Abstract
In 2000, an outbreak of Rift Valley fever virus (RVFV) occurred in the Kingdom of Saudi Arabia (KSA). Since then there have been sparse efforts to monitor for RVFV reemergence. During 2012, we enrolled 300 individuals with ruminant exposure and 50 age-group matched non-exposed controls in southwestern KSA, in a cross-sectional epidemiological study of RVFV. Sera from the participants were screened with an enzyme-linked immunosorbent assay (ELISA) for anti-RVFV IgG antibodies of which 39 (11.1%) were positive. Sixteen (41.0%) of those 39 were also positive by a plaque reduction neutralization assay (PRNT). The PRNT-positive subjects were further studied with an IgM ELISA and one was positive. No RVFV was detected in the 350 sera using real-time reverse transcription polymerase chain reaction. Contact with cattle (odds ratio [OR] = 3.16, 95% confidence interval [CI] 1.01, 9.90) and a history of chronic medical illness (OR = 6.41, 95% CI 1.75, 23.44) were associated with greater odds of RVFV seropositivity by PRNT. The IgM-positive participant was 36 years of age, and reported multiple risk factors for ruminant contact. Although these findings simply may be vestiges of the 2000 epidemic, KSA's frequent visits from pilgrims and importations of live animals from RVFV-endemic areas suggest that more comprehensive surveillance for imported RVFV virus in ruminants, mosquitoes, and travelers is imperative.
Introduction
Rift Valley fever virus (RVFV) is a zoonotic pathogen important to both human and animal health. First isolated in 1930 from diseased sheep in the Rift Valley Province of Kenya, by the end of the 20th century the virus was known to circulate throughout much of sub-Saharan Africa.1 In 2000, RVFV was discovered in the Arabian Peninsula, causing severe animal and human disease, resulting in 883 human cases and 124 deaths in the Kingdom of Saudi Arabia (KSA)2 and 1,328 human cases and 166 deaths in the Republic of Yemen.2
In response to the outbreak, the KSA Ministry of Health (MoH) and Ministry of Agriculture (MoA) implemented multiple control strategies including community education, culling of infected animals, livestock import controls, vector control, and intensive ruminant vaccination programs.3 Additionally, the MoA established a systematic surveillance program, monitoring sentinel herds in high-risk areas for the circulation of RVFV.3 Though there are no active human or mosquito surveillance programs in KSA, RVFV has not been reported to the KSA MoH since 2001, suggesting that perhaps the interventions have been successful.
Since the outbreak in 2000, there have been a number of studies conducted among both animal4–7 and human8–10 populations in KSA, evaluating the prevalence of antibodies against RVFV, though few have assessed human populations with intense ruminant exposure. Hence, we conducted this epidemiological study of ruminant-exposed participants in Jazan Region, the epicenter of the 2000 RVFV outbreak in KSA, to assess the prevalence of antibodies against RVFV and to identify risk factors for previous infection.
Materials and Methods
Study design.
Two institutional review boards (KSA and Western IRB) approved this study. The KSA MoH professionals used an informed consent procedure to enroll adults > 18 years of age with ruminant exposure from the Jazan Region, located in the southwest corner of KSA. Ruminant exposure was defined as having an average of one or more cumulative hours per week exposure to camels, cattle, goats, or sheep, through touching and/or coming within 1 m of such animals during the 12 months before enrollment. Participants enrolled as controls resided in the same areas, denied having such contact, and were age-group and gender-matched to exposed participants based upon the final distribution of exposed study participants. Exclusion criteria for both groups included individuals < 18 years of age, having any form of immunosuppression, or having been identified as medically likely to have greater susceptibility to various infectious agents.
Sample collection.
Upon enrollment, participants completed an enrollment questionnaire, which gathered data about demographics, animal and environmental exposures, and relevant medical information. Participants then permitted a blood sample to be collected, in which sera were separated and preserved at −80°C at the KSA MoH laboratory in Jazan, Saudi Arabia. Later, an aliquot of serum was shipped on dry ice to the University of Florida Global Pathogen Laboratory for serological testing.
Enzyme-linked immunosorbent assay (ELISA) screening for antibodies against RVFV.
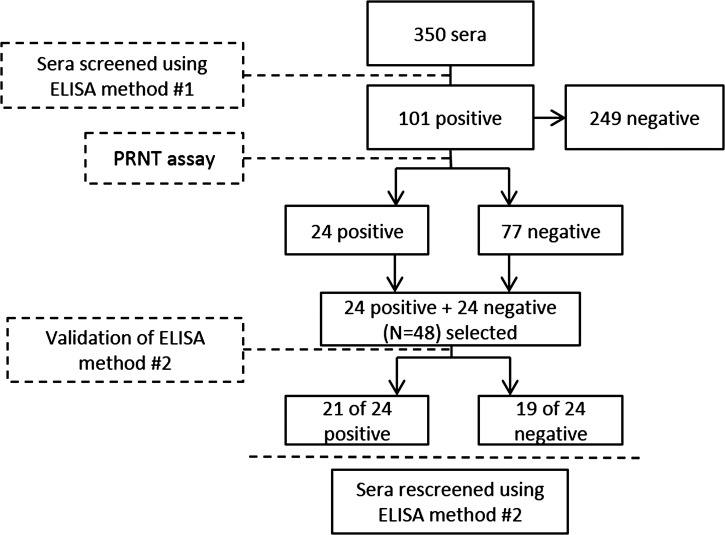
Sera received from KSA MoH were first heat-inactivated for 30 minutes at 56°C and then screened for human antibodies using an indirect capture ELISA adapted in-house using the principles of Paweska and others.11 However, screening sera with this first method resulted in a high percentage of false positives when tested using a confirmatory plaque reduction neutralization assay (PRNT) assay. Hence, we made modifications to the in-house ELISA, validating this second method using 24 PRNT-positive and 24 PRNT-negative specimens selected from the original batch of tested sera. Figure 1 summarizes the assay development and validation plan.
Figure 1.
Flowchart summarizing the validation process of the in-house enzyme-linked immunosorbent assay (ELISA) used to screen sera for antibodies against Rift Valley fever virus (RVFV).
All sera were retested using the second ELISA method described as follows. First, 96-well microtiter plates were coated with a goat anti-human IgG/IgM/IgA antibody (KPL, Inc., Gaithersburg, MD, catalog no. 01-10-07) at a dilution of 1:2000 in sodium bicarbonate buffer (pH = 9.6), covered with plate seals, and incubated at 4°C overnight. Unbound antibody was washed from the well with phosphate buffered saline (PBS) and 0.05% Tween20 (PBS-T), and plates were then blocked with 5% skim milk powder in PBS at room temperature for 2 hours. Test sera, diluted 1:100 in PBS-T with 5% skim milk powder, were added to coated plates and allowed to incubate for 1 hour at 37°C. Gamma-irradiated RVFV antigen, obtained from BEI Resources (NIAID, NIH, Rift Valley Fever Virus, ZH501, Gamma-irradiated, NR-37380), was diluted 1:1000 in PBS-T with 5% skim milk powder, added to the plates, and allowed to incubate for 1 hour at 37°C. Rabbit anti-RVFV polyclonal antibody, obtained from Integrated Biotherapeutics Inc. (Gaithersburg, MD, catalog no. 04-0001), was diluted 1:1000 in PBS-T with 5% skim milk powder, added to the plates and allowed to incubate for 1 hour at 37°C. Extra serum adsorbed horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (KPL, Inc., catalog no. 074-15-061) was diluted 1:2000, added to each well, and incubated for 1 hour at 37°C. All wells were washed 5 times after each incubation step using PBS-T. Each plate contained a “no antigen” negative control well to adjust for background absorbance. A positive control was also run for each set to calculate positive cut-offs for test samples. Chomagenic detection of HRP was performed by the addition of 0.1 mL of the peroxidase substrate 3,3′,5,5′- tetramethylbenzidine (TMB) (KPL, Inc.), at room temperature for 10 minutes and stopped by the addition of 0.1 mL 1N sulfuric acid.12 Absorbance of each well at 450 nm (A450) was measured on a PowerWave HT microplate spectrophotometer (Biotek, Winooski, VT). Sera samples were considered ELISA positive if the average A450 of samples was greater than or equal to 60% of the average A450 of the positive control divided by the average A450 of the negative control wells.
All sera were also tested using a commercial RVFV human IgG ELISA kit obtained from Biological Diagnostic Supplies Limited (BDSL, Scotland, UK) according to the manufacturer's instructions. Briefly, plates were coated with a recombinant nucleocapsid RVFV antigen diluted 1:1000 in sodium bicarbonate buffer (pH = 9.6), covered with plate seals, and incubated at 4°C overnight. Unbound antigen was removed by washing 3 times for 15 s each using PBS-T. Plates were then blocked with 10% skimmed milk in PBS (PBS-SM) at 37°C for 1 hour. Plates were washed, test sera added in duplicate at a dilution of 1:400 in 2% PBS-SM, and incubated for 1 hour at 37°C. Plates were washed once more, and HRP-conjugated anti-human IgG antibody, diluted 1:25,000 in 2% PBS-SM, was added to each well and incubated for 1 hour at 37°C. After a final wash, chromagenic detection of HRP and absorbance measurement was performed as described previously. Negative and positive control sera were included for each plate. Sera samples were considered positive if their optical density (OD) calculation was ≥ 0.29 (Net OD serum/Net Mean OD positive control).
Plaque reduction neutralization test.
All samples testing positive by either the in-house or kit ELISAs were further studied using a PRNT, adapted from methods previously described.13,14 The RVFV MP-12 vaccine strain, propagated in Vero-CCL81 cells, was used in the PRNT assay. Sera were tested in duplicate using six 4-fold dilutions starting with 1:10 and ending at 1:10,240. A back titration of the diluted stock MP-12 virus was performed each time assays were run to ascertain the unneutralized titer of virus stock used (typically, 30–60 plaque-forming units/mL). A neutralization cut-off of 80% reduction, as determined by a corresponding back titration plate, was used to determine sera titration endpoints. The minimum PRNT titer for a serum to be considered PRNT positive was ≥ 1:40.
Testing PRNT-positive samples using IgM ELISA.
To ascertain whether an individual had evidence of an acute infection, PRNT-positive samples were subsequently tested by the previously described indirect capture ELISA method using a goat anti-human IgM antibody (KPL, Inc. catalog no. 01-10-03) at a dilution of 1:2000 instead of the IgG/IgM/IgA antibody originally used for screening. An IgM positive control sample was not available for this assay. Instead, serum samples collected from six individuals with no possible RVFV exposure were collected and included in the assay run. The IgM positivity was defined as any sample with an OD greater than three times the standard deviation plus the average OD of the six negative control sera. All other testing conditions remained the same.
RNA extraction/quantitative real-time polymerase chain reaction (qRT-PCR).
To further assess whether individuals had an acute infection upon enrollment, we also screened each serum sample for RVFV RNA. Nucleic acid extraction from 140 μL of serum was performed using the QIAamp Viral RNA Mini Kit (Qiagen; Valencia, CA), according to manufacturer's instructions. A one-step qRT-PCR protocol was performed using the iScript cDNA synthesis kit (BioRad, Hercules, CA) using primers, probes, and cycling protocol previously described.15 A positive control of extracted RNA from the RVFV MP-12 strain was prepared for each run, and a no template and negative control. All qRT-PCR samples were tested in triplicate.
Statistical methods.
Bivariate χ2 tests of independence or Fisher's exact test were used to examine the association of demographic variables with the PRNT serological outcome. Variables determined by bivariate analyses to be statistically associated with RVFV seropositivity (P < 0.25), were then entered into a multivariable logistic regression model. Backward elimination was performed and covariates with P < 0.05 were retained in the model. Individual predictors retained in the final logistic models were tested for collinearity using bivariate χ2 tests. Finally, Hosmer-Lemeshow χ2 statistics for goodness-of-fit were performed. All demographic statistics, bivariate testing, and logistic modeling were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Study population.
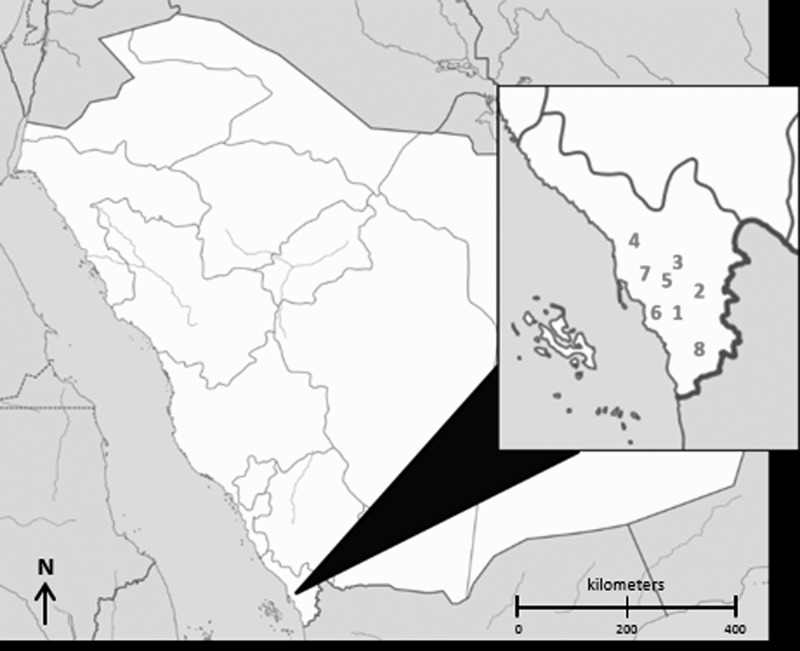
Participants were enrolled in the Jazan Region (location of 2000 RVFV outbreak), which is 11,671 km2 and located in the southwestern corner of KSA (Figure 2). It has a subtropical climate, sharing a border to the south with the Republic of Yemen and ~300 km of coastline along the Red Sea to the west. According to the 2010 census, the population of the province was 1,365,110. Although this represents only 5.2% of the entire population of KSA, it should be noted that Jazan Region has the highest population density compared with other provinces in KSA.
Figure 2.
Map of governorates where participants were enrolled in the southwest region of the Kingdom of Saudi Arabia (1-Abu Arish, 2-Al Aridah, 3-Al Aydabi, 4-Baish, 5-Dhamad, 6-Jazan, 7-Sabya, and 8-Samtah).
In July 2012, field staff from the KSA MoH enrolled a total of 350 participants (300 exposed and 50 controls) from the governorates of Abu Arish, Al Aridah, Al Aydabi, Baish, Dhamad, Jazan, Sabya, and Samtah, within Jazan Region, KSA. All participants, but one, were male with a median age of 37.9 years (Table 1). Participants reported diverse occupations, which included being a student, butcher, health care worker, office worker, and herder. The majority of participants enrolled (43.7%) reported residency in Jazan, the capital city of Jazan Region. Additionally, 16.9% of participants reported they were semi-nomadic.
Table 1.
Demographic characteristics of the study population (N = 350) sampled in southwestern KSA by exposure group
| Demographic variables | No. (%) | Exposure group | |
|---|---|---|---|
| Exposed | Control | ||
| No. (%) | No. (%) | ||
| Exposed vs. control | 300 (85.7%) | 300 (100.0%) | – |
| Exposed* | |||
| Control | 50 (14.3%) | – | 50 (100.0%) |
| Gender | |||
| Male | 346 (98.9%) | 296 (98.7%) | 50 (100.0%) |
| Female | 1 (0.3%) | 1 (0.3%) | – |
| Permanent residence in village or semi-nomadic | |||
| Permanent | 281 (80.3%) | 238 (79.3%) | 43 (86.0%) |
| Semi-nomadic | 59 (16.9%) | 53 (17.7%) | 6 (12.0%) |
| Governorate | |||
| Abu Arish | 41 (11.7%) | 33 (11.0%) | 8 (16.0%) |
| Al Aridah | 30 (8.6%) | 26 (8.7%) | 4 (8.0%) |
| Al Aydabi | 28 (8.0%) | 21 (7.0%) | 7 (14.0%) |
| Baish | 51 (14.6%) | 46 (15.3%) | 5 (10.0%) |
| Dhamad | 8 (2.3%) | 8 (2.7%) | – |
| Jazan | 153 (43.7%) | 137 (45.7%) | 16 (32.0%) |
| Sabya | 18 (5.1%) | 18 (6.0%) | – |
| Samtah | 9 (2.6%) | – | 9 (18.0%) |
Exposure was defined as close contact through touching and/or coming within 1 m of a ruminant animal during the 12 months before enrollment.
KSA = Kingdom of Saudi Arabia.
In-house ELISA validation.
Of the 24 PRNT-positive and 24 PRNT-negative samples tested for the validation of the modified in-house ELISA, 21 and 5 were positive, respectively. This reflected a sensitivity of 87.5% (95% confidence interval [CI] 0.74, 1.0) and specificity of 79.2% (95% CI 0.62, 0.95).
ELISA, plaque reduction neutralization test, and qRT-PCR.
Of 350 samples collected and tested by both ELISA assays, 18 were positive for RVFV antibodies using the in-house ELISA, 26 were positive using the BDSL ELISA kit, and 5 were positive by both the in-house ELISA and BDSL ELISA kit. Of the 39 positive samples by either ELISA, 16 were confirmed positive by the PRNT assay at a sera dilution ≥ 1:40. All the confirmed PRNT-positive samples were from individuals with exposure to ruminants. The titer range for the exposed positives was 1:40 to 1:10,240, with 11 of the 16 (68.8%) having a titer > 1:160 (Table 2). Of the 16 PRNT-positive sera, one specimen with a PRNT titer of 1:640 tested positive by ELISA for IgM antibodies. The sample was from a man 36 years of age with daily reported exposures to sheep, goats, and cattle, who lived in Al Aydabi and had not traveled outside of KSA. This individual also reported monthly slaughtering, butchering, and skinning of animals, and caring for a birthing and sick animal, which included the disposal of an aborted fetus. RVFV RNA was not detected in any of the sera samples.
Table 2.
Titer distribution of positive samples, by exposure group, using the 80% plaque reduction neutralization test
| Titer | Exposed* | Controls |
|---|---|---|
| 1:40 | 2 | 0 |
| 1:160 | 3 | 0 |
| 1:640 | 7 | 0 |
| 1:2560 | 3 | 0 |
| 1:10240 | 1 | 0 |
| Total: | 16 | 0 |
Exposure was defined as close contact through touching and/or coming within 1 m of a ruminant animal during the 12 months before enrollment.
Bivariate and multivariate analysis.
Eight variables were identified as potential statistically significant predictors of RVFV seropositivity (P < 0.25). Unadjusted odds ratios (ORs) with 95% CIs were calculated for each and presented in Table 3. These variables were included in a multivariate model using binary logistic regression, and after performing backward elimination three covariates remained as significant (P < 0.05) predictors of RVFV seropositivity. Adjusted ORs with 95% CIs were calculated and also included in Table 3. No collinearity problems were detected between these three variables. The Hosmer-Lemeshow χ2 statistics for goodness-of-fit indicated that predictors sufficiently described the data.
Table 3.
Unadjusted and adjusted odds ratios for evidence of previous RVFV infection by a positive plaque reduction neutralization test (minimum titer ≥ 1:40)
| Risk factor | No. | Rift Valley fever virus IgG positive | |
|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| History of contact with cattle | 3.2 (1.0, 9.9) | ||
| Yes | 132 | 3.5 (1. 2, 10.4) | Ref. |
| No | 217 | Ref. | |
| History of chronic medical problems | |||
| Yes | 27 | 7.0 (2.2, 22.1) | 6.4 (1.8, 23.4) |
| No | 316 | Ref. | Ref. |
| Sheltered livestock in home | |||
| Yes | 186 | 4.0 (1.1, 14.3) | |
| No | 163 | Ref. | |
| Cared for birthing animal | |||
| Yes | 87 | 4.2 (1.5, 11.6) | |
| No | 261 | Ref. | |
| Disposed of and aborted animal fetus | |||
| Yes | 72 | 3.2 (1.2, 9.0) | |
| No | 278 | Ref. | |
| Cared for a sick animal | |||
| Yes | 88 | 4.2 (1.5, 11.5) | |
| No | 262 | Ref. | |
| Handled an animal carcass | |||
| Yes | 73 | 3.1 (1.1, 8.7) | |
| No | 275 | Ref. | |
| History of poor vision | |||
| Yes | 20 | 4.3 (1.1, 16.5) | |
| No | 329 | Ref. | – |
RVFV = Rift Valley fever virus; OR = odds ratio; CI = confidence interval.
In bivariate analyses, important risk factors for RVFV seropositivity included contact with cattle, history of chronic medical illness, housing livestock in the home, caring for a birthed animal, handling an aborted fetus, caring for a sick animal, discarding an animal carcass, and history of poor vision anytime in the last 12 months before enrollment. After running the multivariate model with backward elimination, statistically significant predictors remaining in the model included contact with cattle during the previous 12 months (OR = 3.16, 95% CI 1.01, 9.90) and history of chronic medical illness (OR = 6.41, 95% CI 1.75, 23.44).
Discussion
In this cross-sectional study in the KSA, we tested sera collected from individuals with exposure to ruminant animals for the presence of antibodies against RVFV, and compared the results with matched non-exposed controls. Additionally, we used questionnaire data to identify significant risk factors associated with a positive PRNT assay against RVFV.
Sixteen (4.6%) of the 350 sera tested, were confirmed positive for elevated antibodies against RVFV by PRNT assay. This prevalence is slightly lower than those reported from other cross-sectional studies of humans conducted in KSA. Al-Azraqi and others, in two more recent works, reported an IgG prevalence of 6.0% among a random sample (N = 2322) of adults, and an IgG prevalence of 4.9% among a random sample (N = 389) of children and adolescents, both tested in late 2008 from southwestern KSA.8,9 One could argue that detectable antibody levels seen in this study are not surprising and may be a result of RVFV exposure during the 2000 outbreak, particularly given that Jazan Region was considered the epicenter. However, although viremia and IgM antibody persistence for RVFV have been well documented, less is known about the long-term persistence of human IgG antibodies against RVFV; therefore, post-outbreak exposure cannot be completely ruled out.15 Furthermore, the detection of IgM antibody in one of the PRNT-positive samples could suggest the possibility of recent exposure to RVFV. More evidence would be necessary, including the collection of prospective exposure data, to better ascertain whether a recent exposure had occurred.
All PRNT-positive samples were collected from individuals reporting ruminant exposure, although none of the controls showed RVFV positivity. This coincides with other studies that have strongly implicated ruminants as an interepidemic reservoir for RVFV in Saudi Arabia, suggesting that importing diseased ruminants could contribute to a reemergence of RVFV in the future.3–6,8 Analysis of multiple possible risk factors in a multivariate model showed contact with cattle and a history of chronic illness in the last 12 months, to be significant predictors of RVFV antibody persistence, after adjusting for other covariates. Notably, caring for a birthing animal, disposing of an aborted fetus, caring for a sick animal, and handling an animal carcass, which are all risk factors that involve possible close contact with animal fluid, were only just below the cut-off for significance in the full model.
This study had several limitations. The population sampled was nearly all male, creating a gender bias in the interpretation of the data. Furthermore, the study design was cross sectional, which is not the best method for determining when an infection has occurred. Instead, a prospective study would be better suited to capture recent infections and antibody response over time. Finally, the RVFV MP-12 vaccine strain, which was used in the PRNT assay, may not be representative of the wild-type strains in southwestern KSA.
The KSA's surveillance for RVFV is currently limited to disease surveillance among ruminants. There is no active surveillance for RVFV in mosquito pools or among human patients with RVFV-like infections. Furthermore, since the control of the outbreak in 2000, ruminant vaccination programs in the region are no longer operational. Considering the 10 million pilgrims who visit KSA to participate in the Hajj or Umruh and the millions of ruminants, which are imported into the Kingdom each year (many from RVFV enzootic areas), it seems quite possible that another epizootic of RVFV could easily occur in KSA. Simple adjustments in RVFV surveillance to include mosquitoes and humans could greatly enhance KSA's protection efforts.
Overall, results from this study show a difference in the detection of neutralizing antibodies against RVFV between individuals exposed to ruminant animals and those who are not. This suggests that those who are exposed may be at a greater risk of RVFV infection and measures should be implemented to educate and monitor these populations for possible RVFV reemergence. If future studies are designed to further explore these findings it would seem prudent to make them prospective in design such that incidence rates could be ascertained and individual risk factors associated with RVFV infection could be further explored.
ACKNOWLEDGMENTS
We thank the following professionals for their much appreciated scientific advice and sharing of viruses: Robert B. Tesh of the University of Texas Medical Branch, Galveston, TX; Douglas M. Watts, University of Texas at El Paso, El Paso, TX; and Kenneth Linthicum of the USDA-Center for Medical, Agricultural and Veterinary Entomology, Gainesville, FL. We also thank Kelli Barr, John Paul Burks, Stephen J. Blazs, Clint McDaniel, John Friary, and Yilun Sun, all formerly with the Global Pathogens Laboratory; Juergen Richt, Igor Morozov, and Karinne K. Cortes of the Center of Excellence for Emerging and Zoonotic Animal Diseases (CEEZAD), Kansas State University, Manhattan, KS, for their numerous contributions and support of this work.
Footnotes
Financial support: This study was made possible through funds and collaborations between the Ministry of Health, Kingdom of Saudi Arabia, Riyadh, Saudi Arabia; and the Global Pathogens Laboratory, University of Florida Emerging Pathogens Institute. Additional funding was provided by the Department of Homeland Security Center of Excellence for Emerging and Zoonotic Animal Diseases (CEEZAD), Grant no. 2010-ST061-AG0001.
Authors' addresses: Ziad A. Memish, College of Medicine, Alfaisal University, Riyadh, Kingdom of Saudi Arabia, and Ministry of Health, Riyadh, Kingdom of Saudi Arabia, E-mail: zmemish@yahoo.com. Malak A. Masri, Public Health Directorate, Ministry of Health, Riyadh, Kingdom of Saudi Arabia, E-mail: malouk22@hotmail.com. Benjamin D. Anderson and Gregory C. Gray, Division of Infectious Diseases and Duke Global Health Institute, Duke University, Durham, NC, E-mails: benjamin.anderson2@duke.edu and gregory.gray@duke.edu. Gary L. Heil, Hunter R. Merrill, and Salah U. Khan, University of Florida, Gainesville, FL, E-mails: glheil@ehs.ufl.edu, hmerrill@ufl.edu, and m.khan@ufl.edu. Ahmad Alsahly, Ministry of Health, Jazan Health Region, Kingdom of Saudi Arabia, E-mail: rvf2002@hotmail.com.
References
- 1.Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J Pathol Bacteriol. 1931;34:545–579. [Google Scholar]
- 2.CDC Outbreak of Rift Valley fever – Saudi Arabia, August–October, 2000. MMWR Morb Mortal Wkly Rep. 2000;49:905–908. [PubMed] [Google Scholar]
- 3.Al-Afaleq AI, Hussein MF. The status of Rift Valley fever in animals in Saudi Arabia: a mini review. Vector Borne Zoonotic Dis. 2011;11:1513–1520. doi: 10.1089/vbz.2010.0245. [DOI] [PubMed] [Google Scholar]
- 4.Elfadil AA, Hasab-Allah KA, Dafa-Allah OM. Factors associated with Rift Valley fever in south-west Saudi Arabia. Rev Sci Tech. 2006;25:1137–1145. [PubMed] [Google Scholar]
- 5.Al-Qabati AG, Al-Afaleq AI. Cross-sectional, longitudinal and prospective epidemiological studies of Rift Valley fever in Al-Hasa Oasis, Saudi Arabia. Journal of Animal and Veterinary Advances. 2010;9:258–265. [Google Scholar]
- 6.Al-Afaleq AI, Hussein MF, Al-Naeem AA, Housawi F, Kabati AG. Seroepidemiological study of Rift Valley fever (RVF) in animals in Saudi Arabia. Trop Anim Health Prod. 2012;44:1535–1539. doi: 10.1007/s11250-012-0100-x. [DOI] [PubMed] [Google Scholar]
- 7.Elfadil AA, Hasab-Allah KA, Dafa-Allah OM, Elmanea AA. The persistence of rift valley fever in the Jazan region of Saudi Arabia. Rev Sci Tech. 2006;25:1131–1136. [PubMed] [Google Scholar]
- 8.Al-Azraqi TA, El Mekki AA, Mahfouz AA. Rift Valley fever in southwestern Saudi Arabia: a sero-epidemiological study seven years after the outbreak of 2000–2001. Acta Trop. 2012;123:111–116. doi: 10.1016/j.actatropica.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Al Azraqi TA, El Mekki AA, Mahfouz AA. Rift Valley fever among children and adolescents in southwestern Saudi Arabia. J Infect Public Health. 2013;6:230–235. doi: 10.1016/j.jiph.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Memish ZA, Albarrak A, Almazroa MA, Al-Omar I, Alhakeem R, Assiri A, Fagbo S, MacNeil A, Rollin PE, Abdullah N, Stephens G. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg Infect Dis. 2011;17:2316–2318. doi: 10.3201/eid1712.110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paweska JT, Burt FJ, Swanepoel R. Validation of IgG-sandwich and IgM-capture ELISA for the detection of antibody to Rift Valley fever virus in humans. J Virol Methods. 2005;124:173–181. doi: 10.1016/j.jviromet.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Antonovics J, Hood ME, Baker CH. Molecular virology: was the 1918 flu avian in origin? Nature. 2006;440:E9. doi: 10.1038/nature04824. [DOI] [PubMed] [Google Scholar]
- 13.Webb PA, Johnson KM, Mackenzie RB. The measurement of specific antibodies in Bolivian hemorrhagic fever by neutralization of virus plaques. Proc Soc Exp Biol Med. 1969;130:1013–1019. doi: 10.3181/00379727-130-33711. [DOI] [PubMed] [Google Scholar]
- 14.Earley E, Peralta PH, Johnson KM. A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med. 1967;125:741–747. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- 15.Bird BH, Bawiec DA, Ksiazek TG, Shoemaker TR, Nichol ST. Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J Clin Microbiol. 2007;45:3506–3513. doi: 10.1128/JCM.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]