Abstract
Circadian rhythms are generated by an endogenously organized timing system that drives daily rhythms in behavior, physiology and metabolism. In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus is the locus of a master circadian clock. The SCN is synchronized to environmental changes in the light:dark cycle by direct, monosynaptic innervation via the retino-hypothalamic tract. In turn, the SCN coordinates the rhythmic activities of innumerable subordinate clocks in virtually all bodily tissues and organs. The core molecular clockwork is composed of a transcriptional/post-translational feedback loop in which clock genes and their protein products periodically suppress their own transcription. This primary loop connects to downstream output genes by additional, interlocked transcriptional feedback loops to create tissue-specific ‘circadian transcriptomes’. Signals from peripheral tissues inform the SCN of the internal state of the organism and the brain’s master clock is modified accordingly. A consequence of this hierarchical, multilevel feedback system is that there are ubiquitous effects of circadian timing on genetic and metabolic responses throughout the body. This overview examines landmark studies in the history of the study of circadian timing system, and highlights our current understanding of the operation of circadian clocks with a focus on topics of interest to the neuroscience community.
Keywords: chronopharmacology, endocrine, feeding, mental health, obesity, sleep
Introduction
Early history
Daily changes in behavior and physiology have been known, most likely, since prehistoric times. Initially, it was believed that daily changes were not endogenously generated but were, instead, driven by external temporal cues. Early evidence for the endogenous nature of circadian rhythms came from a classic study by Jean-Jacques d’Ortous de Mairan (1729) in which he investigated the daily leaf motion in the heliotropic plant, Mimosa pudica (Somers, 1999). In addition to its best-known behavior, where the leaves of M. pudica rapidly fold inward when touched, the foliage of this plant also closes during the night and reopens during the day. To examine whether this rhythm was endogenous, de Mairan placed these plants into constant darkness and monitored leaf movements. Despite having been removed from the light:dark (LD) cycle, the plants in constant darkness continued to show daily leaf movement with a period close to 24 h.
Although the results of de Mairan’s work provided compelling evidence for endogenous daily rhythms, it was argued, quite plausibly, that daily changes in temperature or unspecified geophysical cues could be driving these oscillations. Although it was not their intention, research by Nathanial Kleitman and his colleague, Bruce Richardson, helped to provide further evidence for the endogenous nature of circadian rhythms (Kleitman, 1939). With their goal being to attempt to synchronize their sleep–wake cycle to a 28 h day, Kleitman and Richardson spent over a month in Mammoth Cave, Ken-tucky, 150 feet below ground, where temperature and light were constant. The younger Richardson was capable of modifying his behavior to a 28 h day, whereas Kleitman was not, continuing to sleep on an approximately 24 h schedule. Kleitman noted daily rhythms in his body temperature, with peak efficiency occurring when body temperature was highest. Although inconclusive given the disparity between the two researchers, the fact that Kleitman’s behavior and temperature oscillated with a 24 h cycle in the face of 28 h time cues suggested the existence of an endogenous clock.
Definitions and criteria
In nature, rhythmic responses that oscillate with ultradian (< 24 h), infradian (> 24 h), circannual (~1 year) and circalunar (~29.5 days) periods are known, but the molecular, cellular, network and behavioral processes underlying these oscillations are understood only in the case of circadian rhythms. That said, several criteria must be met in order to confirm that a particular variable is under endogenous circadian control (as opposed to being driven by daily changes in the environment). First, circadian rhythms should persist when animals or tissues are removed from all daily temporal cues. This can be tested by housing animals in constant darkness or by examining tissues in culture. In addition, the response must persist for a minimum of two or more cycles. In general, the first 24 h interval following placement into constant conditions is not part of this assessment, as this first cycle may be a consequence of the change in external conditions or temporary rhythm maintenance following removal from a driving stimulus. Thus, further confidence that a rhythm is endogenous is gained through observing additional cycles under such conditions. Finally, the measured response should be entrained (synchronized) to a daily temporal cue (e.g. the LD cycle) and resynchronized to this entraining agent following phase adjustments. Application of these criteria indicates that circadian rhythms are ubiquitous.
Many molecular, cellular, physiological or behavioral measures exhibit robust circadian rhythmicity. A dramatic example is seen in the circadian oscillation of the liver-enriched transcriptional activator protein, D-site of albumin promoter-binding protein (DBP), which is not detectable in liver nuclei in the morning hours. DBP levels rise during the afternoon and peak at about 20:00 h. During the night, the cellular DBP concentration again decreases below detectability (Wuarin & Schibler, 1990). Although somewhat more difficult to study, there are also circadian oscillations in gene expression in the brain, and these are region-specific. For example, rhythmicity in PER2 expression was described in 18 different brain regions, with clusters of peaks at different times of day (Harbour et al., 2013). Likewise, the transcriptional regulation of ~3–10% of genes in the brain and periphery show daily rhythms (Akhtar et al., 2002; Duffield et al., 2002; Miller et al., 2007; Hughes et al., 2009). In this context, it is not surprising that there are pronounced daily rhythms in cognitive functioning, e.g. the ability to learn and recall in animals held in an LD cycle or constant conditions (reviewed in Smarr et al., 2014).
Implications for the research
As there are significant circadian oscillations in many biological responses, it is important to control for time of day when collecting experimental data, as this can contribute significantly to response variability. Direct assessment of circadian impact entails investigating a phenomenon across the day and night. Without consideration of circadian timing, one might fail to uncover the impact of experimental manipulation. Furthermore, exposure to light, even brief exposure, can lead to pronounced shifts in circadian phase. At night, light in animal facilities, from windows on doors, leakage around door frames, or dim lights used for maintenance, can alter circadian rhythms of gene expression, shift feeding times, increase body mass, reduce glucose tolerance, alter melatonin rhythms and modulate oncogenicity (Minneman et al., 1974; Dauchy et al., 1999; Fonken et al., 2010; Butler et al., 2012). Such observations underscore the importance of taking into consideration the time of day and photic environment when conducting manipulations, tissue collection, or behavioral examinations.
Present goal
The foregoing background describes the phenomenology of circadian rhythms and the criteria used in delineating endogenous controlled processes. Today, it is clear that oscillations in functional state impact broad swaths of neuroscience research. Our goal in the present article is to provide a broad overview of the circadian timing system for non-specialists and to underscore implications for circadian timing in the study of neuroscience and behavior. In addition, we highlight the significance of circadian timing particularly for researchers interested in feeding and metabolism, sleep biology, mental health, sex differences, and the pharmacological treatment of disease. Given the broad nature of this overview, our intention is to point readers to key considerations of circadian timing for research in the neurobiological basis of behavior, and to the recent literature, rather than exhaustively reviewing literature on more limited aspects of circadian rhythmicity.
Landmarks in the study of circadian rhythms
Modern history
Since the findings by de Mairan and Kleitman, numerous converging lines of evidence support the endogenous nature of circadian timing. First, in constant conditions, the period of circadian rhythms is approximately, but not precisely, 24 h. Were rhythms driven by daily cues such as the LD cycle or geophysical signals, these cycles would be precisely 24 h. Second, unlike most biological processes, where increased temperatures hasten biochemical processes and decreased temperatures have the opposite effect, circadian rhythms are temperature compensated, such that the period of the rhythm is unaltered by temperature changes (Pittendrigh & Caldarola, 1973). This result rules out the possibility that daily changes in temperature are responsible for circadian rhythmicity, although some rhythms can be entrained to cycles in temperature. Together, these findings provide strong suggestive evidence for the endogenous regulation of circadian rhythms.
Suprachiasmatic nucleus as a brain master clock
The discovery of the suprachiasmatic nucleus (SCN) and its identification as a master brain clock launched the study of circadian rhythms into a fruitful era of mechanistic studies. In 1972, two laboratories showed that the destruction of a very discrete hypothalamic area, the SCN, led to the permanent loss of circadian rhythms (Moore & Eichler, 1972; Stephan & Zucker, 1972). The finding of a neural locus for circadian timekeeping provides definitive evidence for the endogenous control of circadian rhythmicity. In the two decades following the characterization of the SCN as a master circadian clock, substantial support accumulated for the notion that, in mammals, this hypothalamic nucleus is an internal timekeeper, with a necessary role in circadian timing. The supporting evidence includes proof that, both in vivo (Inouye & Kawamura, 1979) and in vitro (Gillette & Prosser, 1988), the SCN is rhythmic when isolated from the rest of the brain. When transplanted from a fetal donor animal into an SCN-lesioned host, an SCN graft rescues rhythmicity and the restored behavior has the period of the donor rather than the host (Lehman et al., 1987; Ralph et al., 1990). In addition, the molecular mechanisms responsible for rhythm generation at the cellular level have been well characterized, and it has been shown that genetic mutations or knockout of essential clock genes leads to either arrhythmicity or gross deficits in circadian timekeeping of the SCN (Hastings et al., 2014; Partch et al., 2014).
The molecular clockwork
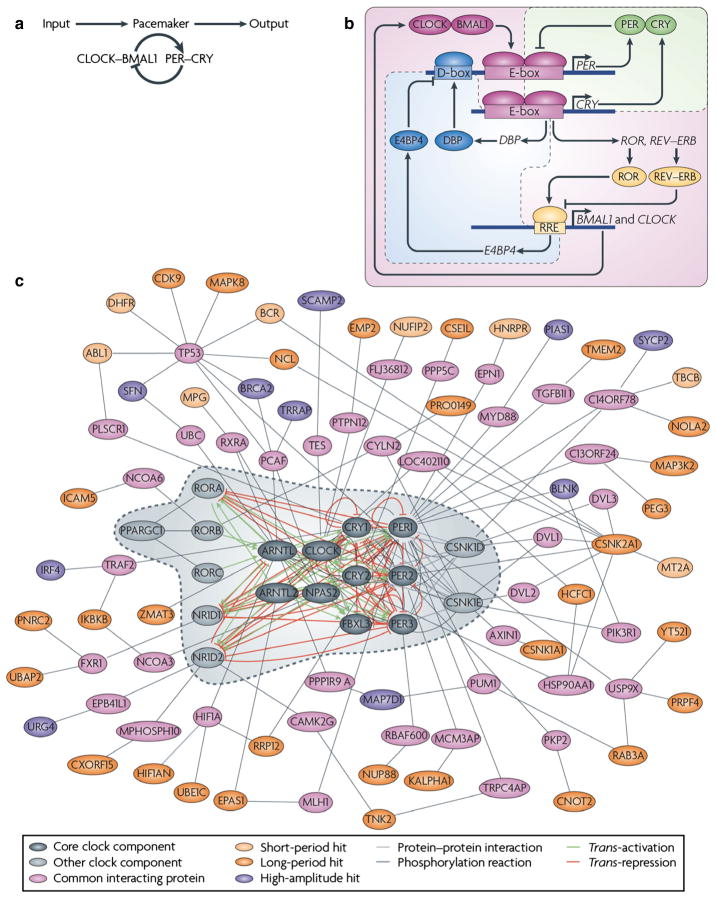
The SCN is comprised of about 20 000 cells that form a highly organized network to produce a coherent, tissue-level clock (Welsh et al., 2010; Partch et al., 2014). At the cellular level, circadian rhythms are generated by interlocked transcriptional/translational feedback loops consisting of ‘clock’ genes and their protein products (reviewed in Zhang & Kay, 2010; Hastings et al., 2014; Partch et al., 2014) (Fig. 1). In mammals, the core feedback loop consists of two transcriptional activators, CLOCK and BMAL1, and two transcriptional repressors, the PERIOD (PER) and CRYPTO-CHROME (CRY) proteins (Huang et al., 2012). In the morning, CLOCK and BMAL1 activate transcription of the Per (Per1, Per2 and Per3) and Cry (Cry1 and Cry2) genes by binding to the E-box (CACGTG) domain on their gene promoters. Over the course of the day, the Per and Cry genes are translated into their respective proteins. PER and CRY proteins form heterodimers late in the day that translocate from the cytoplasm to the cell nucleus to inhibit CLOCK:BMAL1-mediated transcription. The timing of nuclear entry is balanced by regulatory kinases that phosphorylate the PER and CRY proteins, leading to their degradation (Lowrey et al., 2000; Shanware et al., 2011). REV-ERBα/ROR-binding elements (Preitner et al., 2002) act to regulate Bmal1 transcription via a secondary feedback loop. The transcriptional retinoid-related orphan receptor (ROR) is a transcriptional activator of Bmal1, whereas REV-ERBα, an orphan nuclear receptor, negatively regulates Bmal1. The same CLOCK:BMAL1 mechanism controlling Per and Cry gene transcription also controls transcription of REV-ERBα. This secondary feedback loop produces rhythmic expression of BMAL1, further stabilizing the clockwork. The clockwork at the cellular level is functionally similar across taxa, with interacting transcription/translation feedback loops driving rhythms at the cellular level. Importantly, clock genes themselves are not conserved across higher taxa, but transcriptional feedback loops and post-transcriptional controls are common mechanisms for the generation of cell-based oscillation (reviewed in Harmer et al., 2001).
Fig. 1.
A decade ago, our understanding of the pacemaker was limited to a single ‘core’ loop, in which the transcriptional activators circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1) dimerize and induce the expression of repressors PER and CRY, which feedback into the loop to inhibit their own expression. (b) During the first half of the past decade, a triple interlocking loops model was developed, including the PER–CRY loop, which is the primary loop, and the ROR–REV–ERB-associated loop and D-site of albumin promoter-binding protein (DBP)–E4 promoter-binding protein 4 (E4BP4)-associated loop. In addition to the primary loop, which was previously recognized as the core loop (a), transcriptional regulation of CLOCK and BMAL1 is controlled by ROR transcriptional activators and the dimeric REV–ERB repressors [by binding to REV response element (RRE)], the expression of which is governed by CLOCK–BMAL1 activity (through binding to E-box-containing DNA elements). Furthermore, the transcriptional activator DBP (the expression of which is controlled by an E-box) and E4BP4 (the expression of which is controlled by RRE) synergistically regulate the expression of D-box-containing genes, including PER. (c) Hundreds of clock modifiers have now been identified through a genome-wide screen, some of which directly or indirectly associate with known clock components. Genes that affect clock amplitude or period are shown in different colors. The dashed line circle indicates previously known clock genes as shown in the triple interlocking loops (b). Reproduced from Zhang & Kay (2010), with permission (Nature Publishing Group).
Circadian rhythms and temporal niche
Circadian oscillation is key to understanding how organisms are synchronized to their local environments, and species-typical adaptations to their temporal niches are markedly influenced by environmental LD cycles (reviewed in Hut et al., 2012). As noted above, in mammals, photic input from the retina entrains the SCN, but somewhat surprisingly, the phases of SCN electrical, metabolic and molecular rhythms, relative to the light cycle, have the same daytime peaks in diurnally and nocturnally active species (reviewed in Smale et al., 2003). As an example, rhythms of Period gene expression in the SCN peak at approximately the same time of day in diurnal as in nocturnal rodents, suggesting that the phase of clock gene expression in the SCN relative to the LD cycle is conserved across mammalian groups, and implying that the signaling cascade initiating daily activity lay beyond the SCN. This phenomenon has piqued the interest of investigators, especially because there is significant evidence that switching of temporal niches can occur (Mrosovsky & Hattar, 2005; Gattermann et al., 2008). It appears that neural responses to light can mediate acute temporal-niche switching. Thus, a switch from nocturnal to diurnal activity rhythms occurs in wild-type mice transferred from standard intensity to scotopic levels of light in an LD cycle (Doyle et al., 2008). A similar switch from nocturnal to diurnal activity rhythms occurs in double-knockout mice, bearing little rod function, due to a lack of the inner-retinal photopigment melanopsin (OPN4) and of RPE65, a key protein used in retinal chromophore recycling. Interestingly, the rhythm of clock gene expression in the SCN is also reversed in these mice, suggesting that the nocturnal to diurnal switch is due to a change in the neural response to light upstream from the SCN. It is possible that a common mechanism produces phase reversals in Rpe65−/−; Opn4−/− mice and in wild-type mice during dim LD cycles.
Ubiquity of circadian oscillators
The identification of ‘clock genes’ and the invention of reporter gene technology enabled the assessment of rhythmicity in cultured cells and tissues, such as SCN slice preparations. The technical developments and experimental findings based on assessing the activities of specific genes and proteins within cells and tissues has led to a reconceptualization of the circadian organization as a hierarchy of oscillators. This vision has brought the circadian timing system to the attention of a very broad clinical and basic research community. Ignoring circadian effects leads to errors of interpretation in basic research and can result in suboptimal diagnosis and treatments in medicine. Circadian clocks regulate the timing of gene expression in each organ, and the regulated genes are unique to each organ (Akhtar et al., 2002; Duffield et al., 2002; Miller et al., 2007; Hughes et al., 2009; Dibner et al., 2010). Thus, circadian control overlies the normal expression of tissue-specific genes and proteins. Not surprisingly, the maintenance of normal phase relationships among tissues and organs appears to be adaptive. Disrupting the circadian network can produce severe pathology (Litinski et al., 2009; Karatsoreos et al., 2011). Optimizing the circadian timing system for treatment, such as appropriately timing drug administration is a frontier research area (Levi & Schibler, 2007; and see below).
Hierarchical organization of the circadian timing system
Since the discovery of the SCN, and the consistent finding that most circadian rhythms are abolished following its destruction, it was generally assumed that the SCN was the only locus capable of independent circadian rhythm generation. In turn, all circadian rhythms throughout the brain and body were thought to be driven by downstream communication from the SCN. This notion was challenged following the observation that cultured fibroblasts exhibit circadian rhythms in gene expression following a serum shock (Balsalobre et al., 1998). With this experiment, it became clear that the ability to oscillate was a general property of tissues throughout the central nervous system and periphery (Damiola et al., 2000; Yamazaki et al., 2000; Yoo et al., 2004). The discovery that the SCN is not alone in the capacity to express endogenous oscillation was the beginning of a reconceptualization of the internal timekeeping system (Balsalobre et al., 1998).
It is now known that the circadian system is composed of multiple individual cellular oscillators located throughout the body and most of its organs and glands. For example, a role for intrinsic rhythmicity in other tissues has been demonstrated. An example of SCN-independent timekeeping is seen in the olfactory bulb (Granados-Fuentes et al., 2006). SCN lesions eliminate circadian locomotor rhythms, but not odor-induced c-Fos rhythms in the olfactory bulb or piriform cortex. Olfactory bulb oscillators drive rhythms in spontaneous and odor-evoked activity within the bulb and also in its primary synaptic targets in the piriform cortex. In the sense that olfactory bulb oscillators express circadian rhythms in the absence of the SCN, persist in constant darkness and are required for rhythms in the piriform cortex, these oscillators can be considered master circadian pacemakers in the olfactory system. That said, in the intact animal, under unperturbed conditions, the SCN sets the phase of the olfactory bulb and other independent oscillators. The SCN modulates temporal activity in these cellular oscillators, such that each bears regulated phase relationships to SCN pacemakers and hence to each other. Such findings led to the interpretation that the circadian clock mechanism modulates the activity of genes in a tissue-specific manner (Akhtar et al., 2002; Duffield et al., 2002; Miller et al., 2007; Silver & Lesauter, 2008; Hughes et al., 2009). This process can be accomplished either directly by CLOCK: BMAL1 activation through an E-box domain on their gene promoters (i.e. clock-controlled genes) or indirectly via downstream actions of clock-controlled gene products to optimize system-wide functioning on a daily schedule (Fig. 2). For example, the thrombomodulin, a cofactor for thrombin that is expressed on the surface of endothelial cells to reduce blood coagulation, gene contains an E-box domain in its promoter and is directly regulated by the CLOCK: BMAL1 complex (Takeda et al., 2007). The resulting rhythm in thrombomodulin probably contributes to daily changes in the likelihood of cardiovascular events. Generally, the risk of cardiovascular events peaks in the morning and evening; the morning time point is associated with a daily peak in rhythmic cortisol and epinephrine, whereas the night-time peak is associated with peak blood pressure and a trough in cardiac vagal modulation (Scheer et al., 2010). These broad implications expanded the audience of investigators and disciplines attending to the workings of the circadian timing system. Not only were the salient phenomena, such as sleep–wake cycles, of immediate interest, but also the invisible circadian oscillations such as seen in the workings of the heart, or in the timing of cell division. Finally, the occurrence of sex differences in circadian rhythms (Bailey & Silver, 2013) and the demonstration of diseases associated with altered clock gene function rendered it necessary to consider the circadian timing system in a broad array of apparently unrelated disciplines, both applied and basic.
Fig. 2.
The hierarchical organization of the circadian system. The circadian system is a hierarchy of similar cellular clock elements. The master clock of the SCN achieves the ‘master’ role by virtue of the nucleus’s internal circuitry and its ability to send timing signals to the rest of the brain. The SCN itself is entrained to solar time and also receives information from arousal-related activity of the body. Thus, the SCN integrates external and internal information, and coordinates the rhythmic activities of peripheral oscillators to produce coherent timing in behavioral, autonomic, and endocrine functions. Reproduced from Silver & Lesauter (2008), with permission (John Wiley and Sons).
Unique features of the suprachiasmatic nucleus master clock
The finding of extra-SCN oscillators begged the question of the relationship of the brain master clock to these other clocks. What was the unique aspect of the SCN that lent it master clock function? Important discoveries included the finding that the cellular/molecular mechanism of the clock was similar in the SCN and in other tissues (Storch et al., 2002; Kamphuis et al., 2005; Liu et al., 2007; Miller et al., 2007). Thus, clock functioning in cells outside the SCN is equally vulnerable to disruption of the molecular clock (Liu et al., 2007). Although the intracellular core clock molecular mechanisms could not explain SCN master clock function, the unique pattern of its connections appeared to be responsible, i.e. the coupling of its neurons appeared to lend stability to the oscillation of the SCN tissue, and its unique inputs and outputs appeared to be the basis of its capacity to function as a master clock (Liu et al., 2007; Welsh et al., 2010; Hastings et al., 2014). Unlike the SCN, rhythmic clock gene expression in other central and peripheral tissues dampens within a few days in culture, suggesting a loss of coupling among oscillators that results in an inability to detect population-wide rhythmicity (Balsalobre et al., 1998; Abe et al., 2002; Wilsbacher et al., 2002). Indeed, this notion was confirmed by monitoring the single-cell bioluminescence of Per2::luciferase in mouse cultured fibroblasts and establishing that, despite the loss of population-wide rhythms in clock gene expression, single cells continued to show clear rhythms in Per2 expression (Welsh et al., 2004; Leise et al., 2012). These findings suggest that, in vivo, coherence among populations of subordinate oscillators is maintained through SCN communication. Also dramatic is the observation that, although SCN lesions abolish most circadian responses, some rhythms survive the ablation of the SCN. For example, SCN-lesioned animals continue to show circadian rhythms when treated with methamphetamine (Honma & Honma, 2009) and they also continue to show food anticipatory behavior, a response based on circadian timing (Saper, 2006; Patton & Mistlberger, 2013). These latter findings suggest that the methamphetamine-entrainable oscillator and the food-entrainable oscillator might share network coupling properties in common with the SCN.
Although cellular oscillators are virtually ubiquitous, the SCN is unique not only in terms of its ability to maintain rhythmic network-level stability, but also in its direct access to timing information. The SCN receives light information through a direct retino-hypothalamic tract to synchronize the master clock to environmental time (Morin & Allen, 2006). Historically, it was believed that the only photoreceptors present in the retina were rods and cones. This notion was questioned following the finding that mice lacking both rod and cone photoreceptors (retinally degenerate mice) exhibit normal photic entrainment despite being visually blind (Foster et al., 1993). By using a retrograde labeling strategy to identify retinal cells projecting to the SCN, it was discovered that a subset of retinal ganglion cells, expressing the photopigment melanopsin, were intrinsically photosensitive (Berson et al., 2002; Hannibal & Fahrenkrug, 2002; Hattar et al., 2002; Panda et al., 2002). As with the elimination of rod/cone signaling, elimination of melanopsin was not sufficient to abolish entrainment (Ruby et al., 2002; Lucas et al., 2003). Entrainment is only fully prevented in mice doubly mutant for both melanopsin and traditional rod/cone photoreceptors (Hattar et al., 2003; Panda et al., 2003). Underscoring the importance of connectivity, even though all photoreceptive classes can contribute to entrainment, this occurs through the conduit of the intrinsically photosensitive retinal ganglion cells; ablating these cells alone (only ~2% of all retinal ganglion cells) prevents entrainment (Schmidt et al., 2011). Together, these findings suggest that rod/cone photoreceptors project to intrinsically photosensitive retinal ganglion cells that then send projections to the SCN to communicate this integrated light information. Because subordinate oscillators do not have access to light information, their phase relative to external time must be maintained through communication from the master clock in the SCN under light-entrained conditions.
Suprachiasmatic nucleus communication to central and peripheral targets
As indicated previously, temporal harmony is maintained among systems through SCN communication to central and peripheral targets. This coordination is essential for optimizing the timing of behavioral and physiological events and maximizing health. The SCN sets the phase relationship among various tissues via monosynaptic neural targets, projections via the autonomic nervous system, systemic hormone secretions, behavioral cycles of feeding and activity and the rhythmic alterations of body temperature (Kriegsfeld & Silver, 2006; Refinetti, 2010; Kalsbeek et al., 2011; Mavroudis et al., 2012; Patton & Mistlberger, 2013; Sladek & Sumova, 2013). The following section provides a brief overview of the specific means by which information is communicated from the master clock to target systems and considers the implications for physiological and behavioral outcomes.
Neural suprachiasmatic nucleus communication
Prior to the advent of viral tract-tracing techniques, monosynaptic anterograde and retrograde tracers were used to explore the connectivity of the SCN to central targets (Stephan et al., 1981; Watts & Swanson, 1987; Watts et al., 1987; Kalsbeek et al., 1993; Morin et al., 1994; Leak & Moore, 2001; Kriegsfeld et al., 2004). These studies revealed extensive monosynaptic projections proceeding rostrally to the septum and bed nucleus of the stria terminalis, rostrally and dorsally to the thalamus, rostrally and laterally throughout the hypothalamus, and caudally to the posterior paraventricular thalamus, precommissural nucleus and olivary pretectal nucleus. Given these widespread projections, it is likely that the SCN is in a position to communicate with the entire brain through secondary or tertiary synapses originating from these primary target loci. In addition, many of these target loci contain neuroendocrine cells and hypothalamic releasing factors that can be secreted into the cerebrospinal fluid or general circulation, providing pervasive communication to systems throughout the brain and body (van der Beek et al., 1993; Vrang et al., 1995; Kalsbeek et al., 1996; Horvath, 1997; Van der Beek et al., 1997; Buijs et al., 1998; Horvath et al., 1998; Gerhold et al., 2001).
In addition to tract-tracing strategies to reveal SCN outputs, there have been a number of studies to exploit novel behavioral patterns that have been found to correlate with altered SCN rhythms. For example, hamsters will spontaneously ‘split’ and exhibit two rest–activity cycles each day instead of one when housed in constant light. In a classic study, de la Iglesia et al. (2003) showed that, in ‘split’ hamsters, the right and left SCN oscillate out of phase with each other, with each SCN’s molecular rhythms in phase with only one of the two daily peaks of activity. Likewise, examination of Per1::GFP expression in cultured SCNs from split mice shows anti-phase oscillations that can be monitored for several cycles (Ohta et al., 2005). Subsequent work using this split model revealed that, rather than a simple right–left split, each SCN splits into two compartments that oscillate in antiphase (Tavakoli-Nezhad & Schwartz, 2005; Yan et al., 2005). This four-way split means that the split hamsters’ SCNs exhibit 24 h rhythms of PER1 protein that cycles in antiphase between the left and right sides and between core and shell subregions. Associated with this SCN oscillation is a 12 h rhythm of FOS expression in brain regions that receive SCN efferents (Butler et al., 2012). In the target regions examined (medial preoptic area, paraventricular nucleus of the hypothalamus, dorsomedial hypothalamus and orexin-A neurons), the oscillations were in-phase between hemispheres (unlike in the SCN), although with detectable right–left differences in amplitude. Importantly, in all three conditions studied (split and unsplit hamsters in constant light, and control hamsters in LD cycles), the timing of FOS expression in targets occurred at the same time of day and always occurred at a common phase reference point of the SCN oscillation, suggesting that, at a specific internal phase, each SCN signals these targets once daily.
In addition to communication via direct projections to neural loci, the SCN also sends multisynaptic connections, via the autonomic nervous system, to targets in the periphery, setting the phase of subordinate oscillatory systems and controlling their activity. By applying transynaptic, retrograde viral tracers, such as a pseudo rabies virus, to various organs and glands, precise multisynaptic connections from the SCN to the periphery have been established. Early studies employing this technique established that corticosterone secretion is controlled by direct projections to the adrenal gland (Buijs et al., 1999), lipid mobilization via projections to adipose tissue (Bamshad et al., 1998; Bartness & Bamshad, 1998; Bartness et al., 2001; Demas & Bartness, 2001), ovarian function (Gerendai et al., 1998, 2000; Gerendai & Halasz, 2000), and thyroid function (Kalsbeek et al., 2000). It is likely that neural connections from the SCN to peripheral glands and organs may be universal for all targets in the body.
Given the technical limitations of these tracers, it is not surprising that several questions still remain. For example, does the SCN employ the same cell phenotype(s) to communicate to all organs and glands? Does the SCN communicate with one neurochemical mediator, or a combination of neurochemical mediators, to set the phase of subordinate oscillators? Is sympathetic and parasympathetic control of peripheral tissues controlled by the same SCN cell phenotypes? Technical innovations now permit an assessment of projections from specific neuropeptidergic cell phenotypes using viral tracers driven by specific gene promoters. By applying these tools to the SCN, important insight can be gained into the specific modalities by which the SCN communicates to central and peripheral targets.
Diffusible signals
In addition to monosynaptic and multisynaptic neural projections, several early lines of evidence suggested that a diffusible signal from the SCN can sustain behavioral rhythmicity. First, in early studies of SCN-lesioned hamsters, locomotor rhythmicity and rhythmic gnawing behavior are restored following grafting of fetal SCN tissue into the third ventricle of the lesioned host (Lehman et al., 1987, 1995; Ralph et al., 1990; LeSauter & Silver, 1994). Postmortem analysis indicated that few connections were made between the graft and the host brain, suggesting that the re-establishment of rhythmic behavior did not result from the restoration of SCN projections (Aguilar-Roblero et al., 1994; Lehman et al., 1995). Furthermore, when an ‘SCN island’ is created with a Halasz knife, animals recover free-running rhythms, even though efferent fibers from the SCN have been severed (Inouye & Kawamura, 1979). Although it is possible that efferent fibers may have grown across the knife cut to form correct synaptic connections, there is no evidence of such plasticity in the mammalian brain.
More direct evidence for the existence of a diffusible SCN signal was gained by transplanting SCN grafts encapsulated in a semi-porous membrane that permitted diffusible, but not neural, outflow into an SCN-lesioned host (Silver et al., 1996). In cases with viable grafts, circadian locomotor rhythms were restored with the period of the donor animal. These results demonstrate that the transplanted biological clock can regulate rhythmicity by means of a diffusible signal. Whether or not such a diffusible signal drives behavioral rhythms under natural conditions has been a more challenging question. Several candidate diffusible signals have been investigated since these initial findings, including prokineticin-2 (Cheng et al., 2002), transforming growth factor-alpha, and cardiotrophin-like cytokine (Kramer et al., 2001; Cheng et al., 2002; Kraves & Weitz, 2006; Li et al., 2006, 2012). All three proteins are expressed rhythmically in the SCN and their receptors are present in major SCN targets or around the third ventricle. The administration of prokineticin-2 and transforming growth factor-alpha during the night (when levels are typically low) inhibits wheel-running behavior, whereas the administration of cardiotrophin-like cytokine antibody during the day (when levels are typically low) leads to increased daytime locomotor activity. Interestingly, in contrast to behavioral rhythms, endocrine rhythms require neural output (Silver et al., 1996 Nunez & Stephan, 1977; Meyer-Bernstein et al., 1999). It has also been demonstrated that diffusible signals are sufficient to produce oscillations in the SCN of non-rhythmic SCNs from mutant animals. Thus, using a coculture technique in which a wild-type SCN graft was used to examine the restoration of rhythmicity in non-rhythmic mutant SCN, it was demonstrated that paracrine signals, involving vasoactive intestinal polypeptide, arginine vasopressin and gastrin-releasing peptide, were sufficient to restore cellular synchrony and oscillation amplitude (Maywood et al., 2011). Likewise, in Cry double-knockout [Cry1(−/−)/Cry2(−/−)] mice, circadian rhythms are synchronized in neonates but not in adults, indicating a loss of rhythm synchrony in the course of development. Whether a diffusible factor(s) in the SCN contributes to the coupling of cellular circadian rhythms was investigated by coculture of a non-bioluminescent SCN slice with a bioluminescent (PER2::luciferase) SCN slice. Synchronized circadian rhythms in adult Cry1 (−/−)/Cry2(−/−) SCN were restored by coculture of neonatal, but not of juvenile, SCN. The results indicate that the neonatal SCN produces a diffusible signal that supports the development of inter-cellular networks that subserve coherent rhythm expression in adult SCN (Ono et al., 2013).
Implications and consequences for the study of normal physiology and behavior
In order to maximize survival and reproductive success, animals restrict their behavior to optimal times of the day or night, and the circadian system is crucial for this temporal organization. In the absence of a functional circadian system, survival and reproduction are compromised. For example, chipmunks in the Allegheny Mountains were more vulnerable to predation following SCN lesions, presumably due to inappropriate night-time restlessness revealing their location to predators (DeCoursey et al., 2000). In addition, in most spontaneously ovulating female rodents, the SCN is essential for ovulation and sexual behavior (Kriegsfeld & Silver, 2006; Christian & Moenter, 2010; Tolson & Chappell, 2012). In women, disruptions to circadian timing through shift work or jet lag also have pronounced negative consequences for pregnancy and its maintenance (Mahoney, 2010). In addition to affecting reproduction, the circadian system impacts virtually every behavioral or physiological system studied to date, and several well-studied examples are presented below.
Feeding and circadian rhythms
The circadian system coordinates metabolism and food intake to optimize feeding and with daily changes in digestion and nutrient absorption (Tahara & Shibata, 2013). Mice with a mutation of the Clock gene, for example, have greatly reduced daily rhythms in feeding that lead to hyperphagia and obesity associated with elevated lipids, leptin and glucose, and low insulin levels (Turek et al., 2005). Likewise, high-fat-diet-induced obesity can be abrogated by treatment with a Reverb agonist, reducing body fat and hyperglycemia (Solt et al., 2012). Interestingly, the impact of circadian disruption on obesity occurs at the level of fat cells; site-specific deletion of Bmal1 in mouse adipocytes leads to increased daytime feeding and body mass, reduced locomotor activity and decreased circulating levels of polyunsaturated fatty acids (Paschos et al., 2012). Recent findings in humans indicate that sleep deprivation results in an increased desire for high-caloric foods, and decreased frontal and insular cortex activity and increased amygdala activity, as assessed by functional magnetic resonance imaging (Greer et al., 2013). Thus, the extent to which circadian disruptions lead to obesity through disturbances to sleep represents an important opportunity for further enquiry.
Circadian disruptions can arise from exposure to inappropriate photic conditions. Exposure to dim (5 lux) light at night leads to increased alterations in daily feeding and body mass along with reduced rhythms of hypothalamic and liver clock gene expression in mice (Fonken et al., 2013). The adverse impact of dim light at night on metabolism, such as the dim red or white light used for animal maintenance, can be ameliorated through wheel-running exercise or subsequent exposure to dark at night (Fonken et al., 2014). There has been substantial interest in the effect of light intensity and wavelength on metabolic and other responses. In studies examining light, effects controlling intensity, wavelength, and photoreceptor absorption spectra are taken into account. When wavelength is a question of interest, then irradiance (incident power, in W/m2), rather than illuminance (luminous flux, in lux), is assessed. Measures of lux provide a useful approximate mark that can ground a reader, but it is a measure of perceived intensity by humans, a psychophysical number comprising both the photoreceptor absorption of light and the cognitive processing of that light. Because humans have a red-sensitive cone, red that is perceived to be as bright as a reference blue light (equal lux) would be much dimmer compared with the blue to a mouse’s eye that lacks a red cone. Photosensitivity to different wavelengths is therefore better investigated with light sources providing equal photon fluxes (photosensitivity will still be wavelength dependent, but this will be a measure of photoreceptor function and therefore transmission to the brain); a cell is agnostic to wavelength once the photon has been absorbed. Light-emitting diodes with narrow spectral emissions or notch filters facilitate these investigations. Practicalities may force a reliance on incandescent and fluorescent lights but, because of their complex spectra, comparing light of different colors is more difficult. Illuminance measures suffice when wavelength per se is not a central focus.
The photosensitivity of a physiological or behavioral response to light depends on what is being measured. This is important as the photosensitivity of one response cannot be generalized to other functions. As an example, measurements were made of the thresholds of entrainment of wheel-running rhythms at three wavelengths, and these were compared with the thresholds of two other non-image-forming visual system functions, i.e. masking and the pupillary light reflex. Dim light that entrained mice failed to elicit either masking or pupillary light reflex; in general, circadian entrainment is more sensitive by 1–2 log units than other measures of the non-image-forming visual system. In an artificial photic environment, dim light can entrain circadian rhythms even when it fails to produce more easily measurable acute responses to light such as phase shifting and melatonin suppression (Butler & Silver, 2011).
As mentioned previously, not only does the circadian system influence feeding and metabolism, but food cues can also act to entrain circadian rhythms (Saper, 2006; Patton & Mistlberger, 2013). If food presentation is restricted to a short temporal window (typically a few hours), animals exhibit increased activity in anticipation of feeding [food anticipatory activity (FAA)]. Because this synchronization of behavior with feeding persists in the absence of the SCN, a separate designation of the food entrainable oscillator was coined (Stephan et al., 1979). The identification of the neural locus of the food-entrainable oscillator has been challenging. The dorsomedial nucleus of the hypothalamus (DMH) probably plays a role in food entrainment (Gooley et al., 2006; Fuller et al., 2008), although mice and rats can entrain to food cues in the absence of a DMH (Landry et al., 2006, 2007; Acosta-Galvan et al., 2011). In mice, DMH lesions lead to reduced FAA, whereas lesions of both the SCN and DMH result in enhanced FAA (Acosta-Galvan et al., 2011). These findings suggest that the DMH participates in FAA, but is not the sole neural locus of the food-entrainable oscillator. It is likely that metabolic cues from the periphery, communicated to the central nervous system, participate in food entrainment. For example, ghrelin cells in the stomach that signal hunger express clock genes, ghrelin administration leads to increased activity in animals fed ad libitum, and ghrelin and clock gene rhythms in these stomach cells are synchronized to feeding (LeSauter et al., 2009). Consistent with these findings, FAA is greatly reduced in ghrelin receptor knockout mice (Blum et al., 2009; LeSauter et al., 2009). These findings suggest that restricted feeding leads to entrainment of stomach clocks in ghrelin-expressing cells and food-entrained ghrelin signaling feeds back to the central nervous system to drive changes in FAA. Other neuronal systems including the hypocretin arousal system (Akiyama et al., 2004; Mieda et al., 2004) and orexogenic melanocortin system (Sutton et al., 2008; Patton & Mistlberger, 2013) have been implicated in food entrainment, with disruptions to either system causing pronounced deficits in FAA.
Sleep–wakefulness and circadian rhythms
Most salient in daily life is the relationship between sleep and circadian rhythmicity. Associated with the timing of the rest–activity cycles are rhythms in alertness/drowsiness, mood, and other behaviors. These cycles, and the processes that they impact, are an immense and fundamentally important topic, with much work in basic, clinical and pharmacological aspects (reviewed in Murray & Harvey, 2010; Harvey, 2011; Krystal et al., 2013; Saper & Sehgal, 2013). Although the details of sleep–circadian relationships are beyond the scope of this review, we highlight some major aspects.
The relationship between circadian clocks and sleep involves two interacting processes, and is captured in the classical opponent process model of Borbely (1982). The homeostatic component of sleep involves a process whereby the sleep pressure (termed process S) increases the longer that an individual is awake. The neural locus regulating this homeostatic pressure is not well defined, and involves multiple brain regions and transmitters (see below). In contrast, the circadian system that regulates the timing of wakefulness and sleep has its well-characterized anatomical locus in the SCN. The SCN has monosynaptic efferents to a number of nearby hypo-thalamic regions (reviewed in Morin, 2013), and these in turn relay information to a large number of brain regions, including those involved in regulating awake and sleep states.
Sleep circuits
The neural circuits involved in sleep and arousal include the basal forebrain, brainstem, and hypothalamic components. The SCN has relatively few direct outputs to sleep–wake regulatory systems. Most of its output projects to nearby hypothalamic regions that relay signals to sleep and wake regulatory regions. The sleep circuits are comprised of numerous projections of neurons releasing different types of neurotransmitters and neuropeptides (reviewed in Saper et al., 2005) (Fig. 3). Briefly, arousal pathways include cholinergic neurons of the ascending arousal pathway, located in the pedunculopontine and laterodorsal tegmental nucleus, serotoninergic neurons in the dorsal raphe nucleus, noradrenergic neurons in the locus coeruleus, dopaminergic neurons in the median raphe nucleus, and histaminergic neurons in the tuberomammillary nucleus of the hypothalamus. Activity in these neurons promotes alertness and cortical arousal. This system is inhibited by γ-aminobutyric acid neurons from the ventrolateral preoptic nucleus (VLPO) during sleep. Specifically, the locus coeruleus provides norepinephrine-mediated inhibition of the VLPO during wakefulness. During sleep, activity in the ventral and median preoptic nuclei inhibits the ascending arousal system via the inhibitory neurotransmitters γ-aminobutyric acid and galanin. The sleep-regulatory cells in the VLPO are directly and indirectly regulated by SCN input (Novak & Nunez, 2000; Chou et al., 2002; Kriegsfeld et al., 2004). This circuit enables master clock interactions with homeostatic sleep regulatory systems. Among the most important known homeostatic sleep factors is adenosine (reviewed in Porkka-Heiskanen, 2013). The accumulation of adenosine (a powerful somnogen) during wakefulness is associated with increased VLPO activity upon increased adenosine binding. VLPO activation then inhibits the ascending arousal circuits and promotes non-rapid eye movement sleep.
Fig. 3.
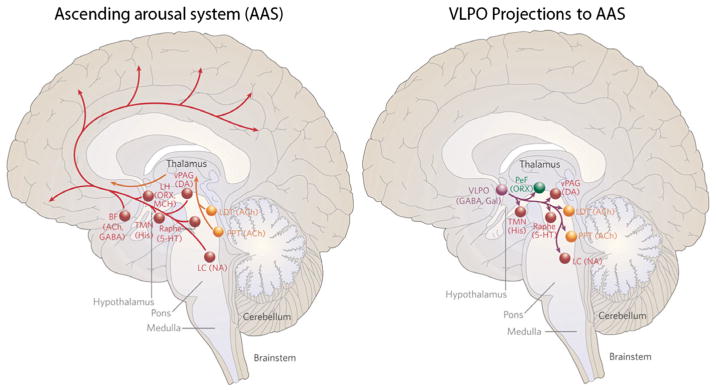
(Left) A schematic drawing showing some key components of the ascending arousal system. A major input to the relay and reticular nuclei of the thalamus (yellow pathway) originates from ACh cell groups in the upper pons, the PPT and LDT. These inputs facilitate thalamocortical transmission. A second pathway (red) activates the cerebral cortex to facilitate the processing of inputs from the thalamus. This arises from neurons in the monoaminergic cell groups, including the tuberomammillary nucleus (TMN) containing histamine (His), the A10 cell group containing DA, the dorsal and median raphe nuclei containing serotonin (5-HT), and the LC containing NA. This pathway also receives contributions from peptidergic neurons in the LH containing ORX or MCH, and from BF neurons that contain γ-aminobutyric acid (GABA) or ACh. Note that all of these ascending pathways traverse the region at the junction of the brainstem and forebrain where von Economo noted that lesions caused profound sleepiness. (Right) A schematic drawing to show the key projections of the VLPO to the main components of the ascending arousal system. It includes the monoaminergic cell groups (red), such as the TMN, the A10 cell group, the raphe cell groups and the LC. It also innervates neurons in the LHA (green), including the PeF ORX neurons, and interneurons in the ACh cell groups (yellow), the PPT and LDT. Note that the VLPO neurons lie within the region outlined by von Economo for the anterior hypothalamic lesion that caused insomnia. ACh, cholinergic; BF, basal forebrain; DA, dopamine; Gal, galanin; LC, locus coeruleus; LDT, laterodorsal tegmental nuclei; LH, lateral hypothalamus; MCH, melanin-concentrating hormone; NA, noradrenaline; ORX, orexin; PAG, periaqueductal gray; PeF, perifornical; PPT, pedunculopontine. Adapted from Saper et al. (2005), with permission (Nature Publishing Group).
Future directions
Sleep disturbances
It is beyond question that the most salient aspect of the circadian system is its role in controlling the timing of sleep. Moreover, environmental disruptions to circadian rhythms, including shift work, travel across time zones, and irregular social schedules, tend to precipitate or exacerbate mood-related episodes. Using shift work or jet lag as a model experimental paradigm, numerous studies, in both humans and animals, have demonstrated the adverse effects of disrupted sleep–wake schedules on health (Wang et al., 2011).
Nearly all people suffering from mood disorders have significant disruptions in circadian rhythms, and altered sleep patterns are one of the major diagnostic criteria for these disorders. Importantly, sleep disorders seem to precede clinical diagnoses of mental illness, and reduction of sleep disturbances improves mental illness (Ritter et al., 2011; Pritchett et al., 2012). It appears that many pathologies are comorbid in both mental illness and neurodegenerative disease; these include poor vigilance and memory, reduced mental and physical reaction times, reduced motivation, depression, insomnia, and obesity, among other states (Wulff et al., 2010). Furthermore, manipulation of the timing of sleep can ameliorate symptoms of major depressive disorder and bipolar disorder (Bunney & Bunney, 2013; Kaplan & Harvey, 2013).
Although chronic sleep deprivation or disruption exacerbates behavioral problems, and may directly affect cognitive function and mood disorders, there is also evidence of mechanistic links between circadian clocks, sleep and mental illness (reviewed in Lamont et al., 2010; Menet & Rosbash, 2011). For example, in humans, casein kinase 1 epsilon and PER2 mutations are associated with familial advanced sleep phase disorder, which causes patients to fall asleep several hours earlier than normal (Toh et al., 2001). In addition, associations for polymorphisms in other circadian genes, including polymorphisms in the Cry2 locus with bipolar disorder (Shi et al., 2008; Sjoholm et al., 2010) and depression (Lavebratt et al., 2010), the Per3, Cry1, and timeless (Tim) genes with schizophrenia and schizoaffective disorder, and Cry1, have been suggested (Mansour et al., 2006; Lamont et al., 2007; Peng et al., 2007; Moons et al., 2011). Bmal1 and Tim are associated with bipolar disorder or schizophrenia (Mansour et al., 2006). Finally and impressively, mistimed sleep in humans disrupts the molecular processes associated with core clock gene expression and disrupts overall temporal organization throughout the body (Archer et al., 2014). In summary, sleep disruption is associated with a wide range of symptoms related to mental health.
Connecting circadian clocks to cellular metabolism
The current view of circadian clocks rests on a model of intracellular interlocked transcriptional and translational feedback loops that generate circadian rhythms, with numerous post-translational and post-transcriptional modifications (Partch et al., 2014). This well-established landscape has started to move in a totally new direction with the discovery of numerous cytosolic circadian loops central to cellular physiology.
Several studies now point to metabolic rhythms that are independent of transcription. These studies led to a search for the ways in which the traditional transcription/translational feedback loops of clock genes and their protein products are integrated with cytosolic and metabolic components of cellular physiology. Over the years, there have been hints of the existence of circadian oscillation in the absence of transcriptional and translational feedback loops. A major breakthrough was the demonstration that circadian oscillation could be reconstituted in a test tube with a purely biochemical oscillator (Nakajima et al., 2005).
A rhythmic, post-translational modification of peroxiredoxin was first reported in mouse liver (Reddy et al., 2006). The dramatic insight came from the discovery of circadian oscillations in human red blood cells, which lack a nucleus and therefore lack the genetic clock mechanism (O’Neill & Reddy, 2011; Edgar et al., 2012). The peroxiredoxin family is part of the cellular defense against reactive oxygen species, specifically H2O2, which are an unavoidable by-product of aerobic metabolism. Red blood cells express peroxiredox-in rhythms that are entrainable by temperature cycles, and are temperature compensated. Circadian rhythms occur in the availability of nicotinamide adenine dinucleotide, a coenzyme for energy conversion in the cell, controlling the timing of oxidative metabolism in mammalian mitochondria (Peek et al., 2013). These data suggest that an underlying rhythmic capacity exists in the cytoplasm, not directly reliant on nascent gene expression. The implication is that, in nucleated cells, at a post-translational level, metabolic rhythms interact reciprocally with transcriptional and translational feedback loop elements known to regulate circadian timekeeping (Rey & Reddy, 2013) (Fig. 4). Circadian cross-talk between metabolic and transcriptional clock components probably co-ordinates genome-wide temporal, cell type-specific programs of gene expression. In this way, circadian clocks exert regulatory control over almost every aspect of physiology, with disruptions leading to disease states, and their understanding lending opportunities for the analysis of novel mechanisms of diagnosis and treatment.
Fig. 4.
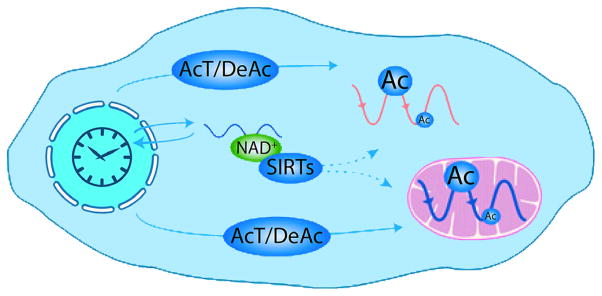
Circadian acetylation in the cell. Scheme representing plausible mechanisms that the circadian clock might use to generate cytosolic and mitochondrial acetylation rhythms. The activity of AcT, including CLOCK, together with DeAc, might be controlled through transcriptional and post-transcriptional mechanisms. In addition, the circadian clock may also use the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases SIRTs to modulate protein Ac, because NAD+ levels are rhythmic in the cytosol. Reproduced from Rey & Reddy (2013). © National Academy of Sciences of the United States of America. Ac, acetylation; AcT, acetyltransferases; DeAc, deacetylases; SIRTs, sirtuins.
Sex differences in circadian rhythms
An aspect of the circadian regulation of genetic, metabolic and cytosolic clocks that has received relatively little attention is how these processes might be affected by sex differences. Also, sex differences may confound the results of studies in which the sex of the animal or cell is not taken into account. Within the brain, widespread sex differences in gene expression and splicing have been detected in all major brain regions and involve 2.5% of all expressed genes (Trabzuni et al., 2013). Furthermore, a diffusion tensor imaging study indicates widespread sex differences in regional and global network characteristics of the brains of youths (Ingalhalikar et al., 2014). The sparsity of circadian studies may be attributed in part to the expense and work load associated with undertaking studies of both males and females, especially when ovulatory cycle-associated changes must also be taken into account (Morin et al., 1977). That said, there are tremendous sex differences in the circadian timing system (reviewed in Bailey & Silver, 2013). A salient example is seen in sleep regulation. Women go to sleep later and later until the age of around 19.5 years, whereas men continue to delay their sleep until around the age of 21 years (Roenneberg et al., 2007). Furthermore, throughout adulthood, men tend to go to sleep later than women. This sex difference disappears at around the age of 50, at around the time of menopause.
A key symptom of major depressive disorder is the disruption of circadian patterns. In a study applying time-of-death analysis to gene expression data from postmortem brains, cyclic patterns of gene expression were much weaker in the brains of patients with major depressive disorders due to shifted peak timing and potentially disrupted phase relationships between individual circadian genes (Li et al., 2013). As noted above, sleep disturbance is associated with major depressive disorders. Turning to the question of sex differences, Plante et al. (2012) found that women, but not men, with major depressive disorders demonstrate significant increases in slow wave activity in multiple cortical areas relative to control subjects. In conclusion, sex differences become important when they can provide clues to the mechanisms conferring protection to one sex or susceptibility to the other, and in those research areas where sex differences are salient, attention to the underlying mechanisms is especially warranted.
Circadian chronotherapeutics
It is now generally accepted that disruptions to the circadian timing system hasten the progression, or contribute to the etiology, of a number of disease states including cancer, cardiovascular disease, neural cell death, peptic ulcers, obesity, rheumatoid arthritis, bipolar disorder, depression and schizophrenia (reviewed in Golombek et al., 2013). In addition to the impact of circadian disturbances on disease, numerous studies in animal models and human clinical trials indicate that there is pronounced impact on the efficacy of a variety of treatments based on the timing of their delivery. Early work in rats and mice, for example, provided evidence that cancer chemotherapy was more efficacious if delivered at times of greatest drug tolerance (Halberg et al., 1980; Levi, 1987; Reinberg et al., 1987). Later, it was recognized that cancer cells exhibit daily rhythms in mitotic activity, and cytotoxic chemotherapeutic agents could be most effectively applied during peak mitotic activity, ideally when cell division is at a nadir in marrow and mucosal cells to avoid damage to healthy tissues (Ortiz-Tudela et al., 2013). Despite repeated clinical trials for a number of cancers revealing enormous increases in response rate and survivorship and decreased negative side-effects, it has been challenging to incorporate a chronotherapeutic strategy into oncological practice. Part of the challenge arises from the fact that sex, lifestyle, and genetic background influence the most appropriate time of delivery across individuals (Ortiz-Tudela et al., 2013). The finding of high-throughput, reliable circadian biomarkers for host and cancerous tissues, along with the implementation of timed drug-delivery systems, is currently being explored to bring chronotherapeutic approaches to the clinic.
More recently, it has become clear that vast improvements in the efficacy of pharmacological, in addition to chemotherapeutic, agents can be gained by considering the timing of delivery. One strategy that has met with success is to administer medication at a time of greatest risk (e.g. myocardial infarction risk is greatest in the morning) or at the daily peak in the manifestation of the ailment (e.g. asthma symptoms exhibit marked daily changes) (Bairy, 2013). A more effective strategy is to consider daily changes in drug pharmacodynamics and to deliver medications at a time when the drug is best tolerated and metabolism and elimination are lowest. For over 300 drugs, prominent daily changes in absorption, distribution, metabolism, and elimination have been noted (reviewed in Levi & Schibler, 2007). By considering these daily changes in pharmacokinetics, striking increases in plasma concentrations of a drug can be achieved simply by altering the timing of administration (e.g. Ollagnier et al., 1987; Smolensky et al., 1987; Bruguerolle, 1998) (Fig. 5). In addition to maximizing the concentration of drugs and minimizing their toxicity, drug targets exhibit daily changes that alter the response, including erythrocyte permeability (Levi et al., 1987; Bruguerolle & Prat, 1989) and receptor numbers/binding affinity (Redfern, 2003). Such strategies have been effectively applied to the treatment of a variety of ailments that show marked daily fluctuations, including hypertension (Nayak et al., 2009; Hermida et al., 2014), asthma (Smolensky et al., 1987; Nainwal, 2012) and rheumatoid arthritis (Cutolo, 2012). Given that differences in the timing of symptoms for many conditions are similar across individuals, implementing chronotherapeutic strategies for the treatment of some diseases is quite feasible and researchers and pharmaceutical companies are developing strategies to effectively deliver medications in a time-dependent fashion thorough time-release oral administration, implants, and pumps (reviewed in Maroni et al., 2010). Given the rapid advances in this emerging knowledge and technology, it will be important to educate the medical community in the magnitude of such effects and practical implementation of chronotherapeutic approaches.
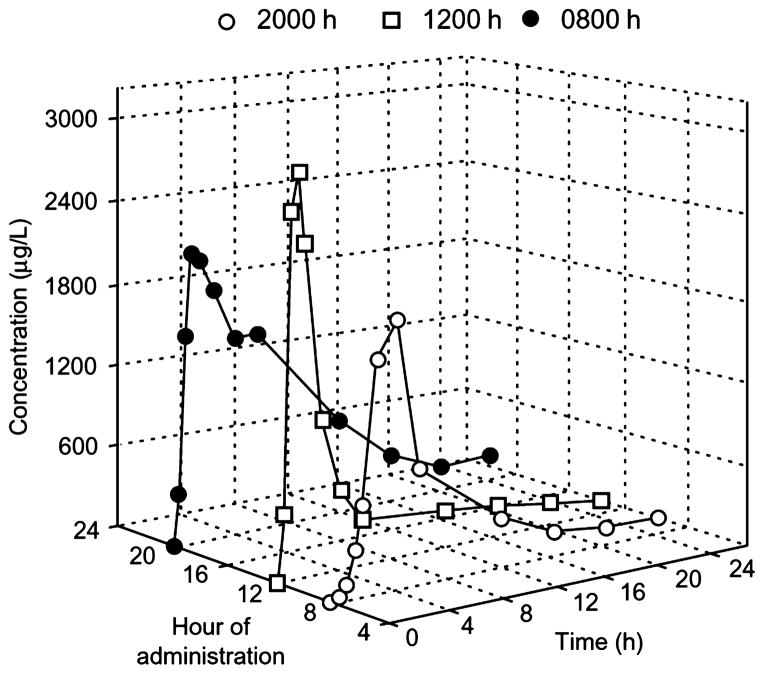
Fig. 5.
Chronopharmacokinetics of indomethacin (75 mg/h) after oral administration of a sustained release preparation; plasma concentrations of indomethacin according to the time of administration in patients. Reproduced from Bruguerolle (1998), with permission (Adis International Limited).
Concluding remarks
The cells of our brains and bodies have evolved in a 24 h solar system in ways that enable optimal coordination of our internal and external circadian cycles. Transcription–translation feedback loops are modified by post-transcriptional regulatory processes, enabling a central master clock to signal peripheral clocks that then exert local control of cellular function specific to each organ and gland. Making optimal use of circadian timing mechanisms within specific brain regions and tissues will enable the understanding of interindividual differences and development of pharmacological modulators of circadian timing identified from high-throughput screens. The hope is that the robustness and resilience of circadian oscillation can be enhanced, dysfunctional clocks can be repaired, and personalized treatment regimens developed for age-related declines and treatment of disease. Further information on mechanisms whereby the SCN signals rhythmic gene expression in the rest of the brain and body requires new genetic, mathematical and statistical tools to understand the spatial and temporal changes in the circadian timing system that underlie its normal and disrupted neural function.
Acknowledgments
We thank Dr Matthew Butler and unidentified reviewers for their comments on earlier drafts of this article. Support during the writing of this review and research from our laboratories reported herein was provided by NSF IOS-1256105 and NIH NS37919 (R.S.), and NIH HD050470 and NSF IOS-1257638 (L.J.K.).
Abbreviations
- Cry
cryptochrome
- DMH
dorsomedial hypothalamus
- FAA
food anticipatory activity
- LD
light dark
- Per
Period
- ROR
retinoid-related orphan receptor
- SCN
suprachiasmatic nucleus
- VLPO
ventrolateral preoptic nucleus
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M, Del Carmen Basualdo M, Escobar C, Buijs RM. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci USA. 2011;108:5813–5818. doi: 10.1073/pnas.1015551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Roblero R, Morin LP, Moore RY. Morphological correlates of circadian rhythm restoration induced by transplantation of the suprachiasmatic nucleus in hamsters. Exp Neurol. 1994;130:250–260. doi: 10.1006/exnr.1994.1203. [DOI] [PubMed] [Google Scholar]
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, Smith CP, Dijk DJ. From the Cover: mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci USA. 2014;111:E682–E691. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2013;35:111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairy LK. Chronotherapeutics: a hype or future of chronopharmacology? Indian J Pharmacol. 2013;45:545–546. doi: 10.4103/0253-7613.121265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythm. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol. 1993;5:137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Bruguerolle B. Chronopharmacokinetics. Current status. Clin Pharmacokinet. 1998;35:83–94. doi: 10.2165/00003088-199835020-00001. [DOI] [PubMed] [Google Scholar]
- Bruguerolle B, Prat M. Temporal variations in the erythrocyte permeability to bupivacaine, etidocaine and mepivacaine in mice. Life Sci. 1989;45:2587–2590. doi: 10.1016/0024-3205(89)90243-9. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Hermes MH, Kalsbeek A. The suprachiasmatic nucleus-paraventricular nucleus interactions: a bridge to the neuroendocrine and autonomic nervous system. Prog Brain Res. 1998;119:365–382. doi: 10.1016/s0079-6123(08)61581-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiat. 2013;73:1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Butler MP, Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. P Roy Soc B-Biol Sci. 2011;278:745–750. doi: 10.1098/rspb.2010.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Rainbow MN, Rodriguez E, Lyon SM, Silver R. Twelve-hour days in the brain and behavior of split hamsters. Eur J Neurosci. 2012;36:2556–2566. doi: 10.1111/j.1460-9568.2012.08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M. Chronobiology and the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:312–318. doi: 10.1097/BOR.0b013e3283521c78. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Gene Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy RT, Blask DE, Sauer LA, Brainard GC, Krause JA. Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett. 1999;144:131–136. doi: 10.1016/s0304-3835(99)00207-4. [DOI] [PubMed] [Google Scholar]
- DeCoursey PJ, Walker JK, Smith SA. A circadian pacemaker in free-living chipmunks: essential for survival? J Comp Physiol A. 2000;186:169–180. doi: 10.1007/s003590050017. [DOI] [PubMed] [Google Scholar]
- Demas GE, Bartness TJ. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol-Reg I. 2001;281:R1499–R1505. doi: 10.1152/ajpregu.2001.281.5.R1499. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Yoshikawa T, Hillson H, Menaker M. Retinal pathways influence temporal niche. Proc Natl Acad Sci USA. 2008;105:13133–13138. doi: 10.1073/pnas.0801728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Melendez-Fernandez OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythm. 2013;28:262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Melendez-Fernandez OH, Weil ZM, Nelson RJ. Exercise attenuates the metabolic effects of dim light at night. Physiol Behav. 2014;124:33–36. doi: 10.1016/j.physbeh.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Foster RG, Argamaso S, Coleman S, Colwell CS, Lederman A, Provencio I. Photoreceptors regulating circadian behavior: a mouse model. J Biol Rhythm. 1993;8(Suppl):S17–S23. [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Letters. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendai I, Halasz B. Central nervous system structures connected with the endocrine glands. findings obtained with the viral trans-neuronal tracing technique. Exp Clin Endocr Diab. 2000;108:389–395. doi: 10.1055/s-2000-8134. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B. Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracing technique. Neuroendocrinology. 1998;68:244–256. doi: 10.1159/000054372. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B. CNS structures presumably involved in vagal control of ovarian function. J Autonom Nerv Syst. 2000;80:40–45. doi: 10.1016/s0165-1838(00)00071-0. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Prosser RA. Circadian rhythm of the rat suprachiasmatic brain slice is rapidly reset by daytime application of cAMP analogs. Brain Res. 1988;474:348–352. doi: 10.1016/0006-8993(88)90449-0. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Casiraghi LP, Agostino PV, Paladino N, Duhart JM, Plano SA, Chiesa JJ. The times they’re a-changing: effects of circadian desynchronization on physiology and disease. J Physiology-Paris. 2013;107:310–322. doi: 10.1016/j.jphysparis.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci. 2006;26:12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Nelson W, Levi F, Culley D, Bogden A, Taylor DJ. Chronotherapy of mammary cancer in rats. Int J Chronobiol. 1980;7:85–99. [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Melanopsin: a novel photopigment involved in the photoentrainment of the brain’s biological clock? Ann Med. 2002;34:401–407. doi: 10.1080/078538902320772151. [DOI] [PubMed] [Google Scholar]
- Harbour VL, Weigl Y, Robinson B, Amir S. Comprehensive mapping of regional expression of the clock protein PERIOD2 in rat fore-brain across the 24-h day. PLoS One. 2013;8:e76391. doi: 10.1371/journal.pone.0076391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Bi. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psycho. 2011;7:297–319. doi: 10.1146/annurev-clinpsy-032210-104550. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Brancaccio M, Maywood ES. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J Neuroendocrinol. 2014;26:2–10. doi: 10.1111/jne.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Smolensky MH, Fernandez JR, Mojon A, Crespo JJ, Rios MT, Moya A, Portaluppi F. Chronotherapeutics of conventional blood pressure-lowering medications: simple, low-cost means of improving management and treatment outcomes of hypertensive-related disorders. Curr Hypertens Rep. 2014;16:412. doi: 10.1007/s11906-013-0412-x. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur J Neurosci. 2009;30:1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- Horvath TL. Suprachiasmatic efferents avoid phenestrated capillaries but innervate neuroendocrine cells, including those producing dopamine. Endocrinology. 1997;138:1312–1320. doi: 10.1210/endo.138.3.4976. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Cela V, van der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res. 1998;795:277–281. doi: 10.1016/s0006-8993(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Huang N, Chelliah Y, Shan Y, Taylor CA, Yoo SH, Partch C, Green CB, Zhang H, Takahashi JS. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science. 2012;337:189–194. doi: 10.1126/science.1222804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. In search of a temporal niche: environmental factors. Prog Brain Res. 2012;199:281–304. doi: 10.1016/B978-0-444-59427-3.00017-4. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci. 2003;23:7412–7414. doi: 10.1523/JNEUROSCI.23-19-07412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111:823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Teclemariam-Mesbah R, Pevet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) J Comp Neurol. 1993;332:293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Yi CX, Cailotto C, la Fleur SE, Fliers E, Buijs RM. Mammalian clock output mechanisms. Essays Biochem. 2011;49:137–151. doi: 10.1042/bse0490137. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem Bioph Res Co. 2005;330:18–26. doi: 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- Kaplan KA, Harvey AG. Behavioral treatment of insomnia in bipolar disorder. Am J Psychiat. 2013;170:716–720. doi: 10.1176/appi.ajp.2013.12050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness as Alternating Phases in the Cycle of Existence. University of Chicago Press; Chicago, USA: 1939. [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat Neurosci. 2006;9:212–219. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468:361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Benca RM, Kilduff TS. Understanding the sleep-wake cycle: sleep, insomnia, and the orexin system. J Clin Psychiat. 2013;74 (Suppl 1):3–20. doi: 10.4088/JCP.13011su1c. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9:333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatr Rel. 2010;47:27–35. [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol-Reg I. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythm. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Sjoholm LK, Soronen P, Paunio T, Vawter MP, Bunney WE, Adolfsson R, Forsell Y, Wu JC, Kelsoe JR, Partonen T, Schalling M. CRY2 is associated with depression. PLoS One. 2010;5:e9407. doi: 10.1371/journal.pone.0009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, LeSauter J, Kim C, Berriman SJ, Tresco PA, Silver R. How do fetal grafts of the suprachiasmatic nucleus communicate with the host brain? Cell Transplant. 1995;4:75–81. doi: 10.1177/096368979500400111. [DOI] [PubMed] [Google Scholar]
- Leise TL, Wang CW, Gitis PJ, Welsh DK. Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of PER2:LUC bioluminescence. PLoS One. 2012;7:e33334. doi: 10.1371/journal.pone.0033334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Suprachiasmatic nucleus lesions abolish and fetal grafts restore circadian gnawing rhythms in hamsters. Restor Neurol Neuros. 1994;6:135–143. doi: 10.3233/RNN-1994-6207. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F. Chronobiology and cancer. Pathol Biol. 1987;35:960–968. [PubMed] [Google Scholar]
- Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- Levi F, Reinberg A, le Fur G, Gueremy C, Uzan A, Benavides J, Quarteronet D, Canton T, Touitou Y, Auzeby A, Sulon J. Circadian rhythm in the membrane of circulating human blood cells: microviscosity and number of benzodiazepine binding sites. A search for regulation by plasma ions, nucleosides, proteins or hormones. Chronobiol Int. 1987;4:235–243. doi: 10.3109/07420528709078530. [DOI] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Hu WP, Zhou QY. The circadian output signals from the suprachiasmatic nuclei. Prog Brain Res. 2012;199:119–127. doi: 10.1016/B978-0-444-59427-3.00028-9. [DOI] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ, Jr, Akil H, Bunney WE. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci USA. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinski M, Scheer FA, Shea SA. Influence of the circadian system on disease severity. Sleep Med Clin. 2009;4:143–163. doi: 10.1016/j.jsmc.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:813764. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, Monk TH, Devlin B, Nimgaonkar VL. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- Maroni A, Zema L, Del Curto MD, Loreti G, Gazzaniga A. Oral pulsatile delivery: rationale and chronopharmaceutical formulations. Int J Pharm. 2010;398:1–8. doi: 10.1016/j.ijpharm.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Entrainment of peripheral clock genes by cortisol. Physiol Genomics. 2012;44:607–621. doi: 10.1152/physiolgenomics.00001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet JS, Rosbash M. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Curr Opin Neurobiol. 2011;21:849–857. doi: 10.1016/j.conb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman KP, Lynch H, Wurtman RJ. Relationship between environmental light intensity and retina-mediated suppression of rat pineal serotonin-N-acetyltransferase. Life Sci. 1974;15:1791–1796. doi: 10.1016/0024-3205(74)90180-5. [DOI] [PubMed] [Google Scholar]
- Moons T, Claes S, Martens GJ, Peuskens J, Van Loo KM, Van Schijndel JE, De Hert M, van Winkel R. Clock genes and body composition in patients with schizophrenia under treatment with antipsychotic drugs. Schizophr Res. 2011;125:187–193. doi: 10.1016/j.schres.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61:391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Diurnal mice (Mus musculus) and other examples of temporal niche switching. J Comp Physiol A. 2005;191:1011–1024. doi: 10.1007/s00359-005-0017-1. [DOI] [PubMed] [Google Scholar]
- Murray G, Harvey A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 2010;12:459–472. doi: 10.1111/j.1399-5618.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Nainwal N. Chronotherapeutics - a chronopharmaceutical approach to drug delivery in the treatment of asthma. J Control Release. 2012;163:353–360. doi: 10.1016/j.jconrel.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- Nayak UY, Shavi GV, Nayak Y, Averinen RK, Mutalik S, Reddy SM, Gupta PD, Udupa N. Chronotherapeutic drug delivery for early morning surge in blood pressure: a programmable delivery system. J Control Release. 2009;136:125–131. doi: 10.1016/j.jconrel.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Novak CM, Nunez AA. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. Neuro Report. 2000;11:93–96. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol. 1977;20:224–234. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Ollagnier M, Decousus H, Cherrah Y, Levi F, Mechkouri M, Queneau P, Reinberg A. Circadian changes in the pharmacokinetics of oral ketoprofen. Clin Pharmacokinet. 1987;12:367–378. doi: 10.2165/00003088-198712050-00003. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono D, Honma S, Honma K. Cryptochromes are critical for the development of coherent circadian rhythms in the mouse suprachiasmatic nucleus. Nat Commun. 2013;4:1666. doi: 10.1038/ncomms2670. [DOI] [PubMed] [Google Scholar]
- Ortiz-Tudela E, Mteyrek A, Ballesta A, Innominato PF, Levi F. Cancer chronotherapeutics: experimental, theoretical, and clinical aspects. Handb Exp Pharmacol. 2013;217:261–288. doi: 10.1007/978-3-642-25950-0_11. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DF, Mistlberger RE. Circadian adaptations to meal timing: neuroendocrine mechanisms. Front Neurosci. 2013;7:185. doi: 10.3389/fnins.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZW, Chen XG, Wei Z. Cryptochrome1 may be a candidate gene of schizophrenia. Med Hypotheses. 2007;69:849–851. doi: 10.1016/j.mehy.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Caldarola PC. General homeostasis of the frequency of circadian oscillations. Proc Natl Acad Sci USA. 1973;70:2697–2701. doi: 10.1073/pnas.70.9.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, Goldstein MR, Riedner BA, Wanger T, Guokas JJ, Tononi G, Benca RM. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density EEG investigation. BMC Psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T. Sleep homeostasis. Curr Opin Neurobiol. 2013;23:799–805. doi: 10.1016/j.conb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Pritchett D, Wulff K, Oliver PL, Bannerman DM, Davies KE, Harrison PJ, Peirson SN, Foster RG. Evaluating the links between schizophrenia and sleep and circadian rhythm disruption. J Neural Transm. 2012;119:1061–1075. doi: 10.1007/s00702-012-0817-8. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Redfern P. Chronotherapeutics. Pharmaceutical Press; London, UK: 2003. [Google Scholar]
- Refinetti R. Entrainment of circadian rhythm by ambient temperature cycles in mice. J Biol Rhythm. 2010;25:247–256. doi: 10.1177/0748730410372074. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Hallek M, Levi F, Touitou Y, Smolensky M. Aspects of chronopharmacology and chronotherapy in pediatrics. Prog Clin Biol Res. 1987;227B:249–258. [PubMed] [Google Scholar]
- Rey G, Reddy AB. Protein acetylation links the circadian clock to mitochondrial function. Proc Natl Acad Sci USA. 2013;110:3210–3211. doi: 10.1073/pnas.1300419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter PS, Marx C, Bauer M, Leopold K, Pfennig A. The role of disturbed sleep in the early recognition of bipolar disorder: a systematic review. Bipolar Disord. 2011;13:227–237. doi: 10.1111/j.1399-5618.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–252. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sehgal A. New perspectives on circadian rhythms and sleep. Curr Opin Neurobiol. 2013;23:721–723. doi: 10.1016/j.conb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanware NP, Hutchinson JA, Kim SH, Zhan L, Bowler MJ, Tibbetts RS. Casein kinase 1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. J Biol Chem. 2011;286:12766–12774. doi: 10.1074/jbc.M111.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, Willour VL, McMahon FJ, Gershon ES, Liu C. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet B. 2008;147B:1047–1055. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Lesauter J. Circadian and homeostatic factors in arousal. Ann NY Acad Sci. 2008;1129:263–274. doi: 10.1196/annals.1417.032. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Sjoholm LK, Backlund L, Cheteh EH, Ek IR, Frisen L, Schalling M, Osby U, Lavebratt C, Nikamo P. CRY2 is associated with rapid cycling in bipolar disorder patients. PLoS One. 2010;5:e12632. doi: 10.1371/journal.pone.0012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek M, Sumova A. Entrainment of spontaneously hypertensive rat fibroblasts by temperature cycles. PLoS One. 2013;8:e77010. doi: 10.1371/journal.pone.0077010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythm. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Jennings JJ, Driscoll JR, Kriegsfeld LJ. A time to remember: the role of circadian clocks in learning and memory. Behav Neurosci. 2014 doi: 10.1037/a0035963. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Scott PH, Harrist RB, Hiatt PH, Wong TK, Baenziger JC, Klank BJ, Marbella A, Meltzer A. Administration-time-dependency of the pharmacokinetic behavior and therapeutic effect of a once-a-day theophylline in asthmatic children. Chronobiol Int. 1987;4:435–447. doi: 10.3109/07420528709083532. [DOI] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE. The physiology and molecular bases of the plant circadian clock. Plant Physiol. 1999;121:9–20. doi: 10.1104/pp.121.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6:2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, Butler AA. The melanocor-tin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Shibata S. Chronobiology and nutrition. Neuroscience. 2013;253:78–88. doi: 10.1016/j.neuroscience.2013.08.049. [DOI] [PubMed] [Google Scholar]
- Takeda N, Maemura K, Horie S, Oishi K, Imai Y, Harada T, Saito T, Shiga T, Amiya E, Manabe I, Ishida N, Nagai R. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem. 2007;282:32561–32567. doi: 10.1074/jbc.M705692200. [DOI] [PubMed] [Google Scholar]
- Tavakoli-Nezhad M, Schwartz WJ. c-Fos expression in the brains of behaviorally “split” hamsters in constant light: calling attention to a dorsolateral region of the suprachiasmatic nucleus and the medial division of the lateral habenula. J Biol Rhythm. 2005;20:419–429. doi: 10.1177/0748730405278443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- Tolson KP, Chappell PE. The changes they are a-timed: metabolism, endogenous clocks, and the timing of puberty. Front Endocrinol (Lausanne) 2012;3:45. doi: 10.3389/fendo.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabzuni D, Ramasamy A, Imran S, Walker R, Smith C, Weale ME, Hardy J, Ryten M North American Brain Expression Consortium. Widespread sex differences in gene expression and splicing in the adult human brain. Nat Commun. 2013;4:2771. doi: 10.1038/ncomms3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Mikkelsen JD. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995;684:61–69. doi: 10.1016/0006-8993(95)00425-p. [DOI] [PubMed] [Google Scholar]
- Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34:452–463. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, Abe M, Block G, Spitznagel E, Menaker M, Takahashi JS. Photic and circadian expression of luciferase in mPeriod1luc transgenic mice invivo. Proc Natl Acad Sci USA. 2002;99:489–494. doi: 10.1073/pnas.012248599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin J, Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990;63:1257–1266. doi: 10.1016/0092-8674(90)90421-a. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci. 2005;25:9017–9026. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Bio. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]