Abstract
In total, 3,145 ticks of the species Haemaphysalis longicornis (3,048; 96.9%), R. microplus (82; 2.6%), H. campanulata (9; 0.3%), and Dermacentor sinicus (5; 0.2%) were collected from animals and vegetation at Yantai in Shandong Province. Both adult and immature ticks were obtained, and all ticks collected from vegetation were unfed. Eggs were obtained from 22 blood-fed female ticks through maintenance at room temperature after collection. Severe fever with thrombocytopenia syndrome virus (SFTSV) viral RNA was identified in H. longicornis and R. microplus, with a prevalence of 4.75 per 100 ticks (95% confidence interval [95% CI] = 3.87–5.63) for ticks collected from animals and 2.24 per 100 ticks (95% CI = 1.27–3.21) for ticks collected from vegetation. The possibility that SFTSV transmission may occur by both the transstadial and transovarial routes was suggested by the fact that viral RNA was detected in H. longicornis at all developmental stages. Tick-derived sequences shared over 95.6% identity with human- and animal-derived isolates. This study provides evidence that implicates ticks as not only vectors but also, reservoirs of SFTSV.
Introduction
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel phlebovirus in the Bunyaviridae family; SFTSV was first identified in China in 20101 and has subsequently been reported in Japan and Korea.2,3 Human SFTSV infections have a high case fatality rate (initial rate of 30%). Since its discovery, the number of SFTS cases has increased significantly, with a current case fatality rate of approximately 10–16% according to the China Information System for Disease Control and Prevention.
Phleboviruses are transmitted by arthropods. Vectors include phlebotomine sandflies, mosquitoes, and ticks. Both the SFTSV and Heartland virus have been detected in ticks, which are likely the vectors of these viruses.4–6 However, the natural maintenance and transmission of SFTSV have not been studied adequately. Epidemiological studies of SFTSV infections in domestic animals have shown high prevalence and incidence, although clinical signs of SFTSV infection have not been observed, and the animals do not develop substantial viremia.7 Thus, ticks may not only be vectors but also, function as reservoirs of SFTSV through transstadial and transovarial transmission. Here, we report the detection of SFTSV in ticks collected from an endemic region in Shandong Province, China. We also provide evidence that SFTSV is maintained in ticks through transstadial and transovarial transmission, thereby allowing ticks to serve as a link between wild and domestic animal and human infections.
Materials and Methods
Tick collection.
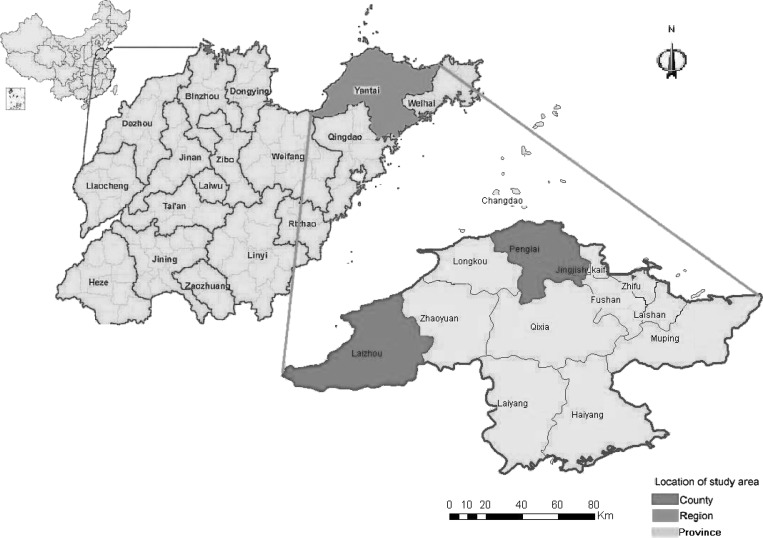
Ticks were collected from both animals and vegetation in the Yantai region, an SFTSV-endemic region of Shandong Province (119°34′–121°57′ E, 36°16′–38°23′ N) (Figure 1). SFTSV human cases have been reported in this region since 2010.
Figure 1.
Map of the Yantai region indicating the locations of the sampling areas. The maps show the location of the Yantai region in Shandong Province, China, where an epidemiological investigation of SFTSV infection in domesticated animals was performed in the study area in 2011. The map at the bottom shows the sampling areas in the Yantai region (red shading), the map in the middle indicates the Yantai region in Shandong Province, and the map at the top shows Shandong Province in China.
Ticks were collected from sheep, cattle, dogs, chickens, and small wild animals in this area. The animals' bodies were examined for ticks. If fewer than 10 ticks were found on one animal, then all ticks were collected; if more than 10 ticks were found, then 10 ticks from each species at each developmental stage were chosen and removed from each animal. The ticks were collected using forceps and placed in perforated tubes containing a moistened piece of filter paper. Labels with unique numbers were placed on each tube to identify the date, location of sample collection, and host from which the ticks had been removed. Engorged ticks were maintained individually, whereas other ticks from the same host were maintained in one tube until species determination. All ticks were maintained at room temperature (20–25°C) for 1 week, after which the engorged female ticks were allowed to oviposit. After oviposition, females were placed individually in 2-mL cryovials, and the cryovials were labeled and stored at −80°C. Eggs from each female that oviposited were immediately placed in a 2-mL cryovial labeled with the same identifying number as that used for the engorged female tick that had laid the eggs followed by an extension of “egg” and stored at −80°C.
Ticks from vegetation were sampled by “the woolen flannel cloth dragging method” as described by Mejlon and Jaenson.8 The size of the woolen flannel cloth was 1 × 1 m. One end of the cloth was attached to a bamboo cane and a string. The method was implemented by dragging the cloth slowly through vegetation for 1 hour in the morning. At 30- to 50-m intervals, the cloth was inspected, and the attached ticks were removed and collected as described above. The tubes were labeled with a unique number to identify the date, location, and collection site.
Ticks collected by both methods were transported to the Chinese National Institute for Viral Diseases Control and Prevention, where they were identified and pooled into groups by site, collection type, host, species, and stage. Adult ticks were placed individually in 2-mL cryovials, whereas nymphs (5–8 per cryovia) and larvae (10 per cryovia) were pooled and stored at −80°C.
RNA extraction and viral RNA detection.
Individual and pooled tick specimens were surface-sterilized with sequential washes with Dulbecco's modified eagle's medium (DMEM) medium containing antibiotics and then homogenized in 500 μL chilled DMEM medium using a tissue homogenizer (TissueLyser; QIAGEN, Hilden, Germany). The tick homogenates were transferred into 1.5-mL microcentrifuge tubes and centrifuged at 4°C and 9,279 × g for 1 minute (Eppendorf, Germany). The clarified supernatants were transferred in 150-μL aliquots to labeled 1.5-mL microcentrifuge tubes for RNA extraction using an RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using 5 μL each aliquot of extracted RNA and a China Food and Drug Administration (CFDA)-approved qRT-PCR kit (DaAn Gene, Guangzhou, China) following the manufacturer's instructions; primer sets and probes targeted the L, M, and S segments of SFTSV as previously described.9 The result was considered positive if a sample had a Threshold cycle (Ct) value below the cutoff for the assay. All real-time qRT-PCR–positive samples were confirmed using qRT-PCR targeting of the three segments of the viral genome. Viral RNA copy numbers were determined by comparison with a standard curve generated by the amplification of positive-control RNA. Two-step conventional RT-PCR was performed for real-time PCR-positive samples using the SuperScript III First-Strand cDNA Synthesis Kit (Invitrogen) to generate cDNA. FastStart High-Fidelity Taq DNA Polymerase (Roche) and primers were used for PCR amplification of the viral genome S segment as previously described.1
Virus isolation.
Tick homogenates that tested positive for viral RNA were tested for the presence of viable virus using Vero cells cultured in six-well plates using a modified version of a previously published protocol.1 Briefly, 100 μL each viral RNA-positive sample was inoculated onto a monolayer of Vero cells. Wells were inspected daily and tested for viral RNA and N-protein antigen on days 6–8 post-infection. If the tests were negative, the culture supernatant was used to inoculate Vero cells for another two passages.
Sequencing and phylogenetic analysis.
RT-PCR amplicons of the S segment of SFTSV were sequenced by the Genewiz Service Company (Genewiz, Beijing, China) using the ABI BigDye Terminator V3.1 Ready Reaction Cycle Sequencing Mix (Applied Biosystems, Carlsbad, CA). Nucleotide sequences were assembled using SeqMan software (DNASTAR) and visual inspection. Alignments were conducted using ClustalW (MEGA 5). Phylogenetic analyses of whole tick-, animal-, and human-derived S segments of SFTSV were conducted with MEGA 5 software using the neighbor-joining (NJ) method with 1,000 replicates for bootstrap testing.10
Statistics.
The prevalence of SFTSV infection in ticks is presented as the maximum likelihood estimate (MLE) per 100 ticks with a 95% confidence interval (95% CI). The MLE was calculated and analyzed using SPSS (v16) software. Viral RNA (vRNA) copy numbers in different species and developmental stages of ticks collected from either animals or plants were analyzed using the Kruskal–Wallis test performed using GraphPad Prism software (version 5.0; GraphPad Software Inc., La Jolla, CA). Values of P < 0.05 were considered statistically significant.
Results
Tick collection.
In total, 2,251 ticks of the species Haemaphysalis longicornis (2,154; 95.7%), Rhipicephalus microplus (83; 3.7%), Haemaphysalis campanulata (9; 0.4%), or Dermacentor sinicus (5; 0.2%) were collected from animal hosts. H. longicornis was the dominant species; ticks of this species at all stages of development were collected (1,342 adults, 652 nymphs, and 138 larvae), and eggs were obtained from 22 H. longicornis adults during maintenance at room temperature for 1 week after detachment (Table 1). Both adult (N = 50) and nymph (N = 33) R. microplus were collected, whereas only adult H. campanulata (N = 9) and D. sinicus (N = 5) were collected. Most of the ticks were collected from sheep (1,718; 76.3%), cattle (254; 11.3%), dogs (194; 8.6%), chickens (61; 2.7%), and hedgehogs (24; 1.1%). In total, 894 H. longicornis ticks were collected by dragging a cloth through vegetation (461 adults, 415 nymphs, and 18 larvae) (Table 1). All ticks collected from vegetation were unfed.
Table 1.
Number of ticks collected by origin, species, and stage
| Origin/species | Adult | Nymph | Larvae | Eggs* | Total |
|---|---|---|---|---|---|
| Animals | 1,406 | 685 | 138 | 22 | 2,251 |
| Sheep | |||||
| H. longicornis | 1,038 | 545 | 102 | 22 | 1,707 |
| R. microplus | 9 | 2 | 0 | 0 | 11 |
| Cattle | |||||
| H. longicornis | 199 | 16 | 0 | 0 | 215 |
| R. microplus | 11 | 28 | 0 | 0 | 39 |
| Dog | |||||
| H. longicornis | 101 | 60 | 0 | 0 | 161 |
| R. microplus | 30 | 3 | 0 | 0 | 33 |
| Chicken | |||||
| H. longicornis | 0 | 25 | 36 | 0 | 61 |
| Hedgehog | |||||
| H. longicornis | 4 | 6 | 0 | 0 | 10 |
| H. campanulata | 9 | 0 | 0 | 0 | 9 |
| D. sinicus | 5 | 0 | 0 | 0 | 5 |
| Vegetation | |||||
| H. longicornis | 461 | 415 | 18 | 0 | 894 |
| Total | 1,867 | 1,100 | 156 | 22 | 3,145 |
Eggs were laid by blood-fed female ticks collected from animals and maintained at room temperature for 1 week after collection. Samples are grouped according to the adult female ticks that laid eggs.
In total, 3,145 ticks from four species were collected in the study region. The ticks were identified and grouped into 2,044 pools by site, collection type, host, species, and stage in preparation for viral RNA detection assays.
Detection of SFTSV RNA in ticks.
Of four species collected, only H. longicornis (larvae, nymphs, and adults) and R. microplus (adults) were positive for SFTSV (Tables 2 and 3). In addition, 2 of 22 of the egg masses oviposited by blood-fed H. longicornis females were positive for SFTSV.
Table 2.
SFTSV infection in ticks by origin, species, and stage
| Origin | Adult (positive/pools/ticks) | Infection rate (95% CI)* | Nymph (positive/ pools/ticks) | Infection rate (95% CI) | Larvae (positive/ pools/ticks) | Infection rate (95% CI) |
|---|---|---|---|---|---|---|
| Animals | 69/1,406/1,406 | 4.91 (3.78–6.04) | 32/91/685 | 4.67 (3.09–6.26) | 4/14/138 | 2.90 (0.06–5.73) |
| H. longicornis | 65/1,342/1,342 | 4.84 (3.69–5.99) | 32/85/1,067 | 3.00 (1.97–4.02) | 4/14/138 | 2.90 (0.06–5.73) |
| Sheep | 56/1,038/1,038 | 5.39 (4.02–6.77) | 29/70/545 | 5.32 (3.43–7.21) | 4/10/102 | 3.92 (0.09–7.75) |
| Cattle | 4/199/199 | 2.01 (0.04–3.98) | 0/3/16 | 0 | − | − |
| Dog | 5/101/101 | 4.95 (0.65–9.25) | 3/8/60 | 5.00 (0–10.68) | − | − |
| Chicken | − | − | 0/3/25 | 0 | 0/4/36 | − |
| Hedgehog | 0/4/4 | 0 | 0/1/6 | 0 | − | − |
| R. microplus | 4/50/50 | 8.00 (0.21–15.79) | 0/33 | 0 | − | − |
| Sheep | 0/9/9 | 0 | 0/1/2 | 0 | − | − |
| Cattle | 1/11/11 | − | 0/4/28 | 0 | − | − |
| Dog | 3/30/30 | − | 0/1/3 | 0 | − | − |
| H. campanulata | − | − | − | − | − | − |
| Hedgehog | 0/9/9 | 0 | − | − | − | − |
| D. sinicus | − | − | − | − | − | − |
| Hedgehog | 0/5/5/ | 0 | ||||
| Vegetation | 6/461/461 | 1.30 (0.26–2.34) | 13/48/415 | 3.13 (1.45–4.82) | 0/2/18 | 0 |
| H. longicornis | 6/461/461 | 1.30 (0.26–2.34) | 13/48/415 | 3.13 (1.45–4.82) | 0/2/18 | 0 |
Infection rate expressed as MLE per 100 ticks. The ranges in parentheses are 95% CIs representing the upper and lower limits.
Table 3.
SFTSV infection in ticks by origin and species
| Tick species/origin | Number positive/number examined | Infection rate (95% CI)* |
|---|---|---|
| H. longicornis | ||
| Sheep | 91/1,685 | 5.28 (4.21–6.35) |
| Cattle | 4/215 | 1.57 (0.03–3.10) |
| Dog | 8/161 | 4.97 (1.58–8.36) |
| Chicken | 0/61 | 0 |
| Hedgehog | 0/10 | 0 |
| Vegetation | 19/894 | 2.13 (1.18–3.07) |
| R. microplus | ||
| Sheep | 0/11 | 0 |
| Cattle | 1/39 | 2.56 (0–7.75) |
| Dog | 3/33 | 9.09 (1–19.44) |
| H. campanulata | ||
| Hedgehog | 0/9 | 0 |
| D. sinicus | ||
| Hedgehog | 0/5 | 0 |
Infection rate expressed as MLE per 100 ticks. The ranges in parentheses are 95% CIs representing the upper and lower limits.
Ticks collected from animals were divided into 1,533 pooled groups and tested; SFTSV RNA was detected in 107 of the pooled samples. The frequencies of infection in ticks were recorded as the MLEs per 100 ticks, with 95% CIs. The MLE of the prevalence of infection in ticks collected from animals was 4.75 per 100 ticks (95% CI = 3.87–5.63) (Table 2). No significant difference in prevalence was observed among adults, nymphs, and larvae (P > 0.1). Of these positive pools, 64.5% (69 of 107) were adults, 29.9% (32 of 107) were nymphs, and 3.7% (4 of 107) were larvae (Table 2). In total, 3 of 22 adult female H. longicornis that oviposited were positive for SFTSV RNA, and two of three egg masses laid by SFTSV-positive females were also positive. We identified 96.3% (103 of 107) of the SFTSV-positive ticks as H. longicornis, whereas the remaining 3.7% (4 of 107) were adult R. microplus (Table 2). No significant difference in the prevalence of SFTSV in H. longicornis was found between ticks collected from sheep and ticks collected from dogs (P > 0.05), but the prevalence in sheep and dogs was both higher than the MLE of the prevalence in ticks collected from cattle (P < 0.05).
In total, 894 H. longicornis ticks were collected by dragging cloths through vegetation. These ticks were examined in 511 pools, of which 6 of 461 adult pools and 13 of 48 nymph pools were SFTSV RNA-positive. The MLE of the prevalence was significantly higher in nymphs (3.13%) than in adults (1.3%; P < 0.05) (Table 2). No viral RNA was detected in two pools of 18 larvae. In total, the MLE of the prevalence in ticks from vegetation was 2.13 per 100 ticks (95% CI = 1.18–3.07) (Table 3), which was significantly lower than that in ticks from animals (P = 0.001).
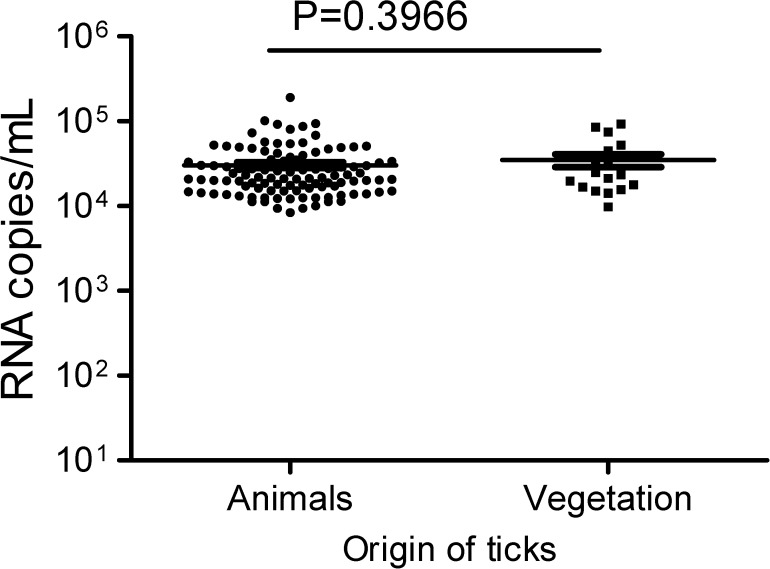
The detected viral RNA concentration was approximately 3.0 × 104 copies/mL (95% CI = 2.6–3.5 × 104) in ticks from animals and 3.5 × 104 copies/mL (95% CI = 2.2–4.8 × 104) in ticks from vegetation. The difference in RNA copy number between ticks collected from animals and ticks collected from vegetation was not statistically significant (P = 0.336) (Figure 2). The S segment of SFTSV was sequenced from 10 pools composed of H. longicornis eggs, nymphs, and adults. Virus could not be grown from any SFTSV-positive tick samples.
Figure 2.
Viral RNA copies in ticks collected from animals and vegetation. Viral RNA copies in ticks were quantified using real-time qRT-PCR; copy numbers for viral RNA-positive ticks are shown. In the scattered dot graphs, each dot depicts one sample, the short line among the dots indicates the geometric mean in ticks, and the P value for the comparison of the number of RNA copies in ticks between animals and vegetation is shown at the top of the graph.
Phylogenetic analysis.
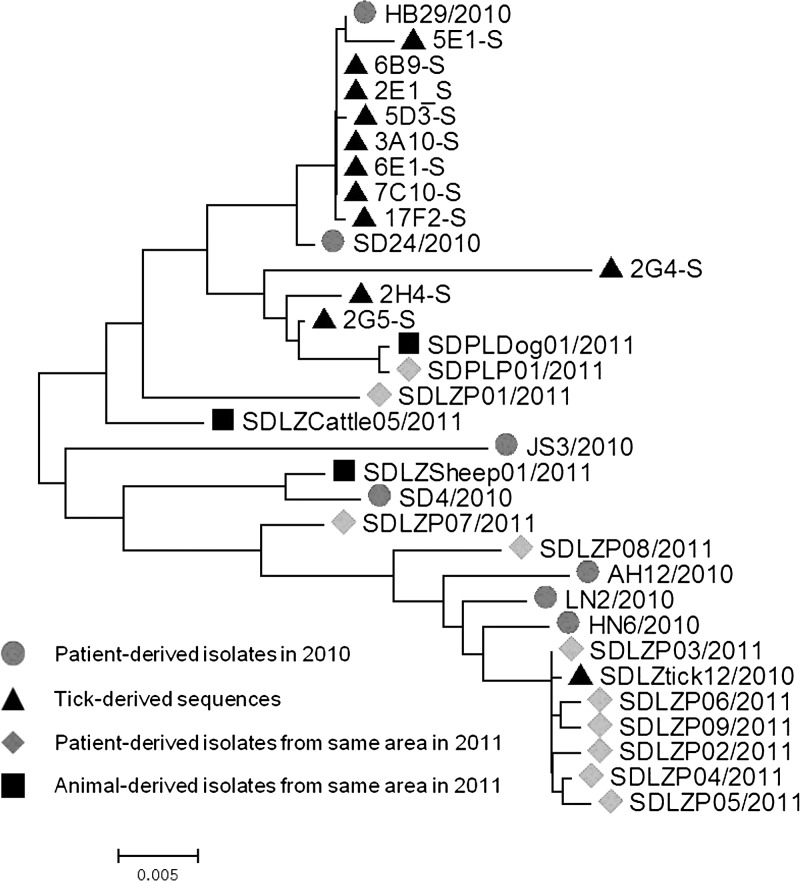
The S segment of SFTSV was amplified and sequenced from 11 of 126 of the RT-PCR–positive tick samples. Phylogenetic analyses were performed, and the S-segment sequences were compared with previously published SFTSV sequences obtained from patients, animals, and ticks. The SFTSV sequences that we obtained from ticks have a 95.6–99.9% nucleotide identity with each other and a 94.6–99.8% identity with sequences reported from patients and animals from both the same area and other provinces. NJ trees constructed with full S-segment sequences showed that SFTSV from local patients, dogs, and cattle clustered with some of the sequences that we obtained from ticks (Figure 3).
Figure 3.
Phylogenetic analysis of SFTSV S-segment sequences amplified from ticks. A phylogenetic tree based on S-segment sequences for representative viruses derived from humans, animals, and ticks inferred by the NJ method using MEGA 5 is shown. The tree was drawn to scale, with branch lengths representing the number of nucleotide substitutions per site. Dots indicate the patient-derived sequences amplified in 2010; triangles indicate the sequences amplified from ticks in this study. Diamonds indicate the sequences isolated from patients, and squares indicate the sequences isolated from domestic animals.
Discussion
SFTSV RNA was detected in H. longicornis at different developmental stages (in larvae, nymphs, and adults) as well as in eggs oviposited by blood-fed adult females. These results suggest that transmission of SFTSV may occur by the transstadial and transovarial routes and show that H. longicornis plays a role in SFTSV maintenance and transmission.
Of four species collected, H. longicornis and R. microplus were the two most frequently encountered ticks on both domestic animals and vegetation in the study region. The tick species and infection rates are similar to those reported from previous tick surveys from areas with SFTS cases in Henan Province and Hubei Province.11 No statistically significant difference was observed for the rate of SFTSV infection among the different stages of H. longicornis, but the prevalence in ticks from animals was significantly higher than that in ticks from vegetation. Furthermore, the infection rate in nymphs was higher than in unfed adults from vegetation. This might imply that blood feeding has an impact on viral RNA detection. Surveillance of SFTSV in ticks, animals, and humans provides epidemiological information about the maintenance and risks associated with epizootic transmission to domestic animals and incidental transmission to humans. All developmental stages of H. longicornis as well as eggs from SFTSV-positive oviposited females were positive for SFTSV, which showed a mode for the maintenance of the virus in vector populations in the absence of feeding on viremic hosts. However, our data were only collected from the field through epidemiologic investigation, and only a few egg masses were collected from SFTSV-positive oviposited females; therefore, additional studies are needed to confirm in the laboratory the transmission of SFTSV in ticks by the transstadial and transovarial routes. In R. microplus, only adults were positive, suggesting that this species may not be effective at maintaining the virus in nature. Although H. campanulata and D. sinicus were SFTSV-negative, their role in SFTSV maintenance and transmission cannot be dismissed, because few ticks of these species were collected for analysis. No viable virus was recovered from ticks in this study; however, this finding cannot be construed as evidence of absence of the virus, and it may, instead, be because of the extremely low titers of the virus in field ticks, which were indicated by the low RNA copy numbers in our qRT-PCR analysis. Several separate field investigations have been performed in areas of China with SFTS cases. One isolate was obtained from 1 of 140 H. longicornis ticks collected in 2010 that possessed genetic and identical serological characteristics similar to those of isolates obtained from humans and domestic animals.4,11
Infectious virus, a competent vector, and susceptible vertebrate hosts are necessary to establish and maintain arbovirus transmission cycles.12 The natural vertebrate reservoirs of SFTSV have not been identified. Domestic animals (including sheep, goats, cattle, and dogs) are considered to be incidental hosts, because they do not develop substantial viremia and long viremic periods have not been observed in these animals.7,13 This finding suggests that the role of viremic domestic animals in transporting SFTSV into new areas to reinfect ticks during a blood meal may be limited. Threshold levels of viremia are usually required for the infection of vectors with virus; however, ticks do not require substantial viremia in an infected host to take up an arbovirus.14–17 While blood-feeding, ticks are attached to their hosts for days, which is crucial for virus transmission in ticks through a mechanism known as cofeeding. During cofeeding, viruses are transferred from one tick to another.18 Adults and immature ticks (either larvae or nymphs) feed on the same host, which may also lead to transmission and maintenance of the arbovirus between different lifecycle stages of the vector.
The risk of SFTSV to humans is related to vector abundance, the infection rate in various habitats, human exposure, tick feeding preferences, and prevention measures used to reduce the frequency of tick bites. The distribution of SFTSV cases is, therefore, shaped by distributions of human populations and behaviors that favor human–tick contact. The high seroprevalence of antibodies to SFTSV detected in sheep (69.5%), cattle (60.4%), dogs (37.9%), and chickens (47.4%)7 may indicate that the virus is more widespread than is evidenced by human cases. It would be beneficial to identify zoonotic hosts that may contribute to the maintenance and distribution of SFTSV.
RNA viruses usually have relatively high mutation rates, which when combined with natural selection, allow viruses to quickly adapt to changes in the host environment. We found that viral sequences from ticks, domestic animals, and local patients could be grouped into different sublineages. The lack of strict phylogenetic linkages among the viral genomic sequences detected in ticks, animals, and human patients from the same geographic location may reflect the evolutionary dynamics of SFTSV, which are observed with other RNA viruses. In addition, the emergence and geographic dispersal of distinct phylogenetic lineages in the same geographic location may be enhanced by the movement of tick-infested animals, which may be introduced into new areas intentionally or through natural migratory activities.
In conclusion, our data provide important epidemiological evidence for SFTSV infection in ticks in an endemic area and suggest the possibility that transstadial transmission of SFTSV may occur among larvae, nymphs, and adults of H. longicornis and that vertical transovarial transmission of SFTSV may occur from infected adult female ticks to eggs and larvae. H. longicornis may act as not only the primary vector of SFTSV but also, a reservoir for this virus.
ACKNOWLEDGMENTS
We are grateful to Mei Jiang from the Yantai City Centers for Disease Control and Prevention (CDC), Haiying Yin from the Laizhou County CDC, Zhidian Wang from the Penglai County CDC, and all who assisted in collecting tick samples.
Disclaimer: The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by China Mega-Project for Infectious Diseases Grants 2011ZX10004-001, 2013ZX10004-101, and 2012ZX10004215; Ministry of Science and Technology and Ministry of Health Natural Science Foundation of China Grant 81102171; and Shandong Medical Science and Technology Development Program Grant 2011HZ055.
Authors' addresses: Shiwen Wang, Jiandong Li, Chuan Li, Quanfu Zhang, Mifang Liang, and Dexin Li, National Institute for Viral Disease Control and Prevention, China Centers for Disease Control and Prevention, Beijing, China, E-mails: wangshiwencdc@163.com, ldong121@126.com, ngy120@163.com, lcehfcdc@163.com, zhqf1960@163.com, mifangl@vip.sina.com, and lidx@chinacdc.cn. Guoyu Niu, National Institute for Viral Disease Control and Prevention, China Centers for Disease Control and Prevention, Beijing, China, and Tianjin International Travel Health Care Center, Tianjin, China, E-mail: ngy120@163.com. Xianjun Wang, Shujun Ding, Xiaolin Jiang, and Zhenqiang Bi, Shandong Province Centers for Disease Control and Prevention, Ji Nan, Shandong, China, E-mails: xjwang62@163.com, dsj_jn@126.com, jxl198607@126.com, and bzq63@163.com.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2014;209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang XL, Wang XJ, Li JD, Ding SJ, Zhang QF, Qu J, Zhang S, Li C, Wu W, Jiang M, Liang MF, Bi ZQ, Li DX. Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals. Chin J Virol. 2012;28:252–257. [PubMed] [Google Scholar]
- 5.Savage HM, Godsey MS, Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 7.Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Wang Z, Li C, Zhang Q, Jin C, Wang X, Ding S, Xing Z, Wang S, Bi Z, Li D. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–763. doi: 10.3201/eid1905.120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mejlon HA, Jaenson TG. Seasonal prevalence of Borrelia burgdorferi in Ixodes ricinus in different vegetation types in Sweden. Scand J Infect Dis. 1993;25:449–456. doi: 10.3109/00365549309008526. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol. 2012;53:48–53. doi: 10.1016/j.jcv.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YZ, Zhou DJ, Qin XC, Tian JH, Xiong Y, Wang JB, Chen XP, Gao DY, He YW, Jin D, Sun Q, Guo WP, Wang W, Yu B, Li J, Dai YA, Li W, Peng JS, Zhang GB, Zhang S, Chen XM, Wang Y, Li MH, Lu X, Ye C, de Jong MD, Xu J. The ecology, genetic diversity and phylogeny of huaiyangshan virus in China. J Virol. 2012;86:2864. doi: 10.1128/JVI.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeffer M, Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit Vectors. 2010;3:35. doi: 10.1186/1756-3305-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu XJ. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg Infect Dis. 2012;18:963–965. doi: 10.3201/eid1806.111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehacek J. Development of animal viruses and Rickettsia in ticks and mites. Annu Rev Entomol. 1965;10:1–24. [Google Scholar]
- 15.Burgdorfer W, Varma M. Trans-stadial and transovarial development of disease agents in arthropods. Annu Rev Entomol. 1967;12:347–376. doi: 10.1146/annurev.en.12.010167.002023. [DOI] [PubMed] [Google Scholar]
- 16.Beasley S, Campbell J, Reid H. In: Threshold problems in infection of Ixodes ricinus with the virus of louping ill. Tick-Borne Diseases and Their Vectors. Wilde J, editor. Edinburgh, UK: Centre for Tropical Veterinary Medicine; 1978. pp. 497–500. [Google Scholar]
- 17.Shepherd AJ, Swanepoel R, Shepherd SP, Leman PA, Mathee O. Viraemic transmission of Crimean-Congo haemorrhagic fever virus to ticks. Epidemiol Infect. 1991;106:373–382. doi: 10.1017/s0950268800048524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph SE, Gern L, Nuttall PA. Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parasitol Today. 1996;12:472–479. doi: 10.1016/s0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]