Abstract
Undifferentiated febrile illnesses (UFIs) are common in low- and middle-income countries. We prospectively investigated the causes of UFIs in 627 patients presenting to a tertiary referral hospital in Kathmandu, Nepal. Patients with microbiologically confirmed enteric fever (218 of 627; 34.8%) randomized to gatifloxacin or ofloxacin treatment were previously reported. We randomly selected 125 of 627 (20%) of these UFI patients, consisting of 96 of 409 (23%) cases with sterile blood cultures and 29 of 218 (13%) cases with enteric fever, for additional diagnostic investigations. We found serological evidence of acute murine typhus in 21 of 125 (17%) patients, with 12 of 21 (57%) patients polymerase chain reaction (PCR)-positive for Rickettsia typhi. Three UFI cases were quantitative PCR-positive for Rickettsia spp., two UFI cases were seropositive for Hantavirus, and one UFI case was seropositive for Q fever. Fever clearance time (FCT) for rickettsial infection was 44.5 hours (interquartile range = 26–66 hours), and there was no difference in FCT between ofloxacin or gatifloxacin. Murine typhus represents an important cause of predominantly urban UFIs in Nepal, and fluoroquinolones seem to be an effective empirical treatment.
Undifferentiated febrile illnesses (UFIs) are a common clinical problem in south Asia.1,2 Defined as a fever without a focus of infection on initial physical examination or in basic laboratory tests, UFIs represent a considerable burden of disease with diagnostic and therapeutic challenges. Empirical broad-spectrum antimicrobials are generally prescribed but with little evidence-based guidance on likely etiologies or potential treatment responses. Previous studies on UFIs, including those originating from our research group in Nepal,3–5 have been limited by a lack of molecular testing, little convalescent serological testing, and few data on treatment outcomes.
We sought to address this knowledge gap by expanding investigations to determine further causes and treatment outcomes of Nepalese patients with UFIs. Diagnostic tests were performed for scrub typhus, murine typhus, Spotted Fever Group (SFG) rickettsiosis, Q fever, leptospirosis, Hantavirus, Brucella, and dengue (Table 1). The patients described in this report were previously enrolled into a randomized, controlled trial (RCT) comparing gatifloxacin with ofloxacin for treating enteric fever in Patan Hospital, a 450-bed teaching hospital within the Kathmandu Valley, Nepal. All patients had UFIs at enrollment and came from predominantly urban regions; the methods and results from patients with subsequent blood culture-confirmed enteric fever have been reported previously.3 Briefly, patients were eligible to enter if they were > 2 years old, had an untreated UFI for > 3 days, and could be treated in the community. Each patient was randomly assigned to 7 days of treatment with either gatifloxacin (10 mg/kg per day in a single oral dose) or ofloxacin (20 mg/kg per day in two divided oral doses). All patients were managed as outpatients, with assessment of fever clearance time (FCT) and collection of acute (day 1) and convalescent (day 30) blood samples by trained community medical auxiliaries. Approval for the study was obtained from the Nepal Health Research Council and the Oxford Tropical Research Ethics Committee. The trial was registered as ISRCTN 63006568. Written informed consent was obtained from all study participants.
Table 1.
Diagnostic tests used for the study
| Organism/diagnostic tests | Supplier | Catalog number | Diagnostic criteria | Methodological reference or validation study | Purpose |
|---|---|---|---|---|---|
| Orientia tsutsugamushi | |||||
| IgM ELISA | NMRC | In house | ≥ 0.2 nett OD | 11 | Serological screening |
| IgG ELISA | NMRC | In house | ≥ 0.2 nett OD | 11 | Serological screening |
| IgM IFA | ARRL | RT-001 | ≥ Fourfold rising titer in paired samples | 12 | Quantitative serological confirmation |
| IgG IFA | ARRL | RT-001 | ≥ Fourfold rising titer in paired samples | 12 | Quantitative serological confirmation |
| Real-time PCR | MORU | In house | 47-kDa gene amplification | 13 | |
| R. typhi | |||||
| IgM ELISA | NMRC | In house | ≥ 0.2 nett OD | 11 | Serological screening |
| IgG ELISA | NMRC | In house | ≥ 0.2 nett OD | 11 | Serological screening |
| IgM IFA | ARRL | RT-001 | ≥ Fourfold rising titer in paired samples | 12 | Quantitative serological confirmation |
| IgG IFA | ARRL | RT-001 | ≥ Fourfold rising titer in paired samples | 12 | Quantitative serological confirmation |
| Real-time PCR | MORU | In house | ompB gene amplification | 14 | Confirmation of infection |
| Rickettsia spp. | |||||
| Real-time PCR | MORU | In house | 17-kDa gene amplification | 13 | Confirmation of infection |
| Coxiella burnetti | |||||
| Phase II IgM ELISA | Serion | ESR1312M | Manufacturer's criteria | Product insert | Serological screening |
| Phase I/II IFA | Fuller | Manufacturer's criteria | Product insert | Quantitative serological confirmation | |
| Hantavirus Puumala | |||||
| IgM ELISA | Serion | ESR145M | Manufacturer's criteria | Product insert | Serological screening |
| Anti-Hantavirus IIFT Mosaic II Test | Euroimmun | Quantitative serological confirmation | |||
| Leptospira | |||||
| IgM ELISA | Serion | ESR125M | Manufacturer's criteria | Product insert | Serological screening |
| Microscopic agglutination test* | QSHL | In house | ≥ Fourfold rising titer in paired samples | 15 | Quantitative serological confirmation |
| Brucella spp. | |||||
| Rose–Bengal | NIAH | In house | Positive agglutination reaction | 16 | Serological screening |
| Dengue | |||||
| SD NS1 Ag ELISA | Alere | 11EK50 | Manufacturer's criteria | 17 | Serological screening |
ARRL = Australian Rickettsial Reference Laboratory; ELISA = enzyme-linked immunosorbent assay; IFA = indirect immunofluorescence assay; Ig = immunoglobulin; IIFT = indirect immunofluorescence test; MORU = Mahidol Oxford Research Unit; nett OD = net optical density (net stands for the difference from baseline to measured values); NIAH = National Institute of Animal Health–Thailand; NMRC = Naval Medical Research Centre; QSHL = Queensland State Health Laboratory; SD NS1 Ag = standard diagnostics non-structural protein number one (refers to Dengue virus protein) antigen.
Leptospira serovars tested: pomona, hardjo, tarassovi, grippotyphosa, celledoni, copenhageni, australis, pyrogenes, canicola, hebdomadis, sari, sarmin, autumnalis, cynopteri, ballum, bataviae, djasiman, javanica, panama, shermani, and pohnpei.
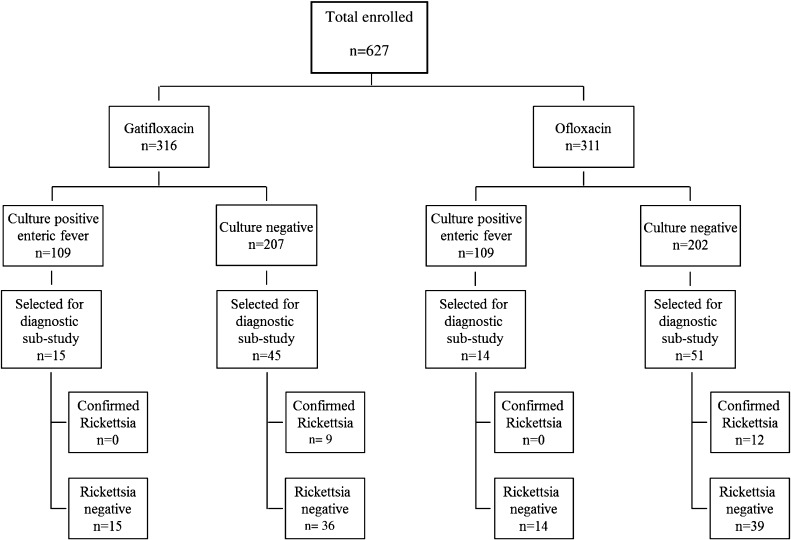
Between July of 2008 and August of 2011, 627 patients with UFIs were enrolled in the RCT: 311 of 627 (49.6%) patients received gatifloxacin, and 316 of 627 (50.4%) patients received ofloxacin (Figure 1). Salmonella Typhi and Salmonella Paratyphi A were cultured from the blood of 109 of 311 (35%) and 109 of 316 (34%) patients in each treatment arm, respectively. The remaining 409 of 627 (65%) patients had UFIs with negative blood cultures. Although no formal sample size calculation was carried out for this study, we randomly selected 125 of 627 (20%) UFI patients for additional diagnostic testing, consisting of 96 of 409 (23%) UFI patients and 29 of 218 (13%) enteric fever patients (Salmonella Typhi, N = 17; Salmonella Paratyphi A, N = 12) (Table 1).
Figure 1.
Flowchart of patients from the RCT and the substudy of UFIs. In total, 627 patients were enrolled into the clinical trial comparing gatifloxacin with ofloxacin in the treatment of enteric fever. In total, 316 patients were randomized to receive gatifloxacin, and 311 patients were randomized to receive ofloxacin. One patient was not randomized. There were 109 culture-confirmed enteric fever cases in each arm, leaving 207 and 202 culture-negative patients in the gatifloxacin and ofloxacin arms, respectively. In total, 125 patients were selected for the UFI diagnostic substudy; 29 of these 125 patients were selected from culture-positive enteric fever group, and an additional 96 patients from the culture-negative groups.
All data were analyzed using Stata v13 (College Station, TX). Kruskal–Wallis tests were used to compare clinical parameters between the enteric fever and rickettsial groups. FCTs were summarized by Kaplan–Meier estimates and compared between groups using a Cox regression model with only one covariate. All tests were performed using two-sided 5% significance.
In total, 21 of 125 (17%) patients were identified with acute murine typhus infection on the basis of at least a fourfold antibody titer rise from day 1 to day 30 (Figure 1); 10 of these cases were confirmed by quantitative polymerase chain reaction (PCR; ompB gene target), and 2 cases were confirmed by conventional PCR/sequencing of the 17-kDa and/or gltA genes. In total, 12 of 21 (57%) PCR-positive murine typhus cases were confirmed. Three cases with a Rickettsia spp.-positive quantitative PCR result could not be further differentiated because of limited sample specimen. However, these specimens have a high probability of being murine typhus cases because of their positive R. typhi serology. The possibility remains that SFG Rickettsia could be responsible for these cases. None of the patients with rickettsial infections were coinfected with Salmonella Typhi or Salmonella Paratyphi A. Additionally, two cases were serologically positive for Hantavirus, and one case was serologically positive for Q fever.
Although the study design allowed for limited comparison, the clinical presentations and basic laboratory values, such as complete blood count, liver function test, and creatinine, of 21 rickettsial patients and 29 enteric fever patients were, in general, similar. However, the FCT was significantly prolonged in the enteric fever patients, with a median of 88 hours (interquartile range [IQR] = 54–116), for both drugs compared with the FCT in those with rickettsial infections, with a median of 44.5 hours (IQR = 26–66; hazard ratio = 3.71; P < 0.001).
Our study has a number of limitations. First, we were unable to test the whole study population for alternative causes of UFI, and the 20% proportion of patients selected may not have been truly representative of the whole population. Second, serological testing for Rickettsia may lack specificity, although we defined acute infection as a greater than or equal to a fourfold rise in reciprocal antibody titers between admission and convalescence sera.
Despite these limitations, our study highlights that Rickettsia spp. are an important cause of UFIs in Nepal6 and that these patients present with similar clinical characteristics to enteric fever. Although the original study was designed to enroll typhoid patients and represents more of an urban population, we detected a 17% murine typhus case rate and a possible 2% Rickettsia spp. infection rate in a random subselection of the study. Notably, we have evidence suggesting that Hantavirus and Q fever contribute to UFIs. The absence of scrub typhus is likely because of the predominantly urban patients enrolled in this study.
The recommended therapy for murine typhus is doxycycline,7 although fluoroquinolones are known to be an effective alternative for the treatment of SFG rickettsioses.8 Without control groups of untreated or doxycycline-treated patients, only tentative conclusions can be drawn, but despite previous reports of poor responses to ciprofloxacin in murine typhus9,10 our findings suggest that gatifloxacin and ofloxacin may be effective empirical treatment choices in Nepalese patients with UFIs.
ACKNOWLEDGMENTS
We are grateful to the Patan Hospital patients and their families for their assistance. Thanks to Krishna Prajapati and Bijaya Karanjit from Patan Hospital for laboratory support. We also thank Ampai Tanganuchitcharnchai and Suthatip Jintawon from Mahidol Oxford Tropical Medicine Research Unit for performing the serological studies.
Footnotes
Financial support: This work was funded by the Wellcome Trust of Great Britain.
Authors' addresses: Corinne N. Thompson, Christiane Dolecek, Stephen Baker, and Guy Thwaites, Hospital for Tropical Diseases, Wellcome Trust Major Overseas Programme, Oxford University Clinical Research Unit–Vietnam, Ho Chi Minh City, Vietnam, E-mails: cthompson@oucru.org, cdolecek@gmail.com, sbaker@oucru.org, and gthwaites@oucru.org. Stuart D. Blacksell, Daniel H. Paris, and Nick Day, Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: stuart@tropmedres.ac, parigi@tropmedres.ac, and nickd@tropmedres.ac. Amit Arjyal, Abhilasha Karkey, Sabina Dongol, Abhishek Giri, and Buddha Basnyat, Oxford Clinical Research Unit–Nepal, Patan Academy of Health Sciences, Patan Hospital, Lalitpur, Nepal, E-mails: amitarjyal@yahoo.com, abhilashakarkey@hotmail.com, dongolsabina@yahoo.com, giriabhishek@hotmail.com, and buddhabasnyat@gmail.com. Jeremy Farrar, Wellcome Trust, London, United Kingdom, E-mail: JeremyJfarrar@gmail.com.
References
- 1.Chrispal A, Boorugu H, Gopinath K, Chandy S, Prakash J, Thomas E, Abraham A, Abraham O, Thomas K. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a teriary care hospital in South India. Trop Doct. 2010;40:230–234. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 2.Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, Scott RM, Basnyat B, Archibald LK, Reller LB. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- 3.Koirala S, Basnyat B, Arjyal A, Shilpakar O, Shrestha K, Shrestha R, Shrestha UM, Agrawal K, Koirala KD, Thapa SD, Karkey A, Dongol S, Giri A, Shakya M, Pathak KR, Campbell J, Baker S, Farrar J, Wolbers M, Dolecek C. Gatifloxacin versus ofloxacin for the treatment of uncomplicated enteric fever in Nepal: an open-label, randomized, controlled trial. PLoS Negl Trop Dis. 2013;7:e2523. doi: 10.1371/journal.pntd.0002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arjyal A, Basnyat B, Koirala S, Karkey A, Dongol S, Agrawaal KK, Shakya N, Shrestha K, Sharma M, Lama S, Shrestha K, Khatri NS, Shrestha U, Campbell JI, Baker S, Farrar J, Wolbers M, Dolecek C. Gatifloxacin versus chloramphenicol for uncomplicated enteric fever: an open-label, randomised, controlled trial. Lancet Infect Dis. 2011;11:445–454. doi: 10.1016/S1473-3099(11)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandit A, Arjyal A, Day JN, Paudyal B, Dangol S, Zimmerman MD, Yadav B, Stepniewska K, Campbell JI, Dolecek C, Farrar JJ, Basnyat B. An open randomized comparison of gatifloxacin versus cefixime for the treatment of uncomplicated enteric fever. PLoS ONE. 2007;2:e542. doi: 10.1371/journal.pone.0000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman MD, Murdoch DR, Rozmajzl PJ, Basnyat B, Woods CW, Richards AL, Belbase RH, Hammer DA, Anderson TP, Reller LB. Murine typhus and febrile illness, Nepal. Emerg Infect Dis. 2008;14:1656–1659. doi: 10.3201/eid1410.080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 7th Ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. pp. 2525–2527. [Google Scholar]
- 8.Raoult D, Drancourt M. Antimicrobial therapy of rickettsial diseases. Antimicrob Agents Chemother. 1991;35:2457–2462. doi: 10.1128/aac.35.12.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laferl H, Fournier P, Seiberl G, Pichler H, Raoult D. Murine typhus poorly responsive to ciprofloxacin: a case report. J Travel Med. 2002;9:103–104. doi: 10.2310/7060.2002.21970. [DOI] [PubMed] [Google Scholar]
- 10.Gikas A, Doukakis S, Pediaditis J, Kastanakis S, Manios A, Tselentis Y. Comparison of the effectiveness of five different antibiotic regimens on infection with Rickettsia typhi: therapeutic data from 87 cases. Am J Trop Med Hyg. 2004;70:576–579. [PubMed] [Google Scholar]
- 11.Richards A, Rahardjo E, Rusjdi A, Kelly D, Dasch G, Church C, Bangs M. Evidence of Rickettsia typhi and the potential for murine typhus in Jayapura, Irian Jaya, Indonesia. Am J Trop Med Hyg. 2002;66:431–434. doi: 10.4269/ajtmh.2002.66.431. [DOI] [PubMed] [Google Scholar]
- 12.Phetsouvanh R, Thojaikong T, Phoumin P, Sibounheuang B, Phommasone K, Chansamouth V, Lee S, Newton P, Blacksell S. Inter- and intra-operator variability in the reading of indirect immunofluorescence assays for the serological diagnosis of scrub typhus and murine typhus. Am J Trop Med Hyg. 2013;88:932–936. doi: 10.4269/ajtmh.12-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Maina AN, Knobel DL, Cleaveland S, Laudisoit A, Wamburu K, Ogola E, Parola P, Breiman RF, Njenga MK, Richards AL. Molecular detection of Rickettsia felis and Candidatus rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550–558. doi: 10.1089/vbz.2012.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry K, Jiang J, Rozmajzl P, Azad A, Macaluso K, Richards A. Development of quantitative real-time PCR assays to detect Rickettsia typhi and Rickettsia felis, the causative agents of murine typhus and flea-borne spotted fever. Mol Cell Probes. 2007;21:17–23. doi: 10.1016/j.mcp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Cole J, Sulzer C, Pursell A. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.OIE . OIE Terrestrial Manual: Bovine Brucellosis. Paris, France: OIE; 2009. [Google Scholar]
- 17.Blacksell S, Jarman R, Gibbons R, Tanqanuchitcharnchai A, Mammen M, Nisalak A, Kalayanarooj S, Bailey M, Premaratna R, de Silva HJ, Day NP, Lalloo DG. Comparison of seven commercial antigen and antibody enzyme-linked immunosorbent assays for detection of acute dengue infection. Clin Vaccine Immunol. 2012;19:804–810. doi: 10.1128/CVI.05717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]