Abstract
GluN3A or NR3A is a developmentally regulated N-methyl-d-aspartate receptor (NMDAR) subunit, showing a unique inhibitory role that decreases NMDAR current and the receptor-mediated Ca2+ influx. In the neonatal brain, GluN3A is shown to associate with synaptic maturation and spine formation and plays a neuroprotective role. Its functional role in the adult brain, however, is largely unknown. We tested the hypothesis that, disrespecting the relatively lower expression level of GluN3A in the adult brain, this inhibitory NMDAR subunit shows a protective action against ischemia-induced brain injury. In littermate wild-type (WT) and GluN3A knockout (KO) mice, focal cerebral ischemia was induced by permanent occlusion of right distal branches of the middle cerebral artery (MCA) plus 10-min ligation of both common carotid arteries (CCAs). Twenty-four hours after focal cerebral ischemia, the infarction volume assessed using 2,3,5-triphenyltetrazolium chloride (TTC) staining was significantly larger in GluN3A KO mice compared with WT mice. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining demonstrated enhanced cell death in GluN3A KO mice. Moreover, the deletion of GluN3A hindered sensorimotor functional recovery after stroke. It is suggested that, although the expression level is relatively lower in the adult brain, GluN3A is still a noteworthy regulator in ischemia-induced excitotoxicity and brain injury.
Keywords: stroke, NMDA receptors, GluN3A, adult brain, functional recovery
the n-methyl-d-aspartate subtype of glutamate receptor (NMDAR) serves critical roles in physiological and pathological processes in the central nervous system (CNS) (7, 9, 17, 20). The receptor activity regulates fast excitatory neurotransmission, contributes to the regulation of neuronal gene expression, and plays key roles in the formation of neural networks and the formation of long-term potentiation (LTP) and memory (18). NMDAR is composed of proteins derived from the GluR1 (NR1) family and the GluN2 (NR2) family of genes. The GluN1 family has a variety of splice variants, and the GluN2 family is comprised of four subunit classes, GluN2A, GluN2B, GluN2C, and GluN2D. The gene expression of both families shows temporal and spatial distributions in the brain (13, 15, 22). NMDAR may also contain an inhibitory subunit, GluN3A (NR3A, NMDAR-L, or chi-1) and GluN3B (NR3B) (2, 6, 19, 26). Incorporation of GluN3A with GluN1/GluN2 subunits suppresses NMDAR activity. For example, coexpression of GluN3A results in decreased NMDAR single channel conductance, Ca2+ permeability, and the receptor Mg2+ sensitivity, which may limit synaptic strengthening and stabilization (3, 6, 8, 25, 26). On the other hand, genetic knockout (KO) of GluN3A in mice results in enhanced NMDA currents and increased dendritic spines in early postnatal cerebrocortical neurons compared with wild-type (WT) mice (8). In addition, increases of intracellular Ca2+ concentration in the neuronal cells from GluN3A KO mice contribute to cell death (3, 5, 14).
It is well known that GluN3A is a developmentally regulated NMDAR subunit. GluN3A mRNA and protein levels peak at the end of the first postnatal week but decrease to a low level by adulthood (6, 26). Based on these findings, studies on GluN3A have been almost exclusively focused on the developing brain of neonates (26, 30). In a hypoxic-ischemic stroke model of neonatal mice, deletion of GluN3A caused enlarged infarct formation while overexpression of GluN3A decreased the brain damage (24). On the other hand, the significance of endogenous GluN3A in the adult brain has been largely ignored, most likely due to the fact that the GluN3A level is downregulated in the adult brain and due to the assumption that the low level GluN3A has no or little functional role. The pathological role of GluN3A in the adult brain, ironically, has never been directly tested, and no quantified experimental data from adult animals have been reported before.
We recently demonstrated that even though the GluN3A expression level declines to lower levels from the neonatal period to the adulthood, significant protein levels of GluN3A are still detectable in the adult brain (21). An important role of GluN3A in locomotion, pain perception, and cognitive functions was observed in adult animals (21). The results indicate that even if at relatively lower expression level, GluN3A still can act as an imperative player in the adult brain. In the present investigation, we extended the examination into the pathological condition of ischemic stroke and tested the hypothesis that a role of GluN3A in the adult stroke brain might have been overlooked before. We also hypothesized that a neuroprotective role of GluN3A in the adult brain could indicate a receptor subunit target for the development of therapeutic treatment of stroke.
METHODS
Animals.
NMDA receptor GluN3A (NR3A) constitutive KO mice (kindly provided by Dr. Stuart A. Lipton and Dr. Nobuki Nakanishi at Stanford University) and littermate WT mice at different postnatal days were used in the experiments. The animals were maintained at a constant temperature of 21°C with a 12-h light-dark cycle in the Laboratory Animal Center for Research, Medicine School of Emory University. All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee at Emory University (Animal Protocol No. 208-2008). No human tissue or sample was tested in this study.
Genotyping.
The genotyping of GluN3A KO and WT animals was performed as described previously (21). DNA for genotyping was extracted from tail snips (∼2–4 mm). The separately primers were 5′-CCA CGG TGA GCT TGG GGA AG (NN494), 5′-GCC TGA AGA ACG AGA TCA GC (NN506), and 5′-TTG GGG AGC GCC CTG CAT GG (NN507; Sigma-Aldrich, St. Louis, MO). The 251-bp product (NN494 and NN506) confutes GluN3A KO mice and 200-bp product (NN494 and NN507) confutes WT mice. DNA (2 μl) was amplified on a thermocycler (MJ minim; Personal Thermal Cycler, Bio-Rad, CA) for 40 cycles (95°C for 60 s, 58°C for 30 s, and 72°C for 60 s). After additional incubation at 72°C for 10 min and being transferred to 4°C, PCR products were subjected to electrophoresis in 1.5% agarose gel with ethidium bromide. Relative intensity of PCR bands was analyzed using the InGenius3 manual gel documentation system (Syngene, Frederick, MD).
Neuronal cell cultures.
The preparation of nearly pure cortical neurons was previously described (31). Briefly, neurons were harvested from the cortex of gestational 14- to 16-day mouse embryos. The pregnant mouse was killed using overdosed isoflurane, and brain dissection was performed soon after heartbeat stopped and no response to pawn stimuli. The cortex was dissociated in trypsin and plated on poly-d-lysine and laminin-coated tissue culture dishes. Cells were maintained in neurobasal media (Invitrogen, Carlsbad, CA) with B27 supplement and l-glutamine until the time of the experiment. Cytarabine (Ara-C) was added on the third day to prevent the proliferation of glial cells. Mature neuronal cultures [12–14 days in vitro (DIV)] were used for cell death assays.
NMDA toxicity and assessment of neuronal cell death.
Excitotoxicity was induced by a 10-min exposure to NMDA (300 μM) alone or in the presence of z-VAD-fmk (100 μM), carried out at room temperature in a HEPES buffer solution (HBSS) containing the following (in mM): 120 NaCl, 5.4 KCl, 0.8 MgCl2, 1.8 CaCl2, 20 HEPES, 15 glucose, and 0.01 glycine (pH 7.4). The exposure solution was then washed away and replaced by media stock (MS) supplemented with 10 μM glycine before returning cultures to the incubator for 20–24 h.
Neuronal cell death was estimated by examination of the cultures under phase-contrast microscopy and quantitated by measurement of lactate dehydrogenase (LDH) release by damaged cells into the bathing medium 24 h following the experimentation (14). The LDH level corresponding to near-complete neuron death (without glial death) was determined by assaying sister cultures exposed to 300 μM NMDA for 24 h in MS supplemented with glycine. The percent neuronal death was normalized to the mean LDH value released 24 h after a sham wash (defined as 0%) and continuous exposure to 300 μM NMDA (defined as 100%). Background LDH levels were determined in sister cultures subjected to sham washing and subtracted from experimental values to yield the signal specific to experimentally induced injury.
Focal cerebral ischemia in mice.
Focal cerebral ischemia, confined to the sensorimotor cortex in the right middle cerebral artery (MCA) territory of WT and GluN3A KO male mice, was induced by permanent occlusions of distal branches of the right MCA and temporary (10 min) ligations of both common carotid arteries (CCAs) (29). In brief, 8- to 10-wk-old male mice (25–28 g) were anesthetized by 4% chloral hydrate (100 mg/kg ip). Chloral hydrate has been widely used as a sedation agent in human and animal surgeries/procedures for a number of years. It was chosen in this investigation due to its stable and well-controlled anesthetic action and negligible effect on NMDA receptors (4). The right MCA branches were permanently ligated using a 10-0 suture (Surgical Specialties, Reading, PA) accompanied by a bilateral 10-min ligation of CCAs. Body temperature was monitored during surgery and maintained at 37.0 ± 0.3°C using a temperature control unit and heating pads. The mortality rate due to ischemic surgery and/or anesthesia failure was ∼7% in these experiments. Free access to food and water was allowed after recovery from anesthesia. All mice were kept in air-ventilated cages with room temperature maintained at 24 ± 0.5°C. Animals were euthanized and decapitated at different time points after ischemic stroke. Brains were immediately removed, mounted in optimal cutting temperature compound (Sakura Finetek, Torrance, CA), and stored at −80°C for further processing.
2,3,5-Triphenyltetrazolium chloride staining of infarct volume measurement.
Twenty-four hours after onset of middle cerebral artery occlusion, animals in different groups were killed for assessment of brain infarct formation. 2,3,5-Triphenyltetrazolium chloride (TTC; Sigma-Aldrich) staining was used to reveal damaged/dead brain tissue as previously described (29). Brains were removed, placed in a brain matrix, and then sliced into 1-mm coronal sections. Slices were incubated in 2% TTC solution at 37°C for 5 min, then stored in 10% buffered formalin for 24 h. Digital images of the caudal aspect of each slice were obtained by a flatbed scanner. Infarct, ipsilateral hemisphere, and contralateral hemisphere areas were measured using ImageJ software (National Institutes of Health, Bethasda, MD). The indirect method (subtraction of residual right hemisphere cortical volume from cortical volume of the intact left hemisphere) will be used for infarct volumes calculation. Infarct measurements were performed under double-blind conditions.
Western blot analysis.
Western blot analysis was used to detect the expression of NMDAR subunits. Brain cortical tissue was lysed in a lysis buffer containing 0.02 M Na4P2O7, 10 mM Tris·HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton, 1 mM EGTA, 2 mM Na3VO4, and a protease inhibitor cocktail (Sigma-Aldrich). The supernatant was collected after centrifugation at 15,000 g for 10 min at 4°C. Protein concentration was determined with a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). Equivalent amounts of total protein were separated by molecular weight on an SDS-polyacrylamide gradient gel and then transferred to a PVDF membrane. The blot was incubated in 5% bovine serum albumin for at least 1 h and then reacted with primary antibodies at 4°C for overnight. The primary antibodies, and the dilutions for each, were as follows: rabbit anti-GluN2A antibody (Cell Signaling, Danvers, MA) at 1:2,000, rabbit anti-GluN2B antibody (Cell Signaling) at 1:2,000, rabbit anti-GluN3A antibody (Abcam, Cambridge, MA) at 1:1,000, and mouse anti-β-actin (Sigma-Aldrich) at 1:5,000. After being washed with Tris-buffered saline with Tween (TBST), membranes were incubated with alkaline phosphatase-conjugated or horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) for 1–2 h at room temperature. After a final washing with TBST, the signals were detected with bromochloroidolylphosphate/nitroblue tetrazolium (BCIP/NBP) solution (Sigma-Aldrich) or film. Signal intensity was measured by ImageJ and normalized to the actin signal intensity.
Immunohistochemical assessment.
Frozen brain tissues were sliced into 10-μm-thick coronal sections using a cryostat vibratome (Leica CM 1950; Leica Microsystems, Buffalo Grove, IL). Sections were dried on the slide warmer for 30 min, fixed with 10% formalin buffer, washed with −20°C precooled ethanol:acetic acid (2:1) solution for 10 min, and finally permeabilized with 0.2% Triton-X 100 solution for 5 min. All slides were washed three times with PBS (5 min each) after each step. Then, tissue sections were blocked with 1% fish gelatin (Sigma-Aldrich) in PBS for 1 h at room temperature and subsequently incubated with the following primary antibodies overnight at 4°C: mouse anti-GluN1 antibody (1:200; Life Technologies, Grand Island, NY), rabbit anti-GluN2A antibody (1:200; Abcam), rabbit anti-GluN2B (1:200; Abcam), rabbit anti-GluN3A (1:200; Abcam), and mouse anti-NeuN (1:400; Millipore, Billerica, MA). The next day, the slides were washed three times with PBS for 5 min, then reacted with the secondary antibodies Alexa Fluor488 goat anti-mouse or -rabbit (1:300; Life Technologies) and Cy3-conjugated donkey anti-rabbit (1:300; Jackson ImmunoResearch Laboratories, West Grove, PA) or Cy5-conjugated donkey anti-mouse or -rabbit (1:400; Jackson ImmunoResearch Laboratories) for 80 min at room temperature. After three washes with PBS, nuclei were stained with Hoechst 33342 (1:20,000; Life Technologies) for 5 min as a counterstain. Then, the brain sections were mounted, coverslipped, imaged, and photographed under a fluorescent microscope (BX51; Olympus) and laser scanning confocal microscope (Carl Zeiss Microimaging, Thornwood, NY).
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining and positive cell quantification.
A terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit was used to examine cell death by detecting fragmented DNA in 10-μm-thick coronal fresh frozen sections as described previously (n = 8 per group) (16). After fixation [10% buffered formalin for 10 min and then ethanol:acetic acid (2:1) solution for 5 min] and permeabilization (0.2% Triton X-100 solution), brain sections were incubated in the equilibration buffer for 10 min. A recombinant terminal deoxynucleotidyl transferase (rTdT) and nucleotide mixture was then added on the slide at 37°C for 60 min in the dark. Reactions were terminated by 2× SSC solution for 15 min. Nuclei were counterstained with Hoechst 33342 (1:20,000; Molecular Probes) for 5 min.
Cell counting.
Cell counting was performed following the principles of design-based stereology (16). Systematic random sampling was employed to ensure accurate and nonredundant cell count. Every section under analysis was at least 90 μm apart from the next. Three 10-μm thick sections spanning the entire region of interest were selected for cell quantification. Counting was performed on five to eight nonoverlapping randomly selected 20× fields per section in the specified region of the cortex. ImageJ (National Institutes of Health) was used to analyze each picture. All analysis was performed in a double-blinded fashion.
Adhesive removal test.
The adhesive removal test measures sensorimotor function as previously described (1, 10). Briefly, a small adhesive dot was placed on each forepaw, and the amount of time (seconds) needed to contact and remove the tape from each forepaw was recorded. Mice were trained three times for 3 days (1–2 trials per day) before ischemia induction to ensure that they were able to remove the tape within 12 s to get a basal level of performance. Mice that show no response or prolonged time to remove the tape (>20 s) during training period were excluded from future experiments. Animals were tested before ischemia and 3 and 7 days after ischemia by an investigator who was blind to the experimental groups. The test was performed four times per mouse, and the average time was used in the analysis. In this behavioral study, 16 animals were randomly assigned to WT and GluN3A KO groups before ischemia and 3 days after ischemia. At 7 days after stroke, six animals were used in each group. All testing trials were conducted during the day time.
Locomotor activity test.
Behavioral changes of experimental mice were monitored and analyzed using the TopScan System (Clever Sys, Reston, VA). Mice were allowed to freely move in an open field container (50 × 50 × 50 cm). For locomotor activity, travelled distance and velocity were recorded for 1 h before surgery and at 3 and 7 days after focal cerebral ischemia. After the recording, the videos were analyzed by the TopScan Realtime Option Version 3.0 (Clever Sys).
Cylinder test.
A unilateral injury to the motor cortex results in an asymmetry in the forelimb used for support the body during rearing, which can be measured using the cylinder test. The mice were placed in a glass cylinder (9.5-cm diameter and 11-cm height), and the number of times each forelimb or both forelimbs were used to support the body on the wall of the cylinder was counted for 5 min. The animals were evaluated before surgery and at 3 and 7 days after focal cerebral ischemia. Two mirrors were placed behind the cylinder to view all directions. The numbers of impaired and nonimpaired forelimb contacts were calculated as a percentage of total contacts.
Statistical analysis.
All results are expressed as means ± SE. Statistical comparisons, using Graph Pad Prism 6 (Graph Pad Software, San Diego, CA), were analyzed by unpaired Student's t-test and Fisher's test with least significant difference to identify significant differences. P < 0.05 was considered significant for all comparisons.
RESULTS
GluN3A expression patterns and cellular distribution in the mouse brain.
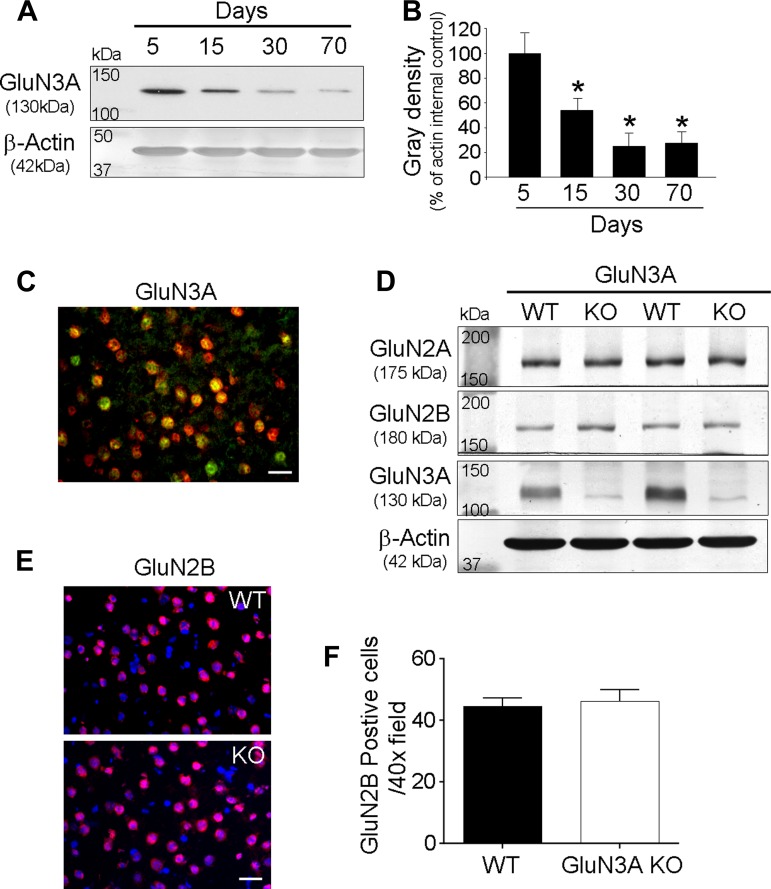
To characterize the expression of GluN3A, we measured the expression level of GluN3A at postnatal day (P)5, 15, 30, and 70 in WT mice using Western blot analysis. As reported before, the highest level of GluN3A was observed in the P5 brain and the GluN3A expression was then declined to lower levels in the following days (Fig. 1, A and B). However, even if the GluN3A level was relatively lower in the adult brain (P30–P70) than that in the neonatal brain (P5–P15), the GluN3A expression was readily detectable. In WT and GluN3A KO brains, Western blotting confirmed that this NMDAR subunit was largely eliminated or undetectable in the KO brain tissues (Fig. 1D). Immunohistochemical analysis revealed that GluN3A was observed in the neuron of cortex including sensorimotor cortex from WT adult brain (Fig. 1C). We and others showed before that deletion of GluN3A does not affect the expression levels of GluN1 and GluN2 (21, 24). Since GluN2 subunits especially GluN2B are specifically linked to excitotoxicity (27), we measured the GluN2A and GluN2B protein levels in the adult WT and GluN3A KO brains and the expression in cortical neurons. There was no change in GluN2A and GluN2B levels in the absence of GluN3A (Fig. 1, D and E). Also, the number of GluN2B-positive cells did not show any difference between WT and GluN3A KO mice (Fig. 1F).
Fig. 1.
Expression patterns of GluN3A in the mouse brain. Expression patterns of GluN3A and its effect on other N-methyl-d-aspartate receptor (NMDAR) subunits were examined using Western blot analysis and immunohistochemical method in the mouse brain. A and B: peak level of GluN3A was detected in the postnatal day (P)5 brain and its expression declines to lower levels in the following days. However, GluN3A expression was still detectable in even P30 and P70 brain. *P < 0.05 vs. P5 brain; n = 4–5 animals in each group. C: GluN3A (green) immunoreactivity, which was colocalized with NeuN (red), was detected in the sensorimotor cortex from wild-type (WT) adult brain. Scale bars = 20 μm. D: Western blotting revealed that the deletion of GluN3A had no effects on the expression levels of other NMDAR subunits such as GluN2A and GluN2B. There was no difference in the expression levels of GluN2A and GluN2B between WT and GluN3A knockout (KO) adult brains. E: it was confirmed using immunohistochemical methods that GluN2B expression (red) was not changed between WT and GluN3A KO mice. F: quantified data from the immunostaining data in E; the number of GluN2B showed no significant difference between WT and GluN3A KO mice.
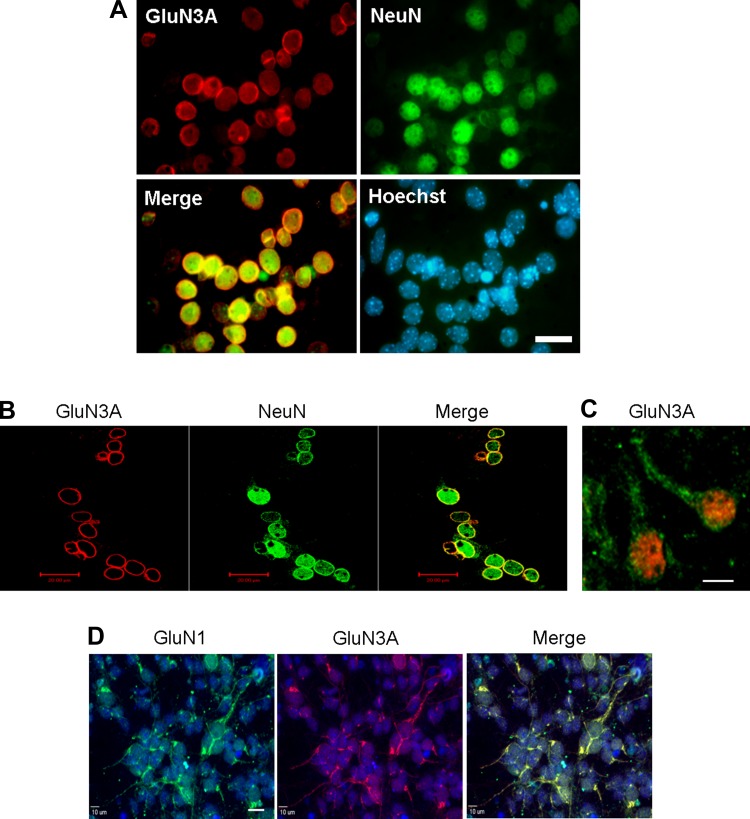
Using immunocytochemical staining, we specifically examined the cellular distribution of GluN3A in cultured cortical neurons. It was clear that GluN3A localized to the cell membrane and dendrites (Fig. 2, A–C). Moreover, they were colocalized with the GluN1 subunit, suggesting formation of functional NMDARs in these locations (Fig. 2D).
Fig. 2.
Cellular distribution of GluN3A in cortical neurons. Immunocytochemistry was used to investigate the distribution of GluN3A expression in primary cortical neurons. A and B: typical results of GluN3A immunoreactivity in primary cortical neurons were shown by the colocalization of the neuronal marker NeuN (red) and GluN3A (green). Total cells were visualized with Hoechst 33342 staining (blue). High levels of GluN3A immunoreactivity were mainly detected in the cell membrane. Scale bars = 20 μm. C: GluN3A (green) immunoreactivity, which was colocalized with NeuN (red), was detected not only in the cell membrane but also in the dendrites. Scale bars = 20 μm. D: immunocytochemistry showed colocalized labeling of GluN1 (green) and GluN3A (red) immunoreactivities in primary cortical neurons. Scale bars = 20 μm.
Neuroprotective effects of GluN3A expression against in vitro ischemic insult in cortical cultures.
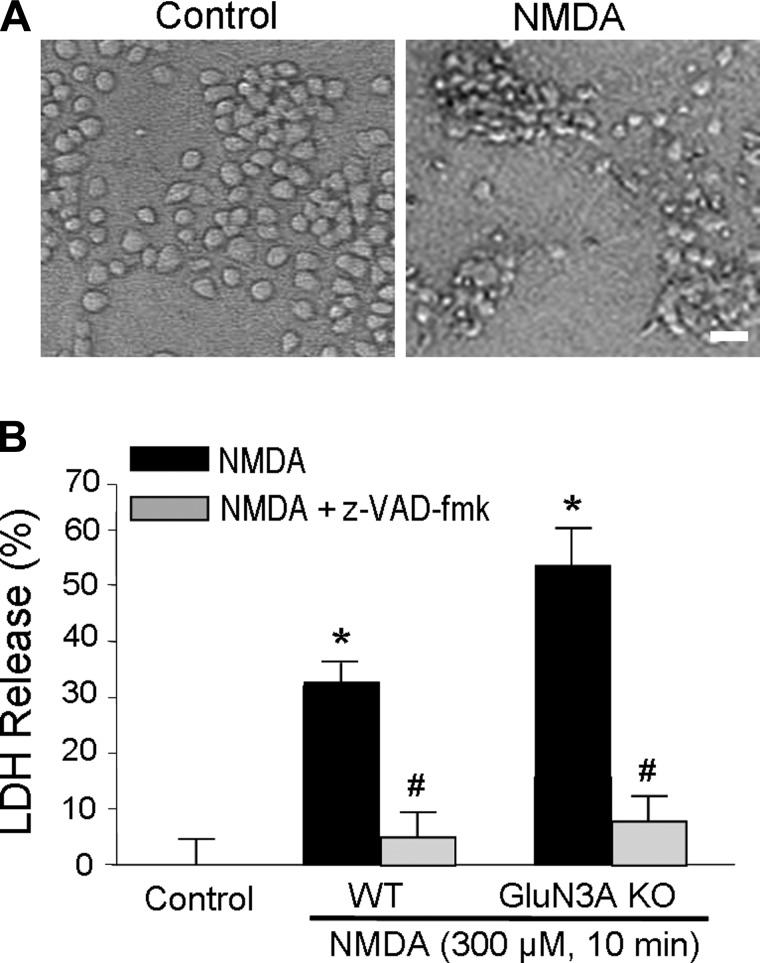
We next examined NMDA-induced neuronal cell death in cortical neuronal cultures of 12–14 DIV. The neuronal culture was exposed to 300 μM NMDA for 10 min and washed out with normal culture media. This short-term NMDA insult caused marked cell death 24 h later. The neuronal cell death showed apoptotic features such as cell body shrinkage and sensitivity to the block by the caspase inhibitor z-VAD-fmk (Fig. 3, A and B). Compared with WT neurons, GluN3A KO neurons showed enhanced vulnerability to the NMDA insult; there was significant more neuronal death in GluN3A KO cultures (Fig. 3B).
Fig. 3.
Neuroprotective effect of GluN3A against excitotoxic insults in cortical neurons. NMDA-induced neuronal death in cortical neurons was examined to determine whether GluN3A expression could protect neurons against excitotoxicity. A: phase contrast photomicrographs of cortical cell cultures 24 h after 10-min exposure to 300 μM NMDA in cortical neurons from WT and GluN3A KO mice. Scale bars = 20 μm. B: cortical cell cultures were exposed to 300 μM NMDA for 10 min, alone or with 100 μM z-VAD-fmk, in cortical neurons from WT and GluN3A KO mice. Neuronal death was analyzed 24 h after by measuring LDH efflux into the bathing medium scaled to the mean LDH value corresponding to complete neuronal death induced by continuous exposure to 300 μM NMDA. *P < 0.05 vs. control; #P < 0.05 vs. NMDA; n = 6–8 culture wells per condition.
Neuroprotective effects of GluN3A expression against ischemic damage in the adult brain.
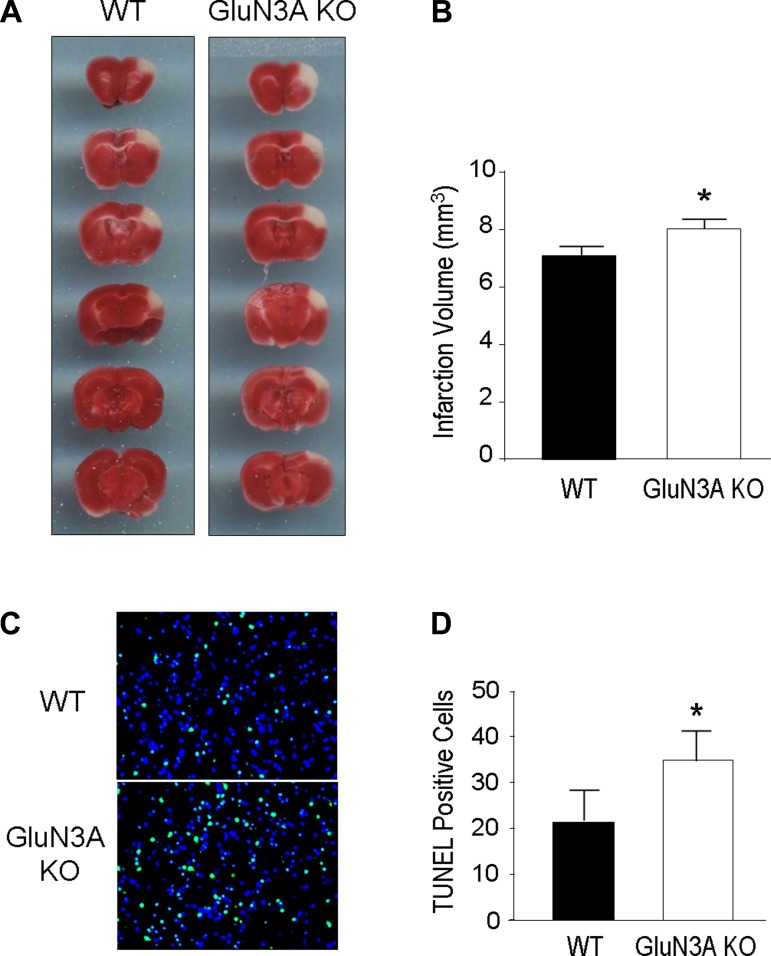
The focal cerebral ischemia in our experiments selectively damaged the somatosensory cortex of adult mice. Twenty four hours after the ischemic insult, brain sections were stained with TTC solution to identify the damaged/died brain tissue (Fig. 4A). The infarction volume of the cerebral cortex in GluN3A KO mice was significantly larger than that in WT mice (Fig. 4B). Using Western blotting, we compared the levels of GluN3A before and after ischemia in WT mice and did not observe significant change (data not shown).
Fig. 4.
Cerebral infarction and cell deaths after focal cerebral ischemia in WT and GluN3A KO mice. Cerebral infarction and cell death were measured 24 h after focal cerebral ischemia in WT and GluN3A KO adult mice. A: 2,3,5-triphenyltetrazolium chloride (TTC)-stained brain sections of WT and GluN3A KO mice 24 h after focal cerebral ischemia. Representative images show brain sections from WT and GluN3A KO mice after focal cerebral ischemia. GluN3A KO mice showed larger infarct volume. B: bar graphs summarize the infarct volume after focal cerebral ischemia in WT and GluN3A KO mice. *P < 0.05 vs. WT mice; n = 13–15 animals in each group. C: terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining revealed DNA damages and cell death 24 h after focal cerebral ischemia in WT and GluN3A KO mice. Total cells were visualized with Hoechst 33342 staining (blue). Massive TUNEL-positive cells (green) were observed in the injured areas from WT and GluN3A KO mice after focal cerebral ischemia. GluN3A KO mice showed more TUNEL-positive cells compared with WT mice. D: TUNEL-positive cells among total Hoechst 33342-positive cells in the penumbra were counted and summarized in the bar graph. There were a significant increased number of TUNEL-positive cells in GluN3A KO mice than that in WT mice 24 h after focal cerebral ischemia. *P < 0.05 vs. WT mice; n = 8 animals in each group. Scale bars = 20 μm.
To confirm the difference in the ischemia-induced injury between the two groups at cellular levels, TUNEL staining was applied to evaluate the cell death in the penumbra region 24 h after focal cerebral ischemia. The number of dead/injured cells measured as TUNEL-positive cells was noticeably higher in GluN3A brain than that in WT stroke mice (Fig. 4, C and D).
Effect of GluN3A on functional recovery after focal cerebral ischemia.
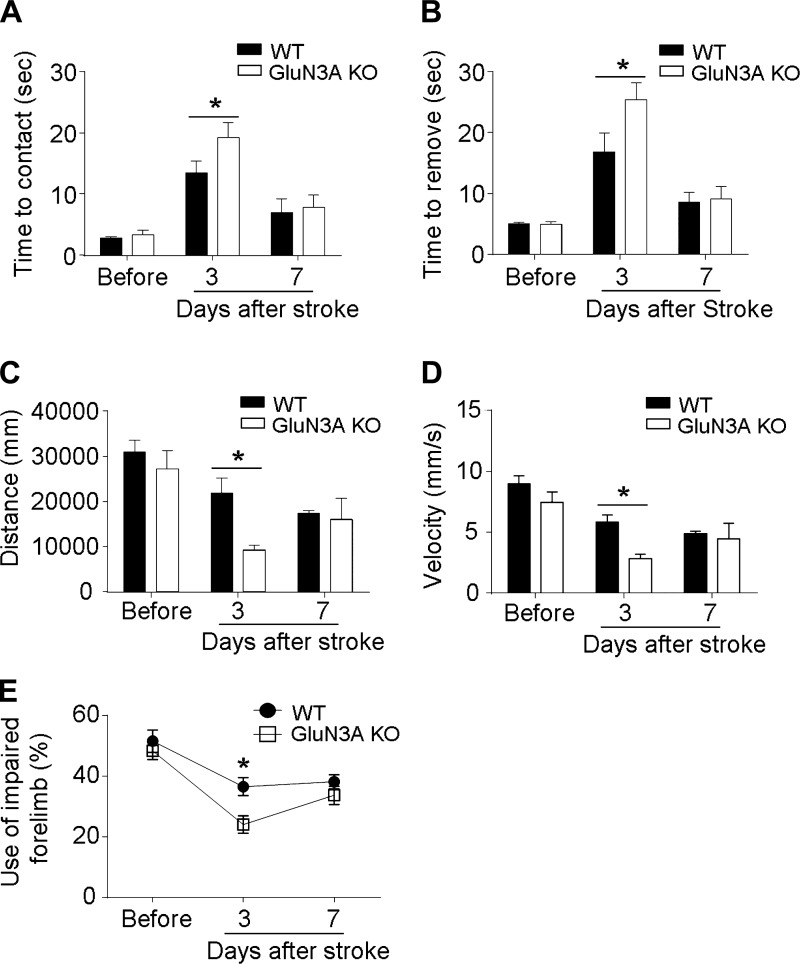
Several behavior tests measured the functional outcomes after focal cerebral ischemia. In the adhesive dot removal test, the latency times to contact and remove the sticky dot from the right and left forelimbs were recorded at 3 and 7 days after focal cerebral ischemia (Fig. 5, A and B). Before ischemic insult, the contact and removal times were similar between WT and GluN3A KO mice. After focal cerebral ischemia, animals showed prolonged times in response to the sticky dot attached to their left paws due to impaired sensorimotor function on the right side of the sensorimotor cortical area. GluN3A KO mice showed significantly hindered performance at 3 days after ischemia on both time to contact and time to remove sticky dot activities (Fig. 5, A and B). TopScan system analysis was used to measure the locomotor activity after focal cerebral ischemia. GluN3A KO mice showed significantly less travelled distance and lower velocity than WT mice at 3 days after focal cerebral ischemia (Fig. 5, C and D). Consistently, GluN3A KO mice showed significant worse performance in cylinder test at 3 days after focal cerebral ischemia (Fig. 5E).
Fig. 5.
GluN3 KO mice hindered functional recovery after focal cerebral ischemia. The adhesive removal test was performed to examine the effect of GluN3A on functional recovery before surgery and at 3 and 7 days after focal cerebral ischemia. A: all animals showed increased time to contact after focal cerebral ischemia, indicating impaired function. GluN3 KO mice exhibited significantly hindered sensorimotor activity at 3 days after focal cerebral ischemia. *P < 0.05 vs. WT mice at each time point; n = 6–16 animals in each group. B: time to remove the sticky dot from left paw was significantly increased in GluN3 KO mice compared with WT time at 3 days after focal cerebral ischemia. *P < 0.05 vs. WT mice at each time point; n = 6–16 animals in each group. C and D: travelled distance and velocity were measured using TopScan System analysis. At 3 days after focal cerebral ischemia, GluN3A KO mice exhibited significant less travelled distance and lower velocity than WT mice, indicating impaired locomotor activity. *P < 0.05 vs. WT mice at each time point; n = 4–5 animals in each group. E: cylinder test showed that forelimb activities of mice with focal cerebral ischemia were impaired during the first few days after focal cerebral ischemia. GluN3 KO mice performed significantly worse than WT mice at 3 days after focal cerebral ischemia. *P < 0.05 vs. WT mice at each time point; n = 4–5 animals in each group.
DISCUSSION
This investigation reveals that, even at a relatively lower level than in the neonatal brain, the NMDAR inhibitory subunit GluN3A in the adult brain exhibits a neuroprotective effect in ischemic stroke. We demonstrate that deletion of this subunit results in enlarged infarct volume and increased cell death in the ischemic cortex of the adult mouse brain. Also, GluN3A KO mice exhibited hindered functional recovery compared with WT mice. These observations, on one hand, are surprising based on the common belief that GluN3A has little, if any, functional role in the adult brain due to its low expression level (26, 30). On the other hand, the result is consistent with our previous investigation showing that GluN3A plays an imperative role in pain perception, locomotion, and cognitive function in adult mice (21). Taking together, it is suggested that although the expression level of GluN3A in the adult brain is downregulated from its peak level at the neonatal stage, this subunit in the adult brain still has a significant impact on multiple brain activities under physiological and pathological conditions such as in ischemic stroke. We noticed that the difference in the infarct volume between WT and GluN3A KO mice is relative moderate, which is consistent with the low level of GluN3A in the adult brain. The present investigation, however, is not an investigation of stroke treatment, which is largely judged by how big the protective effect is. Instead, we aimed to reveal whether GluN3A could play a functional role in cerebral ischemia of the adult brain. The observation provides important information for a previously unidentified function of the GluN3A subunit after ischemic stroke in adults. It is expected that genetic manipulations and other strategies of increasing the GluN3A level in the adult brain can amplify the endogenous defense mechanism against ischemic stroke.
A neuroprotective role of the GluN3A subunit was demonstrated in an earlier investigation in cortical cultures subjected to excitotoxic insults and in a neonatal hypoxia-ischemia model of mice (24). It was mentioned in the abstract of the report that a neuroprotective effect against ischemic stroke was only observed in neonatal but not in adult GluN3A KO mice (24). Unfortunately, no experimental data on the adult stroke experiment were presented in the report (24). On the other hand, the authors did show a neuroprotective effect of the GluN3A subunit in adult animals subjected to NMDA-induced retina injury and in the GluN3A-overexpressing transgenic mice after transient ischemic stroke (24). The protective effect of overexpressing GluN3A was also shown in a recent study on cultured hippocampal neuronal cell death induced by oxygen glucose deprivation (28). We show in adult animals that the basal level of this subunit already shows a moderate neuroprotection, supporting that increasing the GluN3A level can be an effective therapeutic target for the treatment of stroke.
Recent studies raise an intriguing possibility that NMDAR hypofunction in schizophrenia and bipolar disorder may arise from an excess increase or decrease of GluN3A expression. GluN3A mRNA levels are significantly elevated in the dorsolateral prefrontal cortex of schizophrenic patients and decreased in that of bipolar disorder patients (23). Altered GluN3A expression levels regulate the development of spine densities in schizophrenic patients (11, 12). Taken together, these findings suggest that GluN3A expression can be an important regulator on the development of schizophrenia and bipolar disorder and a key target gene for treating the patients with psychotic disorders.
In conclusion, the present study shows a NMDAR subunit-mediated endogenous neuroprotective action after focal cerebral ischemia in the adult brain. Together with the previous reports of the GluN3A regulation on pain sensation, cognitive activities, locomotion, and retina excitotoxicity in adult animals (21, 24), the expression of GluN3A thus shows significant physiological and pathophysiological roles in adults. In this study, we used conventional GluN3A KO mice. It is possible that the loss of GluN3A activity during the development stage may mask and change the role of the gene in the adult stage. Therefore, further studies using conditional KO approach may be needed to provide more inside information on the developmentally regulated neuroprotective mechanism.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-062097, NS-073378, and NS-075338, a Veterans Affairs Merit grant, American Heart Association Grant-in-Aid Grant 12GRNT12060222, and an American Heart Association Established Investigator Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H.L. and S.P.Y. conception and design of research; J.H.L., Z.Z.W., D.C., and X.G. performed experiments; J.H.L., Z.Z.W., D.C., X.G., L.W., and S.P.Y. analyzed data; J.H.L., L.W., and S.P.Y. interpreted results of experiments; J.H.L., Z.Z.W., D.C., X.G., and S.P.Y. prepared figures; J.H.L. and S.P.Y. drafted manuscript; L.W. and S.P.Y. edited and revised manuscript; L.W. and S.P.Y. approved final version of manuscript.
REFERENCES
- 1.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, Freret T. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 4: 1560–1564, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415: 793–798, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Chen ST, Hsu CY, Hogan EL, Juan HY, Banik NL, Balentine JD. Brain calcium content in ischemic infarction. Neurology 37: 1227–1229, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol 21: 259–271, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 13: 171–182, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci 15: 6498–6508, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11: 327–335, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393: 377–381, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999. [PubMed] [Google Scholar]
- 10.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann-Bard P, Boulouard M. Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav Neurosci 123: 224–230, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57: 65–73, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry 58: 203, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Goebel DJ, Poosch MS. NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Brain Res Mol Brain Res 69: 164–170, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Koh JY, Choi DW. Vulnerability of cultured cortical neurons to damage by excitotoxins: differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci 8: 2153–2163, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci 14: 3180–3194, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Wei L, Gu X, Wei Z, Dix TA, Yu SP. Therapeutic effects of pharmacologically induced hypothermia against traumatic brain injury in mice. J Neurotrauma 31: 1417–1430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330: 613–622, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Ma OK, Sucher NJ. Molecular interaction of NMDA receptor subunit NR3A with protein phosphatase 2A. Neuroreport 15: 1447–1450, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda K, Kamiya Y, Matsuda S, Yuzaki M. Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res 100: 43–52, 2002. [DOI] [PubMed] [Google Scholar]
- 20.McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev 74: 723–760, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Mohamad O, Song M, Wei L, Yu SP. Regulatory roles of the NMDA receptor GluN3A subunit in locomotion, pain perception and cognitive functions in adult mice. J Physiol 591: 149–168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res 71: 361–370, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi N, Tu S, Shin Y, Cui J, Kurokawa T, Zhang D, Chen HS, Tong G, Lipton SA. Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci 29: 5260–5265, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AC, Diez-Garcia J, Rodriguiz RM, Lopez IP, Lujan R, Martinez-Turrillas R, Pico E, Henson MA, Bernardo DR, Jarrett TM, Clendeninn DJ, Lopez-Mascaraque L, Feng G, Lo DC, Wesseling JF, Wetsel WC, Philpot BD, Perez-Otano I. Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron 63: 342–356, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci 15: 6509–6520, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology 53: 10–17, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Yan H, Zhang S, Wei X, Zheng J, Li J. The GluN3A subunit exerts a neuroprotective effect in brain ischemia and the hypoxia process. ASN Neuro 5: 231–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LL, Chen D, Lee J, Gu X, Alaaeddine G, Li J, Wei L, Yu SP. Mobilization of endogenous bone marrow derived endothelial progenitor cells and therapeutic potential of parathyroid hormone after ischemic stroke in mice. PLoS One 9: e87284, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otano I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, Lipton SA, Sucher NJ. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol 450: 303–317, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci 22: 1350–1362, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]