Abstract
In recent years, the emergence of fungal resistance has become frequent, partly due to the widespread clinical use of fluconazole, which is minimally toxic and effective in the prevention and treatment of Candida albicans infections. The limited selection of antifungal drugs for clinical fungal infection therapy has prompted us to search for new antifungal drug targets. Calcium, which acts as the second messenger in both mammals and fungi, plays a direct role in controlling the expression patterns of its signaling systems and has important roles in cell survival. In addition, calcium and some of the components, mainly calcineurin, in the fungal calcium signaling pathway mediate fungal resistance to antifungal drugs. Therefore, an overview of the components of the fungal calcium-calcineurin signaling network and their potential roles as antifungal targets is urgently needed. The calcium-calcineurin signaling pathway consists of various channels, transporters, pumps, and other proteins or enzymes. Many transcriptional profiles have indicated that mutant strains that lack some of these components are sensitized to fluconazole or other antifungal drugs. In addition, many researchers have identified efficient compounds that exhibit antifungal activity by themselves or in combination with antifungal drugs by targeting some of the components in the fungal calcium-calcineurin signaling pathway. This targeting disrupts Ca2+ homeostasis, which suggests that this pathway contains potential targets for the development of new antifungal drugs.
INTRODUCTION
Invasive fungal infections have become frequent in severely immunocompromised individuals, such as transplant, cancer chemotherapy, and HIV-infected patients (1, 2). Candida spp., Aspergillus spp., and Cryptococcus spp. are the most pervasive fungal pathogens isolated in invasive fungal infections (3–8). In contrast with bacterial infections, many of which can be treated with multiple classes of antibiotics, the therapeutic options for fungal infections are exceedingly insufficient due to the limited number of antifungal drugs available and their potential toxicity (9, 10). Azoles, especially fluconazole, have frequently been used in clinical practice due to their great efficacy in the prevention and treatment of Candida albicans infections and their reduced toxicity, but their use results in the emergence of drug resistance. Moreover, innately resistant species, such as non-albicans Candida species, are increasingly being isolated, which is a serious problem in the fight against fungal infections (11–13). Therefore, new antifungal drugs or new approaches for coping with invasive fungal infections are urgently needed (14). However, the development of brand-new antifungal drugs is time consuming and costly. Moreover, fungal cells are eukaryotic, and they share the conserved biochemical and molecular biological networks of all eukaryotes, which complicates the identification of fungal-specific targets that are essential for fungal cell growth (9). Thus, antifungal agents with novel modes of action, such as targeting the virulence, filamentation, and biofilm formation of pathogenic fungi, are urgently needed (10).
In recent years, calcium signal transduction in fungi has been the focus of extensive study due to its essential role in the survival of fungi (15–17). One of the regulators of calcium homeostasis, calcineurin (CN), has been identified as a virulence factor in filamentous fungi, and some calcium channel proteins have been found to be responsible for the filamentation of these pathogenic fungi (18–21). Moreover, calcium-mediated and calcineurin-mediated azole resistance has frequently been documented (22–24). Many findings indicate that various components of the calcium signaling pathway play important roles in fungal physiological processes, mediate stress responses, and promote virulence (22, 25). There are also many reports documenting that nonantifungal compounds, such as amiodarone, cyclosporine (CsA), tacrolimus (FK506), the estrogen receptor antagonists tamoxifen and toremifene, and some calcium channel blockers, exhibit antifungal activity alone or in combination with antifungal drugs through interference with the functions of these components. Although some of the components in fungal cells are similar to those of mammalian cells, the subtle structural differences have made them a hot area in the development of new antifungal agents or research into new approaches to resisting invasive fungal infections (26). However, the calcium channels, exchangers, pumps, and downstream signaling components involved in this complex system of fungal cells are not fully understood. Therefore, reviewing the calcium signaling pathway and its regulatory mechanisms is important. The budding yeast Saccharomyces cerevisiae is among the simplest eukaryotic organisms that are widely used as valuable tools for the study of basic cellular processes and pathways. Furthermore, this yeast is an excellent organism for the identification of molecular targets and elucidation of the molecular/cellular mechanisms of sensitivity to various drugs because the major signaling pathways and processes involved in the cellular response to cytotoxic agents are conserved between yeasts and mammalian cells (27). Here, we mainly describe the latest findings concerning the genes, proteins, and enzymes involved in the calcium signaling pathway of Saccharomyces cerevisiae, which is the main yeast model (the calcium-calcineurin signaling pathway is depicted in Fig. 1). The relationship between calcium signaling and fungal cell survival is then analyzed, with the findings implicating a close connection between calcium signaling and fungal resistance. Next, we summarize the compounds that exhibit antifungal activity when used alone or in combination with antifungal drugs by interfering with components in the calcium signaling pathway (Fig. 2). Although some compounds with antifungal activity also show defined effects on mammalian cells, e.g., calcium channel blockers, they have safely been used in the clinic, and the study of their antifungal mechanisms could provide new clues for the identification of drugs with greater fungal specificity or new antifungal targets.
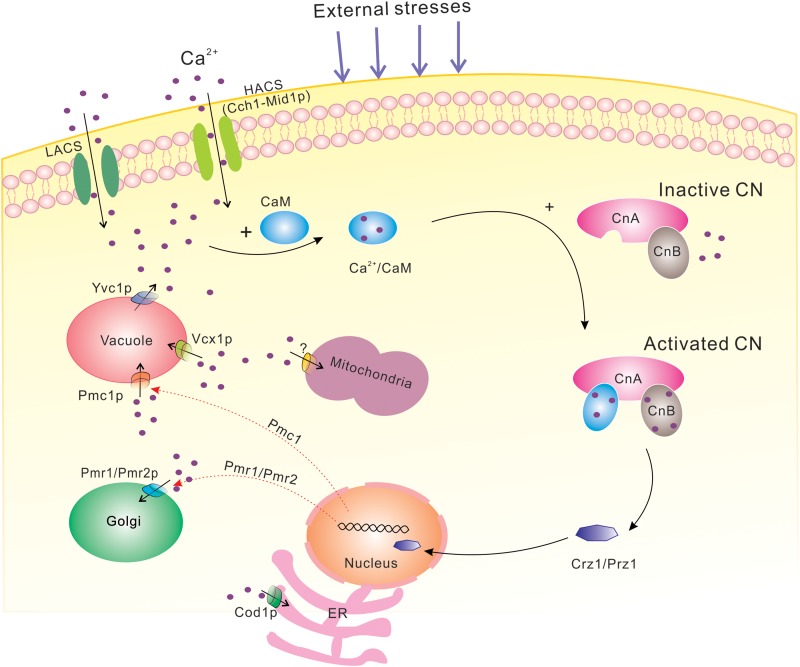
FIG 1.
Description of the calcium-calcineurin signaling pathway in fungal cells. When external stresses are encountered, the plasma membrane Ca2+ influx system (HACS and LACS) is activated, resulting in a rapid influx of Ca2+. Transient increases in intracellular Ca2+ concentrations may also be due to secretion from internal compartments. The increased Ca2+ concentrations are sensed by CaM, and three calcium ions bind to CaM; then, Ca2+-calmodulin specifically binds to subunit A of CN and, simultaneously, Ca2+ binds to the high-affinity Ca2+-binding sites on the B subunit of CN, leading to its activation. Activated CN acts on its downstream targets CRZ1 and PRZ1, inducing their dephosphorylation and translocation from cytoplasm to nucleus. Calcineurin-PRZ1/CRZ1 signaling induces the expression of a set of Ca2+/CN-dependent target genes, including PMC1, PMR1, and PMR2. Subsequently, the intracellular Ca2+ concentration is reduced to basal levels, attributed to the uptake of Ca2+ by organelles. CaM, calmodulin; CN, calcineurin; ER, endoplasmic reticulum; LACS, low-affinity Ca2+ influx system; HACS, high-affinity Ca2+ influx system.
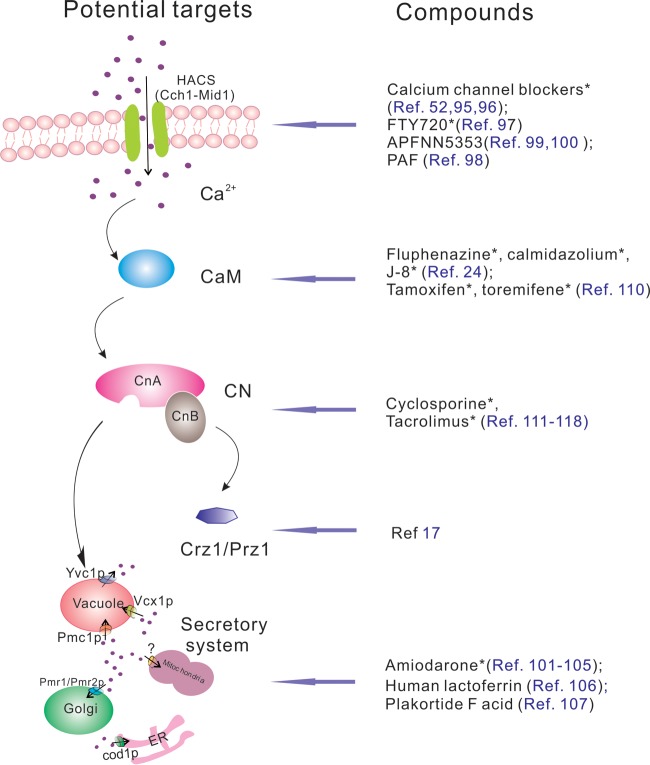
FIG 2.
Potential targets in the calcium-calcineurin signaling pathway and some compounds that exhibit antifungal activity by themselves or in combination with antifungal drugs through interference with these potential targets. CaM, calmodulin; CN, calcineurin; ER, endoplasmic reticulum; *, compound is in clinical therapeutic use.
CALCIUM SIGNALING PATHWAY IN FUNGAL CELLS
Intracellular calcium ions (Ca2+) are important second messengers in all organisms. The concentrations of cytosolic Ca2+ are very low at resting states, ranging from 50 to 200 nM in fungal cells when the environmental Ca2+ concentrations range from <1 μM to >100 mM (26, 28, 29). The calcium homeostasis system, which consists of various calcium channels and pumps, as well as many related proteins and enzymes, plays an important role in maintaining the optimal Ca2+ concentrations in the cytosol and intracellular compartments, such as the vacuole, endoplasmic reticulum (ER), and Golgi apparatus (29–31). In general, the plasma membrane Ca2+ influx system is activated to result in a rapid influx of Ca2+ ions in response to various external stresses, such as store-operated stress, hyperosmotic stress, alkaline stress, cold stress, thermal stress, oxidative stress, and ethanol stress (32–37). The transient increases in the intracellular Ca2+ concentrations may also be elevated by secreting Ca2+ from internal compartments (38). An increased Ca2+ concentration in yeast and filamentous fungal cells affects a wide range of cellular processes, such as cell cycle progression, sporulation, spore germination, oriented hyphal tip growth, hyphal branching, gene expression, and circadian rhythms. This increased concentration also modulates signaling cascades and activates the calcineurin pathway to reduce the Ca2+ concentration to the basal level (36, 39). However, the decrease of the intracellular Ca2+ concentration due to the inhibition of the Ca2+ influx system or efflux of Ca2+ from intracellular compartments to the extracellular space is less well documented. Therefore, we mainly discuss the Ca2+ influx system, the secretory Ca2+ system, and the calcineurin cascades of calcium signaling transduction to characterize the calcium signaling pathway.
CALCIUM INFLUX SYSTEM ON CELL MEMBRANE
Most fungal plasma membranes contain at least two different Ca2+ influx systems, the high-affinity Ca2+ influx system (HACS) and the low-affinity Ca2+ influx system (LACS) (40). The HACS consists of two putative proteins, Cch1p and Mid1p, which are expressed and colocalize to the plasma membrane in a variety of fungi, such as the saprophytes Schizosaccharomyces pombe and Neurospora crassa (41, 42), the animal-pathogenic fungi Candida albicans and Cryptococcus neoformans (43–45), and the plant-pathogenic fungi Gibberella zeae, Claviceps purpurea, and Uromyces appendiculatus (46–49). Notably, Aspergillus species (50, 51) express the putative Ca2+ channel homologs of CCH1 and MID1, namely, cchA and midA, whose topology is similar to the overall topology of Ca2+ voltage-gated channels in higher eukaryotes. These channels play unique and complex roles in low-calcium environments. In Saccharomyces cerevisiae, the sequence and topological structure of Cch1p are similar to those of the pore-forming a1 subunit of mammalian L-type voltage-gated Ca2+ channels (VGCCs) (52), and Mid1p was suggested to be analogous to the a2δ subunit of animal VGCCs because of structural features like N glycosylation, a cysteine-rich domain, and a putative N-terminal signal peptide (53). These two essential subunits form a stable complex that is activated in response to sudden stimulation, allowing the influx of Ca2+ from the extracellular space (20). More importantly, both proteins have been shown to be indispensable for the uptake of extracellular Ca2+ in cells that respond to mating pheromones (54, 55). HACS was found to be regulated by Ecm7p, a member of the PMP-22/EMP/MP20/Claudin superfamily of transmembrane proteins that includes the λ subunits of VGCCs (53). ECM7 is stabilized by MID1, and MID1 is stabilized by CCH1 in nonsignaling conditions, suggesting that all of these proteins interact. Moreover, the ecm7Δ/Δ mutants of Candida albicans were shown to be sensitive to oxidative stress, which resulted in a defect in hyphal development and attenuated the ability of yeast cells to invade and diffuse in mouse kidneys compared with the phenotype of the wild-type strain (20). LACS, whose main important component or regulator is Fig1p (22), displays a 16-fold-lower affinity for Ca2+ than HACS does (40). Many other factors related to polarized morphogenesis and cell fusion, such as Fus1p, Fus2p, Rvs161p, Bni1p, Spa2p, and Pea2p, were also found to be necessary for LACS activity (22). LACS can reportedly produce robust calcium signals in response to pheromones via cch1 and mid1 double mutants that lack HACS. However, LACS and HACS are required for hyphal orientation in response to electric fields and surface topography (21). LACS appears to function only in rich media, such as yeast extract-peptone-dextrose (YPD) or synthetic complete (SC) media, and is insensitive to calcineurin, while HACS is almost undetectable in rich media due to feedback inhibition by calcineurin (56).
Ca2+ SECRETORY SYSTEM ON ENDOMEMBRANE
Like animal cells, fungal cells employ a compartmentalized secretory system that contains numerous Ca2+-dependent proteins and enzymes, such as channels, transporters, or pumps, on the vacuole, Golgi apparatus, mitochondria, and endoplasmic reticulum (ER). The vacuole, an important compartment in fungi, is crucial for differentiation, adaptation to stress, endocytosis, autophagy, and pathogenesis (57). Moreover, this compartment, not the ER, is the major site of intracellular calcium storage in fungal cells (58, 59). Vacuolar calcium channels, which are similar to IP3 or ryanodine receptors in the mammalian ER membrane, are undoubtedly involved in cellular calcium homeostasis and cell response to an environmental stimulus.
Yvc1p, the transient receptor potential (TRP) homolog, has been identified as a vacuole membrane-localized calcium channel protein in some eukaryotic cells (15, 60, 61). Just like the CCH1-MID1 complex, YVC1 also mediates calcium transport and contributes to cytoplasmic calcium fluctuation by releasing calcium from the vacuole into the cytoplasm as a response to an alkaline stimulus (15). However, a sustained increase in the cytosolic Ca2+ concentration is detrimental to fungal cells. Numerous enzymes that catalyze the folding, modification, processing, and trafficking of secretory proteins are activated in response to stress, which results in Ca2+ sequestration in secretory organelles or the initiation of other restorative pathways. Specifically, the Ca2+-ATPase Pmc1p and H+/Ca2+ exchanger Vcx1p, which are located on the vacuole membrane, and the Ca2+ pumps Pmr1p, Cod1p, and Eca1p, which are predominantly located on the Golgi apparatus and ER, are activated to direct the cytosolic Ca2+ to secretory organelles, such as the vacuole, Golgi apparatus, and ER (31, 58, 62–65). However, Vcx1p was identified as the protein complex that is predominantly responsible for restoring cytosolic Ca2+ concentrations after a brief challenge with high extracellular Ca2+ concentrations, while Pmc1p appears to be critical for long-term Ca2+ tolerance (59). Furthermore, most cells express Ca2+ release channels in the endoplasmic reticulum that can be activated by secondary messengers during responses to extracellular stimuli. Rapid Ca2+ release lowers the Ca2+ concentration in the endoplasmic reticulum and elevates the free Ca2+ concentrations in the cytosol, which then can activate various signaling transduction pathways. Because Ca2+ pumps in the plasma membrane compete with secretory organelle pumps for substrates, the intracellular Ca2+ concentration can return to basal levels prior to the refilling of secretory compartments. Thus, in the absence of Ca2+ influx into the cell, the repetitive or continuous activation of Ca2+ release channels will only transiently elevate the intracellular Ca2+ and result in the sustained depletion of the secretory Ca2+ reservoir.
CALCIUM-REGULATING PROTEINS IN THE CYTOPLASM
Calcineurin (CN) is a Ca2+/calmodulin (CaM)-activated protein phosphatase that is highly conserved from fungi to mammals (66) and transmits Ca2+ signals to elicit downstream responses mainly by regulating various transcriptional factors, such as CRZ1 and PRZ1 (19, 67, 68). CN consists of two subunits, a catalytic subunit A (encoded by CNA1 and CNA2) (66, 69) and a regulatory subunit B (encoded by CNB1) (70). The A subunit contains a central calmodulin-binding domain, and the B subunit is identified as a dumbbell-shaped protein with four EF hands, which serve as the high-affinity Ca2+-binding site (71, 72). These two subunits are tightly associated via hydrophobic interactions at a 1:1 ratio in an inactive state (71). However, the Ca2+ sensor protein calmodulin (CaM) detects increases in the intracellular Ca2+ concentrations and binds cytosolic Ca2+ ions at EF-hand motifs of CaM to subsequently activate several Ca2+/CaM-dependent enzymes, such as the phosphatase calcineurin (73). Activated calcineurin acts on its downstream targets CRZ1/TCN1, PRZ1, and other CRZ1 orthologues, which are C2H2-type zinc finger transcription factors, inducing their dephosphorylation and translocation from the cytoplasm to the nucleus (67, 74, 75). Calcineurin-PRZ1/CRZ1 signaling then induces the expression of a set of Ca2+/CN-dependent target genes, including the Ca2+-ATPase genes PMC1, PMR1, and PMR2 (encoding Ca2+-pumping ATPases in the vacuole and Golgi complex) and the glucan synthase gene FKS2, by binding to CN-dependent responsive elements. This signaling also strongly inhibits the function of Vcx1p (74, 76–79). Subsequently, the intracellular Ca2+ level is reduced to the basal level due to the uptake of Ca2+ by organelles and the inhibition of Ca2+ release from the vacuole. While this set of calcineurin targets generally seems to be coordinately regulated, the authors of another report (80) demonstrate that a deletion mutation of any of the components SNF7, SNF8, STP22, VPS20, VPS25, VPS28, and VPS36 of the endosomal sorting complex required for transport (ESCRT) complex activates Ca2+/CN signaling in yeast cells but, surprisingly, reduces the expression of the ER/Golgi calcium pump gene PMR1 by nearly half, independent of calcium stress. Although this finding seems to contradict the well-known fact that Ca2+/CN signaling positively regulates Pmr1, it is consistent with the important role of PMR1, together with PMC1, in preventing the lethal activation of calcineurin under standard (low-Ca2+) conditions (81).
THE ROLES OF CALCIUM SIGNALING IN FUNGAL CELL SURVIVAL
The survival of all organisms depends critically on their interactions with their environment, which are mediated largely by the actions of small molecules, such as reprogramming of gene expression, dephosphorylation of calcineurin, unfolded protein response (UPR), etc. (82, 83). A calcium cell survival (CCS) pathway may be involved in the survival of cells subjected to a variety of cellular stresses, because the activation of a variety of Ca2+ channels, calmodulin, calcineurin, and other factors is necessary for the long-term survival of cells undergoing ER stress, and the genes involved in this pathway are known to be essential in many cell biological processes; these essential components of the whole pathway would likely make good targets for antifungal therapy (19, 56, 84–86).
The major calcium influx system components CCH1 and MID1 have been identified as important factors in the survival of many fungi (45, 48, 50, 87). Previous reports have demonstrated that CCH1 and MID1 are responsible for the resistance of Candida albicans to azoles, as the deletion of the CCH1 and MID1 genes attenuated the strain's resistance to fluconazole and itraconazole, and the cch1Δ/Δ or mid1Δ/Δ mouse models displayed attenuated virulence (85). Moreover, hypha formation and maintenance defects, as well as sensitivity to oxidant agents, were identified in the mutant strains, which demonstrates that CCH1 and MID1 play important roles in morphogenesis, the oxidative stress response, and virulence in Candida albicans (86, 87). CCH1 also plays a role in mediating the virulence of C. neoformans and is required for the growth of C. neoformans at low extracellular Ca2+ concentrations, especially at mammalian body temperatures (45). In aspergilli, the homologs of CCH1 and MID1, cchA and midA, not only have the functional benefits of fast growth but also play important roles in calcium homeostasis and virulence (50, 51).
The components of the Ca2+ secretory system in fungal cells also play critical roles in fungal survival, virulence, and infections. Many reports have demonstrated that VCX1, YVC1, PMC1, and PMR1 are involved in the tolerance and virulence of a variety of fungi, such as Cryptococcus neoformans, Saccharomyces cerevisiae, and Aspergillus fumigatus (51, 88, 89).
Moreover, calcineurin has been demonstrated to be essential for the survival of Candida spp. and required for virulence and stress responses in many other major fungi (18, 84, 90, 91). Zhang et al. found that calcineurin and its downstream target CRZ1 were responsible for Candida lusitaniae's pseudohyphal growth, cell wall integrity, ER stress response, optimal growth in serum, virulence in a murine systemic infection model, and antifungal drug tolerance (19). Another study also demonstrated that the activation of the Ca2+ channel, calmodulin, calcineurin, and other factors was necessary for the long-term survival of cells undergoing ER stress (92). When treated with tunicamycin (TM), an inhibitor of N glycosylation in the ER, yeast strains lacking Cch1p, Mid1p, Ca2+/calmodulin (Cmd1-6 mutants), Ca2+/calmodulin-dependent protein kinases (CMK1 CMK2 double mutants), and calcineurin (CNB1 mutants) died rapidly, whereas mutants lacking the calcineurin-dependent transcription factor Tcn1p behaved similarly to wild-type cells, remaining fully viable for long periods of time in survival assays. Moreover, the calcineurin-binding protein CBP1 and calmodulin direct the morphogenesis and high-temperature growth of Cryptococcus neoformans (36, 93). These findings demonstrated that calcium factors such as Cch1p, Mid1p, calmodulin, and calcineurin promote the long-term survival of cells that suffer ER stress. Furthermore, the authors also demonstrated that the CCS pathway is responsible for the resistance to azole antifungal drugs and operates in pathogenic fungi, such as C. albicans and Candida glabrata.
The CCS pathway is so closely related to the survival of fungal cells that its presence in fungi may provide new opportunities for the treatment of fungal infections.
POTENTIAL ANTIFUNGAL TARGETS IN FUNGAL CALCIUM SIGNALING PATHWAY
Calcium is a highly versatile intracellular signal that can regulate many different cellular functions, such as cell differentiation, division, cell-cell fusion, endocytosis, and mating morphogenesis (94). Therefore, the balance of the flows between the extracellular and intracellular stores that constitute the cytoplasmic concentration of Ca2+ must be maintained at close to 0.1 mM to ensure normal intracellular signaling transduction. Small increases in the intracellular calcium concentration can trigger a variety of cellular responses, such as the activation of pathways that control ion channel activity, secretion, and gene transcription. However, larger, sustained increases can be deleterious to cells and may cause cell death, which highlights the Ca2+-mediated cell death pathway as a promising approach to antifungal drug development. Many of these findings suggest that specific inhibitors of fungal Ca2+ channels, such calmodulin, calcineurin, or other as-yet-unknown components of the CCS pathway, could greatly improve the efficacy of existing antifungal therapies.
TARGETS IN FUNGAL MEMBRANE SYSTEM
The concentration of calcium in fungal cells may increase in response to external or internal stresses, leading to a variety of intracellular responses, such as the opening of calcium channels and exchangers on the plasma membrane or endomembrane system. These calcium channels or exchangers and their genes contribute significantly to the cytosolic calcium concentration fluctuation, and the deletion of some calcium signaling components is detrimental to fungal survival. Thus, interfering with the influx or uptake of calcium through the channels or transporters to disturb the calcium homeostasis may benefit fungicidal activity.
INTERFERENCE WITH CALCIUM INFLUX SYSTEM ON CELL MEMBRANE
Calcium channel blockers, which exert their functions by inhibiting the VGCC on the plasma membrane of mammalian cells, have been the intensive focus of research that examines genes related to the fungal Ca2+ influx system because of the homology of calcium channels in fungi and mammals. For example, the CCH1-MID1 complex is similar to the VGCC of mammals in structure, and reports confirm that it is sensitive to the L-type VGCC blockers nifedipine and verapamil, which decrease the Ca2+ concentration. However, another L-type VGCC blocker, diltiazem, activates Ca2+ entry (52). Fewer reports have documented the antifungal effect of calcium channel blockers used alone, whereas verapamil has been shown to inhibit Candida albicans' hyphal development, adhesion, and gastrointestinal tract colonization, which is related to decreased expression and abnormal transport of the proteins required for morphogenesis (95). Verapamil and fluconazole or tunicamycin have also been observed to exert combined effects, and the results of these previous studies demonstrate synergistic effects on the inhibition of the formation of Candida albicans biofilm. Furthermore, verapamil alone or in combination with fluconazole or tunicamycin significantly decreased the transcriptional level of ALS3, which is essential for biofilm development (96). Because verapamil exerts its inhibitory effects on the plasma membrane calcium channel CCH1 in Saccharomyces cerevisiae (52), calcium channel blockers may be attractive targets for the prevention or eradication of Candida albicans biofilm. Therefore, further studies to observe the effects of other calcium channel blockers used alone or in combination with azole antifungal drugs are needed.
Hagihara et al. (97) provided insight into the molecular mechanisms of fingolimod hydrochloride (FTY720), which is a novel sphingosine 1-phosphate (S1P) receptor modulator that acts on Ca2+ signaling in fission yeast. FTY720 induced a dose-dependent increase in the cytoplasmic Ca2+ levels immediately after the addition of FTY720. This effect was due to an influx of Ca2+ across the Ca2+ machinery on the plasma membrane that is involved in Ca2+ entry, because the addition of EGTA (extracellular Ca2+ chelator) inhibited the peak responses and increased the cytoplasmic Ca2+ levels via FTY720. In addition to the agents discussed above, some secreted antifungal proteins have been reported to disrupt Ca2+ homeostasis by increasing the intracellular Ca2+ concentration. These agents inhibited the growth of a broad range of filamentous fungi (98). PAF is secreted from Penicillium chrysogenum, and it belongs to a family of antifungal peptides. Its elevation of the intracellular Ca2+ resting level within the conidial germ is primarily due to the influx of extracellular Ca2+. Because Ca2+ is a selective chelator, BAPTA [bis-(aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid] ameliorated the PAF toxicity in growth inhibition assays and counteracted the PAF-induced perturbation of Ca2+ homeostasis (98). In contrast, the effects of the L-type Ca2+ channel blocker diltiazem on the response of cells were analyzed, and the results demonstrate that diltiazem and PAF disrupt Ca2+ homeostasis in a similar manner. Moreover, combining diltiazem and PAF had an additive effect on the growth inhibition and change in Ca2+ signatures in response to external stimuli. However, experiments with an aequorin-expressing Δcch1 deletion strain of N. crassa indicated that the L-type Ca2+ channel CCH1 was not responsible for the observed PAF-induced elevation of the intracellular Ca2+ resting level. Thus, the specific mechanism of PAF in Ca2+ homeostasis disruption requires more research. Another antifungal protein, AFPNN5353, which is a defensinlike protein of Aspergillus giganteus, has been examined (99, 100). This protein mediates the germination and growth of filamentous ascomycetes, including important human and plant pathogens, as well as the model organisms Aspergillus nidulans and Aspergillus niger, by inducing the rapid influx of extracellular Ca2+. This influx eventually results in a loss of intracellular Ca2+ homeostasis, because the Ca2+-selective, membrane-impermeable chelator BAPTA did not influence the resting level of intracellular Ca2+ in 12-h-old A. niger cultures, whereas a pretreatment of the samples with 10 mM BAPTA prior to the addition of AFPNN5353 inhibited the protein-specific increase in the intracellular Ca2+ resting level. These findings demonstrate that calcium signaling plays important roles in the mechanistic function of antifungal agents.
INTERFERENCE WITH THE CALCIUM SECRETORY SYSTEM ON THE ENDOMEMBRANE
The calcium secretory system also plays important roles in maintaining normal cytosol Ca2+ concentrations by releasing or sequestrating Ca2+ in a secretory Ca2+ reservoir. Therefore, agents that interfere with the secretory system may impair fungal cells. The antiarrhythmic drug amiodarone (AMD) has been shown to display potent fungicidal activity against not only Saccharomyces but also pathogenic yeasts, such as Candida, Cryptococcus, Fusarium, and Aspergillus species, by interfering with the channel proteins in the calcium secretory system (101–103). Mutants that lack key regulators of calcium homeostasis, including the secretory pathway Ca2+ pump Pmrlp, vacuolar H+-ATPase, and Ca2+/calmodulin-activated protein phosphatase calcineurin, were shown to be hypersensitive to amiodarone, which underlines the important role of Ca2+ in the cellular mechanism of amiodarone toxicity (104, 105). One report (106) indicates that Ca2+ uptake by the mitochondria and Ca2+ release from intracellular stores, such as vacuoles, are crucial in the candidacidal activity of human lactoferrin (hLF), which is an antimicrobial protein. Oxalate, which inhibits Ca2+ release from intracellular stores in various cell types, partially inhibited and a high Ca2+ level completely blocked the hLF-induced killing of Candida albicans. Moreover, ruthenium red interferes with the mitochondrial Ca2+ uniporter to inhibit mitochondrial Ca2+ uptake and block the peptide-induced killing of Candida albicans. However, the specific secretory stores have not been identified. In addition, ruthenium red, oxalate, high extracellular CaCl2, and EGTA completely blocked the hLF-induced change in mitochondrial rhodamine 123 staining, suggesting that mitochondrial Ca2+ uptake and Ca2+ release from intracellular stores are essential for the hLF-induced changes in the mitochondrial membrane potential. Another marine-derived polyketide, endoperoxide plakortide F acid (PFA), was found to elicit a transcriptomic response indicative of a Ca2+ imbalance. This response affected the expression of genes known to be responsive to altered cellular calcium levels and strongly inhibited the opportunistic fungal pathogens Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus (107). The authors showed that calcium transporters, including those with pmr1/pmr1 and pmc1/pmc1 mutations, were sensitive to PFA, and this finding agreed with the transcriptional response to PFA, which appears to be indicative of Ca2+ overload-related stress. In addition, cch1/cch1, mid1/mid1, cna1/cna1, cna2/cna2, cnb1/cnb1, and crz1/crz1 mutants were all hypersensitive to PFA; Ca2+ deprivation in these mutants may result in a compensatory induction of the intracellular Ca2+ levels, and the Ca2+ regulation function deficiency of calcineurin mutants prevented a recovery to normal Ca2+ concentrations, which caused PFA to be more toxic under these conditions.
TARGETS IN FUNGAL CYTOPLASM SYSTEM
The calcium signaling transduction system includes enzymes and proteins, such as calmodulin, calcineurin, and the transcription factors encoded by CRZl/TNC1 and PRZ1, that have been shown to be nonessential for normal growth but critical in mediating cell survival in response to stress (19, 84). Calcineurin-mediated resistance has been considered one of the important factors in the failure of clinical treatment of mycoses (18, 19, 23), and its activation is evoked by the calcium-binding protein calmodulin (73). Therefore, the inhibition of calmodulin and calcineurin activity in order to reverse antifungal resistance and increase the antifungal activity of existing antifungal drugs has been extensively studied.
INTERFERENCE WITH CALMODULIN
Calmodulin is a small calcium-binding protein that participates in the transduction of calcium ions to its effector proteins (108). An increase in the calcium concentration to approximately 10−5 M results in the binding of three calcium ions to fungal calmodulin (109). Ca2+-calmodulin then specifically binds to calcineurin, leading to its activation to regulate the stress response (73). Therefore, preventing calmodulin from exerting its function may perturb Ca2+ homeostasis. Fortunately, Edlind et al. (24) verified this assumption. The authors observed the antifungal effects of three structurally distinct compounds known to be inhibitors of the Ca2+-binding regulatory protein calmodulin: fluphenazine, calmidazolium, and J-8 (W-7 analogue). These three compounds exhibited little or no inhibitory activity of their own, but they all enhanced the activities of azole drugs (miconazole, itraconazole, and terbinafine), and this enhancement varied from 1.6- to >11-fold. To further confirm that these inhibitors are truly specific for calmodulin, strains with calmodulin site-directed mutagenesis were constructed. The results demonstrated that calmodulin mutants showed increased sensitivity to miconazole, terbinafine, or itraconazole compared with the sensitivities of the parent strains. Recently, another report demonstrated that the estrogen receptor antagonists tamoxifen and toremifene exerted their anticryptococcal activity alone or in combination with fluconazole and amphotericin B by directly binding to the essential EF-hand protein calmodulin, which prevented calmodulin from binding to its well-characterized substrate calcineurin and blocked calcineurin activation (110). This finding indicated that calmodulin antagonism contributes to the antifungal activity of this scaffold. More studies that inhibit the function of calmodulin are needed to identify its influence on fungal cell survival in order to discover more efficient antifungal agents.
INTERFERENCE WITH CALCINEURIN
Calcineurin is a major protein phosphatase that is responsible for maintaining calcium homeostasis by activating downstream events, and calcineurin-mediated fungal resistance to fungicides constitutes cause for concern. Therefore, many studies have searched for antifungal agents by inhibiting the activation of calcineurin. An early study demonstrated that the immunosuppressants cyclosporine (CsA) and tacrolimus (FK506) could bind to the receptor cyclopilin (CyP) and FK506 binding protein (FKBP), respectively, in fungal cells and then interact with the regulatory subunit B of calcineurin (111) to exhibit antifungal activity against Cryptococcus neoformans (112, 113), which identified calcineurin as a novel antifungal drug target. Thus, screening for calcineurin inhibitors via different methods may be a new approach for the development of antifungal agents. Uesugi et al. (114) found that inhibiting the calcineurin pathway, such as via the addition of FK506 and CsA to the growth medium or the disruption of the CNB1 and CRZ1 genes in S. cerevisiae, confers tolerance to high-temperature stress on cells with a ubiquitin deletion mutation. Therefore, the authors screened approximately 800 methanol extracts from natural resources for compounds that could restore the growth inhibition of ubiquitin deletion mutant strains following high-temperature treatment and found that some diterpenoid compounds inhibited calcineurin, while their specific antifungal activities and mechanisms require further research. The calcineurin inhibitors FK506 and CsA not only showed antifungal effects when used alone, their combination with antifungal drugs like fluconazole, posaconazole, and itraconazole has also been proposed to treat calcineurin-mediated azole resistance due to their synergistic antifungal effects (115–118).
This fungicidal synergistic interaction deserves further study, as it may be a useful adjunct therapeutic strategy for mycoses. However, FK506 and cyclosporine are not only active in vitro against fungal cells but are also immunosuppressive in the host, which may limit their clinical therapeutic application. Thus, research on the differences between the calcineurins of fungal and mammalian cells is urgently needed to develop antifungal-specific drugs. Fortunately, Juvvadi et al. (119) have found a novel serine-proline-rich region (SPRR) that is evolutionarily conserved and unique to filamentous fungi but completely absent in human calcineurin. The SPRR appears to be required for the phosphorylation of calcineurin that enables it to be active and function well. This finding provides a clue for the development of innovative drugs to fight invasive fungi by harnessing this unique SPRR. In addition, some reports have demonstrated that RTA2, a potential stress-related gene that likely encodes a phospholipid translocase, is responsible for the emergence of calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans (23, 120). The sensitivity of Candida albicans to fluconazole was significantly reduced because calcium-activated calcineurin blocked the impairment of the plasma membrane by fluconazole via Rta2p. Thus, Rta2p may serve as the direct target of antifungal agents.
DISCUSSION AND CONCLUSION
Signaling molecules commonly play critical roles in mediating the cellular stress responses of fungal pathogens. Adverse stimuli activate cellular signaling that prompts fungal cells to respond and adapt to the environment. Calcium, which acts as a secondary messenger molecule, operates over a wide temperature range to regulate many different cellular processes in both fungi and mammals. Similar to mammalian cells, fungal calcium signaling tool kits consist of various signaling molecules that include sensors, such as calmodulin, effectors, such as calcineurin, and the downstream targets of calcineurin, such as CRZ1 and PRZ1. Reports have demonstrated that calcium and calcineurin can mediate the drug resistance of invasive fungal strains. Thus, harnessing these stress responses via the pharmacological inhibition of signaling pathways may provide the foundation for new therapies that could enhance the efficacy of our limited clinically useful antifungal drugs or impede the evolution of antifungal resistance.
Many reports have documented compounds that exhibit antifungal activity alone or in conjunction with antifungal drugs by interfering with the functions of components in the calcium signaling pathway. Although some of these compounds and some combinations have been demonstrated not to be effective in clinical application, their mechanisms of action may provide clues for the search for fungal-specific targets from the calcium signaling pathway. To date, the regulation of Ca2+ homeostasis has not been well studied in all fungal cells, and further searches for safe fungal-specific calcium channel blockers are warranted. The development of biotechnology has allowed transcriptional profiling experiments coupled with genetic and biochemical analyses to be employed to gain insight into the mechanism of action of various antifungal agents, which will delineate the calcium signaling pathway.
ACKNOWLEDGMENTS
This paper was supported by the Department of Science and Technology of Shandong Province, Shandong Provincial Natural Science Foundation, and Shandong Provincial Administration of Traditional Chinese Medicine, China (grants 2013GSF11848, ZR2011HL049, and 2013-196).
The authors declare no competing interests.
REFERENCES
- 1.Kriengkauykiat J, Ito JI, Dadwal SS. 2011. Epidemiology and treatment approaches in management of invasive fungal infections. Clin Epidemiol 3:175–191. doi: 10.2147/CLEP.S12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang HJ, Liu WL, Lin HL, Lai CC. 2014. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS One 9:e99103. doi: 10.1371/journal.pone.0099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wille MP, Guimaraes T, Furtado GH, Colombo AL. 2013. Historical trends in the epidemiology of candidaemia: analysis of an 11-year period in a tertiary care hospital in Brazil. Mem Inst Oswaldo Cruz 108:288–292. doi: 10.1590/S0074-02762013000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumpitazi BF, Lebeau B, Faure-Cognet O, Hamidfar-Roy R, Timsit JF, Pavese P, Thiebaut-Bertrand A, Quesada JL, Pelloux H, Pinel C. 2014. Characteristic and clinical relevance of Candida mannan test in the diagnosis of probable invasive candidiasis. Med Mycol 52:462–471. doi: 10.1093/mmy/myu018. [DOI] [PubMed] [Google Scholar]
- 5.Kazak E, Akin H, Ener B, Sigirli D, Ozkan O, Gurcuoglu E, Yilmaz E, Celebi S, Akcaglar S, Akalin H. 2014. An investigation of Candida species isolated from blood cultures during 17 years in a university hospital. Mycoses 57:623–629. doi: 10.1111/myc.12209. [DOI] [PubMed] [Google Scholar]
- 6.Luzzati R, Cavinato S, Deiana ML, Rosin C, Maurel C, Borelli M. 13 June 2014. Epidemiology and outcome of nosocomial candidemia in elderly patients admitted prevalently in medical wards. Aging Clin Exp Res doi: 10.1007/s40520-014-0251-x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Cela E, Crespo-Sempere A, Gil-Serna J, Porqueres A, Marin S. 19 September 2014. Fungal diversity, incidence and mycotoxin contamination in grapes from two agro-climatic Spanish regions with emphasis on Aspergillus species. J Sci Food Agric doi: 10.1002/jsfa.6876. [DOI] [PubMed] [Google Scholar]
- 8.Cai JP, Liu LL, To KK, Lau CC, Woo PC, Lau SK, Guo YH, Ngan AH, Che XY, Yuen KY. 2014. Characterization of the antigenicity of Cpl1 protein, a surface protein of Cryptococcus neoformans var. neoformans. Mycologia 107:39–45. doi: 10.3852/14-074. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD. 2011. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce CG, Lopez-Ribot JL. 2013. Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs. Expert Opin Drug Discov 8:1117–1126. doi: 10.1517/17460441.2013.807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulu A, Kassu A, Anagaw B, Moges B, Gelaw A, Alemayehu M, Belyhun Y, Biadglegne F, Hurissa Z, Moges F, Isogai E. 2013. Frequent detection of ‘azole’ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis 13:82. doi: 10.1186/1471-2334-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen TC, Chen YH, Chen YC, Lu PL. 2012. Fluconazole exposure rather than clonal spreading is correlated with the emergence of Candida glabrata with cross-resistance to triazole antifungal agents. Kaohsiung J Med Sci 28:306–315. doi: 10.1016/j.kjms.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggero MA, Topal JE. 2014. Development of echinocandin-resistant Candida albicans candidemia following brief prophylactic exposure to micafungin therapy. Transpl Infect Dis 16:469–472. doi: 10.1111/tid.12230. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Hou Y, Chen X, Gao Y, Li H, Sun S. 2014. Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int J Antimicrob Agents 43:395–402. doi: 10.1016/j.ijantimicag.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q, Wang F, Zhao Q, Chen J, Zhang B, Ding X, Wang H, Yang B, Lu G, Zhang B, Li M. 2014. A novel role of the vacuolar calcium channel Yvc1 in stress response, morphogenesis and pathogenicity of Candida albicans. Int J Med Microbiol 304:339–350. doi: 10.1016/j.ijmm.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Bender KW, Dobney S, Ogunrinde A, Chiasson D, Mullen RT, Teresinski HJ, Singh P, Munro K, Smith SP, Snedden WA. 2014. The calmodulin-like protein CML43 functions as a salicylic-acid-inducible root-specific Ca(2+) sensor in Arabidopsis. Biochem J 457:127–136. doi: 10.1042/BJ20131080. [DOI] [PubMed] [Google Scholar]
- 17.Thewes S. 2014. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell 13:694–705. doi: 10.1128/EC.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, Malbas FF Jr, Nett JE, Andes DR, Solis NV, Filler SG, Averette A, Heitman J. 2011. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot Cell 10:803–819. doi: 10.1128/EC.00310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Silao FG, Bigol UG, Bungay AA, Nicolas MG, Heitman J, Chen YL. 2012. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS One 7:e44192. doi: 10.1371/journal.pone.0044192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, Yu Q, Xu N, Wang Y, Cheng X, Qian K, Zhao Q, Zhang B, Xing L, Li M. 2013. Ecm7, a regulator of HACS, functions in calcium homeostasis maintenance, oxidative stress response and hyphal development in Candida albicans. Fungal Genet Biol 57:23–32. doi: 10.1016/j.fgb.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Brand A, Shanks S, Duncan VM, Yang M, Mackenzie K, Gow NA. 2007. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol 17:347–352. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller EM, Mackin NA, Erdman SE, Cunningham KW. 2003. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J Biol Chem 278:38461–38469. doi: 10.1074/jbc.M304089200. [DOI] [PubMed] [Google Scholar]
- 23.Jia XM, Wang Y, Jia Y, Gao PH, Xu YG, Wang L, Cao YY, Cao YB, Zhang LX, Jiang YY. 2009. RTA2 is involved in calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans. Cell Mol Life Sci 66:122–134. doi: 10.1007/s00018-008-8409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edlind T, Smith L, Henry K, Katiyar S, Nickels J. 2002. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol Microbiol 46:257–268. doi: 10.1046/j.1365-2958.2002.03165.x. [DOI] [PubMed] [Google Scholar]
- 25.Patenaude C, Zhang Y, Cormack B, Kohler J, Rao R. 2013. Essential role for vacuolar acidification in Candida albicans virulence. J Biol Chem 288:26256–26264. doi: 10.1074/jbc.M113.494815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui J, Kaandorp JA, Sloot PM, Lloyd CM, Filatov MV. 2009. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res 9:1137–1147. doi: 10.1111/j.1567-1364.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugiura R, Sio SO, Shuntoh H, Kuno T. 2002. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7:619–627. doi: 10.1046/j.1365-2443.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 28.Cui J, Kaandorp JA, Ositelu OO, Beaudry V, Knight A, Nanfack YF, Cunningham KW. 2009. Simulating calcium influx and free calcium concentrations in yeast. Cell Calcium 45:123–132. doi: 10.1016/j.ceca.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui J, Kaandorp JA. 2006. Mathematical modeling of calcium homeostasis in yeast cells. Cell Calcium 39:337–348. doi: 10.1016/j.ceca.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Bonilla M, Cunningham KW. 2002. Calcium release and influx in yeast: TRPC and VGCC rule another kingdom. Sci STKE 2002(127):pe17. doi: 10.1126/stke.2002.127.pe17. [DOI] [PubMed] [Google Scholar]
- 31.Kmetzsch L, Staats CC, Cupertino JB, Fonseca FL, Rodrigues ML, Schrank A, Vainstein MH. 2013. The calcium transporter Pmc1 provides Ca2+ tolerance and influences the progression of murine cryptococcal infection. FEBS J 280:4853–4864. doi: 10.1111/febs.12458. [DOI] [PubMed] [Google Scholar]
- 32.Teng J, Iida K, Imai A, Nakano M, Tada T, Iida H. 2013. Hyperactive and hypoactive mutations in Cch1, a yeast homologue of the voltage-gated calcium-channel pore-forming subunit. Microbiology 159:970–979. doi: 10.1099/mic.0.064030-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Liang Y, Zhang B, Zheng W, Xing L, Li M. 2011. Alkaline stress triggers an immediate calcium fluctuation in Candida albicans mediated by Rim101p and Crz1p transcription factors. FEMS Yeast Res 11:430–439. doi: 10.1111/j.1567-1364.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- 34.Courchesne WE, Vlasek C, Klukovich R, Coffee S. 2011. Ethanol induces calcium influx via the Cch1-Mid1 transporter in Saccharomyces cerevisiae. Arch Microbiol 193:323–334. doi: 10.1007/s00203-010-0673-6. [DOI] [PubMed] [Google Scholar]
- 35.Popa CV, Dumitru I, Ruta LL, Danet AF, Farcasanu IC. 2010. Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J 277:4027–4038. doi: 10.1111/j.1742-4658.2010.07794.x. [DOI] [PubMed] [Google Scholar]
- 36.Kraus PR, Nichols CB, Heitman J. 2005. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot Cell 4:1079–1087. doi: 10.1128/EC.4.6.1079-1087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozubowski L, Aboobakar EF, Cardenas ME, Heitman J. 2011. Calcineurin colocalizes with P-bodies and stress granules during thermal stress in Cryptococcus neoformans. Eukaryot Cell 10:1396–1402. doi: 10.1128/EC.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. 2000. A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast. Mol Cell Biol 20:6686–6694. doi: 10.1128/MCB.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozubowski L, Lee SC, Heitman J. 2009. Signalling pathways in the pathogenesis of Cryptococcus. Cell Microbiol 11:370–380. doi: 10.1111/j.1462-5822.2008.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harren K, Tudzynski B. 2013. Cch1 and Mid1 are functionally required for vegetative growth under low-calcium conditions in the phytopathogenic ascomycete Botrytis cinerea. Eukaryot Cell 12:712–724. doi: 10.1128/EC.00338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tasaka Y, Nakagawa Y, Sato C, Mino M, Uozumi N, Murata N, Muto S, Iida H. 2000. yam8(+), a Schizosaccharomyces pombe gene, is a potential homologue of the Saccharomyces cerevisiae MID1 gene encoding a stretch-activated Ca(2+)-permeable channel. Biochem Biophys Res Commun 269:265–269. doi: 10.1006/bbrc.2000.2278. [DOI] [PubMed] [Google Scholar]
- 42.Lew RR, Abbas Z, Anderca MI, Free SJ. 2008. Phenotype of a mechanosensitive channel mutant, mid-1, in a filamentous fungus, Neurospora crassa. Eukaryot Cell 7:647–655. doi: 10.1128/EC.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller EM, Locke EG, Cunningham KW. 2001. Differential regulation of two Ca(2+) influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics 159:1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brand A, Lee K, Veses V, Gow NA. 2009. Calcium homeostasis is required for contact-dependent helical and sinusoidal tip growth in Candida albicans hyphae. Mol Microbiol 71:1155–1164. doi: 10.1111/j.1365-2958.2008.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M, Du P, Heinrich G, Cox GM, Gelli A. 2006. Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot Cell 5:1788–1796. doi: 10.1128/EC.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavinder B, Hamam A, Lew RR, Trail F. 2011. Mid1, a mechanosensitive calcium ion channel, affects growth, development, and ascospore discharge in the filamentous fungus Gibberella zeae. Eukaryot Cell 10:832–841. doi: 10.1128/EC.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallen HE, Trail F. 2008. The L-type calcium ion channel cch1 affects ascospore discharge and mycelial growth in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot Cell 7:415–424. doi: 10.1128/EC.00248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bormann J, Tudzynski P. 2009. Deletion of Mid1, a putative stretch-activated calcium channel in Claviceps purpurea, affects vegetative growth, cell wall synthesis and virulence. Microbiology 155:3922–3933. doi: 10.1099/mic.0.030825-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhou XL, Stumpf MA, Hoch HC, Kung C. 1991. A mechanosensitive channel in whole cells and in membrane patches of the fungus Uromyces. Science 253:1415–1417. doi: 10.1126/science.1716786. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Cao J, Liu X, Hu H, Shi J, Zhang S, Keller NP, Lu L. 2012. Putative calcium channels CchA and MidA play the important roles in conidiation, hyphal polarity and cell wall components in Aspergillus nidulans. PLoS One 7:e46564. doi: 10.1371/journal.pone.0046564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Castro PA, Chiaratto J, Winkelstroter LK, Bom VL, Ramalho LN, Goldman MH, Brown NA, Goldman GH. 2014. The involvement of the Mid1/Cch1/Yvc1 calcium channels in Aspergillus fumigatus virulence. PLoS One 9:e103957. doi: 10.1371/journal.pone.0103957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teng J, Goto R, Iida K, Kojima I, Iida H. 2008. Ion-channel blocker sensitivity of voltage-gated calcium-channel homologue Cch1 in Saccharomyces cerevisiae. Microbiology 154:3775–3781. doi: 10.1099/mic.0.2008/021089-0. [DOI] [PubMed] [Google Scholar]
- 53.Martin DC, Kim H, Mackin NA, Maldonado-Baez L, Evangelista CC Jr, Beaudry VG, Dudgeon DD, Naiman DQ, Erdman SE, Cunningham KW. 2011. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J Biol Chem 286:10744–10754. doi: 10.1074/jbc.M110.177451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y. 1994. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol Cell Biol 14:8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iida K, Tada T, Iida H. 2004. Molecular cloning in yeast by in vivo homologous recombination of the yeast putative alpha1 subunit of the voltage-gated calcium channel. FEBS Lett 576:291–296. doi: 10.1016/j.febslet.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 56.Bonilla M, Cunningham KW. 2003. Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell 14:4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen I. 2014. Attenuation of virulence with focus on disruption of its vacuole functions. J Oral Microbiol 6:10.3402/jom.v6.23898. doi: 10.3402/jom.v6.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aiello DP, Fu L, Miseta A, Sipos K, Bedwell DM. 2004. The Ca2+ homeostasis defects in a pgm2Delta strain of Saccharomyces cerevisiae are caused by excessive vacuolar Ca2+ uptake mediated by the Ca2+-ATPase Pmc1p. J Biol Chem 279:38495–38502. doi: 10.1074/jbc.M400833200. [DOI] [PubMed] [Google Scholar]
- 59.Forster C, Kane PM. 2000. Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J Biol Chem 275:38245–38253. doi: 10.1074/jbc.M006650200. [DOI] [PubMed] [Google Scholar]
- 60.Chang Y, Schlenstedt G, Flockerzi V, Beck A. 2010. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett 584:2028–2032. doi: 10.1016/j.febslet.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 61.Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y. 2001. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci U S A 98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorin A, Rosas G, Rao R. 1997. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem 272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 63.Fokina AV, Sokolov SS, Kang HA, Kalebina TS, Ter-Avanesyan MD, Agaphonov MO. 2012. Inactivation of Pmc1 vacuolar Ca(2+) ATPase causes G(2) cell cycle delay in Hansenula polymorpha. Cell Cycle 11:778–784. doi: 10.4161/cc.11.4.19220. [DOI] [PubMed] [Google Scholar]
- 64.Lauer Júnior CM, Bonatto D, Mielniczki-Pereira AA, Schuch AZ, Dias JF, Yoneama ML, Pêgas Henriques JA. 2008. The Pmr1 protein, the major yeast Ca2+-ATPase in the Golgi, regulates intracellular levels of the cadmium ion. FEMS Microbiol Lett 285:79–88. doi: 10.1111/j.1574-6968.2008.01214.x. [DOI] [PubMed] [Google Scholar]
- 65.Cronin SR, Rao R, Hampton RY. 2002. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J Cell Biol 157:1017–1028. doi: 10.1083/jcb.200203052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Aguila EM, Silva JT, Paschoalin VM. 2003. Expression of the yeast calcineurin subunits CNA1 and CNA2 during growth and hyper-osmotic stress. FEMS Microbiol Lett 221:197–202. doi: 10.1016/S0378-1097(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 67.Koike A, Kato T, Sugiura R, Ma Y, Tabata Y, Ohmoto K, Sio SO, Kuno T. 2012. Genetic screening for regulators of Prz1, a transcriptional factor acting downstream of calcineurin in fission yeast. J Biol Chem 287:19294–19303. doi: 10.1074/jbc.M111.310615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li F, Wang ZL, Zhang LB, Ying SH, Feng MG. 2015. The role of three calcineurin subunits and a related transcription factor (Crz1) in conidiation, multistress tolerance and virulence in Beauveria bassiana. Appl Microbiol Biotechnol 99:827–840. doi: 10.1007/s00253-014-6124-6. [DOI] [PubMed] [Google Scholar]
- 69.Cyert MS, Kunisawa R, Kaim D, Thorner J. 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci U S A 88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rusnak F, Mertz P. 2000. Calcineurin: form and function. Physiol Rev 80:1483–1521. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe Y, Perrino BA, Chang BH, Soderling TR. 1995. Identification in the calcineurin A subunit of the domain that binds the regulatory B subunit. J Biol Chem 270:456–460. doi: 10.1074/jbc.270.1.456. [DOI] [PubMed] [Google Scholar]
- 72.Shibasaki F, Hallin U, Uchino H. 2002. Calcineurin as a multifunctional regulator. J Biochem 131:1–15. doi: 10.1093/oxfordjournals.jbchem.a003063. [DOI] [PubMed] [Google Scholar]
- 73.Cyert MS. 2001. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu Rev Genet 35:647–672. doi: 10.1146/annurev.genet.35.102401.091302. [DOI] [PubMed] [Google Scholar]
- 74.Karababa M, Valentino E, Pardini G, Coste AT, Bille J, Sanglard D. 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol Microbiol 59:1429–1451. doi: 10.1111/j.1365-2958.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 75.Bodvard K, Jorhov A, Blomberg A, Molin M, Kall M. 2013. The yeast transcription factor Crz1 is activated by light in a Ca2+/calcineurin-dependent and PKA-independent manner. PLoS One 8:e53404. doi: 10.1371/journal.pone.0053404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirayama S, Sugiura R, Lu Y, Maeda T, Kawagishi K, Yokoyama M, Tohda H, Giga-Hama Y, Shuntoh H, Kuno T. 2003. Zinc finger protein Prz1 regulates Ca2+ but not Cl- homeostasis in fission yeast Identification of distinct branches of calcineurin signaling pathway in fission yeast. J Biol Chem 278:18078–18084. doi: 10.1074/jbc.M212900200. [DOI] [PubMed] [Google Scholar]
- 77.Zhang T, Xu Q, Sun X, Li H. 2013. The calcineurin-responsive transcription factor Crz1 is required for conidation, full virulence and DMI resistance in Penicillium digitatum. Microbiol Res 168:211–222. doi: 10.1016/j.micres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev 11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunningham KW, Fink GR. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16:2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y, Du J, Xiong B, Xu H, Jiang L. 2013. ESCRT components regulate the expression of the ER/Golgi calcium pump gene PMR1 through the Rim101/Nrg1 pathway in budding yeast. J Mol Cell Biol 5:336–344. doi: 10.1093/jmcb/mjt025. [DOI] [PubMed] [Google Scholar]
- 81.Cunningham KW, Fink GR. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol 124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferreira RT, Silva AR, Pimentel C, Batista-Nascimento L, Rodrigues-Pousada C, Menezes RA. 2012. Arsenic stress elicits cytosolic Ca(2+) bursts and Crz1 activation in Saccharomyces cerevisiae. Microbiology 158:2293–2302. doi: 10.1099/mic.0.059170-0. [DOI] [PubMed] [Google Scholar]
- 83.Cronin SR. 2002. I'll simply die without my calcium: Ca2+ signaling and surviving cellular stress. Mol Interv 2:284–285. doi: 10.1124/mi.2.5.284. [DOI] [PubMed] [Google Scholar]
- 84.Blankenship JR, Heitman J. 2005. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect Immun 73:5767–5774. doi: 10.1128/IAI.73.9.5767-5774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H, Lu G, Yang B, Wang F, Yu Q, Xu N, Cheng X, Xing L, Li M. 2012. Effect of CCH1 or MID1 gene disruption on drug tolerance and pathogenesis of Candida albicans. Sheng Wu Gong Cheng Xue Bao 28:726–736. (In Chinese.) [PubMed] [Google Scholar]
- 86.Yu Q, Wang H, Cheng X, Xu N, Ding X, Xing L, Li M. 2012. Roles of Cch1 and Mid1 in morphogenesis, oxidative stress response and virulence in Candida albicans. Mycopathologia 174:359–369. doi: 10.1007/s11046-012-9569-0. [DOI] [PubMed] [Google Scholar]
- 87.Roberts SK, McAinsh M, Widdicks L. 2012. Cch1p mediates Ca2+ influx to protect Saccharomyces cerevisiae against eugenol toxicity. PLoS One 7:e43989. doi: 10.1371/journal.pone.0043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mielniczki-Pereira AA, Hahn AB, Bonatto D, Riger CJ, Eleutherio EC, Henriques JA. 2011. New insights into the Ca2+-ATPases that contribute to cadmium tolerance in yeast. Toxicol Lett 207:104–111. doi: 10.1016/j.toxlet.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 89.Pittman JK, Cheng NH, Shigaki T, Kunta M, Hirschi KD. 2004. Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol Microbiol 54:1104–1116. doi: 10.1111/j.1365-2958.2004.04332.x. [DOI] [PubMed] [Google Scholar]
- 90.Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 91.Blankenship JR, Wormley FL, Boyce MK, Schell WA, Filler SG, Perfect JR, Heitman J. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell 2:422–430. doi: 10.1128/EC.2.3.422-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonilla M, Nastase KK, Cunningham KW. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J 21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fox DS, Heitman J. 2005. Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans. Eukaryot Cell 4:1526–1538. doi: 10.1128/EC.4.9.1526-1538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 95.Yu Q, Ding X, Zhang B, Xu N, Jia C, Mao J, Zhang B, Xing L, Li M. 2014. Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS Yeast Res 14:633–641. doi: 10.1111/1567-1364.12150. [DOI] [PubMed] [Google Scholar]
- 96.Yu Q, Ding X, Xu N, Cheng X, Qian K, Zhang B, Xing L, Li M. 2013. In vitro activity of verapamil alone and in combination with fluconazole or tunicamycin against Candida albicans biofilms. Int J Antimicrob Agents 41:179–182. doi: 10.1016/j.ijantimicag.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Hagihara K, Kita A, Mizukura A, Yao M, Kitai Y, Kunoh T, Masuko T, Matzno S, Chiba K, Sugiura R. 2013. Fingolimod (FTY720) stimulates ca(2+)/calcineurin signaling in fission yeast. PLoS One 8:e81907. doi: 10.1371/journal.pone.0081907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Binder U, Chu M, Read ND, Marx F. 2010. The antifungal activity of the Penicillium chrysogenum protein PAF disrupts calcium homeostasis in Neurospora crassa. Eukaryot Cell 9:1374–1382. doi: 10.1128/EC.00050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Binder U, Bencina M, Eigentler A, Meyer V, Marx F. 2011. The Aspergillus giganteus antifungal protein AFPNN5353 activates the cell wall integrity pathway and perturbs calcium homeostasis. BMC Microbiol 11:209. doi: 10.1186/1471-2180-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ouedraogo JP, Hagen S, Spielvogel A, Engelhardt S, Meyer V. 2011. Survival strategies of yeast and filamentous fungi against the antifungal protein AFP. J Biol Chem 286:13859–13868. doi: 10.1074/jbc.M110.203588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta SS, Ton VK, Beaudry V, Rulli S, Cunningham K, Rao R. 2003. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J Biol Chem 278:28831–28839. doi: 10.1074/jbc.M303300200. [DOI] [PubMed] [Google Scholar]
- 102.Courchesne WE, Ozturk S. 2003. Amiodarone induces a caffeine-inhibited, MID1-depedent rise in free cytoplasmic calcium in Saccharomyces cerevisiae. Mol Microbiol 47:223–234. doi: 10.1046/j.1365-2958.2003.03291.x. [DOI] [PubMed] [Google Scholar]
- 103.Courchesne WE. 2002. Characterization of a novel, broad-based fungicidal activity for the antiarrhythmic drug amiodarone. J Pharmacol Exp Ther 300:195–199. doi: 10.1124/jpet.300.1.195. [DOI] [PubMed] [Google Scholar]
- 104.Muend S, Rao R. 2008. Fungicidal activity of amiodarone is tightly coupled to calcium influx. FEMS Yeast Res 8:425–431. doi: 10.1111/j.1567-1364.2008.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang YQ, Rao R. 2007. Global disruption of cell cycle progression and nutrient response by the antifungal agent amiodarone. J Biol Chem 282:37844–37853. doi: 10.1074/jbc.M707593200. [DOI] [PubMed] [Google Scholar]
- 106.Lupetti A, Brouwer CP, Dogterom-Ballering HE, Senesi S, Campa M, Van Dissel JT, Nibbering PH. 2004. Release of calcium from intracellular stores and subsequent uptake by mitochondria are essential for the candidacidal activity of an N-terminal peptide of human lactoferrin. J Antimicrob Chemother 54:603–608. doi: 10.1093/jac/dkh385. [DOI] [PubMed] [Google Scholar]
- 107.Xu T, Feng Q, Jacob MR, Avula B, Mask MM, Baerson SR, Tripathi SK, Mohammed R, Hamann MT, Khan IA, Walker LA, Clark AM, Agarwal AK. 2011. The marine sponge-derived polyketide endoperoxide plakortide F acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob Agents Chemother 55:1611–1621. doi: 10.1128/AAC.01022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fries T, Frank R, Bailer SM. 2011. The Saccharomyces cerevisiae ubiquitin E3 ligase Asr1p targets calmodulin for ubiquitylation. Biochem Biophys Res Commun 411:197–201. doi: 10.1016/j.bbrc.2011.06.136. [DOI] [PubMed] [Google Scholar]
- 109.Cunningham KW, Fink GR. 1994. Ca2+ transport in Saccharomyces cerevisiae. J Exp Biol 196:157–166. [DOI] [PubMed] [Google Scholar]
- 110.Butts A, Koselny K, Chabrier-Rosello Y, Semighini CP, Brown JC, Wang X, Annadurai S, DiDone L, Tabroff J, Childers WE Jr, Abou-Gharbia M, Wellington M, Cardenas ME, Madhani HD, Heitman J, Krysan DJ. 2014. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 5(1):e00765–13 doi: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li W, Handschumacher RE. 1993. Specific interaction of the cyclophilin-cyclosporin complex with the B subunit of calcineurin. J Biol Chem 268:14040–14044. [PubMed] [Google Scholar]
- 112.Odom A, Del Poeta M, Perfect J, Heitman J. 1997. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother 41:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cruz MC, Del Poeta M, Wang P, Wenger R, Zenke G, Quesniaux VF, Movva NR, Perfect JR, Cardenas ME, Heitman J. 2000. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob Agents Chemother 44:143–149. doi: 10.1128/AAC.44.1.143-149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uesugi S, Watanabe D, Kitajima M, Watanabe R, Kawamura Y, Ohnishi M, Takagi H, Kimura K. 2014. Calcineurin inhibitors suppress the high-temperature stress sensitivity of the yeast ubiquitin ligase Rsp5 mutant: a new method of screening for calcineurin inhibitors. FEMS Yeast Res 14:567–574. doi: 10.1111/1567-1364.12143. [DOI] [PubMed] [Google Scholar]
- 115.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother 44:2373–2381. doi: 10.1128/AAC.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Onyewu C, Afshari NA, Heitman J. 2006. Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob Agents Chemother 50:3963–3965. doi: 10.1128/AAC.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun S, Li Y, Guo Q, Shi C, Yu J, Ma L. 2008. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob Agents Chemother 52:409–417. doi: 10.1128/AAC.01070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shirazi F, Kontoyiannis DP. 2013. The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against Mucorales via apoptosis. Eukaryot Cell 12:1225–1234. doi: 10.1128/EC.00138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Juvvadi PR, Gehrke C, Fortwendel JR, Lamoth F, Soderblom EJ, Cook EC, Hast MA, Asfaw YG, Moseley MA, Creamer TP, Steinbach WJ. 2013. Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog 9:e1003564. doi: 10.1371/journal.ppat.1003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia Y, Tang RJ, Wang L, Zhang X, Wang Y, Jia XM, Jiang YY. 2012. Calcium-activated-calcineurin reduces the in vitro and in vivo sensitivity of fluconazole to Candida albicans via Rta2p. PLoS One 7:e48369. doi: 10.1371/journal.pone.0048369. [DOI] [PMC free article] [PubMed] [Google Scholar]