Abstract
The diagnostic performance of histidine-rich protein 2 (HRP-2)–based malaria rapid diagnostic test (RDT) was evaluated in a mesoendemic area for malaria, Kaduna, Nigeria. We compared RDT results with expert microscopy results of blood samples from 295 febrile children under 5 years. Overall, 11.9% (35/295) tested positive with RDT compared with 10.5% (31/295) by microscopy: sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 100%, 98.5%, 88.6%, and 100%, respectively. The RDT sensitivity was not affected by transmission season, parasite density, and age. Specificity and positive PV decreased slightly during the high-transmission season (97.5% and 83.3%). The RDT test positivity rates in the low- and high-transmission seasons were 9.4% and 13.5%, respectively. Overall, the test performance of this RDT was satisfactory. The findings of a low proportion of RDT false positives, no invalid and no false-negative results should validate the performance of RDTs in this context.
Introduction
Globally, an estimated 207 million cases of malaria and 627,000 deaths occurred in 2012 with 80% of cases and 90% of deaths in the African region.1 In Nigeria, there are estimated 1.5 malaria episodes per child per year.2 It is responsible for 30% under-five mortality and 300,000 childhood deaths annually.3 Plasmodium falciparum is responsible for over 94% of all parasitologically confirmed malaria parasite infections among asymptomatic and symptomatic cases of malaria in children under 5 years.4 In many developing countries, treatment of malaria is often based on clinical diagnosis instead of parasite-based diagnosis as now recommended by the World Health Organization (WHO).5 Therefore, decisions on treatment are often taken without the benefit of test results because of long delays due to other significant workload and inadequate manpower.6 This practice has resulted in overprescription of antimalarial medicines, which may lead to drug resistance.7,8 Rapid diagnostic tests (RDTs) are now recommended by WHO for timely diagnosis for malaria case management in all age groups, including children.5 RDTs can expand malaria diagnostics to areas where good quality microscopy cannot be maintained. It requires a short training period and provides an opportunity for improved fever case management at lower levels of the health system.9–11
Malaria diagnosis based on microscopy depends on the availability of adequate reagents and equipment and, most importantly, well-trained and well-supervised technicians. These conditions are not met at peripheral levels of the health care system limiting accessibility to malaria diagnosis.
Until recently, the national policy exempted children under 5 years from parasite-based diagnosis for malaria treatment.9 The use of malaria RDT is growing, and its access is currently being expanded in primary health care facilities where routine microscopy is not available and in secondary and tertiary facilities to complement microscopy for rapid parasitological confirmation of all suspected malaria cases in line with the WHO and National recommendations.5,9,12 At the outset, procured RDTs were rolled out at a ratio of 7:2:1 at primary, secondary (general hospitals), and tertiary facilities, respectively.12
However, the performance of RDTs in test-based therapy is a matter of concern for many health professionals because of lack of access to readily available indigenous data on diagnostic performance of malaria RDTs. The risk of missing a true malaria case can lead to low compliance with negative RDT test results.13,14 There is a need to grade the body of evidence on the diagnostic performance of the HRP-2-based malaria RDT in children less than 5 years in the light of the revised case management policy of universal confirmation for all suspected malaria cases.15,16 We evaluated the diagnostic performance of Standard Diagnostic (SD) Bioline malaria Plasmodium falciparum RDT (Pf RDT) in febrile children under the age of 5 years in Kaduna state, Nigeria, a mesoendemic malaria transmission area (an area with season-dependent intense transmission), and determined parasite-specific (species and density) and seasonal factors that could influence the test performance against expert microscopy as a gold standard or reference test.
Materials and Methods
Study area.
The study was conducted across rainy and dry seasons at Makarfi General Hospital, a 51-bed hospital situated in Makarfi, a rural and semi-urban local government area in Kaduna state, Nigeria, with an average monthly patient load of about 50 children aged less than 5 years. The high-transmission season for malaria is between June and October, whereas low transmission is between November and May. Kaduna state is within the North West geopolitical zone with a reported malaria prevalence of 48% by microscopy in a population survey of children aged less than 5 years.4,9
Patients.
Overall, 295 febrile children aged 6–59 months, who presented with an axillary body temperature of ≥ 37.5°C or history of febrile illness, whose parents consented to participate in the study, were selected consecutively and enrolled. We excluded patients with signs and symptoms of severe malaria and those with a history of any form of bleeding disorder.
Sample size.
A minimum sample size of 175 was calculated using a standard formula17
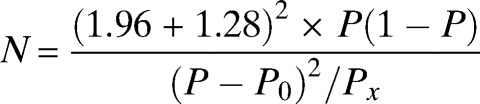 |
with a malaria prevalence (Px) of 48%,3 an estimated sensitivity (P) of 85% for SD Bioline malaria RDT specific for HRP-2, and WHO-recommended minimum sensitivity (P0) of ≥ 95% at ≥ 100 parasites/μL and a 5% precision.18
Data and sample collection.
A designated study team trained on the use of the questionnaire and collection of blood sample was assigned to this study. Standardized structured and pre-tested questionnaires were interviewer administered to caregivers of febrile children under five years at the Medical Outpatient Department and enrolled in line with the eligibility criteria. The questionnaire was translated into the local “Hausa” language—the predominant spoken language— and back-translated into English to avoid any ambiguity. Clinical/pre-treatment history data were obtained for each patient. Finger-prick blood samples were collected under aseptic conditions from eligible patients for the performance of the malaria RDT and for the preparation of thick and thin smears for microscopy. Laboratory data were recorded on a case report form.
Rapid diagnostic test.
The malaria RDT evaluated was the HRP-2-based SD Bioline malaria Pf RDT (Standard Diagnostics, Kyonggi-do, South Korea), which is one of the malaria RDTs that had considerably high performance in the World Health Organization-Foundation for Innovative New Diagnostics (WHO-FIND) annual RDT evaluation program and approved for public sector procurement by the National Malaria Control Program.19 The SD Bioline malaria Pf RDT is an immunochromatographic test, which has one test band and one control band, and detects HRP-2 antigen that is specific for P. falciparum. The RDT tests were performed and the results examined independently by the same person, a trained laboratory scientist with over 5 years' experience, who did not participate in smear microscopy. The malaria RDT kits were stored at room temperature and in a non-humid condition devoid of direct sunlight throughout the study period to ensure compliance with the manufacturer's storage condition. Each RDT pack was examined for expiration, its desiccant was examined for color change, and presence of control line on test band was ensured for validity of each test result. The tests were conducted according to manufacturer's instructions and standardized RDT job aid. Used RDT cassettes, blood transfer devices, and other non-sharp wastes were discarded in a waste bin, and lancets in sharps container. Malaria RDT post-shipment lot testing before study commencement and post-distribution lot testing during the study were carried out at the College of Medicine, University of Lagos, Nigeria—a facility that is part of the WHO-FIND laboratory network for malaria RDT quality assurance. The quality control (QC) check of the RDT was done with highly characterized positive (dilutions at 200 and 2000 parasites/μL of blood) and negative (zero parasitemia confirmed by polymerase chain reaction [PCR]) QC samples prepared using WHO/TDR/FIND standard protocols. All positive QC dilutions were positive (100%) and the negative controls were negative for the SD Bioline HRP-2-based RDT that was used in the study. Furthermore, long-term testing was conducted at 3-month intervals using the highly characterized QC panels.
Microscopic diagnosis.
Microscopic examination of Giemsa-stained thin and thick blood films was carried out at the Ahmadu Bello University Teaching Hospital, Zaria, Nigeria, by two trained, independent expert malaria microscopists (with over 10 years' experience and are national trainers for National Malaria Control Programs on malaria microscopy) and a third QC microscopist who served as a tiebreaker. Smears were read within 24 hours. The parasite density of each patient was determined by counting the number of asexual parasites against 200–500 leukocytes in a positive thick film, and parasite counts were computed and reported in parasites/μL blood using the standard 8,000 leukocytes.20–22 A blood slide was considered negative when the examination of 100 high-power fields did not show the presence of asexual forms of P. falciparum. Essentially, the malaria microscopists were blinded from the RDT results as well as the parasite counts of the individual microscopists. The microscopy reading was coordinated by a slide coordinator who took both readings and computed the mean parasitemia for each patient. The discordance level for the acceptance of the parasite counts of both microscopists was set at 20%, whereas discordant parasite counts above 20% were rejected and sent for a re-reading by both microscopists and a final third microscopist (the tiebreaker).
Quality assurance.
After pre-testing, errors in data collection tools were corrected and ambiguous questions were refined appropriately. The principal investigator carried out regular supervision and spot checks on data collectors. The study was conducted in the context of routine public health care.
Patient management.
All patients with positive RDT test results were treated with artemether–lumefantrine or artesunate–amodiaquine according to the national guidelines immediately after the RDT test. Patients with negative results received further assessment, and an appropriate treatment was given by the attending clinician.9
Data processing and analysis.
Double data entry, use of check codes, and data cleaning and analysis were conducted using Epi-info version 3.5.3 and OpenEpi version 2.3. Five patients were excluded because of incomplete data. The sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), likelihood ratio for a positive test, and likelihood ratio for a negative test of SD Bioline Pf RDT were calculated as recommended by WHO.17 The results were described with proportions and their 95% confidence intervals (CI). χ2 and two sample t tests were used to compare means. We used the following formula: sensitivity = true positives/(true positives + false negatives), specificity = true negatives/(true negatives + false positives), NPV = true negatives/(true negatives + false negatives), PPV = true positives/(true positives + false positives), likelihood ratio for a positive test = sensitivity/(1 − specificity), and likelihood ratio for a negative test = (1 − sensitivity)/specificity.
Ethical consideration.
The study was approved by the Ethical Committee of the Ahmadu Bello University Teaching Hospital, Zaria, Nigeria; Kaduna state Ministry of Health; and the National Health Research Ethics Committee (Approval Number NHREC/01/01/2007-31/12/2010). Informed consent was sought from caregivers and confidentiality of information given was maintained. The perceived benefit of the study to the caregivers of under-5-year-old children was an instant diagnosis of malaria with RDT at no cost, and immediate treatment of those with positive RDT results.
Results
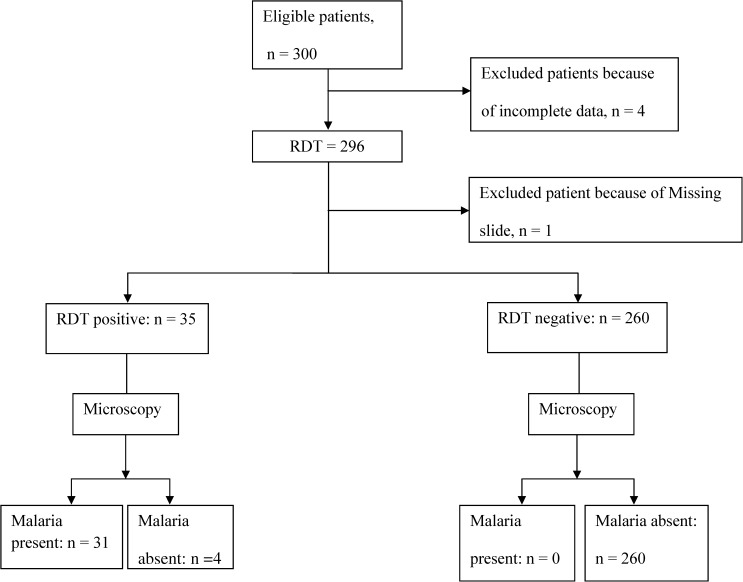
Data were collected from 300 eligible febrile children aged under 5 years enrolled from December 2010 to August 2011. Two-hundred and ninety-five results of blood smear examination and malaria rapid tests were considered for data analysis and interpretation; the remaining results were incomplete and thus excluded (Figure 1). The participants' median age was 24 months (inter-quartile range: 14–47), and 55% was males (Table 1).
Figure 1.
Flow chart showing total patients recruited, rapid diagnostic test (RDT), and blood smear results.
Table 1.
Demographic characteristics and pretreatment history of febrile children presenting at Makarfi General Hospital, Kaduna State, Nigeria, December 2010–August 2011
| Characteristics (N = 295) | Frequency | Percent |
|---|---|---|
| Age (months) | ||
| 6–11 | 58 | 19.7 |
| 12–23 | 62 | 21.0 |
| 24–35 | 55 | 18.6 |
| 36–47 | 46 | 15.6 |
| 48–59 | 74 | 25.1 |
| Child's sex | ||
| Male | 163 | 55.3 |
| Pre-treatment history | ||
| No pre-treatment | 95 | 32.2 |
| Antimalarials | 24 | 8.1 |
| Other treatments | 176 | 59.7 |
P. falciparum was detected by microscopy in 10.5% (31/295) compared with 11.9% (35/295) by RDT (Table 2). P. falciparum was detected by microscopy in 11.2% (20/178) in high-transmission season (June–August) compared with 9.4% (11/117) in low-transmission season (December–May). P. falciparum was detected by RDT in 13.5% (24/178) in high- transmission season compared with 9.4% (11/117) in low-transmission season. Only P. falciparum was detected in all the slides examined. The overall mean parasite density was 8,723 (standard deviation [SD]: 13,655 parasites)/μL blood. Children enrolled during high- and low-transmission seasons had mean parasitemia of 11,171 (SD: 14,645), and 4, 273 (SD: 10,870) parasites/μL blood, respectively (P > 0.05). Parasite density was ≥ 159 parasites/μL blood in all smear-positive cases, and these cases were also tested positive by RDT. The RDT false-positive rate was 1.4% (4/295), and these were not among the 24 children that had been earlier treated with antimalarials before testing at presentation (Table 1). There were no invalid RDT results.
Table 2.
Diagnostic accuracy of Standard Diagnostic Bioline malaria rapid diagnostic in febrile children at Makarfi General Hospital, Kaduna State, Nigeria, December 2010–August 2011
| Measure of diagnostic accuracy | Point estimate (95% CI) | |
|---|---|---|
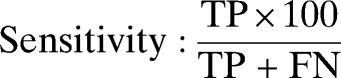 |
31/(31 + 0) × 100 | 100.0% (86.3–100.0) |
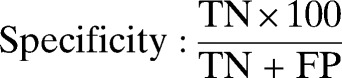 |
260/(4 + 260) × 100 | 98.5% (95.9–99.5) |
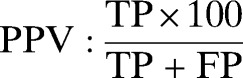 |
31/(31 + 4) × 100 | 88.6% (72.3–96.3) |
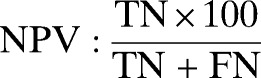 |
260/(0 + 260) × 100 | 100.0% (98.2–100.0) |
| Likelihood ratio of positive test | 31/(31 + 0)/4/(4 + 260) | 66 |
| Likelihood ratio of negative test | 0/(31 + 0)/260/(4 + 260) | 0 |
TP = true positives; TN = true negatives; FP = false positives, FN = false negatives.
The sensitivity and NPV of SD Bioline malaria Pf RDT were constant (100%), irrespective of the parasite density and seasons (Table 3). The specificity dropped from 100% during low transmission to 97.6% (95% CI: 93.6–99.2) during high transmission (P > 0.05), and the PPV went from 100% to 83.3% (95% CI: 61.8–94.5) during low- to high-transmission seasons (P > 0.05) (Table 3).
Table 3.
Diagnostic accuracy of Standard Diagnostic Bioline malaria rapid diagnostic test by transmission season, Makarfi General Hospital, Kaduna State, Nigeria, December 2010–August 2011
| Variable (season) | Parameters for measures of diagnostic accuracy of malaria RDT (n) | Diagnostic accuracy of malaria RDT (%) | Microscopy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FN | FP | TN | SN | SP | PPV | NPV | Mean parasite density (SD)/μL | |
| Low transmission (December–May) | 11 | 0 | 0 | 106 | 100.0 | 100.0 | 100.0 | 100.0 | 4,077 (10,926) |
| High transmission (June–August) | 20 | 0 | 4 | 154 | 100.0 | 97.5 | 83.3 | 100.0 | 10,647 (14,967) |
SN = sensitivity; SP = specificity; SD = standard deviation.
Discussion
In febrile children less than 5 years of age, the sensitivity and specificity of HRP-2-based rapid diagnostic test for P. falciparum used in a clinical setting in Nigeria met the WHO recommendation of ≥ 95% and ≥ 90%, respectively, when compared with expert microscopy. Specificity decreased slightly (97.5%) during the high-transmission season but remained well above 90%. Peripheral blood film microscopy showed only P. falciparum. The test positivity rates of 9.4% (low-transmission season) and 13.5% (high-transmission season) were less than expected in an area of northern Nigeria classified as mesoendemic.
The sensitivity of 100% assessed in our study was not influenced by the transmission season and parasite density. It was comparable to the sensitivity found in a mesoendemic area in Uganda (91% for HRP-2 RDT).23 The sensitivity recorded in this study could be explained by the consistently high parasite density of > 100 parasites/μL.24
The overall specificity of the RDT was consistent with the WHO recommendations and higher than reported by the studies in areas with a moderate malaria transmission in Nigeria (62.9%) and Uganda (65%).23,25 The specificity of the RDT decreased during the high-transmission season. The false-positive RDT results that led to lower specificity during high transmission are consistent with the Nigeria National Malaria Indicator Survey and could be attributed to persistence of HRP-2 antigens because of delayed clearance of HRP-2 after treatment.16,23,24,26,27 HRP-2 antigens can remain in blood for over 30 days after clearance of the parasites, and persistence of HRP-2 has been shown to depend on the presence of the antigen in erythrocytes and parasite density at the initiation of treatment.23 In this study, none of the false positives was among the 24 children that were treated with an antimalarial prior to presenting with fever (Table 1). A lower specificity and PPV of the HRP-2 RDT was reported in a mesoendemic area and higher values for both measures in a holoendemic area during a study conducted during the high-transmission seasons in Senegal.28 However, Bisoffi et al.14 in their study reported a higher PPV during the high-transmission season. Rapid diagnostic tests have high variability in performance that is likely due to inadequate quality of manufacturing, incorrect storage and handling, and sometimes poor study methods, analysis, and reporting.24,25,29–34 In addition, there are possibilities of variation in human users and human errors.35 In Nigeria, “the influence of the epidemiological situation (i.e., the proportion of P. falciparum and other Plasmodium species) in febrile children less than 5 years on the diagnostic accuracy of rapid test result is not known.”20,25 Previous evaluations of diagnostic accuracy of RDT for parasitological diagnosis of malaria carried out in clinical settings in areas of varying endemicity in Nigeria have shown variable results.20,25,33 Moreover, field studies are needed to assess the sensitivity and specificity of RDT in relevant populations to make an informed procurement decision.34
Expert microscopy detected only P. falciparum, which is different from the findings of the National Malaria Indicator Survey among children less than 5 years that reported a national prevalence of 95% for P. falciparum, 6% for Plasmodium ovale, 10% for Plasmodium malariae, and 10% for mixed infections.4 This has implications for the choice of antigen-based RDT for mass deployment to health facilities in the country.4,5,36,37 Evidently in the study area, P. falciparum infection rate implies that HRP-2 RDT is likely to be appropriate for accurate diagnosis, in line with the national policy on diagnosis and treatment of malaria.9
Using the positivity rate of RDT and slide positivity rate of microscopy as a proxy for malaria prevalence, these are lower compared with previous findings of 76.3% and 76.4% (mesoendemic areas) and of 84.7% (holoendemic area) in the same age group surveyed across the seasons by microscopy in Nigeria.20,25,38 A possible explanation could be a decreasing prevalence of malaria given the increased utilization of long-lasting insecticidal nets and increased access to artemisinin-based combination therapies (ACTs) in children less than 5 years in Kaduna state.4,39 This finding may mean malaria is not as common as earlier presumed; if these findings are reproduced, there may be no place for presumptive antimalarial treatment.13 The absence of false negatives implies that the risk of missing a true malaria case is very low, and withholding ACTs in RDT negative cases, provided the RDT is quality assured, will improve management of fever cases and help to ensure rational antimalarial use.40
Other factors influencing diagnostic accuracy such as storage related (humidity, temperature) and user performance were not evaluated in this study. The use of PCR as a second gold standard could have helped to re-classify discordant cases (positive RDT result/negative microscopy) accurately with possible influence on measures of diagnostic performance of the RDT.35,41
Conclusions
This RDT demonstrated a good diagnostic performance in a clinical setting in a mesoendemic area in northern Nigeria. The results provided additional information on the local conditions of malaria transmission as suggested by the WHO for procurement decisions on RDT.9,34 The good diagnostic performance may encourage health workers to trust RDT results, and probably look for other causes of febrile illness when RDT results are negative, and thus reduce dispensing and wastage of ACTs. This has an implication for proposed use of RDT in diagnosing all malaria fevers at the community level irrespective of the season.9 HRP-2 RDT is recommended for malaria diagnosis in Nigeria.
ACKNOWLEDGMENTS
We acknowledge and appreciate the staff and patients of Makarfi General Hospital and members of Makarfi and Kuruntumawa communities, Makarfi Local Government Area, Kaduna state for their immense support and cooperation toward the success of this study. We are indebted to AFENET/USAID/CDC for providing financial support. The malaria rapid test kit used in this study was provided at no cost by the standard diagnostics South Korea through Codix Pharmaceuticals Limited, Nigeria. We have not received any financial incentives from these organizations.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Olufemi Ajumobi, Nigeria Field Epidemiology and Laboratory Training Programme, Abuja, Nigeria, and National Malaria Control Programme, Federal Ministry of Health, Abuja, Nigeria, E-mail: femiajumobi@gmail.com. Kabir Sabitu and Jacob Kwaga, Ahmadu Bello University, Zaria, Nigeria, E-mails: kssabitu@yahoo.com and jacobkwaga@yahoo.com. Patrick Nguku and Gabriele Poggensee, Nigeria Field Epidemiology and Laboratory Training Programme, Abuja, Nigeria, E-mails: drnguku@yahoo.com and gapo.nigeria@gmail.com. Godwin Ntadom, National Malaria Control Programme, Federal Ministry of Health, Abuja, Nigeria, E-mail: ntadomg@yahoo.com. Sheba Gitta, African Field Epidemiology Network, Kampala, Uganda, E-mail: sgitta@afenet.net. Rutebemberwa Elizeus, Makerere University Kampala, Uganda, E-mail: ellie@musph.ac.ug. Wellington Oyibo, College of Medicine, University of Lagos, Nigeria, E-mail: wellao@yahoo.com. Peter Nsubuga, Global Health Solutions, Atlanta, GA, E-mail: pnsubuga@globalphsolutions.com. Mark Maire, Division of Global Health Protection and Division of Parasitic Diseases and Malaria, Center for Global Health, Center for Disease Control and Prevention, Atlanta, GA, E-mail: vlq8@cdc.gov.
References
- 1.World Health Organization . World Malaria Report. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 2.Federal Ministry of Health . A Road Map for Malaria Control in Nigeria Strategic Plan 2009–2013: National Malaria Control Programme. Abuja, Nigeria: Federal Ministry of Health; 2008. [Google Scholar]
- 3.National Population Commission, National Malaria Control Programme . Nigeria Malaria Indicator Survey 2010. Abuja, Nigeria: ICF International; 2012. p. 2. [Google Scholar]
- 4.National Population Commission, National Malaria Control Programme . Abuja, Nigeria: ICF International; 2012. Nigeria Malaria Indicator Survey 2010; p. 66. [Google Scholar]
- 5.World Health Organization . WHO Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 6.World Health Organization . New Perspectives: Malaria Diagnosis. Report of a Joint WHO/USAID Informal Consultation, October 25–27, 1999. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 7.World Health Organization . Global Plan for Artemisinin Resistance Containment. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 8.World Health Organization . Emergency Response to Artemisinin Resistance in the Greater Mekong Subregion: Regional Framework for Action 2013–2015. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 9.National Malaria and Vector Control Division, Federal Ministry of Health . National Policy on Malaria Diagnosis and Treatment. Abuja, Nigeria: 2011. pp. 15–22.http://www.nmcp.gov.ng/document Available at. [Google Scholar]
- 10.World Health Organization . Universal Access to Malaria Diagnostic Testing: An Operational Manual. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 11.D'Acremont V, Malila A, Swai N, Tyllia R, Kahama-Maro J, Lengeler C, Genton B. Withholding antimalarials in febrile children with a negative rapid diagnostic test is safe in moderately and highly endemic areas of Tanzania: a prospective longitudinal study. Clin Infect Dis. 2010;51:506–511. doi: 10.1086/655688. [DOI] [PubMed] [Google Scholar]
- 12.Federal Ministry of Health . Implementation Guide for Parasite-Based Diagnosis of Malaria. Abuja, Nigeria: National Malaria Control Programme, Federal Ministry of Health; 2011. [Google Scholar]
- 13.Hamer DH, Ndhlovu M, Zurovac D, Fox M, Yeboah-Antwi K, Chanda P, Sipilinyambe N, Simon JL, Snow RW. Improved diagnostic testing and malaria treatment practices in Zambia. JAMA. 2007;297:2227–2231. doi: 10.1001/jama.297.20.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisoffi Z, Sirima SB, Menten J, Pattaro C, Angheben A, Gobbi F, Tinto H, Lodesani C, Neya B, Gobbo M, Van den Ende J. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar J. 2010;9:192. doi: 10.1186/1475-2875-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayang CSG, Tahar R, Basco LK, Gazin P, Moyou-Somo R, Delmont J. Use of a histidine-rich protein 2-based rapid diagnostic test for malaria by health personnel during routine consultation of febrile outpatients in a peripheral health facility in Yaounde, Cameroon. Am J Trop Med Hyg. 2009;81:343–347. [PubMed] [Google Scholar]
- 16.Laurent ASJ, Shirima K, Ketende SC, Alonso PL, Mshinda H, Tanner M, Schellenberg D. Performance of HRP-2 based rapid diagnostic test for malaria and its variation with age in an area of intense malaria transmission in southern Tanzania. Malar J. 2010;9:294. doi: 10.1186/1475-2875-9-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2006;4:S20–S32. doi: 10.1038/nrmicro1570. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Geneva, Switzerland: World Health Organization; 2000. New Perspectives: Malaria Diagnosis. Report of a Joint WHO/USAID Informal Consultation, October 25–27, 1999. [Google Scholar]
- 19.World Health Organization . Malaria Rapid Diagnostic Tests Evaluation Programme. Manila, Philippines: Western Pacific Regional Office, World Health Organization; 2005. [Google Scholar]
- 20.Olasehinde GI, Ajayi AA, Taiwo SO, Adekeye BT, Adeyeba OA. Prevalence and management of falciparum malaria among infants and children in Ota, Ogun State, southwestern Nigeria. Afr J Clin Exp Microbiol. 2010;11:159–163. [Google Scholar]
- 21.World Health Organization . Basic Malaria Microscopy. Geneva: World Health Organization; 2010. [Google Scholar]
- 22.Van Den Broek IHO, Gordillo F, Angarita B, Hamade P, Counihan H, Guthmann JP. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. Am J Trop Med Hyg. 2006;75:1209–1215. [PubMed] [Google Scholar]
- 23.Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, Rapuoda B, Greenwood B, Cox J. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malar J. 2008;7:202. doi: 10.1186/1475-2875-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tidi SK, Akogun OB. Effectiveness of rapid test technique (RTT) in malaria diagnosis and chloroquine treatment failures in Yola, Nigeria. Nig J Parasitol. 2005;26:49–54. [Google Scholar]
- 26.Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008;21:97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent A, Schellenberg J, Shirima K, Ketende SC, Alonso PL, Mshinda H, Tanner M, Schellenberg D. Performance of HRP-2 based rapid diagnostic test for malaria and its variation with age in an area of intense malaria transmission in southern Tanzania. Malar J. 2010;9:294. doi: 10.1186/1475-2875-9-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly AB, Tall A, Perry R, Baril L, Badiane A, Faye J, Rogier C, Toure A, Sokhna C, Trape JF, Michel R. Use of HRP-2-based rapid diagnostic test for Plasmodium falciparum malaria: assessing accuracy and cost-effectiveness in the villages of Dielmo and Ndiop, Senegal. Malar J. 2010;9:153. doi: 10.1186/1475-2875-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason DP, Kawamoto F, Lin K, Laoboonchai A, Wongsrichanalai C. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop. 2002;82:51–59. doi: 10.1016/s0001-706x(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 30.Moody A, Hunt-Cooke A, Gabbett E, Chiodini P. Performance of the OptiMAL malaria antigen capture dipstick for malaria diagnosis and treatment monitoring at the Hospital for Tropical Diseases, London. Br J Haematol. 2000;109:891–894. doi: 10.1046/j.1365-2141.2000.01974.x. [DOI] [PubMed] [Google Scholar]
- 31.Bell DR, Wilson DW, Martin LB. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg. 2005;73:199–203. [PubMed] [Google Scholar]
- 32.Endeshaw T, Gebre T, Ngondi J, Graves PM, Shargie EB, Ejigsemahu Y, Ayele B, Yohannes G, Teferi T, Messele A, Zerihun M, Genet A, Mosher AW, Emerson PM, Richards FO. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malar J. 2008;7:118. doi: 10.1186/1475-2875-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tagbo O, Henrietta UO. Comparison of clinical, microscopic and rapid diagnostic test methods in the diagnosis of Plasmodium falciparum malaria in Enugu, Nigeria. Niger Postgrad Med J. 2007;14:285–289. [PubMed] [Google Scholar]
- 34.World Health Organization . Malaria Rapid Diagnostic Test Performance—Results of WHO Product Testing of Malaria RDT s: Round 3 (2010–2011) Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 35.Samadoulougou S, Kirakoya-Samadoulougou F, Sarrassat S, Tinto H, Bakiono F, Nebié I, Robert A. Paracheck® rapid diagnostic test for detecting malaria infection in under five children: a population-based survey in Burkina Faso. Malar J. 2014;13:101. doi: 10.1186/1475-2875-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization . WHO Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 37.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samdi L, Oguche S, Molta NB, Kalu MK, Watila IM, Anyanwu GI, Agomo PU. A comparative longitudinal study of seasonal variation of malaria parasite and vector densities in the Sahel, northeastern Nigeria. Niger J Exp Appl Biol. 2005;6:77–85. [Google Scholar]
- 39.Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, Fall IS, Snow RW. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383:1739–1747. doi: 10.1016/S0140-6736(13)62566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bastiaens GJ, Bousema T, Leslie T. Scale-up of malaria rapid diagnostic tests and artemisinin-based combination therapy: challenges and perspectives in sub-Saharan Africa. PLoS Med. 2014;11:e1001590. doi: 10.1371/journal.pmed.1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkes M, Conroy A, Opoka R, Namasopo S, Liles W, John C, Kain K. Use of a three-band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malar J. 2014;13:43. doi: 10.1186/1475-2875-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]