Abstract
Either calorie restriction [CR; consuming 60–65% of ad libitum (AL) intake] or acute exercise can independently improve insulin sensitivity in old age, but their combined effects on muscle insulin signaling and glucose uptake have previously been unknown. Accordingly, we assessed the independent and combined effects of CR (beginning at 14 wk old) and acute exercise (3–4 h postexercise) on insulin signaling and glucose uptake in insulin-stimulated epitrochlearis muscles from 30-mo-old rats. Either CR alone or exercise alone vs. AL sedentary controls induced greater insulin-stimulated glucose uptake. Combined CR and exercise vs. either treatment alone caused an additional increase in insulin-stimulated glucose uptake. Either CR or exercise alone vs. AL sedentary controls increased Akt Ser473 and Akt Thr308 phosphorylation. Combined CR and exercise further elevated Akt phosphorylation on both sites. CR alone, but not exercise alone, vs. AL sedentary controls significantly increased Akt substrate of 160 kDa (AS160) Ser588 and Thr642 phosphorylation. Combined CR and exercise did not further enhance AS160 phosphorylation. Exercise alone, but not CR alone, modestly increased GLUT4 abundance. Combined CR and exercise did not further elevate GLUT4 content. These results suggest that CR or acute exercise independently increases insulin-stimulated glucose uptake via overlapping (greater Akt phosphorylation) and distinct (greater AS160 phosphorylation for CR, greater GLUT4 for exercise) mechanisms. Our working hypothesis is that greater insulin-stimulated glucose uptake in the combined CR and exercise group vs. CR or exercise alone relies on greater Akt activation, leading to greater phosphorylation of one or more Akt substrates other than AS160.
Keywords: glucose transport, glucose transporter, insulin signaling, insulin resistance, physical activity, aging
because multiple age-related diseases are linked to the development of whole body insulin resistance (19), identifying and understanding interventions that can elevate insulin-stimulated glucose disposal during old age has important implications for health. Skeletal muscle, which accounts for the largest amount of insulin-stimulated blood glucose clearance (18), is a prime target for interventions to enhance insulin sensitivity. Moderate calorie restriction [CR; chronically consuming ∼20–40% below ad libitum (AL) intake] and exercise can each independently enhance insulin-stimulated glucose uptake by muscle in old rats (9, 11, 17, 40, 49). However, the combined effects of CR and acute exercise on muscle glucose uptake and insulin signaling by skeletal muscle during old age are unknown.
Although the combined effects of CR and acute exercise on glucose uptake are uncertain, earlier research has addressed potential mechanisms for enhanced insulin-mediated glucose uptake caused by either CR or acute exercise alone. The complex insulin-signaling pathway leading to insulin-stimulated glucose transport (12, 46) begins when insulin binds its receptor, inducing site-specific tyrosine phosphorylation of the receptor, which in turn leads to site-specific tyrosine phosphorylation of the insulin receptor substrate-1 (IRS-1). Tyrosine-phosphorylated IRS-1 associates with phosphatidylinositol 3-kinase (PI3K), and increased PI3K activity is required for insulin-stimulated glucose transport. Subsequently, the Ser/Thr protein kinase Akt binds to membranes that are enriched in lipids that were phosphorylated by IRS-1-PI3K, leading to greater Akt phosphorylation on Thr308 and Ser473. Akt catalyzes the phosphorylation of a Rab GTPase-activating protein known as Akt substrate of 160 kDa (AS160; also called TBC1D4) on several sites, including Thr642 and Ser588, that are important for insulin-stimulated glucose transport.
Current knowledge about the independent effects of CR or acute exercise on the role of insulin signaling in the regulation of muscle glucose uptake during old age is limited, and apparently nothing has been published about the combined effects of CR and acute exercise on insulin signaling in muscles, regardless of age. Accordingly, the current study was designed to provide new insights into the mechanisms for both the independent and combined benefits of CR and one exercise session on insulin signaling and glucose uptake by insulin-stimulated muscle in old age. The specific aims were to determine in isolated epitrochlearis muscle from 30-mo-old rats the effects of chronic CR (initiated at 14 wk of age) and/or acute exercise on 1) insulin-stimulated glucose uptake, 2) activation of key insulin-signaling steps that regulate glucose uptake (including IRS-1/PI3K activity, Akt Ser473 and Thr308 phosphorylation, and AS160 Ser588 and Thr642 phosphorylation), 3) Akt's association with three protein-binding partners that can influence Akt phosphorylation [protein phosphatase 2A (PP2A), heat shock protein of 90 kDa (HSP90), and adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif 1 (Appl1)], 4) abundance of glucose transporter 4 (GLUT4) and hexokinase II, which are the proteins responsible for muscle glucose transport and phosphorylation, respectively, and 5) phosphorylation of AMP-activated protein kinase (AMPK).
EXPERIMENTAL PROCEDURES
Materials.
Unless otherwise noted, all chemicals were purchased from Fisher Scientific (Hanover Park, IL) or Sigma-Aldrich (St. Louis, MO). Reagents and apparatus for SDS-PAGE and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Bicinchoninic acid protein assay and Pierce MemCode Reversible Protein Stain Kit were purchased from Thermo Fisher (Waltham, MA). Anti-phospho-Akt Thr308 (p-Akt Thr308; no. 9275), anti-phospho-Akt Ser473 (p-Akt Ser473; no. 9272), anti-phospho-AS160 Ser588 (p-AS160 Ser588; no. 8730), anti-phospho-AMPKα Thr172 (p-AMPK Thr172; no. 2531), anti-phospho-insulin receptor Tyr1146 (p-IRTyr1146; no. 3021), anti-Akt (no. 4691), anti-AMPKα (no. 5831), anti-hexokinase II (no. 2867), and anti-rabbit IgG horseradish peroxidase conjugate (no. 7074) and ATP (mo. 9804) were from Cell Signaling Technology (Danvers, MA). Anti-AS160 (no. ABS54) and anti-GLUT4 (no. CBL243) were from EMD Millipore (Billerica, MA). Anti-filamin C (FLNc; no. sc-48496), anti-mouse IgG horseradish peroxidase conjugate (no. sc-2060), and anti-goat IgG horseradish peroxidase conjugate (no. sc-2020) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-FLNc Ser2231 (p-FLNcSer2231; no. PB-131) was from Kinasource (Dundee, Scotland, UK). p-AS160 Thr642 (no. 3028-P) was from Symansis (B-Bridge International, Mountain View, CA). Anti-HSP90 (no. 610419) and anti-PP2A (no. 610556) were from BD Bioscience (San Jose, CA). Anti-Appl1 (no. ab59592) was from Abcam (Cambridge, MA). Anti-AffiniPure Sheep IgG horseradish peroxidase conjugate (no. 713-035-147) was from Jackson ImmunoResearch Laboratories (West Grove, PA). 2-Deoxy-d-[3H]glucose ([3H]2-DG), [14C]mannitol, and [32ATP]-ATP were from Perkin-Elmer (Boston, MA).
Animal treatment.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Fischer-344 × Brown Norway rats (both CR rats and their AL controls) were obtained at ∼29 mo of age from the National Institute of Aging (NIA) calorie-restricted rodent colony. Calorie restriction was initiated at 14 wk of age in the CR group by the NIA. Rats were housed at the University of Michigan for approximately 1 mo prior to experimentation. During this time, the rats were housed individually in shoebox cages and maintained on a 12:12-h light-dark cycle (lights out at 1700) in specific pathogen-free conditions. Rats were provided chow (AL: NIH31 chow; CR: NIH31/NIA fortified chow) and maintained on their respective feeding protocols (AL: free access to chow; CR: ∼60–65% of AL consumption). The muscle glucose uptake experiment was performed when the rats were ∼30 mo of age. Rats were fasted at ∼1900 on the night before the terminal experiment. The following morning at ∼0700, exercised rats swam in a barrel filled with water (35°C; ∼45 cm depth, 6 rats swimming at time, 3 AL and 3 CR). The exercise protocol consisted of nine bouts of swimming (10-min duration/bout), with 10-min rest intervals separating each exercise bout. After 90 min of total exercise, exercising rats were dried and returned to their cages without food, and epitrochlearis muscles were dissected from anesthetized time-matched sedentary and exercised rats 3–4 h after the exercising group had completed the protocol.
Muscle dissection and incubation.
Muscle dissection and incubation procedures have been described previously (42). The two longitudinal muscle strips prepared from each epitrochlearis were placed in vials containing the appropriate media, shaken at 45 oscillations/min, continuously gassed (95% O2-5% CO2), and heated (35°C) in a reciprocating water bath. Muscles were initially incubated in vials containing 2 ml of Krebs Henseleit buffer (KHB) supplemented with 0.1% bovine serum albumin (BSA), 2 mM sodium pyruvate, 6 mM mannitol, and either no insulin (basal) or a submaximally effective concentration of insulin (0.6 nM) for 30 min. Muscles were then transferred to another vial containing 2 ml KHB-BSA, the same concentration of insulin as the previous step, 0.1 mM 2-DG (including a final specific activity of 2.25 mCi/mmol [3H]2-DG), and 5.9 mM mannitol (including a final specific activity of 0.022 mCi/mmol [14C]mannitol) for 20 min. After this step, muscles were blotted on filter paper moistened with ice-cold KHB, trimmed, freeze-clamped using aluminum tongs cooled in liquid nitrogen, and stored at −80°C for later processing and analysis.
Muscle lysate preparation.
Frozen muscles were weighed and homogenized in ice-cold lysis buffer (1 ml/muscle strip) using a TissueLyser II homogenizer (Qiagen, Valencia, CA). For the samples analyzed for 2-DG uptake and immunoblotting, the lysis buffer contained T-PER Tissue Protein Extraction Reagent (no. PI-78510; Thermo Scientific, Rockford, IL) supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate (NaPP), 1 mM sodium vanadate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, and 1 mM PMSF. For the samples analyzed for IRS-1/PI3K activity and Akt coimmunoprecipitation, the lysis buffer contained 50 mM HEPES, pH 7.5, 150 mM sodium chloride, 1% octylphenoxy poly(ethyleneoxy)ethanol a (IGEPAL), 10% glycerol, 10 mM sodium fluoride, 2 mM EDTA, 10 mM NaPP, 1 mM magnesium chloride, 1 mM calcium chloride, 1 mM PMSF, 5 μg/ml leupeptin, 1 tablet/10 ml PhosSTOP (no. 04906837001; Roche, Indianapolis, IN), and 0.1 mM potassium bisperoxo(1,10-phenanthroline)oxovanadate (no. 203695; Millipore).
2-DG uptake.
The calculation of [3H]2-DG uptake by skeletal muscle has been described previously (8, 26). Briefly, [14C]mannitol counts/min, determined by liquid scintillation counting of aliquots from muscle homogenates, were used to determine extracellular space. The intracellular [3H]2-DG of muscle was calculated as the difference between the total [3H]2-DG in muscle and the [3H]2-DG in the extracellular space.
Immunoblotting.
Western blotting procedures have been described previously (42). An equal amount of protein of each sample was mixed with 6× Laemmli buffer, boiled for 5 min, and separated using SDS-PAGE (7% resolving gel) before being transferred to polyvinyl difluoride membranes. The MemCode protein stain was used to confirm equal loading (3). Membranes were blocked in 5% BSA in TBST (Tris-buffered saline, pH 7.5, plus 0.1% Tween-20) for 1 h at room temperature and transferred to 5% BSA-TBST with the appropriate primary antibody overnight at 4°C. Membranes were washed three times for 5 min in TBST and incubated with secondary antibody for 1 h at room temperature. Blots were washed three times for 5 min in TBST and three times for 5 min in TBS and then subjected to enhanced chemiluminescence (Luminata Forte Western horseradish peroxidase substrate no. WBLUF0100; Millipore) to visualize protein bands. Immunoreactive proteins were quantified by densitometry (AlphaEase FC; Alpha Innotech, San Leandro, CA). Values are expressed relative to the normalized average of all of the samples on each blot.
IRS-1-associated PI3K activity.
Muscle IRS-1/PI3K activity was determined as described previously (42). After the addition of 2 μg of anti-IRS-1 antibody to 300 μg of supernatant protein from each muscle sample, the immunocomplexes were allowed to form overnight at 4°C with slow rotation. Then, 100 μl of protein A-Sepharose beads (50% slurry, catalog no. 17-0469-01; GE Healthcare, Piscataway, NJ) was added to each aliquot, and samples were rotated for 2 h at 4°C. Samples were centrifuged at 3,000 g to pellet the protein A-Sepharose immunocomplex. Each immunopellet was washed three times with buffer 1 (phosphate-buffered saline, pH 7.5, containing 1% IGEPAL and 100 μM sodium vanadate), three times with buffer 2 (100 mM Tris, pH 7.5, 500 mM lithium chloride, and 100 μM sodium vanadate), and twice with buffer 3 (10 mM Tris, pH 7.5, 100 mM sodium chloride, 1 mM EDTA, and 100 μM sodium vanadate). After the immunopellet was washed, all of the buffer was removed, and the immunopellet was resuspended in 40 μl of the 10 mM Tris-1 mM EDTA, pH 7.5, buffer containing 10 μg of phosphatidylinositol (Avanti Polar Lipids, Alabaster, AL) and 100 mM magnesium chloride. The reaction was initiated at room temperature by the addition of 5 μl of a phosphorylation mixture containing 880 μM ATP and 30 μCi of γ-[32P]ATP. After 20 min with continuous rotation at 37°C, the reaction was stopped by sequential addition of 20 μl of 8 N hydrochloric acid and 160 μl of chloroform-methanol (1:1). The reaction mixture was vortexed for 5 min and then centrifuged at 3,000 g for 5 min; 50 μl of the organic phase containing the reaction products was spotted onto a thin-layer chromatography (TLC) plate (Whatman, Piscataway, NJ). The products were resolved in a chloroform-methanol-water-ammonium hydroxide (60:47:11.3:2) solution and visualized by autoradiography. The spots corresponding to the phosphatidylinositol-phosphorylated product were scraped from the TLC plate and counted in a scintillation counter.
Coimmunoprecipitation of HSP90, PP2A, and Appl1 with Akt.
For evaluation of Akt association with other proteins, 300 μg of protein from each sample was combined with a 1:1,000 titer of Akt antibody and rotated overnight at 4°C. After initial antibody incubation, 50 μl of protein G magnetic beads (no. 10004D; Life Technologies, Grand Island, NY) was added to the lysate-antibody mixture and rotated for 2 h at 4°C. The immunoprecipitation matrix (bead-antibody-antigen) for each sample was washed three times with lysis buffer, with complete aspiration of buffer after the final wash, and 30 μl of 2× Laemmli buffer was added. Samples were boiled for 5 min and centrifuged, and supernatants were subjected to 10% SDS-PAGE and blotted for HSP90, PP2A, and Appl1.
Statistical analysis.
Two-way analysis of variance (ANOVA) was used to assess the main effects of diet (AL or CR) and exercise (sedentary or 3 h postexercise) and the diet × exercise interaction within each insulin level (minus or plus insulin), and the Tukey test was used for post hoc analysis to identify the source of significant variance (SigmaPlot version 11.0; Systat Software, San Jose, CA). Data lacking normal distribution and/or equal variance were mathematically transformed to achieve normality and equal variance prior to two-way ANOVA being run. Kruskal-Wallis one-way ANOVA on ranks was used if transformation failed to normalize the data, and post hoc analysis was performed by Dunn's method. The Spearman Rank Order Correlation was used to evaluate associations between measured outcomes. Data are presented as means ± SE. A P value of ≤0.05 was accepted as statistically significant.
RESULTS
2-DG uptake.
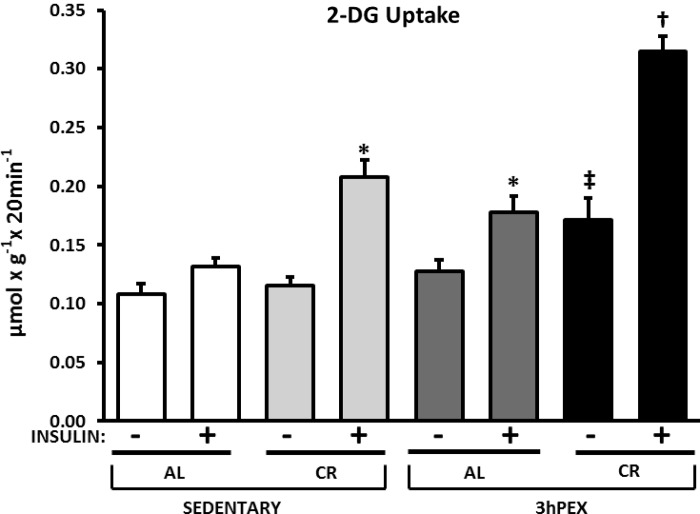
For 2-DG uptake in muscles incubated without insulin (Fig. 1), the 3 h postexercise and CR (3hPEX-CR) group exceeded the sedentary and AL (SED-AL) group (P < 0.05). For 2-DG uptake in muscles incubated with insulin (Fig. 1), there were significant (P < 0.001) main effects of both diet (CR > AL) and exercise (3hPEX > SED) as well as a significant diet × exercise interaction (P < 0.05). Post hoc analysis revealed that 2-DG uptake with insulin in the SED-CR group (P < 0.001) and 3 h postexercise and AL (3hPEX-AL) group (P < 0.05) each exceeded the SED-AL group. The 3hPEX-CR group was greater than both the sedentary and CR (SED-CR) and 3hPEX-AL groups (P < 0.001).
Fig. 1.
Rates of 2-deoxy-d-glucose (2-DG) uptake in muscles from sedentary-ad libitum (SED-AL), sedentary-calorie-restricted (SED-CR), 3 h postexercise-ad libitum (3hPEX-AL), and 3 h postexercise-calorie restricted (3hPEX-CR) rats. Results for 2-DG uptake without insulin were analyzed using 1-way ANOVA on ranks because these data were not distributed normally. Data for 2-DG uptake with insulin were analyzed using 2-way ANOVA. Post hoc analysis indicated for muscles without insulin: ‡3hPEX-CR > SED-AL (P < 0.05). Post hoc analysis indicated for muscles with insulin: *SED-CR (P < 0.001) and 3hPEX-AL (P < 0.05) > SED-AL; †3hPEX-CR > 3hPEX-AL and SED-CR (P < 0.001). Values are means ± SE; n = 8–11/treatment group.
IRS-1-associated PI3K activity.
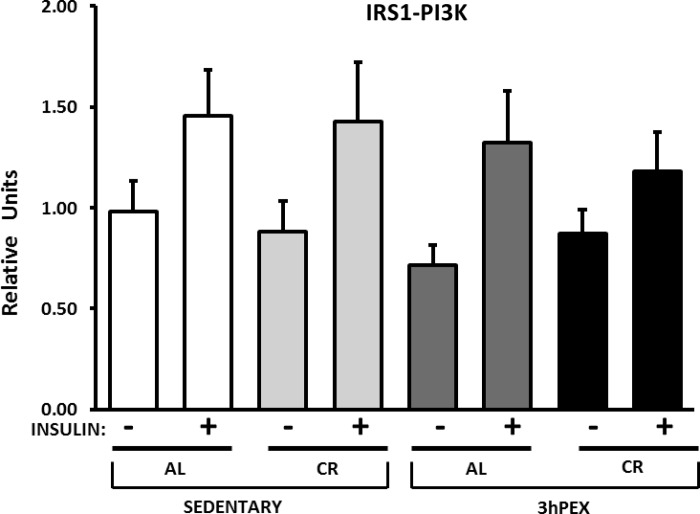
There were no significant effects of diet on IRS-1/PI3K activity in the absence or presence of insulin (Fig. 2). There were also no significant effects of exercise on IRS-1/PI3K activity regardless of insulin concentration.
Fig. 2.
Insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase (PI3K) activity in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. Data were analyzed using 2-way ANOVA within each insulin level (without or with insulin). Values are means ± SE; n = 8–11/treatment group.
Immunoblotting.
Equal loading of samples was confirmed on the basis of the MemCode results. For all of the phosphorylated proteins, the data were expressed as a ratio of the phosphorylated/total protein values. Expressing the results as phosphorylated/total protein ratio rather than as the phosphorylated protein without dividing by the total protein value did not change the interpretation of the results for any of the proteins that were assessed.
Akt.
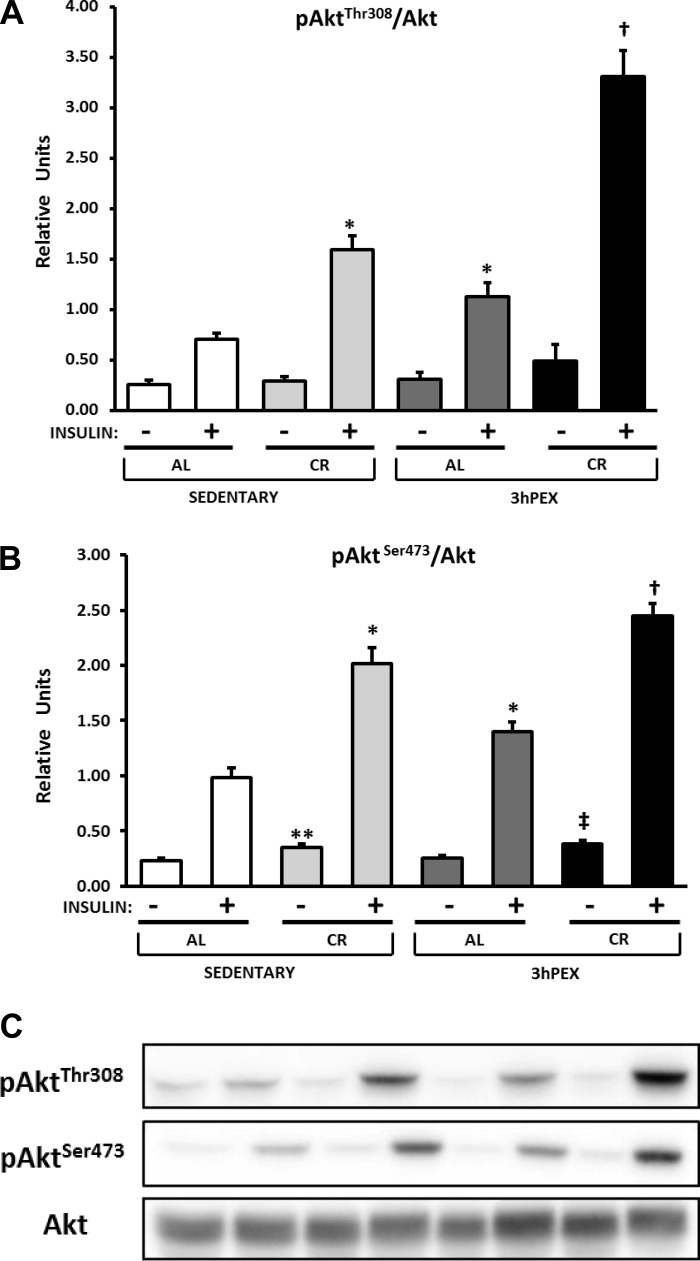
There was a small (∼11%) but significant diet effect (AL > CR, P < 0.01; data not shown) and a diet × exercise interaction (P < 0.05) on total Akt abundance in the muscles incubated without insulin, and post hoc analysis indicated that SED-AL values exceeded SED-CR values (P < 0.001). For p-Akt Thr308/Akt ratio in the absence of insulin, there were no significant diet or exercise effects (Fig. 3A). For p-Akt Thr308/Akt ratio in the presence of insulin, there were significant effects of diet (CR > AL, P < 0.001) and exercise (3hPEX > SED, P < 0.001) and a significant diet × exercise interaction (P < 0.05; Fig. 3A). Post hoc analysis revealed that both the SED-CR (P < 0.001) and 3hPEX-AL groups exceeded the SED-AL group (P < 0.05), and the 3hPEX-CR group was greater than both the 3hPEX-AL and SED-CR groups (P < 0.001). For the p-Akt Ser473/Akt ratio in the absence of insulin, there was a significant main effect of diet (CR > AL, P < 0.001; Fig. 3B), and post hoc analysis revealed that the SED-CR exceeded the SED-AL group (P < 0.01), and the 3hPEX-CR group was greater than the 3hPEX-AL group (P < 0.01). In the presence of insulin, there were significant main effects of diet (CR > AL, P < 0.001) and exercise (3hPEX > SED, P < 0.001) on the p-Akt Ser473/Akt ratio (Fig. 3B). Post hoc analysis demonstrated that the SED-CR group (P < 0.001) and the 3hPEX-AL group (P < 0.05) were each greater than the SED-AL group, and the 3hPEX-CR group exceeded both the 3hPEX-AL (P < 0.001) and the SED-CR (P < 0.01) groups.
Fig. 3.
A and B: phosphorylated (p)-Akt Thr308/Akt (A) and p-Akt Ser473/Akt (B) in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. C: representative Western blots. Results for Akt Thr308/Akt without insulin were analyzed using 1-way ANOVA on ranks because these data were not distributed normally. All other data were analyzed using 2-way ANOVA within each insulin level (without or with insulin). Post hoc analysis indicated for muscles without insulin: **SED-CR > SED-AL, (P < 0.01); ‡3hPEX-CR > 3hPEX-AL (P < 0.01). Post hoc analysis indicated for muscles with insulin: *SED-CR (P < 0.001) and 3hPEX-AL (P < 0.05) > SED-AL; †3hPEX-CR > 3hPEX-AL (P < 0.001) and SED-CR (P < 0.01). Values are means ± SE; n = 8–11/treatment group.
AS160.
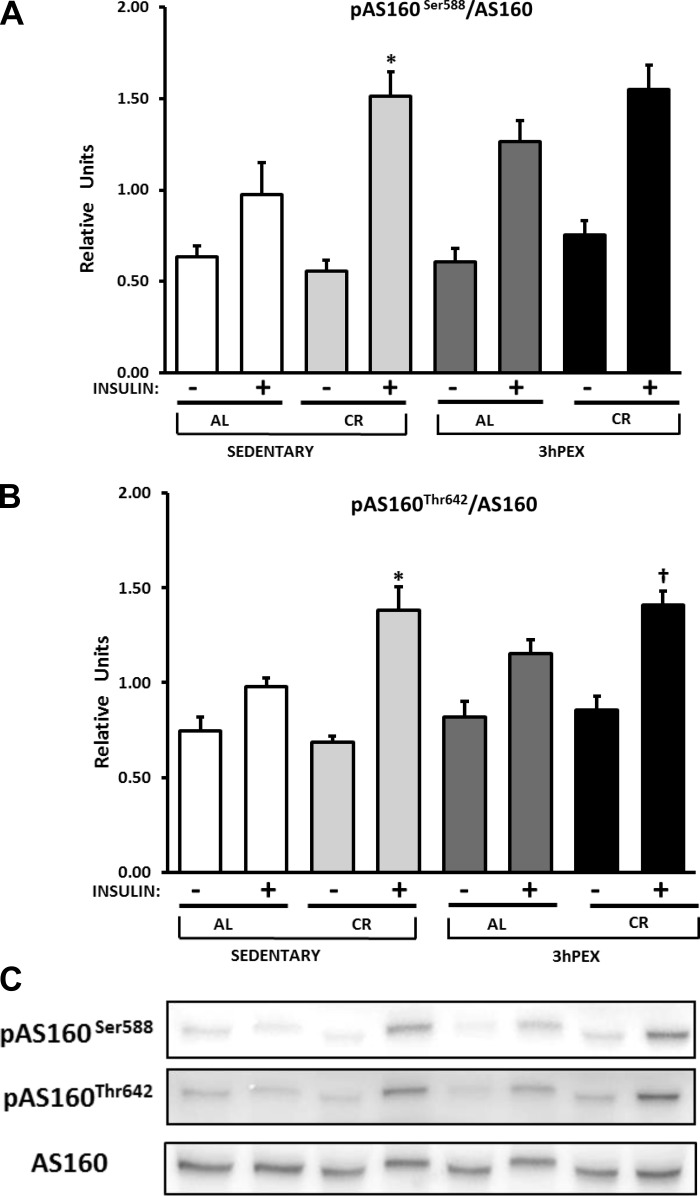
There were no significant effects of diet or exercise on AS160 total abundance (data not shown). For the ratio of pAS160 Ser588/AS160 in the absence of insulin, there were no significant effects of diet or exercise (Fig. 4A). For the ratio of pAS160 Ser588/AS160 in the presence of insulin, there was a significant effect of diet (CR > AL, P < 0.01; Fig. 4A). Post hoc analysis revealed that SED-CR values exceeded SED-AL values (P < 0.05). For the ratio of p-AS160 Thr642/AS160 in the absence of insulin, there were no significant effects of diet or exercise (Fig. 4B). For the ratio of p-AS160 Thr642/AS160 in the presence of insulin, there was a significant effect of diet (CR > AL, P < 0.001; Fig. 4B). Post hoc analysis revealed that SED-CR values exceededed SED-AL values (P < 0.01) and that 3hPEX-CR values (P < 0.05) exceeded 3hPEX-AL values.
Fig. 4.
A and B: p-AS160 (Akt substrate of 160 kDa) Ser588/AS160 (A) and p-AS160 Thr642/AS160 (B) in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. C: representative Western blots. Data were analyzed using 2-way ANOVA within each insulin level (without or with insulin). Post hoc analysis indicated for muscles with insulin: *SED-CR > SED-AL (P < 0.05 for AS160 Ser588, P < 0.01 for AS160 Thr642); †3hPEX-CR > 3hPEX-AL (P < 0.05). Values are means ± SE; n = 8–11/treatment group.
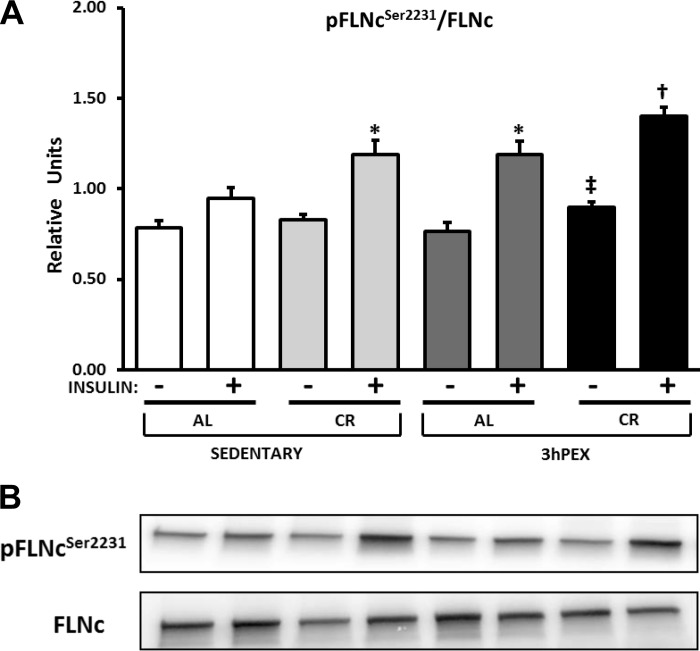
FLNc.
FLNc is an Akt substrate (21, 31). CR was recently found to result in greater FLNc Ser2231 phosphorylation in muscles from both 9- and 24-mo-old rats (41, 43), but the effects of exercise alone or exercise combined with CR had not been reported previously. For FLNc in the absence of insulin, there was a small (∼6%) but significant diet effect on total abundance (AL > CR, P < 0.005; data not shown) as well as a significant diet × exercise interaction (P < 0.05), and post hoc analysis indicated that SED-AL values were greater than SED-CR values (P < 0.001) and 3hPEX-AL values (P < 0.01). For the FLNc Ser2231/FLNc ratio in the absence of insulin, there was a significant diet effect (CR > AL, P < 0.05; Fig. 5A), and post hoc analysis revealed that the 3hPEX-CR values exceeded 3hPEX-AL values (P < 0.05). For the FLNc Ser2231/FLNc ratio in the presence of insulin, there were significant diet (CR > AL,; P = 0.002) and exercise (3hPEX > SED, P < 0.005) effects (Fig. 5A). Post hoc analysis indicated that the SED-CR and 3hPEX-AL groups were greater than the SED-AL group (P < 0.05), and the 3hPEX-CR group was ∼18% greater than the 3hPEX-AL and SED-CR groups (P < 0.05).
Fig. 5.
A: p-FLNc (filamin C) Ser2231/FLNc in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. B: representative Western blots. Data were analyzed using 2-way ANOVA for samples with insulin. Post hoc analysis indicated for muscles without insulin: ‡3hPEX-CR > 3hPEX-AL (P < 0.05). Post hoc analysis indicated for muscles with insulin: *SED-CR and 3hPEX-AL > SED-AL (P < 0.05); †3hPEX-CR > 3hPEX-AL and SED-CR (P < 0.05). Values are means ± SE; n = 8–11/treatment group.
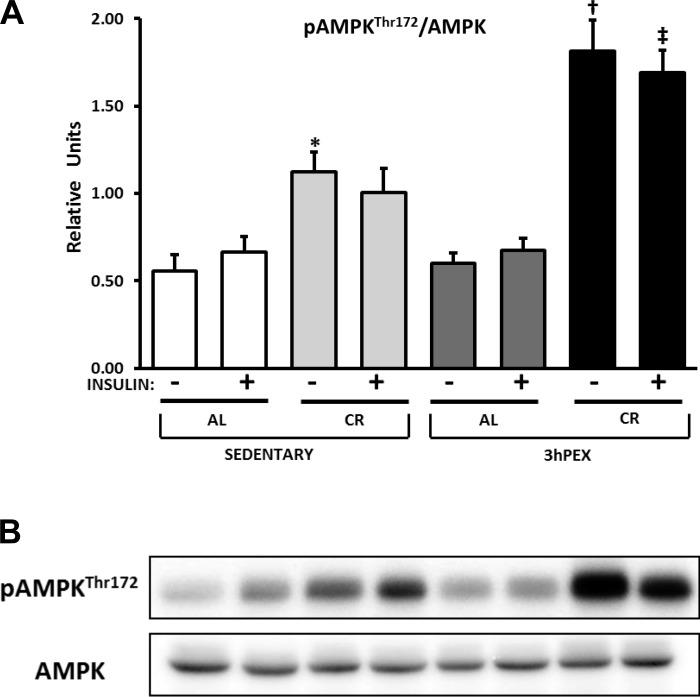
AMPK.
For total AMPK abundance, there was a small (∼11%) but significant effect of diet (AL > CR) either without insulin (P < 0.001) or with insulin (P < 0.005) (data not shown). Post hoc analysis indicated that in the absence of insulin, SED-AL exceeded SED-CR (P < 0.001). For the p-AMPK Thr172/AMPK ratio in the absence of insulin, there were significant effects of diet (CR > AL, P < 0.001) and exercise (3hPEX > SED, P < 0.01), and a significant diet × exercise interaction (P < 0.01; Fig. 6A). Post hoc analysis indicated that SED-CR exceeded SED-AL values (P < 0.01) and that 3hPEX-CR exceeded both SED-CR and 3hPEX-AL values (P < 0.001). For p-AMPK Thr172/AMPK ratio in the presence of insulin, there were significant effects of diet (CR > AL, P < 0.001) and exercise (3hPEX > SED, P < 0.005) and a significant diet × exercise interaction (P = 0.005; Fig. 6A). Post hoc analysis revealed that 3hPEX-CR values were greater than 3hPEX-AL and SED-CR values (P < 0.001).
Fig. 6.
A: p-AMPK (AMP-activated protein kinase) Thr172/AMPK in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. B: representative Western blots. Data were analyzed using 2-way ANOVA within each insulin level (without or with insulin). Post hoc analysis indicated for muscles without insulin: *SED-CR > SED-AL (P < 0.01); †3hPEX-CR > SED-CR and 3hPEX-AL (P < 0.001). Post hoc analysis indicated for muscles with insulin: ‡3hPEX-CR > 3hPEX-AL and SED-CR (P < 0.001). Values are means ± SE; n = 8–11/treatment group.
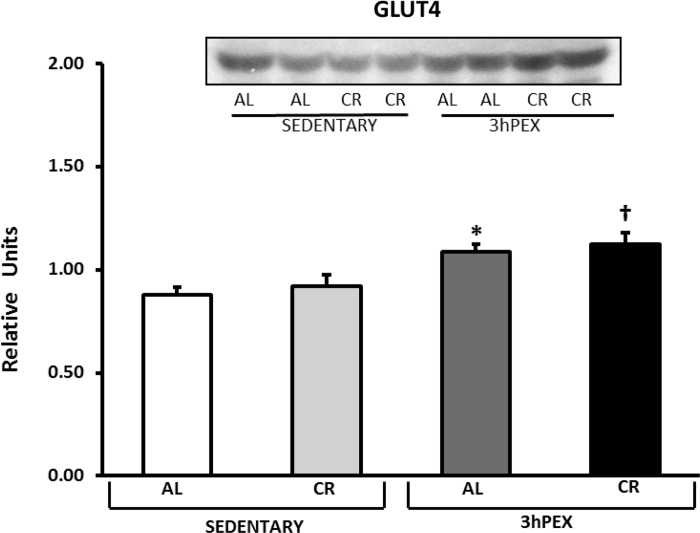
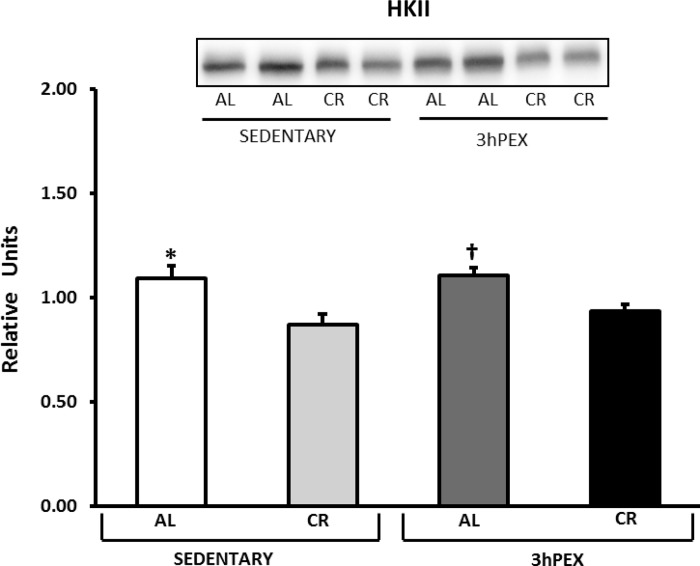
GLUT4 and hexokinase II.
There was a moderate (∼22%) but significant (P < 0.001) exercise effect on GLUT4 abundance (3hPEX > SED; Fig. 7), and post hoc analysis indicated that 3hPEX-AL exceeded SED-AL (P < 0.01) and 3hPEX-CR exceeded SED-CR (P < 0.01). There was also a small (∼15%) but significant (P < 0.001) diet effect on hexokinase II abundance (AL > CR; Fig. 8), and post hoc analysis indicated that SED-AL exceeded SED-CR (P < 0.01) and 3hPEX-AL exceeded 3hPEX-CR (P < 0.01).
Fig. 7.
Glucose transporter 4 (GLUT4) abundance in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. Data were analyzed using 2-way ANOVA. Post hoc analysis indicated: *3hPEX-AL > SED-AL (P < 0.01); †3hPEX-CR > SED-CR (P < 0.01). Values are means ± SE; n = 8–11/treatment group.
Fig. 8.
Hexokinase II (HKII) abundance in muscles incubated from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. Data were analyzed using 2-way ANOVA. Post hoc analysis indicated: *SED-AL > SED-CR (P < 0.01); †3hPEX-AL > 3hPEX-CR (P < 0.01). Values are means ± SE; n = 8–11/treatment group.
HSP90, APPL1, and PP2A abundance and association with akt.
There were no significant effects of diet or exercise on Appl1 or PP2A abundance (data not shown). There was a small (∼9%) but significant main effect of diet (AL > CR, P < 0.01) on HSP90 abundance, and post hoc analysis indicated that SED-AL exceeded SED-CR (P < 0.05; data not shown).
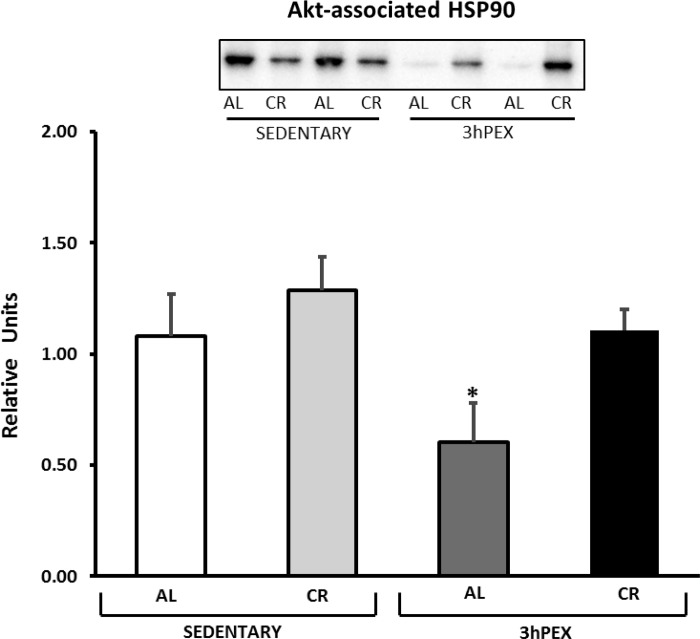
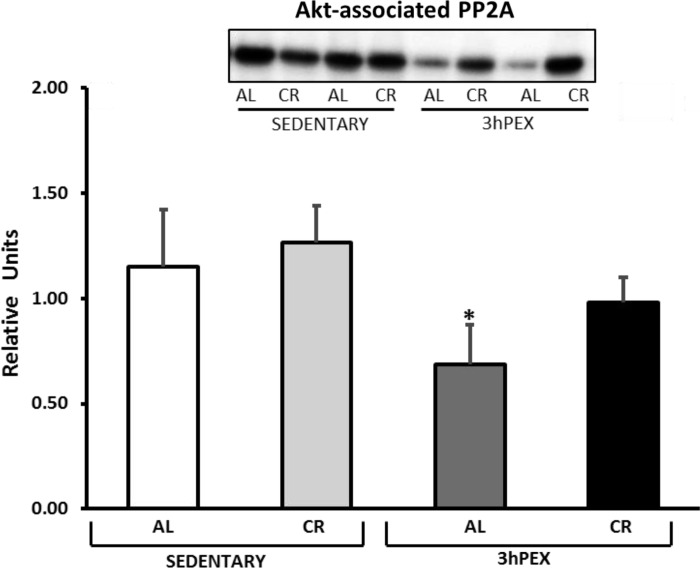
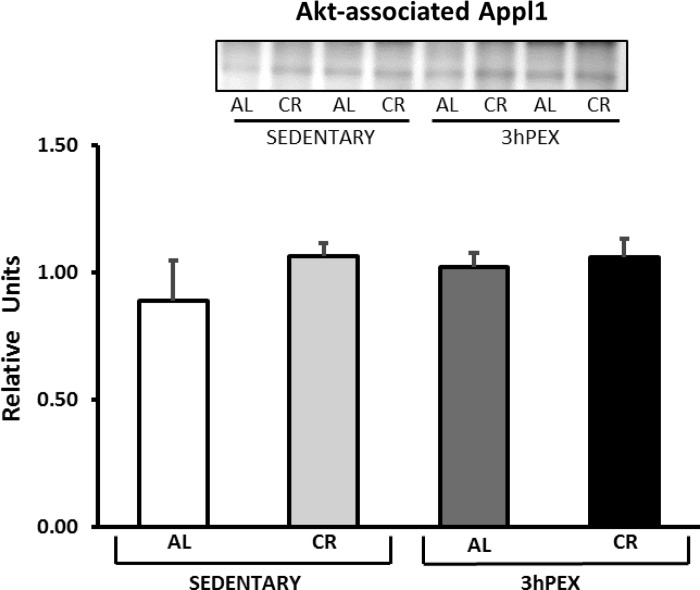
There was a significant main effect of diet (CR > AL, P < 0.05) for HSP90 associated with Akt, and post hoc analysis indicated that 3hPEX-CR exceeded 3hPEX-AL (P < 0.05; Fig. 9). There was a significant main effect of exercise (SED > 3hPEX, P < 0.01) for PP2A associated with Akt, and post hoc analysis indicated that SED-AL exceeded 3hPEX-AL (P < 0.05; Fig. 10). There were no significant effects of diet or exercise on Appl1 associated with Akt (Fig. 11).
Fig. 9.
Heat shock protein of 90 kDa (HSP90) coimmunoprecipitated with Akt in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. Data were analyzed using 2-way ANOVA. Post hoc analysis indicated: *3hPEX-CR > 3hPEX-AL (P < 0.05). Values are means ± SE; n = 8–11/treatment group.
Fig. 10.
Protein phosphatase 2A (PP2A) coimmunoprecipitated with Akt muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. Data were analyzed using 2-way ANOVA. Post hoc analysis indicated: *SED-AL > 3hPEX-AL (P < 0.05). Values are means ± SE; n = 8–11/treatment group.
Fig. 11.
Adaptor protein containing pleckstrin homology domain, phosphotyrosine domain, and leucine zipper motif 1 (Appl1) coimmunoprecipitated with Akt in muscles from SED-AL, SED-CR, 3hPEX-AL, and 3hPEX-CR rats. Data were analyzed using 2-way ANOVA. Values are means ± SE; n = 4–6/treatment group.
Correlations.
For insulin-stimulated muscles, 2-DG uptake was significantly correlated with either p-Akt Thr308/Akt (r = 0.800, P < 0.0001) or p-Akt Ser473/Akt (r = 0.793, P < 0.0001). The 2-DG uptake of insulin-stimulated muscles was also significantly correlated with p-AS160 Ser588/AS160 (r = 0.547, P < 0.0001), p-AS160 Thr642/AS160 (r = 0.599, P < 0.0001), or FLNc Ser2231/FLNc (r = 0.657, P < 0.0001).
DISCUSSION
The current study was the first to assess both the independent and combined effects of CR and acute exercise on insulin-stimulated glucose uptake and insulin signaling in skeletal muscles from old rats. The most important new observation was that glucose uptake by insulin-stimulated muscles from the combined CR and exercise treatment substantially exceeded the values for either CR or exercise alone. However, to interpret the results of the combined CR and exercise intervention, it is necessary to first consider the independent consequences of CR and exercise on glucose uptake and insulin signaling.
CR alone led to enhanced insulin-stimulated glucose uptake that was accompanied by greater insulin-stimulated Akt phosphorylation on both Ser473 and Thr308 in muscle from sedentary CR vs. AL rats. These observations are consistent with previous research on the effects of CR (6, 16, 28–30, 40, 42, 43). Earlier research in 9-mo-old rats that used a highly selective Akt inhibitor to eliminate the CR effect on Akt phosphorylation revealed that the CR-induced increase in Akt phosphorylation was essential for CR's enhancement of insulin-stimulated glucose uptake (43). In this context, it seems likely that greater Akt phosphorylation was required for greater insulin-stimulated glucose uptake in the CR-alone group in the current study.
Consistent with earlier research on young adult (∼5- or 9-mo-old) rats (16, 42), the enhanced Akt phosphorylation in old CR rats compared with AL controls was not accompanied by elevated IRS-1/PI3K activity. However, in an earlier study, greater IRS-1/PI3K activity was reported for insulin-stimulated muscles from ∼12-yr-old monkeys after CR for 4 yr compared with AL controls (48), and phosphotyrosine-associated PI3K activity was greater for insulin-stimulated muscles from ∼3-mo-old CR (8-wk CR duration) vs. AL mice (38). In both of these studies, greater insulin-stimulated glucose uptake and greater Akt phosphorylation were found in the muscles from CR compared with AL animals. Although some differences have been reported with regard to CR effects on PI3K activity, CR-related improvement in insulin sensitivity for glucose uptake have been consistently found together with enhanced Akt phosphorylation across multiple species.
Akt phosphorylation is not determined solely by PI3K activity. For example, it can also be influenced by its binding to several proteins, including HSP90, PP2A, and Appl1. An earlier study reported that CR resulted in greater HSP90 associated with Akt in epitrochlearis muscle from 9-mo-old rats (42). This result was notable because elevated Akt association with HSP90 can oppose PP2A-mediated Akt dephosphorylation (37). There was a trend for CR alone vs. AL sedentary controls to have greater HSP90 association with Akt, but this trend did not reach statistical significance. There was not a significant effect of CR alone on PP2A or Appl1 binding with Akt in muscle.
A key mechanism linking insulin stimulation of Akt to glucose uptake is the phosphorylation of AS160 on Ser588 or Thr642, the two crucial sites implicated in the control of insulin-stimulated glucose transport. The enhanced Akt phosphorylation with CR alone was accompanied by greater AS160 Ser588 and AS160 Thr642 phosphorylation in old rats in the current study. Similarly, in young adult rats, increased Akt phosphorylation caused by CR is accompanied by greater AS160 phosphorylation on both of these sites (42, 43). However, earlier research reported greater insulin-stimulated Akt phosphorylation in epitrochlearis muscles of 24-mo-old CR rats without significant CR-related changes in phosphorylation of AS160 on Ser588 (41) or Thr642 (40). Two differences in the experimental methods of the current study compared with the earlier research were the insulin concentrations (0.6 vs. 1.2 nM) and the ages of the rats (30 vs. 24 mo old). Although there were differences with regard to CR effects on AS160 phosphorylation, each of the earlier studies in old rats as well as the current study reported CR-related elevations in insulin-stimulated Akt phosphorylation and glucose uptake (40, 41).
FLNc is an Akt substrate and actin-binding protein that is selectively expressed in skeletal muscle (21, 31). Actin filament remodeling is implicated in the subcellular distribution of signaling proteins and GLUT4 glucose transporter vesicles (50), and GLUT4 abundance is highly correlated with FLNc abundance in single fibers from rat epitrochlearis muscles (13). Furthermore, insulin leads to increased Ser2231 phosphorylation of FLNc (41, 43). In this context, it is notable that CR led to greater FLNc Ser2231 phosphorylation in the current study as well as in two earlier studies on 9-mo-old and 24-mo-old rats (41, 43). Furthermore, a specific Akt inhibitor that eliminated the CR effects on insulin-stimulated Akt phosphorylation and glucose uptake was accompanied by elimination of the CR effect on FLNc phosphorylation (43). Although these results are interesting, there is currently no evidence that causally links FLNc phosphorylation to insulin-stimulated glucose uptake.
Earlier research has differed with regard to CR's effect on the activation of muscle AMPK. Several studies reported no CR effect (15, 25, 28, 38, 42), and others reported that CR was characterized by elevated AMPK activation in muscle (1, 33, 34, 47). In the current study, CR alone compared with AL controls had greater AMPK Thr172 phosphorylation. AMPK is widely believed to regulate insulin-independent glucose uptake (2, 7, 10, 22, 35), but AMPK has also been proposed to indirectly modify insulin sensitivity (32, 36). It is uncertain whether AMPK played a role in the CR-induced increase in insulin-stimulated glucose uptake.
Increased expression of GLUT4 and/or hexokinase II in skeletal muscle can lead to elevated insulin-stimulated glucose uptake (35). However, in the current study, CR did not produce greater GLUT4 protein abundance in muscle. This observation is consistent with several (11, 24, 40, 44) but not all (4) previous studies. The small reduction in hexokinase II abundance in muscle of CR vs. AL rats clearly demonstrates that the CR-related increase in insulin-stimulated glucose uptake was not attributable to greater hexokinase II expression.
Acute exercise alone resulted in a subsequent increase in insulin-stimulated glucose uptake by epitrochlearis muscle from 30-mo-old rats, in agreement with earlier results in 24- to 25-mo-old rats (9, 49). Earlier research indicated that acute exercise did not elevate insulin receptor tyrosine phosphorylation in muscle from old rats (49). The current results were the first to demonstrate that acute exercise did not alter IRS-1/PI3K activity in insulin-stimulated muscle from old rats, implicating a more distal insulin signaling step to induce the increased insulin-stimulated glucose uptake.
In this context, it was notable that Akt phosphorylation on both Ser473 and Thr308 was increased in insulin-stimulated muscles after exercise, and this effect was consistent with earlier results for 24-mo-old rats after a similar exercise protocol (49). The greater Akt phosphorylation postexercise was not attributable to increased IRS-1/PI3K activity or greater association of Akt with Appl1. The reduced HSP90 associated with Akt that was observed postexercise would be expected to favor reduced Akt phosphorylation, but the concomitantly reduced PP2A association with Akt would be predicted to favor greater Akt phosphorylation. Some studies of ∼2-mo-old rats have reported that acute exercise can enhance the subsequent Akt Thr308 phosphorylation in insulin-stimulated muscle (5, 23, 39), but other studies of young rats have observed increased insulin-stimulated glucose uptake in muscle without postexercise effects on either Akt Thr308 or Akt Ser473 phosphorylation in insulin-stimulated muscles (14, 20, 27, 45). It is possible that the different results reported for Akt phosphorylation in old vs. young rats is related to age differences (∼2 compared with 24–30 mo of age). However, it is important to recognize that the exercise protocols also differed in the studies with old rats (8–9 bouts of 10–20 min of exercise for a total of 90 min of exercise) compared with the studies with young rats (either 60–180 min of continuous exercise or 4 bouts of 30 min for 120 min of exercise).
Neither AS160 Thr642 nor AS160 Ser588 phosphorylation was significantly increased in muscles after exercise. Xiao et al. (49) reported significantly increased AS160 Thr642 phosphorylation, with a nonsignificant trend for an increase in AS160 Ser588 phosphorylation in insulin-stimulated muscles ∼3–4 h after acute exercise. A single exercise session has previously been reported to increase insulin-stimulated glucose uptake by muscle in young adult rats, concomitant with greater AS160 Ser588 and AS160 Thr642 phosphorylation (7, 14, 23, 39). The current results revealed that, at least in 30-mo-old rats, significantly enhanced AS160 phosphorylation on Ser588 or Thr642 is not essential for enhanced insulin-stimulated glucose uptake by muscle after acute exercise.
The lack of greater AMPK phosphorylation as a result of exercise alone (i.e., 3hPEX-AL vs. SED-AL group) in the current study corresponds with earlier observations from ∼2-mo-old rats at a similar time after acute exercise (5, 23, 39). AMPK is implicated in the insulin-independent increase in glucose uptake, so the lack of elevated AMPK phosphorylation several hours after exercise alone is consistent with the observation of no increase in insulin-independent glucose uptake in the 3hPEX group. The lack of increased insulin-independent glucose uptake in the current study is also in agreement with earlier research on old rats (9, 49).
To the best of our knowledge, the current study is the first to test and find that exercise leads to a subsequent increase in FLNc Ser2231 phosphorylation in insulin-stimulated muscles. Akt and FLNc were the only phosphorylated proteins that were increased in insulin-stimulated muscles after exercise alone compared with sedentary controls in the current study. Because FLNc is an Akt substrate, it seems likely that the exercise-related enhancement of Akt activation led to greater FLNc phosphorylation in the insulin-stimulated muscles postexercise.
Increased hexokinase II abundance can result in greater insulin-stimulated glucose uptake by muscle (35), but acute exercise did not alter hexokinase II content. There was a modest but significant increase in GLUT4 protein abundance after exercise in the current study, whereas earlier research did not detect any effect of a similar exercise protocol on epitrochlearis GLUT4 protein content in either 3.5- or 24-mo-old rats (9, 49). However, the current results were not unprecedented, as previous studies have reported that GLUT4 protein content can be moderately increased several hours after one exercise session (35). Greater GLUT4 abundance would be predicted to favor greater insulin-stimulated glucose uptake, but it may not fully account for the greater insulin-stimulated glucose uptake that was observed postexercise.
The primary goal of the current study was to elucidate the mechanisms for the combined effects of CR and acute exercise on muscle glucose uptake in old rats. The most striking outcome was that both Ser473 and Thr308 phosphorylation of Akt were greater in the combined CR and exercise group compared with either CR or exercise treatment alone. There was no evidence that this result was attributable to altered IRS-1/PI3K activity, and the influence of combined CR and exercise on Akt phosphorylation compared with AL sedentary controls was not accounted for by greater changes in Akt binding to HSP90, PP2A, or Appl1. CR alone, but not exercise alone, significantly increased AS160 Ser588 and AS160 Thr642 phosphorylation compared with AL sedentary controls, and no further increase in AS160 phosphorylation was found on either phosphosite when CR was combined with exercise. Other than Akt phosphorylation, the only signaling outcomes found to be greater for muscles from the combined CR and exercise group vs. either the CR or exercise-alone groups were the phosphorylations of AMPK Thr172 and FLNc Ser2213. However, the functional consequences of the greater AMPK and FLNc phosphorylation remain to be established. There were independent effects of CR and exercise on hexokinase II (reduced by CR alone) and GLUT4 (increased by exercise alone). However, combined CR and exercise did not alter these independent effects, suggesting that the further increase in glucose uptake in the combined group was secondary to other changes (e.g., insulin signaling and/or GLUT4 vesicle trafficking).
In conclusion, insulin-stimulated glucose uptake was increased by either CR or exercise alone, and combining these treatments produced an additional increase in insulin-stimulated glucose uptake. Analysis of the results for the independent and combined interventions (CR, exercise, or CR combined with exercise) revealed a close correspondence between the increases in glucose uptake and Akt phosphorylation. These results provide evidence that the improved insulin-stimulated glucose uptake may be secondary, at least in part, to greater activation of Akt, presumably as a consequence of greater phosphorylation of one or more Akt substrates. Greater AS160 phosphorylation was evident with CR alone, but the results for exercise alone or combined CR and exercise revealed the need to consider other Akt substrates. In this context, it was interesting that greater FLNc phosphorylation tracked with the levels of both Akt phosphorylation and insulin-stimulated glucose uptake in all treatment groups. The current results provide evidence for investigating the functional significance of this relationship. Our working hypothesis is that, in insulin-stimulated muscles from old rats, greater insulin-stimulated glucose uptake induced by CR and/or exercise depends on greater activation of Akt. It will be important to identify both of the mechanisms responsible for greater Akt activation and the specific Akt substrate(s) that couples the enhanced Akt activation to elevated glucose uptake.
GRANTS
This research was supported by National Institute on Aging Grant AG-010026 to G. D. Cartee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S. and G.D.C. conception and design of research; N.S., H.W., E.B.A., and C.M.C. performed experiments; N.S., H.W., and E.B.A. analyzed data; N.S., H.W., E.B.A., and G.D.C. interpreted results of experiments; N.S., H.W., and G.D.C. drafted manuscript; N.S., H.W., E.B.A., C.M.C., and G.D.C. edited and revised manuscript; N.S., H.W., E.B.A., C.M.C., and G.D.C. approved final version of manuscript; H.W. prepared figures.
ACKNOWLEDGMENTS
Present address of C. M. Castorena: Internal Medicine, Div. of Hypothalamic Research, Univ. of Texas Southwestern Medical Center, Dallas, TX.
REFERENCES
- 1.Al-Regaiey KA, Masternak MM, Bonkowski MS, Panici JA, Kopchick JJ, Bartke A. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci 62: 18–26, 2007. [DOI] [PubMed] [Google Scholar]
- 2.An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes 59: 1358–1365, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antharavally BS, Carter B, Bell PA, Krishna Mallia A. A high-affinity reversible protein stain for Western blots. Anal Biochem 329: 276–280, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Argentino DP, Dominici FP, Muñoz MC, Al-Regaiey K, Bartke A, Turyn D. Effects of long-term caloric restriction on glucose homeostasis and on the first steps of the insulin signaling system in skeletal muscle of normal and Ames dwarf (Prop1df/Prop1df) mice. Exp Gerontol 40: 27–35, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 3: e2264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 58: 19–30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol 75: 972–978, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Cartee GD, Funai K. Exercise and insulin: Convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev 37: 188–195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartee GD, Kietzke EW, Briggs-Tung C. Adaptation of muscle glucose transport with caloric restriction in adult, middle-aged, and old rats. Am J Physiol Regul Integr Comp Physiol 266: R1443–R1447, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Castorena CM, Arias EB, Sharma N, Bogan JS, Cartee GD. Fiber type effects on contraction-stimulated glucose uptake and GLUT4 abundance in single fibers from rat skeletal muscle. Am J Physiol Endocrinol Metab 308: E223–E230, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes 63: 2297–2308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui M, Yu H, Wang J, Gao J, Li J. Chronic caloric restriction and exercise improve metabolic conditions of dietary-induced obese mice in autophagy correlated manner without involving AMPK. J Diabetes Res 2013: 852754, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson RT, Arias EB, Cartee GD. Calorie restriction increases muscle insulin action but not IRS-1-, IRS-2-, or phosphotyrosine-PI 3-kinase. Am J Physiol Endocrinol Metab 282: E270–E276, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Dean DJ, Cartee GD. Brief dietary restriction increases skeletal muscle glucose transport in old Fischer 344 rats. J Gerontol A Biol Sci Med Sci 51: B208–B213, 1996. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 86: 3574–3578, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Fujita M, Mitsuhashi H, Isogai S, Nakata T, Kawakami A, Nonaka I, Noguchi S, Hayashi YK, Nishino I, Kudo A. Filamin C plays an essential role in the maintenance of the structural integrity of cardiac and skeletal muscles, revealed by the medaka mutant zacro. Dev Biol 361: 79–89, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes 58: 1096–1104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazdag AC, Sullivan S, Kemnitz JW, Cartee GD. Effect of long-term caloric restriction on GLUT4, phosphatidylinositol-3 kinase p85 subunit, and insulin receptor substrate-1 protein levels in rhesus monkey skeletal muscle. J Gerontol A Biol Sci Med Sci 55: B44–B46; discussion B47–B48, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab 287: E1032–E1037, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol 76: 979–985, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Iwabe M, Kawamoto E, Koshinaka K, Kawanaka K. Increased postexercise insulin sensitivity is accompanied by increased AS160 phosphorylation in slow-twitch soleus muscle. Physiol Rep 2: pii: e12162, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54: 1349–1356, 2005. [DOI] [PubMed] [Google Scholar]
- 29.McCurdy CE, Davidson RT, Cartee GD. Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle. Am J Physiol Endocrinol Metab 285: E693–E700, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCurdy CE, Davidson RT, Cartee GD. Calorie restriction increases the ratio of phosphatidylinositol 3-kinase catalytic to regulatory subunits in rat skeletal muscle. Am J Physiol Endocrinol Metab 288: E996–E1001, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Murray JT, Campbell DG, Peggie M, Mora A, Cohen P. Identification of filamin C as a new physiological substrate of PKBalpha using KESTREL. Biochem J 384: 489–494, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Neill HM. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab J 37: 1–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 1: 771–783, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pires RC, Souza EE, Vanzela EC, Ribeiro RA, Silva-Santos JC, Carneiro EM, Boschero AC, Amaral ME. Short-term calorie restriction improves glucose homeostasis in old rats: involvement of AMPK. Appl Physiol Nutr Metab 39: 895–901, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93: 993–1017, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123: 2764–2772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA 97: 10832–10837, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121: 4281–4288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweitzer GG, Arias EB, Cartee GD. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J Appl Physiol 113: 1852–1861, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sequea DA, Sharma N, Arias EB, Cartee GD. Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats. J Gerontol A Biol Sci Med Sci 67: 1279–1285, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sequea DA, Sharma N, Arias EB, Cartee GD. Greater filamin C, GSK3alpha, and GSK3beta serine phosphorylation in insulin-stimulated isolated skeletal muscles of calorie restricted 24 month-old rats. Mech Ageing Dev 134: 60–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma N, Arias EB, Bhat AD, Sequea DA, Ho S, Croff KK, Sajan MP, Farese RV, Cartee GD. Mechanisms for increased insulin-stimulated Akt phosphorylation and glucose uptake in fast- and slow-twitch skeletal muscles of calorie-restricted rats. Am J Physiol Endocrinol Metab 300: E966–E978, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma N, Arias EB, Sequea DA, Cartee GD. Preventing the calorie restriction-induced increase in insulin-stimulated Akt2 phosphorylation eliminates calorie restriction's effect on glucose uptake in skeletal muscle. Biochim Biophys Acta 1822: 1735–1740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma N, Sequea DA, Castorena CM, Arias EB, Qi NR, Cartee GD. Heterogeneous effects of calorie restriction on in vivo glucose uptake and insulin signaling of individual rat skeletal muscles. PLoS One 8: e65118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka S, Hayashi T, Toyoda T, Hamada T, Shimizu Y, Hirata M, Ebihara K, Masuzaki H, Hosoda K, Fushiki T, Nakao K. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism 56: 1719–1728, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 20: 271–284, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Zhang RY, Song J, Guan YF, Xu TY, Du H, Viollet B, Miao CY. Loss of AMP-activated protein kinase-alpha2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle. Diabetes 61: 1051–1061, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZQ, Floyd ZE, Qin J, Liu X, Yu Y, Zhang XH, Wagner JD, Cefalu WT. Modulation of skeletal muscle insulin signaling with chronic caloric restriction in cynomolgus monkeys. Diabetes 58: 1488–1498, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao Y, Sharma N, Arias EB, Castorena CM, Cartee GD. A persistent increase in insulin-stimulated glucose uptake by both fast-twitch and slow-twitch skeletal muscles after a single exercise session by old rats. Age (Dordr) 35: 573–582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J 413: 201–215, 2008. [DOI] [PubMed] [Google Scholar]