as a prognostic marker, and increased TS expression is associated with worse OS.19,20 Studies assessing the association of TS expression with survival in patients with advanced gastric cancer have been limited, and, to date, only 3 small studies have examined TS expression in resected gastric cancer. One study demonstrated that decreased TS expression was associated with improved survival,21 whereas the remaining 2 studies identified no prognostic association between TS expression and OS.16,22 Given the heterogeneous and conflicting results of prior investigations and the inclusion of patients with metastatic disease in many of those studies, we sought to determine if resected GAC exhibited differential tumor expression of ERCC1 or TS and whether expression patterns of these 2 biomarkers had prognostic value for OS.
MATERIALS AND METHODS
A prospectively maintained institutional database was used to identify all patients who underwent resection for GAC with curative intent between January 1, 2000 and June 1, 2011. Patients who underwent palliative resection of advanced metastatic disease or who did not have tissue available for immunohistochemistry (IHC) were excluded from this analysis. Permission from the Emory University Institutional Review Board was obtained, and all research activities were conducted in compliance with the Health Insurance Portability and Accountability Act of 1996.
Medical records were reviewed to identify patient demographics, preoperative medical comorbidities, operative details, and pathologic features, including tumor size, tumor grade, margin status, the presence of lymphovascular invasion (LVI) or perineural invasion (PNI), and lymph node status. Treatment variables, including neoadjuvant and/or adjuvant chemotherapy regimens and radiation therapy, also were collected.
Survival outcomes were calculated from the date of surgery to the date of last follow-up or the date of death from any cause. The Social Security Death Index was used to supplement the medical record for evidence of patient mortality. Patients who died within 30 days of resection (n=1) were excluded from the OS analyses.
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples of tumor tissue from each patient were reacted with anti-ERCC1 (clone 8F1; Thermo Fisher Scientific, Waltham, Mass) and anti-TS monoclonal antibodies (clone TS106; Dako Inc., Philadelphia, Pa) to determine tumor expression levels of ERCC1 and TS, respectively. IHC staining was quantified by a single pathologist (K.E.F.), who was blinded to outcomes, using a previously described, validated method for scoring IHC specimens on the basis of the percentage and intensity of cellular staining.23 All IHC scoring was subsequently reviewed and confirmed by a second, senior pathologist (A.B.F.), who also was blinded to patient outcomes. Overall scores were assigned on a 0 to 4 scale, and patients were dichotomized into a high-expression group (scores >2) and a low-expression group (scores ≤2).10
Statistical Analysis
Data analysis was conducted with Statistical Package for the Social Sciences 19.0 software (SPSS, Inc., Chicago, Ill). The primary outcome was OS. The association of ERCC1 or TS tumor expression with clinicopathologic features was assessed by using chi-square analysis for categorical variables and the Fisher exact test for continuous variables. Kaplan-Meier log-rank survival analysis was performed to evaluate the association of tumor expression of ERCC1 or TS with patient survival. Univariate and multivariate Cox regression analyses were performed to evaluate the prognostic association of known adverse pathologic factors, as well as biomarker expression profiles, with OS. Variables that reached an association of P ≤.10 on univariate regression analysis were included in the multivariate model. Subset analyses with separate univariate and multivariate Cox regression models were performed for those patients who received perioperative therapy in addition to surgery and for those patients who underwent resection only. Statistical significance was defined as P ≤.05.
RESULTS
Demographic and pathologic characteristics are summarized in Table 1. The median patient age was 64 years (range, 23–85 years), and 60 patients (55%) were men. At the time of last follow-up, 55 patients (50%) had died. The median OS for all patients was 28.8 months, and the median follow-up for survivors was 21.2 months. One patient died within 30 days of the index operation and was excluded from survival analyses.
TABLE 1.
Demographic and Clinicopathologic Characteristics, n = 109
| Variable | No. of Patients (% of Total Cohort) |
|---|---|
| Sex | |
| Men | 60 (55) |
| Women | 49 (45) |
| Race | |
| White | 50 (45.9) |
| Black | 45 (41.3) |
| Other | 14 (12.8) |
| Age: Median [range], yrs | 63.6 [23.5–84.8] |
| ASA classification | |
| 2 | 24 (22) |
| 3 | 82 (75.2) |
| 4 | 3 (2.8) |
| Perioperative characteristics | |
| Operation type | |
| Total | 40 (36.7) |
| Subtotal | 69 (63.3) |
| Lymph node dissection type | |
| D0 | 7 (6.4) |
| D1 | 14 (12.8) |
| D2 | 88 (80.7) |
| Pathologic characteristics | |
| Tumor location | |
| GE junction | 14 (12.8) |
| Cardia | 2 (1.8) |
| Body | 64 (58.7) |
| Antrum | 28 (26.6) |
| Tumor size: Median [range], cm | 4.0 [0.2–15.0] |
| Resection margin | |
| R0 | 102 (93.6) |
| R1 | 7 (6.4) |
| Tumor grade | |
| 1: Well differentiated | 5 (4.6) |
| 2: Moderately differentiated | 30 (27.5) |
| 3: Poorly differentiated | 74 (67.9) |
| Tumor type | |
| Diffuse | 18 (16.4) |
| Intestinal | 31 (28.2) |
| Signet ring histology | 57 (52.3) |
| Tumor classification: AJCC 7th edition | |
| T1a | 4 (3.7) |
| T1b | 15 (13.8) |
| T2 | 13 (11.9) |
| T3 | 39 (35.8) |
| T4a | 30 (27.5) |
| T4b | 8 (7.3) |
| TNM stage: AJCC 7th edition | |
| I | 24 (22) |
| II | 31 (28.4) |
| III | 53 (48.6) |
| Lymphovascular invasion | 37 (33.9) |
| Perineural invasion | 25 (22.9) |
| Lymph node-positive disease | 68 (62.4) |
| Perioperative Treatment characteristics | |
| Any adjuvant treatment: Preoperative or postoperative | 73 (66.9) |
| Neoadjuvant chemotherapy | 16 (14.7) |
| Neoadjuvant radiotherapy | 3 (2.7) |
| Adjuvant chemotherapy | 70 (64.2) |
| Adjuvant radiotherapy | 50 (45.8) |
Abbreviations: ASA, American Society of Anesthesiologists; GE, gastroesophageal.
Seventy-three patients (67%) received some form of perioperative chemotherapy; 16 patients (15%) received neoadjuvant chemotherapy, and 70 (64%) received adjuvant chemotherapy. Of those patients who received chemotherapy, 69 (94.5%) received 5-FU or capecitabine, and 28 (38%) received a platinum agent, such as cisplatin or oxaliplatin. Three patients (3%) received neoadjuvant radiation therapy, whereas 50 patients (46%) received adjuvant radiation therapy. All patients who received radiation therapy also received concurrent chemotherapy.
Forty patients (37%) underwent a total gastrectomy, and the remaining 69 patients (63%) underwent a subtotal gastrectomy. The majority of patients (n =88, 81%) underwent a D2 lymph node dissection.
Sixty-four patients (59%) had tumors located in the body of the stomach, and 14 (13%) had tumors of the gastroesophageal junction (Table 1). Regarding pathologic features, 74 patients (68%) had poorly differentiated tumors, 68 patients (62%) had positive lymph nodes, 57 patients (52%) had tumors that exhibited signet cell features, 37 patients (34%) had LVI present, 25 patients (23%) had PNI present, and 7 patients (6%) had microscopically positive resection margins. The median tumor size was 4.0 cm (range, 0.2–15.0 cm). Regarding tumor classification,24 19 tumors were T1 (17.4%), 13 were T2 (11.9%), 39 were T3 (35.8%), and 38 were T4 (34.9%).
Biomarker Expression
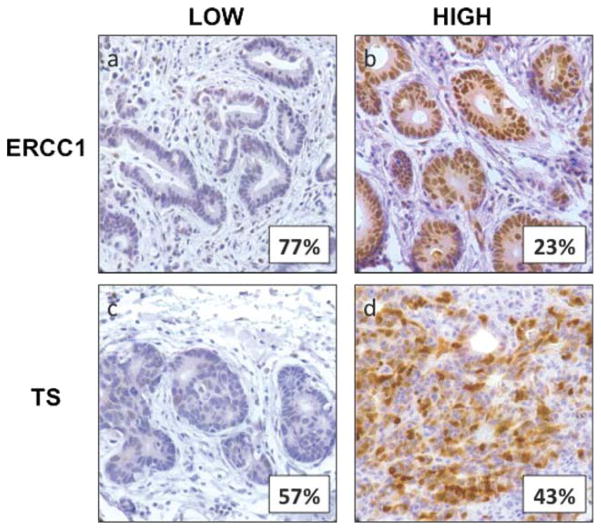
On the basis of the previously defined categories for high and low expression,10 both ERCC1 and TS exhibited differential expression among resected gastric cancers. Twenty-five patients (23%) had resected tumors that exhibited high ERCC1 expression, and 48 patients (43%) had tumors with high TS expression. Neither ERCC1 expression nor TS expression demonstrated a significant association with any clinicopathologic variables (patient sex, age, American Society of Anesthesiology classification, tumor location, tumor size, histologic grade, Lauren classification, signet ring histology, T classification, LVI, PNI, and lymph node involvement) on the chi-square test for categorical variables or the Fisher exact test for continuous variables. Representative images of tumors that demonstrated high and low ERCC1 and TS expression by IHC are depicted in Figure 1.
Figure 1.
Tumor expression levels of excision repair cross-complementing gene-1 (ERCC1) and thymidylate synthase (TS) were evaluated using immunohistochemistry. Representative images are depicted (original magnification ×400). (a) Low ERCC1 expression was observed in 84 patients (77%), (b) high ERCC1 expression was observed 25 patients (23%), (c) low TS expression was observed in 61 patients (57%), and (d) high TS expression was observed in 48 patients (43%).
Survival Analysis
On Kaplan-Meier log-rank survival analysis of the entire cohort, high ERCC1 tumor expression was not associated with OS (18.9 months vs 27.2 months; P =.72) (Fig. 2a). Similarly, high TS expression also was not associated with OS (24.3 months vs 28.8 months; P =.88) (Fig. 2b). On planned subset analysis of the 73 patients who received some form of additional therapy, 16 patients (22%) exhibited high tumor ERCC1 expression. Within that group, high ERCC1 expression was associated with significantly decreased OS (16.7 months vs 53.8 months; P =.03) (Fig. 3a). After evaluating the known, adverse pathologic factors of tumor size, margin, grade, T classification, lymph node involvement, and the presence of LVI or PNI, and including those which were identified as significant on univariate analysis, the negative prognostic value of high ERCC1 expression persisted on multivariate Cox regression analysis (hazard ratio [HR], 2.2; 95% confidence interval [CI], 1.05–4.98; P =.048) (Table 2). In a similar subset analysis of those patients who underwent resection only (n =35), high ERCC1 expression demonstrated a trend toward improved OS (40.4 months vs 12.7 months; P =.10) (Fig. 3b). This positive prognostic value of high ERCC1 expression also was present on multivariate analysis (HR, 0.20; 95% CI, 0.05–0.84; P =.03) (Table 3). TS expression was not associated with survival on subset analysis.
Figure 2.
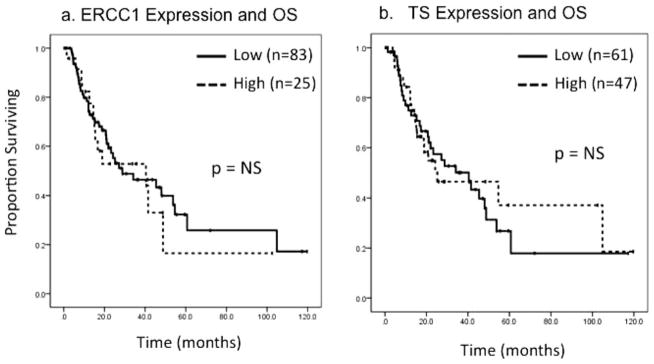
(a) Tumor expression of excision repair cross-complementing gene-1 (ERCC1) and overall survival (OS) are illustrated for all patients (n =108). NS indicates nonsignificant. (b) Tumor thymidylate synthase (TS) expression and OS are illustrated for all patients (n =108).
Figure 3.
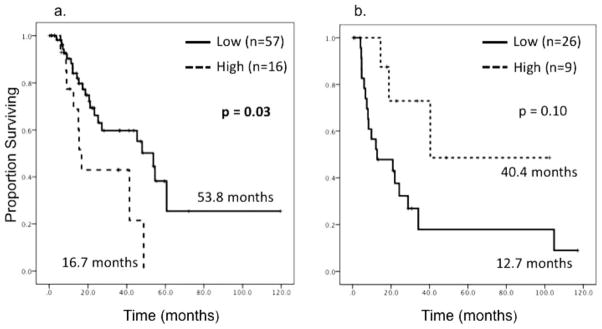
These Kaplan-Meier survival curves illustrate a subset analysis of tumor excision repair cross-complementing gene-1 (ERCC1) expression and overall survival (OS). Median OS is reported. (a) ERCC1 expression and OS is illustrated for patients who received additional therapy (n =73). (b) ERCC1 expression and OS are illustrated for patients who underwent resection only (n=35).
TABLE 2.
Univariate and Multivariate Cox Regression Models for Overall Survival in Patients Receiving Additional Therapy, n = 73
| Variable | Univariate Analysis
|
Multivariate Analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Tumor size | 1.04 (0.93–1.17) | .45 | ||
| Positive margin | 7.84 (2.75–22.34) | <.001a | 7.31 (2.53–21.13) | <.001a |
| Positive lymph node(s) | 1.92 (0.78–4.69) | .15 | ||
| Perineural invasion | 1.31 (0.56–3.09) | .53 | ||
| Lymphovascular Invasion | 1.02 (0.47–2.24) | .95 | ||
| Tumor grade | 1.55 (0.67–3.62) | .31 | ||
| Tumor classification | 2.83 (0.78–10.19) | .11 | ||
| High ERCC1 expression | 2.39 (1.08–5.33) | .03a | 2.22 (1.05–4.98) | .048a |
Abbreviations: CI, confidence interval; ERCC1, excision repair cross-complementing gene-1; HR, hazard ratio.
These P values indicate statistical significance.
TABLE 3.
Univariate and Multivariate Cox Regression Models for Overall Survival in Patients Undergoing Resection Only, n = 35
| Variable | Univariate Analysis
|
Multivariate Analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Tumor size | 1.17 (1.02–1.33) | .03a | 1.24 (1.05–1.47) | .01a |
| Positive margin | 3.24 (0.71–14.89) | .13 | ||
| Positive lymph node(s) | 1.91 (0.80–4.58) | .15 | ||
| Perineural invasion | 1.71 (0.62–4.76) | .30 | ||
| Lymphovascular invasion | 4.33 (1.72–10.89) | .002a | 3.35 (1.19–9.40) | .02a |
| Tumor grade | 1.79 (0.73–4.38) | .20 | ||
| Tumor classification | 4.27 (1.24–14.69) | .02a | 2.33 (0.54–10.05) | .26 |
| High ERCC1 expression | 0.41 (0.14–1.24) | .10a | 0.20 (0.05–0.84) | .03a |
Abbreviations: CI, confidence interval; ERCC1, excision repair cross-complementing gene-1; HR, hazard ratio.
These P values indicate statistical significance.
A separate subset analysis was performed on the basis of ERCC1 expression. Within the subset of 25 patients who exhibited high tumor ERCC1 expression, patients who received some form of additional therapy (n =16) demonstrated a trend toward decreased OS compared with those who underwent resection only (n =9; 16.7 months vs 40.4 months; P =.06) (Fig. 4a). On univariate and multivariate regression analysis examining the above-mentioned adverse pathologic factors within the high ERCC1 expression subset, the administration of additional therapy also demonstrated a trend toward an association with decreased OS (HR, 3.40; 95% CI, 0.85–13.55; P =.08) (Table 4). Within the subset of 83 patients who had low tumor ERCC1 expression, the patients who received some form of additional therapy (n =57) demonstrated significantly improved OS compared with the patients who underwent resection only (n=26), (53.8 months vs 12.7 months; P =.004) (Fig. 4b). The positive prognostic association of additional therapy within the low ERCC1 expression subset persisted on univariate and multivariate regression analyses (HR, 0.27; 95% CI, 0.13–0.55; P <.001) (Table 5).
Figure 4.
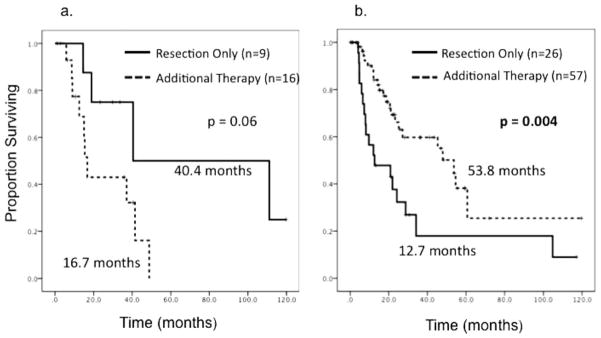
These Kaplan-Meier survival curves illustrate a subset analysis of overall survival (OS) according to treatment modality for patients who underwent surgery and also received additional therapy versus patients who underwent resection only. (a) OS is illustrated for patients who had high excision repair cross-complementing gene-1 (ERCC1) tumor expression (n =25) according to treatment modality. (b) OS is illustrated for patients who had low ERCC1 tumor expression (n =83) according to treatment modality.
TABLE 4.
Univariate and Multivariate Cox Regression Models for Overall Survival in Patients With High Expression of ERCC1, n = 25
| Variable | Univariate Analysis
|
Multivariate Analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Tumor size | 1.06 (0.91–1.25) | .46 | ||
| Positive margin | 15.31 (2.12–110.42) | .01a | 11.46 (1.47–89.48) | .02a |
| Positive lymph node(s) | 1.50 (0.45–4.93) | .51 | ||
| Perineural invasion | 2.66 (0.70–10.12) | .10a | 3.73 (0.86–16.22) | .08 |
| Lymphovascular invasion | 1.30 (0.33–4.89) | .72 | ||
| Tumor grade | 2.12 (0.56–7.95) | .27 | ||
| Tumor classification | 2.52 (0.55–1.55) | .24 | ||
| Any additional therapy | 3.26 (0.89–11.98) | .08a | 3.40 (0.85–13.55) | .08 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
These P values indicate statistical significance.
TABLE 5.
Univariate and Multivariate Cox Regression Models for Overall Survival in Patients With Low Expression of ERCC1, n = 83
| Variable | Univariate Analysis
|
Multivariate Analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Tumor size | 1.11 (0.99–1.23) | .06a | 1.12 (0.99–1.26) | .06 |
| Positive margin | 5.12 (1.89–13.89) | .005a | 3.08 (1.08–8.76) | .04a |
| Positive lymph node(s) | 1.79 (0.91–3.54) | .09a | 1.99 (0.92–4.33) | .08 |
| Perineural invasion | 1.25 (0.58–2.66) | .57 | ||
| Lymphovascular invasion | 1.93 (1.01–3.69) | .05a | 1.63 (0.83–3.21) | .16 |
| Tumor grade | 1.26 (0.64–2.45) | .50 | ||
| Tumor classification | 1.99 (0.93–4.26) | .08a | 1.72 (0.75–3.93) | .20 |
| Any additional therapy | 0.41 (0.22–0.77) | .005a | 0.27 (0.13–0.55) | <.001a |
Abbreviations: CI, confidence interval; HR, hazard ratio.
These P values indicate statistical significance.
DISCUSSION
Given the high rates of recurrence and poor survival still associated with resected GAC, prognostic factors that can guide treatment decisions are needed. Expression patterns of molecular biomarkers offer a potentially improved means of providing prognostic information beyond that of traditional clinicopathologic data. In the current study, we demonstrated the differential expression profiles of ERCC1 and TS in resected gastric cancer and the potential prognostic value of ERCC1 expression in select populations.
TS holds promise as a prognostic biomarker because of its role as the molecular target of 5-FU, a commonly used chemotherapeutic agent in gastric cancer. Similar studies of TS expression in advanced colorectal cancer demonstrated its prognostic value for survival,19,25 but limited studies in gastric cancer to date have not produced similar results.12,21 In the current study, TS expression failed to demonstrate prognostic value for survival among all patients or on subsequent subset analysis of patients who received adjuvant therapy versus those who underwent resection alone.
It is known that ERCC1 expression correlates with resistance to platinum-based chemotherapy, and it has been demonstrated that increased tumor ERCC1 expression has an association with decreased survival after resection of pancreatic adenocarcinoma.10 In the current study, ERCC1 expression was not associated with survival on initial analysis of all patients but did demonstrate prognostic value in subset analyses. High tumor expression of ERCC1 was associated significantly with decreased OS among patients who received adjuvant treatment along with resection. On univariate and multivariate analysis evaluating other known adverse pathologic factors of tumor size, positive margin status, lymph node involvement, PNI, LVI, tumor grade, and pathologic T classification, high ERCC1 expression retained its significant association with decreased OS. Among patients who underwent resection only, high ERCC1 tumor expression had the opposite effect, demonstrating a trend toward improved OS. When evaluating patient subsets on the basis of high versus low ERCC1 tumor expression, a similar dichotomous correlation emerged. Among patients who had high ERCC1 tumor expression, those who received any therapy in addition to surgery demonstrated a trend toward decreased OS compared with those who underwent resection only. Conversely, in patients who had low ERCC1 tumor expression, the addition of other therapy was associated with significantly improved OS versus resection alone.
This dichotomous association of tumor ERCC1 expression and survival, depending on treatment modality, has been observed previously in patients with non-small cell lung cancer (NSCLC). In a study by Simon et al evaluating ERCC1 expression patterns in patients who underwent resection only for NSCLC, high tumor expression of ERCC1 was associated with significantly improved OS.26 Those authors hypothesized that increased ERCC1 expression may indicate preservation of a functional DNA nucleotide excision repair pathway in these tumors, thus preventing excessive accumulation of mutations and maintaining a more indolent tumor phenotype associated with greater OS. In a large cohort of patients from the International Adjuvant Lung Cancer Trial, Olaussen et al examined the expression patterns of ERCC1 in patients with resected NSCLC who were randomized to receive adjuvant cisplatin-based chemotherapy versus observation.27 Although ERCC1 expression did not demonstrate significant prognostic value among the entire cohort, subset analyses of the 2 treatment arms demonstrated that, in patients who received adjuvant cisplatin, low ERCC1 expression was associated with improved OS. Conversely, in patients who underwent resection only, similar to what was reported by Simon et al,26 high ERCC1 expression was associated with better OS.27 The findings from our study in resected gastric cancer mirror the findings in NSCLC. These data suggest that low tumor ERCC1 expression may be advantageous for patients who receive adjuvant therapy, whereas high tumor ERCC1 expression may be protective for patients who undergo surgery alone.
In a recent prospective trial in which patients with advanced NSCLC were randomized to treatment with a chemotherapy regimen that was tailored to the patient’s specific expression pattern of ERCC1 and other biomarkers versus standard chemotherapy regimen, a survival advantage was demonstrated for this “personalized” therapeutic approach.28 In gastric cancer, stratification of patients by biomarker expression also may allow for the identification of populations that will most benefit from adjuvant therapy, potentially better guiding the use of perioperative chemotherapy and/or postoperative chemoradiotherapy beyond endoscopic ultrasound staging and standard histopathologic tumor and lymph node classifications, respectively.
A potential limitation of the current study is the semiquantitative nature of IHC analysis of biomarker expression levels. In an attempt to minimize scoring discrepancies, 2 pathologists, both blinded to patient outcomes, scored all specimens using a previously validated IHC scoring system. Although the measurement of bio-marker messenger RNA levels by polymerase chain reaction can provide additional information, IHC analysis is more widely available and more easily incorporated into clinical practice. An additional limitation of the study is the relatively short follow-up time, although it does approach 2 years. Given the wide range of several confidence intervals, it is difficult to project whether these confidence intervals may narrow or widen with longer follow-up. The study sample size also was relatively small, especially on subset analyses, although our cohort was comparable or larger than other similar biomarker expression profile studies, given the rarity of resected GAC.
The conclusions of the study were limited by its retrospective nature, which did not allow for examination of the predictive value of ERCC1 or TS expression for specific treatment regimens including platinum or 5-FU, respectively. Also, because ours is a tertiary academic referral center, many patients who are treated at our institution are referred from community oncology practices across a large geographic area, making standardization of a single neoadjuvant or adjuvant regimen difficult and continued patient follow-up challenging. Because most patients did not receive a platinum-based regimen, the predictive value of ERCC1 expression in gastric cancer for response to platinum agents could not be evaluated. Another limitation is that 16 patients received neoadjuvant chemotherapy, and the potential effect that such treatment may have on expression levels of ERCC1 and TS in the resected tumor is unknown. Future studies with a more homogenous treatment population should evaluate the role of ERCC1 expression for predicting the response to platinum therapy. Moving forward, an ideal prospective trial would assess ERCC1 expression on initial diagnostic biopsy specimens before patients with locoregionally advanced disease receive neoadjuvant chemotherapy with a standardized platinum-containing regimen. After surgical resection, ERCC1 expression should then be re-evaluated in the resected tumor specimen to investigate the effect of chemotherapy-induced changes on the expression profile.
Our current findings suggest that tumor ERCC1 expression may have a valuable prognostic role in patients with resectable gastric cancer. The effect of ERCC1 expression may be related to the treatment modality administered, similar to what has been observed in NSCLC. Given the study design, the results of this study are not definitive but, rather, are hypothesis-generating and, thus, warrant further exploration. Future prospective investigations are needed to evaluate the role of ERCC1 expression in guiding treatment decisions for patients with resected GAC.
Acknowledgments
FUNDING SUPPORT
This study is supported in part by the Katz Foundation. Dr. Fisher is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Lim do H, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Andrews J, Yeh P, Pao W, Horn L. Molecular predictors of response to chemotherapy in non-small cell lung cancer. Cancer J. 2011;17:104–113. doi: 10.1097/PPO.0b013e318213f3cf. [DOI] [PubMed] [Google Scholar]
- 8.Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev. 2007;33:565–577. doi: 10.1016/j.ctrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 10.Maithel SK, Coban I, Kneuertz PJ, et al. Differential expression of ERCC1 in pancreas adenocarcinoma: high tumor expression is associated with earlier recurrence and shortened survival after resection. Ann Surg Oncol. 2011;18:2699–2705. doi: 10.1245/s10434-011-1610-x. [DOI] [PubMed] [Google Scholar]
- 11.Fisher SB, Patel SH, Bagci P, et al. An analysis of human equilibrative nucleoside transporter-1, ribonucleoside reductase subunit M1, ribonucleoside reductase subunit M2, and excision repair cross-complementing gene-1 expression in patients with resected pancreas adenocarcinoma: implications for adjuvant treatment. Cancer. 2013;119:445–453. doi: 10.1002/cncr.27619. [DOI] [PubMed] [Google Scholar]
- 12.Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–509. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara J, Nishina T, Yamada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer. 2008;98:832–839. doi: 10.1038/sj.bjc.6604211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnenblick A, Rottenberg Y, Kadouri L, et al. Long-term outcome of continuous 5-fluorouracil/cisplatin-based chemotherapy followed by chemoradiation in patients with resected gastric cancer. Med Oncol. 2012;29:3035–3038. doi: 10.1007/s12032-012-0302-0. [DOI] [PubMed] [Google Scholar]
- 15.Fareed KR, Al-Attar A, Soomro IN, et al. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastrooesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer. 2010;102:1600–1607. doi: 10.1038/sj.bjc.6605686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KH, Kwon HC, Oh SY, et al. Clinicopathologic significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers. 2011;16:74–82. doi: 10.3109/1354750X.2010.533284. [DOI] [PubMed] [Google Scholar]
- 17.Baek SK, Kim SY, Lee JJ, Kim YW, Yoon HJ, Cho KS. Increased ERCC expression correlates with improved outcome of patients treated with cisplatin as an adjuvant therapy for curatively resected gastric cancer. Cancer Res Treat. 2006;38:19–24. doi: 10.4143/crt.2006.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidelberger C. On the rational development of a new drug: the example of the fluorinated pyrimidines. Cancer Treat Rep. 1981;65(suppl 3):3–9. [PubMed] [Google Scholar]
- 19.Maithel SK, Gonen M, Ito H, et al. Improving the clinical risk score: an analysis of molecular biomarkers in the era of modern chemotherapy for resectable hepatic colorectal cancer metastases. Surgery. 2012;151:162–170. doi: 10.1016/j.surg.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Tsourouflis G, Theocharis SE, Sampani A, Giagini A, Kostakis A, Kouraklis G. Prognostic and predictive value of thymidylate synthase expression in colon cancer. Dig Dis Sci. 2008;53:1289–1296. doi: 10.1007/s10620-007-0008-x. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi W, Tanabe S, Azuma M, et al. Impacts of fluorouracil-metabolizing enzymes on the outcomes of patients treated with S-1 alone or S-1 plus cisplatin for first-line treatment of advanced gastric cancer. Int J Cancer. 2010;126:162–170. doi: 10.1002/ijc.24726. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, Kim MA, Kim TM, et al. Biomarker analysis in stage III–IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer. 2009;100:732–738. doi: 10.1038/sj.bjc.6604936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher SB, Fisher KE, Patel SH, et al. Excision repair cross-complementing gene-1, ribonucleotide reductase subunit M1, ribonucleotide reductase subunit M2, and human equilibrative nucleoside transporter-1 expression and prognostic value in biliary tract malignancy. Cancer. 2013;119:454–462. doi: 10.1002/cncr.27739. [DOI] [PubMed] [Google Scholar]
- 24.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer Staging Manual. 7. New York: Springer; 2009. [Google Scholar]
- 25.Kim SH, Kwon HC, Oh SY, et al. Prognostic value of ERCC1, thymidylate synthase, and glutathione S-transferase pi for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am J Clin Oncol. 2009;32:38–43. doi: 10.1097/COC.0b013e31817be58e. [DOI] [PubMed] [Google Scholar]
- 26.Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest. 2005;127:978–983. doi: 10.1378/chest.127.3.978. [DOI] [PubMed] [Google Scholar]
- 27.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 28.Simon GR, Schell MJ, Begum M, et al. Preliminary indication of survival benefit from ERCC1 and RRM1-tailored chemotherapy in patients with advanced nonsmall cell lung cancer: evidence from an individual patient analysis. Cancer. 2012;118:2525–2531. doi: 10.1002/cncr.26522. [DOI] [PMC free article] [PubMed] [Google Scholar]