Abstract
Oral vaccines appear less effective in children in the developing world. Proposed biologic reasons include concurrent enteric infections, malnutrition, breast milk interference, and environmental enteropathy (EE). Rigorous study design and careful data management are essential to begin to understand this complex problem while assuring research subject safety. Herein, we describe the methodology and lessons learned in the PROVIDE study (Dhaka, Bangladesh). A randomized clinical trial platform evaluated the efficacy of delayed-dose oral rotavirus vaccine as well as the benefit of an injectable polio vaccine replacing one dose of oral polio vaccine. This rigorous infrastructure supported the additional examination of hypotheses of vaccine underperformance. Primary and secondary efficacy and immunogenicity measures for rotavirus and polio vaccines were measured, as well as the impact of EE and additional exploratory variables. Methods for the enrollment and 2-year follow-up of a 700 child birth cohort are described, including core laboratory, safety, regulatory, and data management practices. Intense efforts to standardize clinical, laboratory, and data management procedures in a developing world setting provide clinical trials rigor to all outcomes. Although this study infrastructure requires extensive time and effort, it allows optimized safety and confidence in the validity of data gathered in complex, developing country settings.
Background and Rationale
Oral vaccines have been shown to be less effective in low-income and developing countries, limiting the optimal benefits of vaccination in populations with the greatest disease burden. Rotavirus diarrhea and poliomyelitis are vaccine-preventable diseases of high public health priority; however, oral rotavirus vaccine is 58% effective at preventing severe rotavirus diarrhea in Nicaraguan children and 46% in Bangladeshi children, compared with > 98% efficacy in Finland.1–3 A similar trend is seen in oral polio vaccine (OPV), where > 95% children with paralytic polio due to wild-type poliovirus infection in India reported receiving more than the standard three doses of OPV, and 77% more than seven doses.4,5
There are several plausible biologic explanations for the observation of vaccine underperformance, including environmental enteropathy (EE), a poorly-defined disorder of the small intestine marked by increased intestinal inflammation and impaired gut immune function.6 Other factors that may contribute to oral vaccine underperformance as part of or independent from EE include malnutrition, concurrent enteric coinfections, infant immunologic maturity, variability in the intestinal microbiome, interference of maternal antibodies, genetic factors, and socioeconomic realities.7,8
The rationale for the design of the “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study was to carefully evaluate factors that could interfere with oral vaccine efficacy in an environment characterized by poverty, urban overcrowding, and poor sanitary conditions. Given the complexity of the hypotheses involved, as well as our priority to apply the highest standards in human subjects protection in working with this vulnerable population (infants in a developing-world context), a prospective randomized clinical trial design was chosen as the platform on which the highest-quality data for all outcomes could be achieved and within which the safest research could be conducted. Consultations with experts in the fields of rotavirus and polio vaccines and reviews of the relevant literature informed decisions on the choice of vaccine interventions on which variables affecting vaccine performance could be best tested. PROVIDE evaluated the efficacy of a delayed-dosing (weeks 10, 17) schedule of oral rotavirus vaccine and an IPVon OPV schedule in children in Bangladesh. On this framework, variables contributing to vaccine underperformance, including EE, were explored in detail. Herein, we describe the design, methodological approach, and baseline population results from the Bangladeshi cohort of the PROVIDE study.
Methods
The primary objectives of the PROVIDE study (in Bangladesh) were to determine: 1) the efficacy of a 2-dose Rotarix® oral rotavirus vaccine (given at 10 and 17 weeks of age) to prevent rotavirus diarrhea in the first year of life and 2) OPV efficacy when a single inactivated polio vaccine (IPV) dose replaced the fourth dose of trivalent OPV (tOPV). The secondary objective was to determine whether EE, measured by lactulose/mannitol testing, was associated with reduced efficacy of oral vaccines for polio and rotavirus among infants. Multiple exploratory objectives included exploration of variables that might impact oral vaccine function, including socioeconomic status (SES), micronutrient deficiency, the presence of enteric co-pathogens, other measures of EE, and interference of transplacental maternal or breast milk antibodies.
Hypotheses and primary endpoints.
Please see Table 1. For the Rotavirus vaccine trial group, the null hypothesis was non-superiority of vaccination versus the alternative (no vaccine) that a 2-dose regimen of orally administered Rotarix vaccine at 10 and 17 weeks of age reduces the incidence of rotavirus-associated diarrhea during the entire first year of life. The dichotomous endpoint was the occurrence of ≥ 1 episodes of rotavirus diarrhea by 1 year of age. Rotavirus diarrhea was defined as a case of diarrhea within which rotavirus antigen was detected by enzyme-linked immunoassay (ELISA) in stool.
Table 1.
PROVIDE study primary analyses
| Objective | Hypothesis | Endpoint measure | Planed primary analysis |
|---|---|---|---|
| Polio and Rotavirus vaccination objectives | |||
| Determine impact of IPV dose on the efficacy of OPV | Substituting IPV for fourth dose of OPV results in improved mucosal immunity/reduced poliovirus shedding | Presence of fecal shedding of any OPV vaccine virus strains following final OPV dose | Intention-to-treat analysis to assess the superiority of the tOPV + IPV over the standard of care |
| Determine efficacy of Rotarix vaccine to prevent rotavirus diarrhea in the first year of life | Two doses of oral rotavirus vaccine at 10 and 17 weeks reduces the risk of rotavirus diarrhea in the first year of life | Any rotavirus diarrhea by 1 year of age (Yes/No) | Intention to Treat analysis to assess the superiority of rotavirus vaccination over no rotavirus vaccination |
| Environmental enteropathy (EE) and exploratory objectives | |||
| Determine effect of EE on oral polio vaccine efficacy | EE impairs oral polio vaccine efficacy | Polio serum neutralizing antibody of (OPV 1–3) at week 18 | Association of Polio serum neutralizing antibody at week 18 or incidence of rotavirus diarrhea by week 52 with biomarker panel analyzed by: |
| Determine effect of EE on oral rotavirus vaccine efficacy | EE impairs oral rotavirus vaccine efficacy | One or more episodes of rotavirus diarrhea by 1 year of age (Yes/No) | • Individual univariable analysis |
| • Best subsets multivariable analysis | |||
| • Regression analysis with unbiased approach for variable selection and internal penalization for the use of multiple variables (e.g., smoothly clipped absolute deviation [SCAD]) | |||
For the poliovirus vaccine trial group, the null hypothesis was non-superiority of an injectable inactivated polio vaccine (IPV) replacing the fourth tOPV dose (at 39 weeks) versus the standard 4-dose OPV series. The dichotomous endpoint for the Poliovirus vaccine trial group was the presence of fecal shedding of any one of the three Sabin poliovirus vaccine types at any assayed time point following a challenge tOPV dose at 52 weeks: days 0 pre-vaccination, or days 4, 11, 18, or 25 post-vaccination. Poliovirus detection for the primary endpoint was determined by cell culture assay.
Secondary endpoints evaluated the hypothesis that oral rotavirus or OPV(s) would have decreased efficacy in infants with EE. The lactulose/mannitol (L/M) ratio, based on urine testing following ingestion of L/M solution, was the primary biomarker of EE; however, an expanded set of biomarkers of gut inflammation and function were added as additional exploratory biomarkers of EE. For analysis of this hypothesis, polio vaccine efficacy was measured by polio serum neutralizing antibody after three doses of OPV at 18 weeks of life.
Study design.
The PROVIDE study was a randomized controlled clinical trial with two vaccine interventions, which enrolled a birth cohort of 700 children and their mothers, and followed the children for their first 2 years of life. The study had a 2 × 2 factorial design (Table 2). The rotavirus vaccine treatment group was open-label and non-placebo controlled. The poliovirus treatment group was an open-label with an active control group. In both groups, laboratory personnel and non-clinic site investigators were masked to poliovirus and rotavirus trial arm assignments.
Table 2.
Vaccines administered to PROVIDE subjects in 2 × 2 factorial study design
| Vaccines administered | No Rotarix | Rotarix | Total |
|---|---|---|---|
| OPV dose at 39 weeks | 175 | 175 | 350 |
| IPV dose at 39 weeks | 175 | 175 | 350 |
| Total | 350 | 350 | 700 |
OPV = oral polio vaccine.
Randomization scheme.
Study subjects were randomized to one of four 2 × 2 treatment groups using permuted blocks with random block size selection (4 or 8). Before enrollment, the Data Coordinating Center produced sealed envelopes, one per subject identification number (SID), containing the random treatment group assignment. SIDs, and thus randomized group assignment, were assigned sequentially to each infant/mother pair during the enrollment process. The sealed envelopes were opened at each infant's week 6 study visit.
Sample size and power.
The study had a fixed enrollment of 700 infants. Children who withdrew prior to completion were not replaced, and study power estimates allowed up to 20% loss to follow-up. For the rotavirus trial group, we assumed 50% vaccine efficacy.9 For the polio trial group, it was assumed that OPV virus shedding rates would be 15–20% with a drop to 5% in the IPV dose group.10,11 For the EE analyses, the sample size allowed > 80% power to detect at least a 20% increase in oral rotavirus and OPV efficacy in children without and with EE under the following assumptions: 1) 50% infants have EE within the first 2 years of life, 2) rotavirus infection affects 26% infants in their first year of life, and 3) serum neutralizing antibody responses following three doses of OPV are 90% and 68% for Sabin types 1 and 3, respectively (assumption based on unpublished, preliminary data in 2010; published in 2014).12
Vaccines schedule.
The Rotavirus vaccine intervention was the administration of two doses of oral rotavirus vaccine (Rotarix) at 10 and 17 weeks of age to half of the study population, per the randomization assignments. Rotavirus vaccine was not included in the national Bangladesh Expanded Program on Immunization (EPI) during the conduct of the trial. Choice of rotavirus vaccine dosing schedule was determined by expert consultation with leaders in the field of rotavirus vaccination. The polio vaccine intervention was the administration of an injected, inactivated polio vaccine (IPV) dose replacing the fourth dose of tOPV at 39 weeks of age. In addition to the vaccine interventions, study children received all standard EPI vaccines through the study clinic. The full immunization schedule is shown in Table 3.
Table 3.
PROVIDE study intervention and routine immunization schedule
| Vaccine(s) | 0 | 6 | 10 | 12 | 14 | 17 | 24 | 39 | 40 | 52 | 65 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPI vaccines* | √ | √ | √ | √ | √ | √ | |||||
| tOPV | √ | √ | √ | √ | √ | ||||||
| tIPV arm | √ | ||||||||||
| Rotarix arm | √ | √ | |||||||||
| Lactulose/mannitol solution | √ | √ | √ |
tOPV = trivalent oral polio vaccine; tIPV = trivalent inactivated polio vaccine.
The national Bangladesh Expanded Program on Immunizations (EPI) schedule includes BCG at birth; pentavalent vaccine (DPT, HepB, Hib) at 6, 10, and 14 weeks; bivalent Measles-Rubella at 40 weeks; and monovalent Measles at 65 weeks. OPV is also included in the EPI, but is listed separately here as a study vaccine.
Study site and population.
This study was performed with experienced senior investigators at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b).13 The study population was drawn from the Mirpur area of Dhaka, Bangladesh. Mirpur is a densely populated region of Dhaka with over 1 million inhabitants and a broad range of SES. Families were recruited from three of Mirpur's northern wards, which are predominantly low-SES households living in slum conditions. A parallel trial with a modified study design ran concurrently at the National Institute of Cholera and Enteric Diseases (NICED) in Kolkata, India, and will be described elsewhere.
Study development and training.
Prior to the start of enrollment, the clinical team, data management teams, and select laboratory leaders at the icddr,b were trained in Good Clinical Practice (GCP) and study-specific procedures. GCP training was delivered by a World Health Organization (WHO)–Tropical Disease Research (TDR)-certified trainer.14 A study initiation visit was performed by the operations team and included study-specific training on the protocol and Manual of Procedures (MOPs), documentation, quality assurance, monitoring, recruitment and enrollment, diarrhea surveillance, and safety reporting. Clinic and data management staff received additional visit-specific training in advance of the first occurrence of each scheduled study visit in the protocol, and following protocol amendments. Refresher trainings were delivered as needed based on monitoring outcomes and clinic management evaluations. Two years after initial training, follow-up GCP training was completed. All trainings integrated specific discussion of best practices in ethical clinical research relevant to this population, including proper consenting procedures for illiterate study participants; the nature of “informed” consent in the Bangladeshi context and conducting study comprehension assessments prior to signing consent; and preventing coercion in recruitment, consenting, and study practices.
Community census and recruitment.
Following a week-long intensive training, the three wards in the study area were divided among 14 female Bangladeshi field research assistants (FRAs) who conducted a complete door-to-door community census covering over 28,000 households and more than 150,000 inhabitants with a goal of identifying pregnant women. FRAs engaged interested pregnant women in a discussion about the study and offered regular follow-up through delivery. Every household with a pregnant woman was assigned a household identification number (HHID), and mothers' interest was recorded for follow-up. A new census was conducted every 6 months during the enrollment period to record new pregnancies in the study area and to inform mothers-to-be about the study. With literacy rates around 50% in the study population, word-of-mouth played an important role in the recruitment efforts for this study.15
Study performance.
Informed consent.
Within 7 days after giving birth, screening for eligibility and study consenting occurred in the household by trained FRAs. Informed consent was obtained for all participating mothers and infants. The screening process consisted of a review of the inclusion and exclusion criteria and was done in a manner appropriate for each mother's literacy level (see Supplemental Table 1). An assessment of comprehension of the study was done using scripted points and open-ended questions. Once consented, the FRA completed enrollment and socioeconomic questionnaires with the mother and measured the infant's weight and height. In a subset of children (N = 381), a trained study medical officer also conducted a gestational age assessment (GAA) at enrollment using the Dubowitz–Ballard assessment scale.16
Clinic methods.
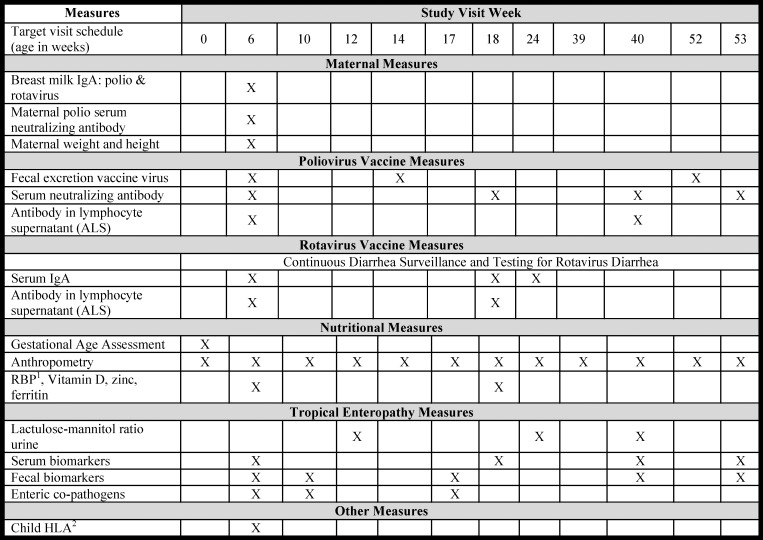
Fifteen scheduled follow-up clinic visits and comprehensive primary care were given through the study clinic for enrollees and primary health care was offered to their families. Figure 1 summarizes the overall work plan for the study through age 1 year.
Figure 1.
The PROVIDE study work plan and schedule of events. 1 RBP = retinol-binding protein; 2 HLA = human leukocyte antigen.
Diarrhea and diarrhea surveillance.
Biweekly diarrhea surveillance occurred in the homes by field research assistants (FRAs). The FRAs used a structured questionnaire, including: history and frequency of diarrhea and vomiting since last home visit, body temperature at visit, baby feeding history, and use of antibiotics and oral rehydration solution (ORS). Diarrhea was defined as three or more abnormally loose stools in 24 hours according to the mother. Diarrheal episodes were separated by at least 72 diarrhea-free hours. Children with active diarrhea were referred to the study clinic for evaluation and treatment, and a diarrheal stool specimen was collected for each episode of diarrhea. Assessment for diarrheal severity was performed by a study medical officer using a Vesikari scale.2 Severe diarrhea was defined as diarrhea with a Vesikari scale ≥ 11. To determine fecal shedding of vaccine strain following OPV given at weeks 14 and 52, stools were collected at pre-vaccination and days 4, 11, 18, and 25 post-vaccination.
Vaccinations.
Continuous monitoring of temperature and expiry data was performed and documented for vaccines administered.
Nutritional status.
Children's nutritional status was followed by anthropometry at every study visit using a calibrated digital baby scale and standardized supine length measurement equipment. At each time point, trained FRAs recorded two complete sets of measurements (both length and height) on source documents, then the average of the two measurements was used. Malnourished children > 3 standard deviation (SD) under the mean weight-for-age were treated according to icddr,b guidelines, which included referral to specialized nutrition centers for malnutrition management after 6 months of age.
Core Laboratory Methods
Rotavirus antigen detection.
Diarrheal stool specimens were tested for rotavirus antigen at the icddr,b using the ProSpecT (Oxoid Ltd., Hampshire, United Kingdom) ELISA.
Rotavirus plasma IgA.
Rotavirus vaccine immunogenicity was measured by rotavirus-specific plasma IgA or IgG at three time points: pre-vaccination (week 6), 1 week after second vaccination (week 18), and 1.5 months post–second vaccination (week 24); methods have been previously described.17 To examine the role of maternal antibody interference in rotavirus vaccine response, rotavirus-specific plasma IgG at weeks 6 and 18 and maternal breast milk IgA at week 6 were analyzed. Consistent with the rotavirus literature, seropositivity was defined as antibody titers ≥ 20 U/mL. Seroconversion was defined as subjects who were seronegative pre-vaccination and seropositive after vaccination.
Fecal shedding of OPV.
The poliovirus vaccine fecal shedding assays were performed at the National Polio Reference Laboratory, Institute of Public Health, Government of Bangladesh using WHO-qualified cell culture based methods, which used two cell lines to determine shedding of polio and/or non-polio enteroviruses.18 Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was used for confirmatory testing and determination of Sabin-specific strains, as described.18
OPV serum neutralizing antibodies.
Poliovirus serotype 1, 2, and 3 serum neutralizing antibody assays were conducted at the Centers for Disease Control and Prevention (CDC), using WHO-standardized microneutralization assays for all three serotypes.19
L/M ratio.
L/M solution was mixed at the icddr,b in sterile water containing 50 mg/mL mannitol and 250 mg/mL lactulose. The solution was transported to the field clinic under cold chain. At study visit weeks 12, 24, 40, and 104, children were given the L/M solution at 2 mL/kg of weight up to 20 mL, and urine was collected for 2 hours using pediatric urine collection bags. Analysis of the urine specimens was conducted using high-performance ion chromatography (HPIC), as described.20
Regulatory, Safety, And Scientific Oversight
The protocol and informed consent (English and Bangla) and all amendments were reviewed and approved by the Research Review Committee (RRC) and Ethics Review Committee (ERC) at the icddr,b and at Institutional Review Boards (IRBs) at the Universities of Virginia and Vermont prior to implementation. Trial registration: ClinicalTrials.gov NCT01375647. All participants were re-consented following approvals of amendments, and these data were subject to review by the clinical monitor. Supplemental Table 2 summarizes the amendments of the PROVIDE study.
A comprehensive regulatory binder was maintained to GCP standards. All adverse events following interventions (vaccines and L/M) were captured for 48 hours following each intervention and were scored for probable, possible, or unlikely relationship to each intervention. All missing protocol-defined events were captured as protocol deviations and reported to all IRBs annually. Comprehensive safety reports were submitted semiannually to the study's Independent Medical Monitor (IMM) and to the Data and Safety Monitoring Board (DSMB). All serious adverse events (SAEs) captured in the study clinic were reported to the local IRB within 24 hours and assessed for severity and relationship to study participation by the site principal investigator. No SAEs were determined (internally or by the IMM or DSMB) to be directly related to study participation. Subjects with SAEs were followed closely by study staff until SAE resolution. External clinical and safety monitoring visits occurred semiannually to validate research subject safety, clinical data integrity, verify vaccine accountability and cold chain documentation, and to ensure the study was conducted and reported in accordance with the protocol, GCP, and all regulatory requirements. Scientific progress reports for the study were made directly to the study sponsor at least annually. An External Advisory Board (EAB) composed of experts in vaccine research, infectious disease, mucosal immunology, and pediatrics also provided scientific advice and oversight.
Data Management
The Data Coordinating Center (DCC) was based at the University of Virginia. The DCC designed, developed, and maintained the study database and web-based Case Report Form data entry and reporting system using the web-based Multi-Schema Information Capture (MuSIC) platform.21,22 The DCC controlled which data were released to investigators for specific exploratory analyses of non-endpoint data. Periodic data freezes were scheduled to coincide with safety reports, continuing review, annual study meetings, and primary analyses. All clinical data were recorded in the study clinic in Mirpur and duplicate case report forms were transported daily to the data management team at the icddr,b for double data entry. Monthly diarrheal surveillance forms were maintained by field workers until complete, after which the data were entered batchwise. Laboratory worksheets were uploaded periodically to the study database based on assay batching.
Quality Management
Clinical data.
Clinical procedures were carried out according to the study's MOPs and study-specific standard operation procedures (SOPs) following staff training, as above. Routine quality control (QC) measures were articulated in SOPs and strictly followed by clinic staff, including up to 100% review of study documents prior to data entry. After double data entry, the data manager ran daily data reconciliations, and discrepancies were resolved in real time between the clinic and data management teams according to strict resolution procedures. The overall double data entry keystroke error rate was 0.08% based on randomly selected source document verification.
Led by the DCC, a final layer of QC for clinical data occurred immediately preceding periodic data freezes and included extensive data queries and reconciliation.
Laboratory data.
All assays conformed to SOPs outlined in the study-specific MOP, and each had a QC component. Each involved laboratory had a designated person responsible for QC of assays. Standardized controls and/or standards were used, and laboratory data were accepted if within expected ranges. To ensure data quality, all data documented on standardized worksheets were 100% reviewed and verified by laboratory supervisors at the appropriate laboratories. Laboratory monitoring was conducted approximately three times per year. Objectives of monitoring visits were to validate laboratory data integrity and verify proper storage and accountability for all biological specimens, clinical specimens, and reagents.
Data Analysis
Primary analysis of the trial endpoints used an “intention-to-treat” (ITT) approach that included all randomized participants in the groups to which they were randomized, regardless of adherence to the protocol. This approach minimizes bias due to subjective inclusion or exclusion, and is a conservative approach to assessing the intervention as superior to the standard of care. Secondary “per protocol” analysis sets and exploratory analyses were defined for the two trials and will be discussed in the individual manuscripts. The statistical test of superiority of the primary endpoint measure in each trial was assessed against an overall two-sided significance level of 0.05. This constrains the type I error rate for the one-sided test of superiority at the conventional type I error used in two-sided tests, as well as allowing suggestive inference of an intervention that might be harmful in the opposing tail, and conforms to FDA and ICH E9 standards.
Missing data for primary endpoints was addressed using imputation methods (see Table 1).
Biannual safety reporting required unmasked analysis of terminations, protocol deviations, adverse events, and serious adverse events, and was conducted by the DCC. No interim analyses of primary endpoints were performed; however, secondary analyses of pooled non-endpoint data were permitted to facilitate exploratory analyses and support non-trial study goals. No type 1 error rate adjustment was made for these analyses since trial enrollment was fixed with no provision for alteration based on interim power calculations or estimates of secondary and exploratory endpoints.
Results
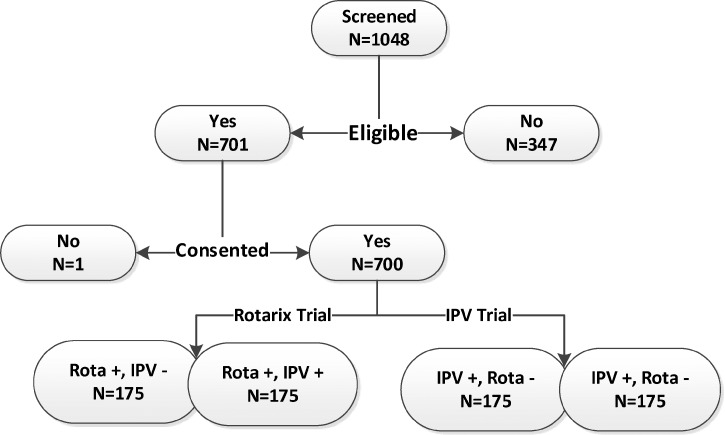
The PROVIDE study preparations (year 1) included development of the full protocol and consent forms (English and Bangla), all IRB approvals, all SOPs and the full MOPs, development of study documentation and secure online database, laboratory technology transfers, clinic site setup, staff training, and initial community census of 28,000 households. The on-study phase spanned May 2011–November 2014. As shown in Figure 2, a total of 1,048 mother and infant pairs were screened for study participation. Of these, 347 pairs were ineligible, primarily due to refusing child blood draws, and 700 eligible children were enrolled into the trial.
Figure 2.
Diagram for screening and enrollment in the PROVIDE study.
Baseline characteristics.
Table 4 shows key baseline characteristics of the study population collected at enrollment. All characteristics are based on 700 child–mother pairs, except GAA, which was introduced by approved protocol and consent form amendment in January 2012 and conducted on the last 381 children enrolled.
Table 4.
Baseline characteristics of PROVIDE study population
| Baseline characteristics | Mean ± SD | Range |
|---|---|---|
| Neonatal | ||
| Age at enrollment (days) | 4.9 ± 1.69 | 1–7 |
| Female gender (%) | 47.4 | |
| Weight at enrollment (kg) | 2.8 ± 0.37 | 1.7–4.1 |
| Length at enrollment (cm) | 48.69 ± 1.75 | 43.1–55.4 |
| HAZ at enrollment | −0.90 ± 0.90 | −3.67 to 2.88 |
| WAZ at enrollment | −1.29 ± 0.84 | −4.00 to 1.24 |
| Gestational age ≤ 36 weeks (%) | 32 | |
| Exclusive breastfeeding at birth (%) | 94.6 | |
| Home birth (%) | 25.9 | |
| BCG given at birth (%) | 2.3 | |
| Maternal | ||
| Age at enrollment (years) | 24.65 ± 4.6 | 18–41 |
| Age at first pregnancy (years) | 18.8 ± 2.9 | 12–35 |
| Total live births | 2.1 ± 1.2 | 1–10 |
| Vaginal delivery (%) | 77.1 | |
| Height (cm) | 150.33 ± 5.5 | 134–187 |
| Postpartum weight (kg) | 49.31 ± 9.41 | 30–80 |
| Postpartum BMI | 21.76 ± 3.7 | 14.23–36.57 |
| Children under 5 years of age | 0.3 ± 0.5 | 0–2 |
| Mother illiterate (%) | 28.9 | |
| Mother homemaker (%) | 85.9 | |
| Household/socioeconomic status | ||
| Total monthly income (Taka)* | 12,762.39 ± 9,409.9 | 3,000–77,000 |
| Piped municipal water source (%) | 96.9 | |
| Toilet (septic tank) (%) | 52.4 | |
| Dwelling size equals one room (%) | 72.4 | |
| Household members | 5.2 ± 2.2 | 1–18 |
BCG = bacille Calmette-Guerin vaccine for tuberculosis disease; BMI = body mass index; HAZ = height-for-age Z score; WAZ = weight-for-age Z score.
1 Bangladeshi Taka = $0.013 during the study period.
Protocol compliance.
Among children who completed study participation, protocol compliance was above 95% for all study visits, procedures, and specimen collection, except diarrheal stools. Out of 700 children enrolled, 105 children were lost to follow-up, or 15% of the study population. Reasons for drop out are presented in Table 5. A total of 4,121 diarrheal stool specimens were collected, representing > 80% reported episodes. Among episodes for which a diarrheal stool was not collected, 82% were of short duration: 1–2 days.
Table 5.
Reasons for study termination
| Reason for termination | N (% of cohort) |
|---|---|
| Consent withdrawn/voluntary withdrawal | 54 (7.7) |
| Moved out of study area | 23 (3.3) |
| Mother unreachable more than 60 days in first year | 23 (3.3) |
| Death of subject | 4 (0.6) |
| Investigator discretion | 1 (0.1) |
| Total | 105 (15) |
Conclusions
The PROVIDE study was designed to evaluate a poorly understood and biologically multifaceted problem: why oral vaccines underperform in developing country settings. Performance of large, complicated field trials in infants in resource-poor settings represents significant operational challenges. The methods presented herein describe our efforts to recruit and follow 700 Bangladeshi children and their mothers in a complex 2-year randomized 2 × 2 trial of vaccine efficacy and variables impacting vaccine performance, including measures of EE. Based on national data,15 our study population is similar to other slum-dwelling populations in Bangladesh with high rates of malnutrition early in life, illiteracy, unemployment, low family income: challenging living conditions common to children globally in whom oral vaccines underperform. Despite a complex study work plan (Figure 1), PROVIDE was able to efficiently enroll and follow our required population with less-than-anticipated drop out and clearly benefited from the strength of our collaborations, expertise of investigators at the icddr,b as well as the experienced and well-run field site.
More important than the feasibility of this work, however, are the lessons learned in study design and execution that help confirm both the validity and generalizability of our research data as well as our responsibility to perform safest possible human-subjects research in this vulnerable population. Although the goal of our work was not to bring a product to licensure, our choice of an interventional clinical trial design was chosen both so that our primary, secondary, and exploratory research questions could have the benefit of measures of efficacy (i.e., have control groups) and high data quality, and also to capitalize on practices that include safety oversight and data quality frameworks that are more systematically applied and standardized in the conduct of interventional clinical trials.
Toward the goal of subject safety, our study protocol was written using a standardized format for clinical trials, we required GCP training of all staff, site-initiation and training visits, and added additional layers to assure informed consent (i.e., tests of comprehension). We performed scheduled safety monitoring visits to monitor regulatory documents, consent forms, protocol deviations as well as documentation of the capture and reporting of adverse events. Safety oversight structures were also established (independent medical monitor, data safety monitoring board). To assure data quality, data management practices were centralized. Data from the clinic went through QC procedures before double data entry was performed and centralized checks of QC were additionally performed by the DCC. Database freezes and locks were established and maintained for all efficacy data. Data analyses for primary and secondary outcomes were planned in advance of data release.
Our work had several limitations and opportunities for improvement. The additional time, cost, and effort needed to perform extensive advanced planning and ongoing monitoring of data and safety are easily underestimated and may not be possible in all settings. This recognition may argue for standardized templates and procedures that could be shared between field sites. Second, although we performed extensive monitoring of safety, clinical, and laboratory data, this process could be further improved by use of completely external and independent monitors. Finally, our work was also challenged throughout with an imperfect method of measuring EE.
Areas for improvement in future studies include the recognition of when flexibility is needed in the conduct of interventional trials in similar settings. For example, we were overambitious with documentation of protocol deviations for issues that were not safety related (i.e., missed collection of scheduled fecal specimens). In addition, although several protocol amendments were necessary for unanticipated issues, such as standardizing the care of children with severe malnutrition and adding in new biomarkers for the measures of enteropathy, each amendment required submission to multiple IRBs and the re-consenting of all children: time-consuming and laborious processes. At a minimum, acceptance of a single IRB representing multiple institutions should be encouraged.
In addition to careful primary and secondary outcomes for the two vaccine interventions, the study evaluated multiple exploratory variables that might contribute to oral vaccine underperformance. These variables included measures of EE, malnutrition and micronutrient deficiency, enteric pathogen coinfections, breast milk antibodies, maternally derived antibodies, microbiota, as well as socioeconomic and genetic factors. We believe that the research subject safety and data quality from this work has been optimized under the umbrella of a clinical trials infrastructure and that expansion of similar approaches encouraging more uniform standards of clinical research in the developing world will permit markedly improved quality, generalizability, and comparability of data. In the meantime, we hope that other investigators will benefit from the description of our methods. We are optimistic that our forthcoming data will help to advance knowledge of the biologic basis for oral vaccine underperformance and facilitate rapid identification of concrete actions to improve the health of these young children.
Supplementary Material
ACKNOWLEDGMENTS
We thank the families of Mirpur for their participation in and support for the study, the entire study staff; R. Bradley Sack, Johns Hopkins University, Independent Medical Monitor; The Gates Foundation, and the PROVIDE external advisory board.
Footnotes
Financial support: Support for this work was from the Bill and Melinda Gates Foundation.
Authors' addresses: Beth D. Kirkpatrick, E. Ross Colgate, Dorothy M. Dickson, Marya P. Carmolli, Mary Claire Walsh, and Sean A. Diehl, Department of Medicine and Vaccine Testing Center, The University of Vermont College of Medicine, Burlington, VT, E-mails: beth.kirkpatrick@med.uvm.edu, ross.colgate@med.uvm.edu, marya.carmolli@med.uvm.edu, dorothy.dickson@med.uvm.edu, mary-claire.walsh@med.uvm.edu, and sean.diehl@med.uvm.edu. Josyf C. Mychaleckyj, Uma Nayak, Mami Taniuchi, Caitlin Naylor, Jennie Z. Ma, and William A. Petri Jr., Department of Medicine, The University of Virginia, Charlottesville, VA, E-mails: jcm6t@virginia.edu, un8x@eservices.virginia.edu, mt2f@cms.mail.virginia.edu, cgn5q@virginia.edu, jzm4h@eservices.virginia.edu, and wap3g@cms.mail.virginia.edu. Rashidul Haque, Firdausi Qadri, Masud Alam, the PROVIDE study teams, the ICDDRB, Dhaka, Bangladesh, E-mails: rhaque@icddrb.org, fqadri@icddrb.org, and masud@icddrb.org.
References
- 1.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, Gonzalez A, Malespin O, Amador JJ, Umaña J, Balmaseda A, Perez MC, Gentsch J, Kerin T, Hull J, Mijatovic S, Andrus J, Parashar U. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 2.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schödel F, Steele AD, Neuzil KM, Ciarlet M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM. Rotavirus Efficacy and Safety Trial (REST) Study Team Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 4.CDC Progress toward interruption of wild poliovirus transmission—worldwide, 2009. MMWR. 2010;59:545–550. [PubMed] [Google Scholar]
- 5.CDC Progress toward poliomyelitis eradication—India, January 2007–May 2009. MMWR. 2009;58:719–723. [PubMed] [Google Scholar]
- 6.Korpe PPW. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parashar UD, Glass RI. Rotavirus vaccines–early success, remaining questions. N Engl J Med. 2009;360:1063–1065. doi: 10.1056/NEJMp0810154. [DOI] [PubMed] [Google Scholar]
- 8.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200((Suppl 1)):S39–S48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondal DMJ, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri W. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;42:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol. 1999;162:5011–5018. [PubMed] [Google Scholar]
- 11.Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, Aylward RB. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J Infect Dis. 2009;200:794–801. doi: 10.1086/605330. [DOI] [PubMed] [Google Scholar]
- 12.Haque R, Snider C, Liu Y, Ma JZ, Liu L, Nayak U, Mychaleckyj JC, Korpe P, Mondal D, Kabir M, Alam M, Pallansch M, Oberste MS, Weldon W, Kirkpatrick BD, Petri WA., Jr Oral polio vaccine response in breast fed infants with malnutrition and diarrhea. Vaccine. 2014;32:478–482. doi: 10.1016/j.vaccine.2013.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2014. http://www.icddrb.org Available at. Accessed August 8, 2014.
- 14.http://www.who.int/tdr/en/ Available at. Accessed August 8, 2014.
- 15.National Institute for Population Research and Training MaA, Macro International . Bangladesh Demographic and Health Survey 2007. Dhaka, Bangladesh and Calverton, MD: 2009. [Google Scholar]
- 16.Ballard JKK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95:769–774. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 17.Azim T, Ahmad SM, Sefat EK, Sarker MS, Unicomb LE, De S, Hamadani JD, Salam MA, Wahed MA, Albert MJ. Immune response of children who develop persistent diarrhea following rotavirus infection. Clin Diagn Lab Immunol. 1999;6:690–695. doi: 10.1128/cdli.6.5.690-695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . IVB Polio Laboratory Manual. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 19.World Health Organization . Manual for the Virological Investigation of Poliomyelitis. Geneva, Switzerland: WHO; 1997. [Google Scholar]
- 20.Galpin L, Manary MJ, Fleming K, Ou CN, Ashorn P, Shulman RJ. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040–1045. doi: 10.1093/ajcn/82.5.1040. [DOI] [PubMed] [Google Scholar]
- 21.Harrison JHRJ, Skully K, Lyman JA. AMIA Summit on Clinical Research Informatics; San Francisco, CA: 2011. Rapid development of research databases with Web access, flexible schemas, and linkage to local data resources. [Google Scholar]
- 22.Lyman JARJ, Harrison JH. Washington, DC: 2010. Development of a dynamic Web interface tool for research databases. Conference abstract: AMIA 34th Annual Symposium on Biomedical and Health Informatics. November 13--17, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.