Abstract
A nationwide survey was conducted to obtain an estimate of Chagas disease prevalence among pregnant women in Ecuador. As part of a national probability sample, 5,420 women seeking care for delivery or miscarriage at 15 healthcare facilities were recruited into the study. A small minority of participants reported knowing about Chagas disease or recognized the vector. A national seroprevalence of 0.1% (95% confidence interval [95% CI] = 0.0–0.2%) was found; cases were concentrated in the coastal region (seroprevalence = 0.2%; 95% CI = 0.0–0.4%). No cases of transmission to neonates were identified in the sample. Seropositive participants were referred to the National Chagas Program for evaluation and treatment. Additional studies are necessary to determine if areas of higher prevalence exist in well-known endemic provinces and guide the development of a national strategy for elimination of mother-to-child transmission of Chagas disease in Ecuador.
Chagas disease, caused by Trypanosoma cruzi, is spread mainly by hematophagous triatomine vectors, blood transfusion, and congenital transmission.1 It is estimated that 200,000 people are infected with T. cruzi in Ecuador,2 where most provinces located on the Pacific coast as well Loja in the southern highlands have, for many decades, been known to be endemic for Chagas disease and continue to be active transmission foci.3,4 Meanwhile, sylvatic foci were detected in the northern Ecuadorian Amazon region during the 1990s,5 and additional studies have shown that this region is very active for T. cruzi transmission.6–8 However, despite punctual knowledge at the local scale, the global epidemiological situation of Chagas disease in Ecuador remains largely uncharacterized: no country-wide study exists to provide a global estimate of prevalence for T. cruzi infection, many areas remain unstudied, and particularly little is known regarding congenital transmission.
Five percent of T. cruzi-infected pregnant women transmit Chagas disease to their offspring congenitally,9 and over 15,000 such cases are estimated to occur yearly in Latin America.10 Screening of pregnant women in endemic areas for infection with T. cruzi is recommended.11 Furthermore, because of human migration, congenital Chagas transmission also constitutes a threat in the United States, Europe, and other non-endemic countries,11 and screening of migrant Latin American women in such settings has also been found to be cost-effective.12 However, parasitological treatment to infected women must be deferred until after pregnancy and breastfeeding, because nifurtimox and benznidazol (the only drugs available for treating Chagas disease) are potentially teratogenic and also, frequently cause undesirable secondary effects.11 Nonetheless, when newborns are treated before 1 year of age, infection is cleared in almost all cases without the secondary effects common among adults.11,13 Diagnosis in neonates is possible immediately after birth through parasitological methods, such as visualization of trypomastigotes on peripheral or cord blood, as well as blood concentration techniques (Strout or microhematocrit14), whereas serologic diagnosis is possible starting at 8 months after birth, when potentially confounding maternal antibodies have disappeared.11 Additionally, recent data suggest that parasitological treatment of young infected women may help prevent congenital transmission later in life.15,16
During 2011 and 2012, under the coordination of the Pan-American Health Organization (PAHO) and the Ecuadorian Ministry of Health, a study was launched to obtain nationally representative estimates of human immunodeficiency virus (HIV) and syphilis prevalence among Ecuadorian pregnant women as well as estimates of coverage of preventive antenatal services.17 Several other national entities as well as academic institutions collaborated in the study. An opportunity thus arose to measure the prevalence of T. cruzi infection in the same sample and gain insight into the epidemiology of congenital Chagas disease transmission in Ecuador.
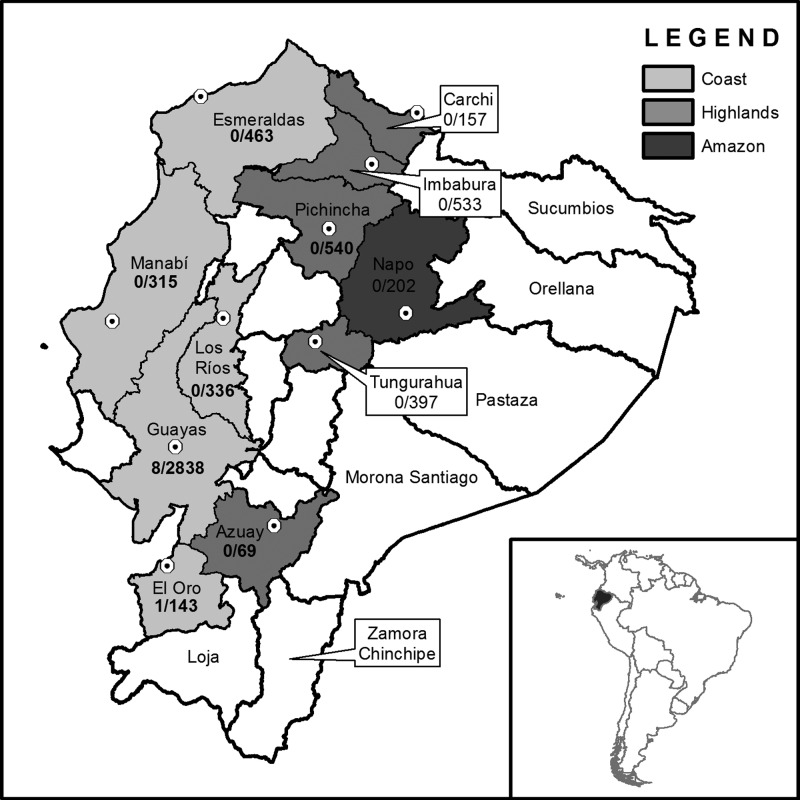
A transversal descriptive study with a two-stage cluster sampling strategy and a target sample size of 6,000 women was designed and implemented (details are provided elsewhere17). Briefly, healthcare facilities listed in the registry of deliveries and miscarriages of the Ecuadorian Ministry of Health were arranged according to region and province (to improve geographical representation), and smaller facilities (those reporting less than 400 births/miscarriages per year) were excluded. Using a systematic sampling with probability of selection proportional to the number of live births and miscarriages reported for each healthcare facility in 2008, 15 facilities were selected, including some within each of three major geographic regions of Ecuador: coastal region (8 facilities), highlands (6 facilities), and Amazon basin (1 facility). The number of participants recruited in each location was proportional to the number of pregnant women who received care at the study site (Figure 1). A design effect of 1.25 was considered because of the multistage sampling design. Other statistical analysis details are provided in the work by Sanchez-Gomez and others.17
Figure 1.
Sampling frame and screening results. Sampled provinces are colored according to geographic region (as per the legend). Dots indicate the location of sampled health facilities. A single facility was sampled in each province, except for Guayas (four facilities) and Pichincha (two facilities) provinces in Guayaquil and Quito cities, respectively. The number of seropositive mothers and the sample size for each province are indicated by fractions. Uncolored provinces were not sampled.
Informed consent was obtained from all human adult participants and parents or legal guardians of infants. Parental permission to participate in the study for pregnant women who were minors was waived by the ethics committee. Protocol was approved by the Bioethics Committee of Universidad Central del Ecuador (Cobi-UCE, Federalwide Assurance FWA00002482 IEC ORG0001932 IRB00002438). In total, 5,988 women were recruited into the study: 51% of them were from the coastal region, 38.4% were from the highlands, and 9% were from the Amazon region. Most (96.7%) of these women completed the survey section dealing with Chagas disease (results are summarized in Table 1), and approximately 90% provided specimens for Chagas disease tests. In the survey, less than 10% of women reported having ever heard of Chagas disease, and among them, only one-half (50.1%) knew that it could be transmitted from mother to infant during pregnancy. Additionally, around 20% of women were able to recognize images of the vector, and 12.8% of women reported having seen it on their homes or peridomiciles.
Table 1.
Knowledge regarding Chagas disease among women participating in the study
| n/N | Percentage* | 95% Confidence interval* | |
|---|---|---|---|
| Have heard about Chagas disease | 573/5,793 | 9.2 | 6.6–11.9 |
| Know Chagas disease can be transmitted from mother to baby (among those who know Chagas) | 265/505 | 50.1 | 41.3–59.0 |
| Report a family member or self has Chagas disease (among those who know Chagas) | 51/455 | 9.3 | 2.715.9 |
| Recognize the vector | 1,338/5,685 | 18.8 | 10.8–26.8 |
| Report seeing the vector at home or nearby | 836/5,470 | 12.8 | 5.1–20.5 |
Weighted values.17
A 1-mL serum aliquot from each participating woman was submitted to the national reference laboratory (Instituto Nacional de Salud Pública e Investigación [INSPI]; formerly Instituto Nacional de Higiene y Medicina Tropical Leopoldo Izquieta Pérez [INHMT]) in Guayaquil, where serological tests were performed according to national guidelines: an initial serological screening (recombinant enzyme-linked immunosorbent assay [Chagatest ELISA recombinante v3.0, WeinerLab, Rosario, Argentina]) was followed by a second serological test (immunoagluttination, Chagatest HAI, WeinerLab, Rosario, Argentina) performed on reactive samples. Seropositivity in both tests was considered diagnostic of T. cruzi infection. Discordant results were resolved with a third independent test by a different serological method (i.e., indirect immunofluorescence [IFA], Inmuno-Con Chagas, WAMA Diagn?stica, Sao Paulo, Brazil), where reactivity in two of three tests was considered diagnostic.
In total, 9 of 5,420 participating women were found to be seropositive for T. cruzi infection, corresponding to 0.1% prevalence (Table 2). All cases were found in the coastal region: eight cases in Guayas and one case in El Oro, two provinces that are well-known to be endemic for Chagas disease. Interestingly, among seropositive women, only two knew about Chagas disease before the study, and only one of them knew that it could be transmitted congenitally. Additionally, one other woman recognized the vector. None of them reported ever receiving blood transfusions, suggesting that they most likely were infected by other means. None of them answered positively to any other item of the Chagas disease survey.
Table 2.
Seropositivity for T. cruzi infection among women participating in the study
| n/N | Percentage (95% confidence interval)* | |
|---|---|---|
| National | 9/5,420 | 0.1 (0.0–0.2) |
| Coastal region | 9/3,564 | 0.2 (0.0–0.4) |
Weighted values.17
Contact information provided by the seropositive women at their healthcare facilities was used by the National Chagas Program to either contact them by telephone or visit them at their domiciles for follow-up. Six seropositive women had provided valid contact information (five from Guayas and one from El Oro), and infection with T. cruzi was systematically studied for all of their offspring, including infants born during the study as well as all of their siblings (eight infants total). The sole exception was one woman from Guayas, whose neonate had died soon after delivery and who had no other offspring.
At the parasitology laboratories of the INHMT in Guayaquil, blood concentration techniques (microStrout) and serological tests (same techniques used for the mothers) were performed for all infants. Serology tests took place when the infants were at least 8 months of age. No evidence of presence of T. cruzi or anti-T. cruzi antibodies was found. Mothers were referred to the National Chagas Program for evaluation and treatment.
This is the first study reporting prevalence of Chagas disease among a nationally representative sampling of pregnant women in Ecuador. Our data suggest a surprisingly low prevalence of infection by T. cruzi among women seeking medical attention for delivery or miscarriage in the Ecuadorian healthcare system. Consistent with our results, previous studies in which migrant Ecuadorian women were screened for Chagas disease in Spain did not report any cases.18,19 From among the provinces randomly included in our study, the concentration of seropositive cases in Guayas and El Oro is not surprising, because both of them have traditionally been known as endemic for Chagas. Additionally, given the small number of seropositive women encountered (9 of 5,420) and the low transmission rate of T. cruzi through the congenital route (5%), the absence of congenitally transmitted cases in our sample is not unexpected.
However, our results must be interpreted with caution, because some limitations related to our sampling strategy, which was originally designed to obtain HIV and syphilis estimates, could influence the estimated Chagas disease prevalence. For example, the study is not representative of women who do not seek skilled healthcare for delivery or miscarriage; these women are estimated to constitute as much as 25% of deliveries in the country.20 In Ecuador, evidence from the most recent Demographic and Health Survey available, conducted in 1987, indicated that this percentage is significantly larger in rural areas.21 The higher proportion of home births in rural areas and its potential impact over the lack of detection of congenital Chagas cases have been recently highlighted in Bolivia.22 However, smaller healthcare facilities (fewer than 400 deliveries and miscarriages/year), which are also common in rural areas, were excluded from the sampling frame because of logistic issues. Therefore, the rural population is underrepresented in our study. Chagas disease is known to be more prevalent in these settings, and therefore, the prevalence at national level could be underestimated.23
Additional studies should target all relevant geographic areas where vectorial transmission occurs. These include provinces in the Ecuadorian Amazon region, such as Sucumbíos, Orellana, Pastaza, Morona-Santiago, and Zamora-Chinchipe, where the data available from the National Chagas Program/Ministry of Health as well as reports from academic groups5–8 show that Chagas disease is an emerging threat. Furthermore, they should include Loja province, a key active endemic region in the highlands.4 Generating epidemiological information from the high-risk areas should be a priority to inform the most cost-effective strategies aimed at eliminating mother-to-child transmission of Chagas disease in Ecuador. Finally, an education component designed to inform the population about Chagas disease and the risk of congenital transmission is required, which is highlighted by the results of our surveys.
Footnotes
Financial support: This project was supported by funds from the Pan-American Health Organization/Latin American Center for Perinatology and Human Development (PAHO/CLAP), the United Nations Children's Fund (UNICEF), and the Ecuadorian Ministry of Health.
Authors' addresses: Jaime A. Costales and César A. Yumiseva, Centro de Investigación en Enfermedades Infecciosas, Pontificia Universidad Católica del Ecuador, Quito, Ecuador, E-mails: jacostalesc@puce.edu.ec and cayumiseva@puce.edu.ec. Amaya Sánchez-Gómez, Pan-American Health Organization, Quito, Ecuador, E-mail: amayasgomez@gmail.com. Luis C. Silva-Aycaguer, National School of Public Health, Havana, Cuba, E-mail: lcsilvaa@yahoo.com. William Cevallos, Biomedical Center, Universidad Central del Ecuador, Quito, Ecuador, E-mail: wcevallos@uce.edu.ec. Susana Tamayo, National STI/HIV-AIDS Program, Ministry of Public Health, Quito, Ecuador, E-mail: st_suzette@hotmail.com. Jerry O. Jacobson, Pan-American Health Organization, Bogota, Colombia, E-mail: jerryojacobson@gmail.com. Luiggi Martini and Caty A. Carrera, Instituto Nacional de Salud Pública e Investigación, Guayaquil, Ecuador, E-mails: luiggimartini8@hotmail.com and c_carrera2008@hotmail.com. Mario J. Grijalva, Tropical Disease Institute, Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH, E-mail: grijalva@ohio.edu.
References
- 1.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar VH, Abad-Franch F, Racines VJ, Paucar CA. Epidemiology of Chagas disease in Ecuador. A brief review. Mem Inst Oswaldo Cruz. 1999;94((Suppl 1)):387–393. doi: 10.1590/s0074-02761999000700076. [DOI] [PubMed] [Google Scholar]
- 3.Black CL, Ocana S, Riner D, Costales JA, Lascano MS, Davila S, Arcos-Teran L, Seed JR, Grijalva MJ. Household risk factors for Trypanosoma cruzi seropositivity in two geographic regions of Ecuador. J Parasitol. 2007;93:12–16. doi: 10.1645/GE-899R.1. [DOI] [PubMed] [Google Scholar]
- 4.Black CL, Ocana-Mayorga S, Riner DK, Costales JA, Lascano MS, Arcos-Teran L, Preisser JS, Seed JR, Grijalva MJ. Seroprevalence of Trypanosoma cruzi in rural Ecuador and clustering of seropositivity within households. Am J Trop Med Hyg. 2009;81:1035–1040. doi: 10.4269/ajtmh.2009.08-0594. [DOI] [PubMed] [Google Scholar]
- 5.Amunarriz M, Chico ME, Guderian RH. Chagas disease in Ecuador: a sylvatic focus in the Amazon region. J Trop Med Hyg. 1991;94:145–149. [PubMed] [Google Scholar]
- 6.Grijalva MJ, Escalante L, Paredes RA, Costales JA, Padilla A, Rowland EC, Aguilar HM, Racines J. Seroprevalence and risk factors for Trypanosoma cruzi infection in the Amazon region of Ecuador. Am J Trop Med Hyg. 2003;69:380–385. [PubMed] [Google Scholar]
- 7.Chico M, Sandoval C, Guevara A, Calvopina M, Cooper PJ, Reed SG, Guderian RH. Chagas disease in Ecuador: evidence for disease transmission in an indigenous population in the Amazon region. Mem Inst Oswaldo Cruz. 1997;92:317–320. doi: 10.1590/s0074-02761997000300002. [DOI] [PubMed] [Google Scholar]
- 8.Guevara AG, Atherton RD, Wauters MA, Vicuna Y, Nelson M, Prado J, Kato H, Calvopina MH, Hashiguchi Y. Seroepidemiological study of chagas disease in the southern Amazon region of Ecuador. Trop Med Health. 2013;41:21–25. doi: 10.2149/tmh.2012-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22–33. doi: 10.1111/1471-0528.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PAHO . Quantitative Estimation of Chagas Disease in the Americas. Washington, DC: Panamerican Health Organization; 2006. [Google Scholar]
- 11.Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, Albajar Vinas P. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis. 2011;5:e1250. doi: 10.1371/journal.pntd.0001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sicuri E, Munoz J, Pinazo MJ, Posada E, Sanchez J, Alonso PL, Gascon J. Economic evaluation of Chagas disease screening of pregnant Latin American women and of their infants in a non endemic area. Acta Trop. 2011;118:110–117. doi: 10.1016/j.actatropica.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics. 2011;127:e212–e218. doi: 10.1542/peds.2010-1172. [DOI] [PubMed] [Google Scholar]
- 14.Feilij H, Muller L, Gonzalez Cappa SM. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J Clin Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosa-Estani S, Cura E, Velazquez E, Yampotis C, Segura EL. Etiological treatment of young women infected with Trypanosoma cruzi, and prevention of congenital transmission. Rev Soc Bras Med Trop. 2009;42:484–487. doi: 10.1590/s0037-86822009000500002. [DOI] [PubMed] [Google Scholar]
- 16.Murcia L, Carrilero B, Munoz-Davila MJ, Thomas MC, Lopez MC, Segovia M. Risk factors and primary prevention of congenital Chagas disease in a nonendemic country. Clin Infect Dis. 2013;56:496–502. doi: 10.1093/cid/cis910. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Gomez A, Grijalva MJ, Silva-Aycaguer LC, Tamayo S, Yumiseva CA, Costales JA, Jacobson JO, Chiriboga M, Champutiz E, Mosquera C, Larrea M, Cevallos W. HIV and syphilis infection in pregnant women in Ecuador: prevalence and characteristics of antenatal care. Sex Transm Infect. 2014;90:70–75. doi: 10.1136/sextrans-2013-051191. [DOI] [PubMed] [Google Scholar]
- 18.Munoz J, Coll O, Juncosa T, Verges M, del Pino M, Fumado V, Bosch J, Posada EJ, Hernandez S, Fisa R, Boguna JM, Gallego M, Sanz S, Portus M, Gascon J. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clin Infect Dis. 2009;48:1736–1740. doi: 10.1086/599223. [DOI] [PubMed] [Google Scholar]
- 19.Ramos JM, Pinargote H, Andreu M, Sastre J, Torrus D, Martinez-Escoriza JC, Portilla J. Prevalence of Trypanosoma cruzi infection in Latin American pregnant women and level of compliance of the Valencian Health Programme in the city of Alicante. Epidemiol Infect. 2014;142:888–890. doi: 10.1017/S0950268813001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CEPAR . Salud materna y salud del niño-niña. In: Yépez ME, Sotomayor JO, Velasteguí AV, Valdivieso NO, Morán LR, Chicaiza RA, Pérez EA, editors. Informe de Endemain 2004. Quito, Ecudaor: Centro de Estudios de Población y desarrollo Social–CEPAR; 2005. p. 4. [Google Scholar]
- 21.CEPAR . Encuesta Demográfica y de Salud Familiar. Quito, Ecuador: Centro de Estudios de Población y Paternidad Responsable–CEPAR; 1987. [Google Scholar]
- 22.Romero M, Postigo J, Schneider D, Chippaux JP, Santalla JA, Brutus L. Door-to-door screening as a strategy for the detection of congenital Chagas disease in rural Bolivia. Trop Med Int Health. 2011;16:562–569. doi: 10.1111/j.1365-3156.2011.02746.x. [DOI] [PubMed] [Google Scholar]
- 23.Briceno-Leon R. Chagas disease in the Americas: an ecohealth perspective. Cad Saude Publica. 2009;25((Suppl 1)):S71–S82. doi: 10.1590/s0102-311x2009001300007. [DOI] [PubMed] [Google Scholar]