Abstract
Human islet research is providing new insights into human islet biology and diabetes, using islets isolated at multiple US centers from donors with varying characteristics. This creates challenges for understanding, interpreting, and integrating research findings from the many laboratories that use these islets. In what is, to our knowledge, the first standardized assessment of human islet preparations from multiple isolation centers, we measured insulin secretion from 202 preparations isolated at 15 centers over 11 years and noted five distinct patterns of insulin secretion. Approximately three quarters were appropriately responsive to stimuli, but one quarter were dysfunctional, with unstable basal insulin secretion and/or an impairment in stimulated insulin secretion. Importantly, the patterns of insulin secretion by responsive human islet preparations (stable Baseline and Fold stimulation of insulin secretion) isolated at different centers were similar and improved slightly over the years studied. When all preparations studied were considered, basal and stimulated insulin secretion did not correlate with isolation center, biological differences of the islet donor, or differences in isolation, such as Cold Ischemia Time. Dysfunctional islet preparations could not be predicted from the information provided by the isolation center and had altered expression of genes encoding components of the glucose-sensing pathway, but not of insulin production or cell death. These results indicate that insulin secretion by most preparations from multiple centers is similar but that in vitro responsiveness of human islets cannot be predicted, necessitating preexperimental human islet assessment. These results should be considered when one is designing, interpreting, and integrating experiments using human islets.
Keywords: human, islet, function
the availability of human islets for basic and translational research has increased markedly over the past decade, fueling insights into human islet biology and diabetes. Studies of human islets have provided insight into human islet morphology, β-cell proliferation (2, 5, 6, 12), epigenetics (2, 4–6, 12, 22), regulation of insulin secretion (9, 25, 28, 29), nutrient-induced toxicity (10, 23, 36), transcription factor regulation (10, 14, 16), and transplantation of human islet cells (1, 5, 26). For example, human β-cells proliferate at much lower frequency in vivo than mouse β-cells (24, 27) and respond to different regulators than mouse β-cells (2, 18). Such advances in our understanding have spurred interest in research with human islets and the demand for human islet tissue.
In the United States, human islets are currently available for research via the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-supported Integrated Islet Distribution Program (IIDP; https://iidp.coh.org), which replaced the National Islet Cell Resource Center Consortium (ICR) in 2009. Human islets isolated at IIDP-affiliated isolation centers are shipped overnight to recipient laboratories (20). Both the number of investigators applying to receive human islets and the total number of requested islets have risen dramatically (21). Along with each islet preparation, the isolation center provides metrics of the isolation procedure, e.g., cold ischemic time, estimated culture time, and purity and viability of the islets, as well as deidentified donor information (age, Sex, BMI, race) that is released by the organ procurement organization.
Currently, there are no accepted standards to uniformly evaluate and report the health and function of human islets prior to experiments, to present data from multiple islet preparations from different isolation centers, or to compare the human islet data from different laboratories. Human islet research uses islets from donors of different ages, sexes, BMIs, and races, that are isolated at isolation centers with different personnel and then shipped to investigators across the US. Certain donor attributes and isolation conditions may correlate with higher or lower islet yield upon isolation (19, 32, 37), but little is known about these potential relationships. Furthermore, how or whether to assess the health and function of health of islet preparations prior to study is not standardized. Some laboratories assess human islet health and/or function using methods such as measurement of oxygen consumption (7, 35) or insulin release in response to stimuli (3, 10), among others (15, 16). Although studies have examined the relationship between donor attributes and insulin release in the context of a single isolation center (9), little is known about insulin secretion compared among islet preparations from different isolation centers, which reflects how human islet research is currently being conducted in most research laboratories.
To define the functional variability in human islet preparations being used for research in the United States, we analyzed categorical and functional data from 202 human islet preparations distributed by the IIDP/ICR, many which were also used by other investigators. Functional data were obtained via islet perifusion, a method that assesses integrated β-cell function with high temporal resolution and in sequential response to multiple secretagogues (3). Our studies indicate that the majority of islet preparations from 15 centers are functional, appropriately secreting insulin in response to two stimuli. However, a sizeable minority of preparations was dysfunctional. These studies suggest necessary considerations for conducting, reporting, and interpreting research with human islets.
RESEARCH DESIGN AND METHODS
Human islet acquisition and assessment.
Human islets were received by overnight shipment from centers supported by the National Institutes of Health (NIH), the Integrated Islet Distribution Program (IIDP, iidp.coh.org), Juvenile Diabetes Research Foundation (JDRF), or the Islet Cell Resource Centers (icr.coh.org), during the years 2002–2013. Islet preparations originated from the centers listed in the section below (Isolation centers). Decisions regarding which islet offers to accept were made based on experimental needs of our laboratory; we did not favor, request, or decline islets from a particular center. Islets were shipped overnight to Vanderbilt (20) and plated into 15 ml of CMRL1066 medium at a density of 12,000–15,000 IEQ per 10-cm nontreated tissue culture dish (Corning, Corning, NY; cat. no. 430591). From 2002 to 2007, 100 islets were perifused, and in each case an islet equivalent (IEQ) value was calculated (10). Since 2006, 60 islets of 180 μm diameter (∼104 IEQs) have been used. Data from all years were normalized to 100 IEQ. Islets were handled and perifused as described (10). Importantly, all human islet preparations were hand-picked in our laboratory prior to perifusion, making the purity of human islets during perifusion higher than the purity reported by the isolation center. Human insulin was measured with an insulin radioimmunoassay from Millipore (cat. no. RI-13K) (10). Some perifusion profiles were part of previously published datasets (5, 6, 10, 14).
Individual isolation centers have performed static culture of isolated human islets and now regularly report these data to the IIDP (some of these data are presented in Fig. 4H, thanks to the assistance of Barbara Olack). The IIDP-published protocol for this static culture (QA-005 Potency Test: Glucose Stimulated Insulin Release Assay) can be found at https://iidp.coh.org/investigator_sops.aspx. Static culture assays performed in our laboratory (Fig. 4I) measured insulin secretion from 60 size-matched islet into RPMI medium over 1 h at 37°C. Previously published data points (open squares) reflect the stimulation index of secretion at 11 mM glucose divided by secretion at 5 mM glucose (10). New data points (closed squares) reflect the stimulation index of secretion at 16.7 mM glucose divided by secretion at 5.6 mM glucose.
Fig. 4.
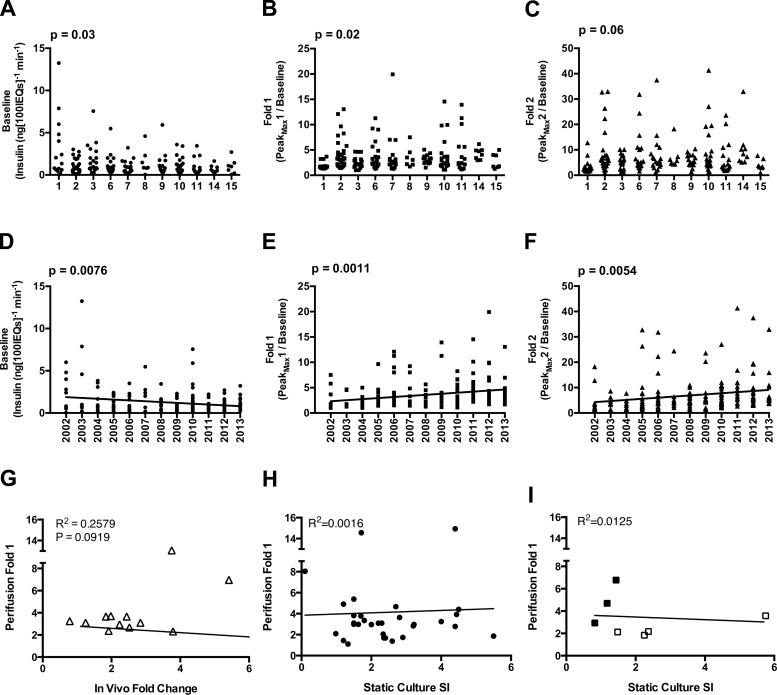
Effects of isolation Year and Center on in vitro and in vivo responsiveness. A–C: univariate analyses of Center vs. Baseline (A), Fold 1 (B), and Fold 2 (C). D–F: linear regression analysis of Year vs. Baseline (D), Fold 1 (E), and Fold 2 (F). G: plot of Fold 1 values from perifusion (Perifusion Fold 1) against in vivo Fold change, measured via glucose-arginine stimulation. Basal human insulin values measured in mouse plasma after 6-h fast. Stimulated insulin values measured 15 min after injection of glucose-arginine; n = 12. H: Perifusion Fold 1 values graphed against static culture stimulation index (SI), the ratio of insulin secretion at high glucose to secretion at low glucose, as reported by isolation centers to the IIDP; n = 30. I: plot of Perifusion Fold 1 vs. Static Culture SI from static culture performed in our laboratory. Previously published data points are represented by open squares (10); newly procured data points are closed squares (see research design and methods).
Isolation centers.
Islets in these studies were procured from the following isolation centers (in alphabetical order): Emory University (Atlanta, GA), National Institutes of Health (Bethesda, MD), Northwestern University (Chicago, IL), Scharp Lacy Research Institute (Irvine, CA), Southern California Islet Consortium (City of Hope, Duarte, CA), University of Alabama (Birmingham, AL), University of Colorado (Denver, CO), University of Illinois (Chicago, IL), University of Massachusetts (Worcester, MA), University of Miami (Miami, FL), University of Minnesota (Minneapolis, MN), University of Pennsylvania (Philadelphia, PA), University of Pittsburgh (Pittsburgh, PA), University of Wisconsin (Madison, WI), and Washington University (St. Louis, MO). The order of this list has no relation to Centers 1–15.
Definition of donor and islet attributes.
“Donor attributes,” characteristics of the human pancreas donor reported by the Organ Procurement Organization (OPO) to the islet isolation center, and “Islet attributes,” characteristics of isolated islet preparations that the IIDP/ICR reports to investigators, are listed in Table 1. Protocols for viability and purity quantification are available on the IIDP website at http://iidp.coh.org/investigator_sops.aspx.
Table 1.
Donor and islet attributes and possible values
| Attribute | Possible Values | Actual Value Range |
|---|---|---|
| Donor attributes | ||
| Center | #1–15 | |
| Year | 2002–2013 | |
| Age (of donor) | Continuous (years) | 7–74 yr |
| Sex | Male/Female | |
| Race* | Caucasian, African American, Hispanic | |
| BMI | Continuous (kg/m2) | 15.2–53.2 kg/m2 |
| Islet attributes | ||
| Cause of Death | Categorical | |
| Ischemic Time† | Continuous (hours) | 2–23 h |
| Culture Time‡ | Continuous (days) | 0–7 days |
| Viability§ | Continuous, 1–100% | 64–99% |
| Purity‖ | Continuous, 1–100% | 40–99% |
Reported as Caucasian, African American, Hispanic, or Asian.
Time of cold ischemia, from time of aortic cross clamp to either pancreas trimming, initial collagenase injection, or start of digestion.
Estimated culture time prior to shipment.
Percentage of viable cells in preparation;
Percentage of dithizone-positive cells in preparation. All information provided by islet isolation centers.
Definition of perifusion attributes.
“In vitro responsiveness” was defined by Baseline (the insulin concentration of the last fraction collected before introduction of 16.7 mM glucose), Peak1Max (highest point of the Peak in response to 16.7 mM glucose), Peak2Max (highest point of the Peak in response to 16.7 mM glucose + IBMX), Fold 1 (Peak1Max/Baseline), Fold 2 (Peak2Max/Baseline), and Peak Difference (Peak2Max − Peak1Max). A Peak has a collected fraction with an insulin concentration more than 1.5 times that of the Baseline value.
Statistical analyses.
The criterion of isolation centers with seven or more islet preparations for further analysis was established by examining the distribution of islet preparations per center. We chose the cut-off point of seven as it provided the best balance between observations per center and number of different centers examined. To study the full spectrum of individual attributes at each site required a control for any potential effect of the center and a sufficient number of islet preps from each center. For univariate analyses, we used a Wilcoxon rank sum test to assess differences between the distributions of islet attributes for each donor attribute. This nonparametric test makes no assumptions about the normality of the islet attribute. We compared our univariate results to an analysis of variance (ANOVA) of log-transformed islet attributes and found no meaningful differences; thus, for all further adjusted analyses, we continued with the log-transformed ANOVA approach, which provides easier interpretation and the ability to adjust for covariates. We also examined the impact of variable missingness on all islet attributes, treating missingness as a categorical variable; this revealed no relationships between missingness and islet attributes. All categorical variables (Center, Race, Sex, Cause of Death, Estimated Culture Time, and Year) were treated as factors. Continuous variables (Cold Ischemia Time, age, BMI, Viability, and Purity) were modeled as a continuous variable in linear regression and were also binned into quantiles and treated as a categorical variable in an ANOVA. For univariate analyses, statistical significance (calculation of a P value) was assessed by a Student's t-test of the regression coefficient. For adjusted analyses, significance was assessed by a one-degree-of-freedom likelihood ratio test comparing a full model (with covariate and variable of interest) to a reduced model (with the covariate only). Polytomous regression was conducted to examine pairwise differences between categorical outcomes. Individual perifusion data points (24 per islet preparation, fractions 7–30) were modeled using a nonlinear mixed-effects model with an eight-knot spline function. Eight knots were chosen to optimally capture the characteristic features of the islet response curve while preserving degrees of freedom. This analysis hierarchically fits an islet response curve separately within each categorical group of the analysis, allowing qualitatively different curve fits within each group. The spline analyses were performed assessing the effects of categorical variables, allowing different insulin secretion response curves to be fitted within different categories of the variable. Continuous variables were not modeled in this way, because selecting cut-off values to generate categorical “bins” would not be biologically informed and would significantly reduce statistical power. Statistical significance for these analyses was assessed by a likelihood ratio test comparing a full model (with one random effect for the variable of interest and one for the individual) to a reduced model (with a random effect for individual alone). All statistical analyses were conducted using R 3.0.1, packages (nlme, lmeSplines), and functions (lm, glm, aov, and lme). The procedures used for this modeling are available upon request. Linear regression analyses for Fig. 4 and ANOVAs for Fig. 6 were performed using Prism v. 6.0d.
Fig. 6.
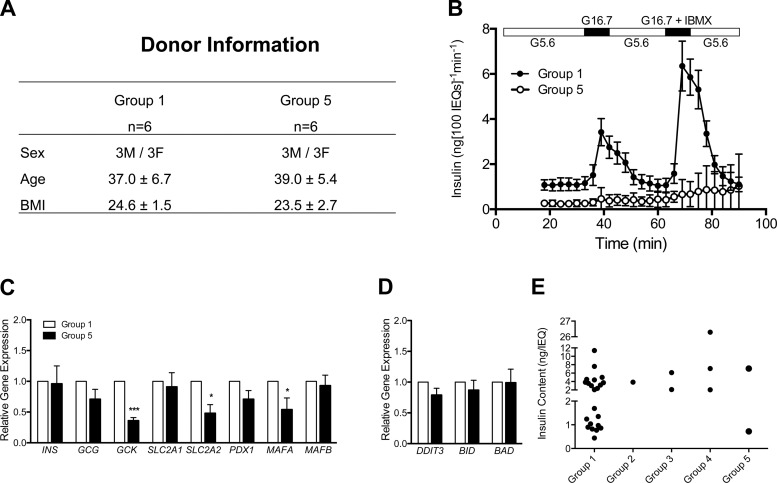
Gene expression in Group 1 and Group 5 islets. A: islets from 6 Group 1 and 6 Group 5 preparations were matched for Sex, Age, and BMI. B: perifusion results. Plotted insulin concentration (ng/100 IEQ/min) values for all collected media fractions, n = 6 for each group. These Group 1 preparations were a subset of a previously published data set (10). C and D: expression of islet-enriched (C) and apoptosis (D) genes quantified by RT-PCR. Gene transcript levels expressed relative to Group 1 values, n = 6 for each group. E: insulin content (ng/IEQ) of aliquots from 30 human islet preparations, separated by response group (Groups 1–5) (not same 30 islet preparations as in Fig. 4H).
Human islet transplantation and assessment of in vivo function.
Human islets (ranging from 500 to 2,000 islet equivalents) were transplanted into immunodeficient mice as described previously (5). These transplantation studies were performed for other projects, not specifically for these analyses. In vivo insulin secretion by human islets (2–5 wk after transplantation) was assessed both before and 15 min after glucose-arginine IP injection following a 6-h fast. Each animal received a 500-μl injection containing 62.5 mg dextrose and 62.5 mg l-arginine (Sigma-Aldrich, St. Louis, MO). All in vivo insulin secretion data used in these analyses reflect human islets that were transplanted into normoglycemic mice on regular chow diet. Plasma human insulin content in blood samples was measured using a human-specific radioimmunoassay (Millipore cat. no. HI-14K) by the Vanderbilt Hormone Assay & Analytical Services Core or by our laboratory and normalized to the number of islet equivalents transplanted. All animal studies were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Quantitative RT-PCR.
Quantitative RT-PCR of total cellular RNA was performed as previously described (10, 14) using Applied Biosystems primers, following the MIQE guidelines (17).
RESULTS
Influence of donor and islet attributes.
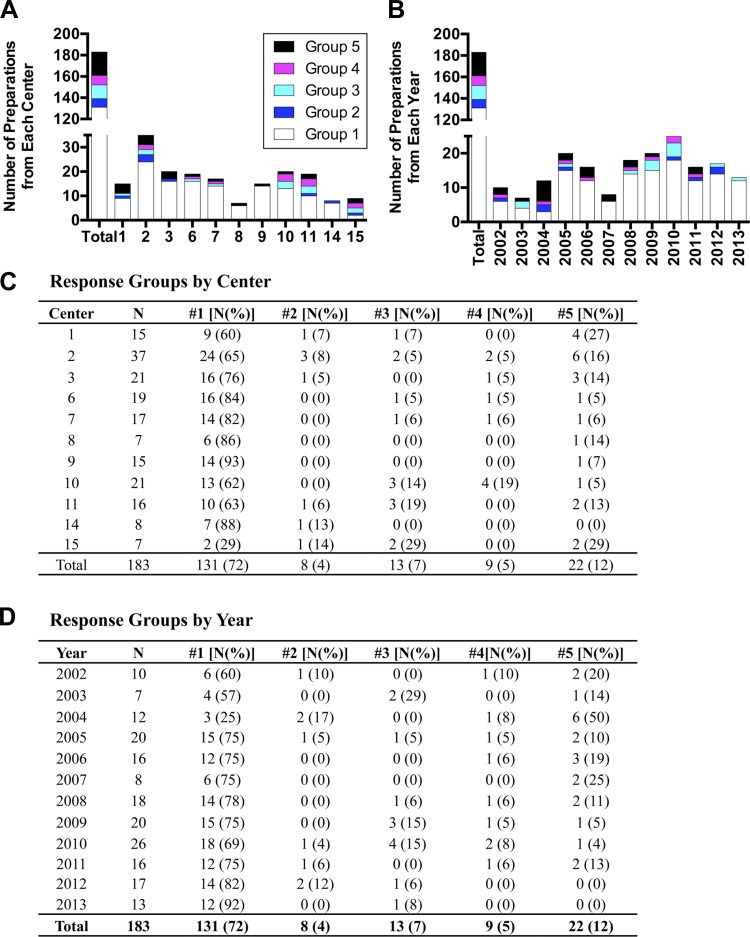
To characterize the influence of donor and islet attributes on human islet preparations used for studies in our laboratory, we hand picked (Fig. 1B) and assessed the insulin-secretory profile of 202 human islet preparations from 15 islet isolation centers during the years 2002–2013, using a dynamic cell perifusion system (Fig. 1A). We first grouped and examined attribute values (Table 1) by isolation center. In the 202 islet preparations, most donor (Table 2) and islet attributes (Table 3) had similar values and ranges among the majority of centers, with one or two centers contributing significant variation. To reduce bias from differences in sample size across centers, we chose for further analysis (beyond statistical summarization) only centers that provided seven or more preparations, leaving 183 islet preparations from 11 centers (Centers 1, 2, 3, 6, 7, 8, 9, 10, 11, 14, and 15) for subsequent analysis.
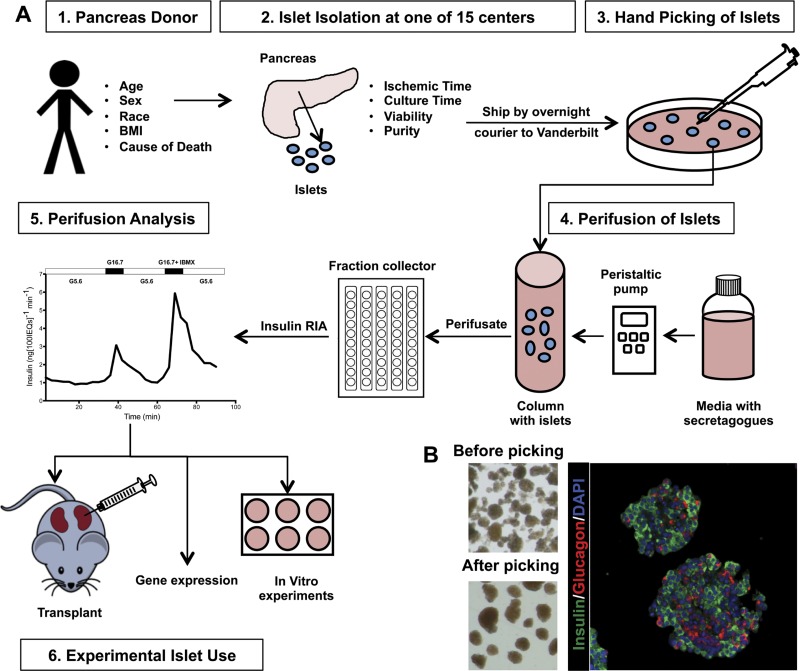
Fig. 1.
Order of events for assessing human pancreatic islets. A: islets isolated from donor pancreata at isolation centers were shipped by overnight courier to Vanderbilt, where they were hand-picked for further purity and IEQ quantification. Islets were perifused to assess in vitro function. Islets were used for subsequent studies that are not part of the current report. B: images showing a human islet preparation before (top left) and after (top right) hand picking. Immunolabeling of human islets for DAPI (blue), insulin (green), and glucagon (red) embedded in collagen gel (far right).
Table 2.
Summary of donor attributes
| Center | Total n | Age, yr*† | n | Sex (M/F)‡ | Race (C/A/H) | BMI, (kg/m2)* | n |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 54.0 ± 2.8 | 2 | 0/2 | 0/0/0 | 33 | 1 |
| (52–56) | |||||||
| 2 | 37 | 38.5 ± 12.6 | 35 | 19/16 | 25/3/3 | 28.8 ± 6.1 | 35 |
| (20–64) | (16.9–46.6) | ||||||
| 3 | 21 | 49.2 ± 10.9 | 12 | 4/8 | 6/0/2 | 26.6 ± 4.2 | 11 |
| (23–65) | (18.0–34.2) | ||||||
| 4 | 6 | 40.4 ± 13.4 | 5 | 3/2 | 4/0/1 | 31.4 ± 5.1 | 5 |
| (24–53) | (24.1–37.9) | ||||||
| 5 | 6 | 42.4 ± 12.3 | 5 | 2/1 | 2/0/0 | 33.2 ± 1.7 | 3 |
| (22–53) | (31.4–34.6) | ||||||
| 6 | 19 | 42.2 ± 13.4 | 18 | 10/8 | 2/0/0 | 28.5 ± 8.3 | 18 |
| (18–58) | (16.1–45.0) | ||||||
| 7 | 17 | 48.2 ± 12.4 | 17 | 9/7 | 9/0/1 | 29.6 ± 5.6 | 17 |
| (26–69) | (22.3–40.9) | ||||||
| 8 | 7 | 31.6 ± 14.1 | 6 | 2/3 | 4/1/0 | 33.4 ± 9.9 | 6 |
| (17–48) | (23.3–48.4) | ||||||
| 9 | 15 | 45.8 ± 16.2 | 12 | 6/6 | 10/0/2 | 36.1 ± 11.8 | 12 |
| (19–64) | (21.6–52.4) | ||||||
| 10 | 21 | 40.2 ± 14.7 | 19 | 10/9 | 15/1/2 | 27.0 ± 4.9 | 19 |
| (11–56) | (18.3–37.8) | ||||||
| 11 | 16 | 50.4 ± 15.4 | 16 | 6/6 | 4/0/1 | 28.5 ± 5.1 | 16 |
| (20–74) | (20.3–39.3) | ||||||
| 12 | 3 | 53.0 ± 5.6 | 3 | 1/2 | 1/2/0 | 25.2 ± 4.2 | 3 |
| (48–59) | (21.4–29.8) | ||||||
| 13 | 4 | 18.0 ± 9.6 | 3 | 3/1 | 0/2/0 | 21.0 ± 8.2 | 2 |
| (7–25) | (15.2–26.8) | ||||||
| 14 | 8 | 43.7 ± 15.7 | 7 | 4/3 | 7/0/0 | 35.7 ± 8.5 | 7 |
| (26–60) | (28.7–53.2) | ||||||
| 15 | 7 | 39.8 ± 6.9 | 4 | 3/1 | 1/0/0 | 31.6 ± 4.2 | 4 |
| (28–46) | (26.0–37.4) | ||||||
| Total | 202 | 42.9 ± 14.1 | 163 | 82/74 | 90/9/12 | 29.6 ± 7.1 | 158 |
| (7–74) | (15.2–53.2) |
Values are means ± SD;
range (min-max);
n, number of preparations with that attribute reported, used to calculate mean, SD, and range for that attribute.
Table 3.
Summary of islet attributes
| Center | Total n | Cold Ischemic Time, h*† | n‡ | Est. Culture Time, days | n‡ | Viability, % | n‡ | Purity, % | n‡ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 13.0 ± 1.4 | 2 | NR§ | 0 | NR | 0 | NR | 0 |
| (12–14) | |||||||||
| 2 | 37 | 12.9 ± 5.2 | 34 | 2.2 ± 1.4 | 31 | 90.4 ± 6.0 | 27 | 87.3 ± 9.9 | 34 |
| (3–23) | (1–7) | (70–99) | (40–97) | ||||||
| 3 | 21 | 14.2 ± 3.2 | 10 | 2.1 ± 0.7 | 10 | 89.3 ± 8.1 | 10 | 84.5 ± 7.3 | 11 |
| (11–20) | (1–3) | (70–96) | (70–95) | ||||||
| 4 | 6 | 6.0 ± 2.8 | 4 | 3.0 ± 2.4 | 5 | 77.4 ± 12.1 | 5 | 60.6 ± 7.4 | 5 |
| (2–8) | (1–7) | (64–97) | (55–73) | ||||||
| 5 | 6 | 8.4 ± 4.4 | 5 | 2.5 ± 0.6 | 4 | 85.5 ± 5.8 | 4 | 72.5 ± 9.6 | 4 |
| (2–13) | (2–3) | (77–90) | (60–80) | ||||||
| 6 | 19 | 10.1 ± 3.2 | 17 | 1.5 ± 0.6 | 4 | 88.5 ± 8.8 | 8 | 72.2 ± 7.1 | 9 |
| (5–15) | (1–2) | (70–98) | (60–85) | ||||||
| 7 | 17 | 4.0 ± 2.8 | 2 | 1.8 ± 1.6 | 11 | 92.8 ± 6.2 | 13 | 77.5 ± 13.1 | 15 |
| (2–6) | (0–6) | (75–98) | (40–90) | ||||||
| 8 | 7 | 9.3 ± 1.3 | 5 | 4.6 ± 0.9 | 6 | 97.7 ± 2.3 | 4 | 75.0 ± 12.3 | 6 |
| (8–11) | (4–6) | (95–99) | (60–90) | ||||||
| 9 | 15 | 6.8 ± 2.5 | 11 | 2.4 ± 1.7 | 12 | 97.1 ± 2.9 | 8 | 80.8 ± 19.7 | 12 |
| (3–11) | (1–7) | (91–99) | (40–99) | ||||||
| 10 | 21 | 8.6 ± 2.9 | 19 | 1.6 ± 1.1 | 18 | 90.9 ± 4.7 | 17 | 85.6 ± 6.0 | 19 |
| (3–16) | (1–5) | (79–97) | (70–95) | ||||||
| 11 | 16 | 10.4 ± 2.7 | 15 | 3.2 ± 1.5 | 10 | 93.0 ± 3.3 | 15 | 74.5 ± 16.5 | 15 |
| (4–14) | (2–7) | (86–96) | (43–95) | ||||||
| 12 | 3 | 5.7 ± 3.1 | 3 | 4.0 ± 1.0 | 3 | 91.3 ± 6.5 | 3 | 82.5 ± 17.7 | 2 |
| (3–9) | (3–5) | (85–98) | (70–95) | ||||||
| 13 | 4 | 10.0 | 1 | 2.7 ± 3.1 | 3 | 96.0 ± 14.1 | 2 | 95.0 | 2 |
| (0–6) | (95–97) | ||||||||
| 14 | 8 | 6.7 ± 1.9 | 7 | 1.8 ± 1.8 | 8 | 93.4 ± 4.0 | 7 | 92.1 ± 5.7 | 7 |
| (3–9) | (1–6) | (88–98) | (80–95) | ||||||
| 15 | 7 | 10.3 ± 1.9 | 3 | 1.6 ± 0.6 | 4 | 94.0 ± 1.2 | 4 | 89.2 ± 2.4 | 4 |
| (9–13) | (1–2) | (92–95) | (85–91) | ||||||
| Total | 202 | 10.1 ± 4.3 | 137 | 2.3 ± 1.5 | 128 | 91.2 ± 6.8 | 126 | 81.6 ± 13.0 | 144 |
| (2–23) | (0–7) | (64–99) | (40–99) |
Values are means ± SD;
range (min-max);
n, number of preparations with that attribute reported, used to calculate mean, SD, and range for that attribute; §NR, not reported.
Grouping of islet preparations by in vitro response.
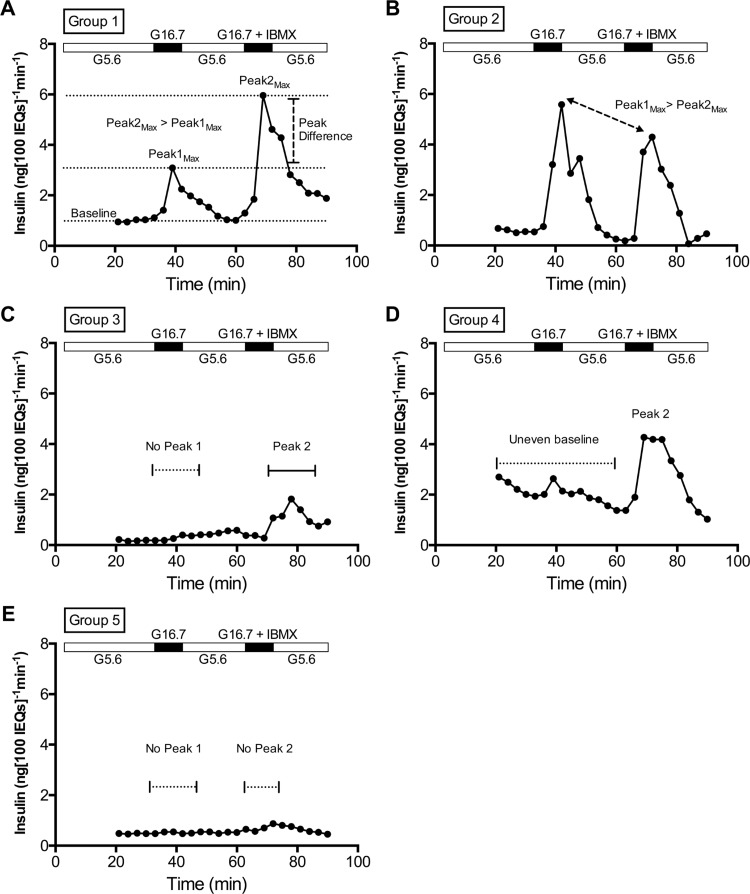
The shape of the perifusion response (insulin secretion) curves varied among preparations, suggesting that combining all data sets could veil biological differences and the contribution of other factors to islet secretion. Among 183 preparations, we noted five recurring in vitro insulin secretion patterns, defined as follows (Fig. 2): Group 1 preparations: stable Baseline at 5.6 mM glucose, well-defined Peaks in response to both 16.7 mM glucose and 16.7 mM glucose + IBMX (denoted by both Fold 1 and Fold 2 exceeding 1.5), and a Peak2Max that was higher than Peak1Max (Fig. 2A). Group 2: Peak1Max that was higher than Peak2Max (Fig. 2B); Group 3: no Peak in response to 16.7 mM glucose (Fold 1 < 1.5) but a Peak in response to 16.7 mM glucose + IBMX (Fold 2 > 1.5) (Fig. 2C); Group 4: unstable Baseline at 5.6 mM glucose (Fig. 2D); Group 5: no response to either stimulus (Fold 1 and Fold 2 < 1.5) (Fig. 2E). The majority of preparations (72%) were in Group 1 (Fig. 3, C and D) despite representing a variety of Centers, Years, donor attributes, and islet attributes. However, 12% of preparations were in Group 5, and the remaining 16% were in Groups 2, 3, or 4 (Fig. 3, C and D). Thus, caution is appropriate when making assumptions about performance of a specific human islet preparation.
Fig. 2.
Definitions of in vitro response groups. Perifusion of human islets with the following order of stimuli in media: 5.6 to 16.7 mM glucose, back to 5.6 mM glucose, then to 16.7 mM glucose with IBMX. From the entire body of perifusion data, 5 general response groups emerged. A–E: real curves from representative preparations, illustrating characteristics of each Group. A: Group 1 had 2 stimulation peaks (16.7 mM glucose + IBMX induces a higher Peakmax than 16.7 mM glucose alone) and a stable Baseline. B: Group 2 differed from Group 1 by having a higher Peak1max (in response to 16.7 mM glucose) than Peak2max (in response to 16.7 mM glucose + IBMX). C: Group 3 had no Peak1 but did have a Peak2. D: Group 4 had an uneven Baseline but has one or both Peaks. E: Group 5 was considered nonresponsive, because it had neither Peak1 nor Peak2.
Fig. 3.
Distribution of response groups among isolation centers and across year of isolation. Distribution of response groups by Center (A) or Year (B), and actual values for Center (C) and Year (D).
Distribution of islet response groups.
We next examined potential reasons for the variability in stimulated insulin secretion. The distribution of preparations among response Groups 1–5 was not influenced by Center (Fig. 3A) or Year (Fig. 3B). To determine whether donor or islet attributes correlates with a particular response group, we compared Group 1 with Group 5 and then searched for attributes associated with an increased probability of any group. Race was the only factor that influenced probability of Group 1 vs. Group 5 (P = 0.007), which was also demonstrated by polytomous regression analysis (Fig. 5D). No other donor or islet attributes influenced group.
Fig. 5.
Fitted spline analysis of perifusion data. A–C: smoothed average curve fits for response by (A) Center, (B) Year, and (C) Cause of Death. D: fitted differences between Group 1 and Group 5 islet preparations by Race (Caucasian and African American). HT, head trauma; ICHem, intracerebral hemorrhage; SAHem, subarachnoid hemorrhage; Anox, anoxia; CVA, cerebrovascular accident; MVA, motor vehicle accident; GSW, gunshot wound; GT, general trauma; CArr, cardiac arrest.
Univariate analysis of donor and islet variables.
To assess whether donor and islet attributes affected in vitro secretion, we focused on Group 1 islet preparations, because attributes of Groups 2–5 (uneven Baseline or lack of Peak1, Peak2, or both) might obscure an association between donor or islet attributes and secretion response. Within Group 1 preparations specifically, both Center and Year influenced Baseline (Fig. 4, A and D, respectively), and Center influenced Fold 1 (Fig. 4B). The relationship between Center and Fold 2 suggested a trend but did not meet statistical significance (Fig. 4C; P = 0.06). There was a linear relationship of decreasing Baseline and increasing Fold 1 and Fold 2 as Year increased (Fig. 4, D–F). Linear regression analysis revealed that these relationships were significant (P = 0.008, 0.001, and 0.005, respectively).
We adjusted all subsequent analyses for Center or Year, removing the contribution of each variable (adjusting for both Center and Year simultaneously was not possible due to loss of statistical power). When adjusted for Center, Baseline was influenced by Purity (P = 0.048) and Year (P = 0.005), Fold 1 was marginally influenced by Year (P = 0.051), and Fold 2 was influenced by Cause of Death (0.016). When adjusted for Year, Fold 2 was influenced by BMI (P = 0.045). These results indicate that (1) Baseline and Fold 2 may be influenced by separate variables, (2) Baseline decreases and Fold changes increase linearly with increasing Year, and (3) when Center or Year is controlled for, only Purity, Cause of Death, and BMI influenced any measure of the in vitro response. This suggests that in vitro responses of islet preparations available for research are improving over time (becoming more similar to a Group 1 curve, with low Baseline and large Fold increases).
In vitro stimulated insulin secretion does not correlate with in vivo function of responsive islet preparations.
To address whether in vitro stimulated insulin secretion predicts in vivo function, we assessed 12 Group 1 preparations that were transplanted as part of other projects. For the 12 transplanted preparations, the average in vivo fold change from basal to stimulated insulin was 2.46 ± 0.38. There was a poor linear relationship between Fold 1 (derived from perifusion) and in vivo fold change (derived from in vivo glucose-arginine stimulation; Fig. 4G) among these 12 preparations. Twelve transplanted islet preparations did not provide sufficient statistical power to detect an effect of donor or islet attributes on measures of in vivo insulin secretion.
Comparison of static culture and perifusion measures of stimulation insulin release.
To compare perifusion and static culture as methods for functional islet assessment, we plotted static culture stimulation indexes (reported by the isolation centers) against Perifusion Fold 1 responses (measured in our laboratory) of 30 human islet preparations. We observed no linear correlation between the two measures (Fig. 4H), indicating that static culture does not predict stimulated insulin secretion by islet perifusion. We then analyzed seven preparations for which both assays were conducted in our laboratory, on the same day (Fig. 4I). This analysis yielded the same result, namely that stimulated insulin values do not correlate well between perifusion and static culture.
Modeling of insulin secretion as assessed by perifusion.
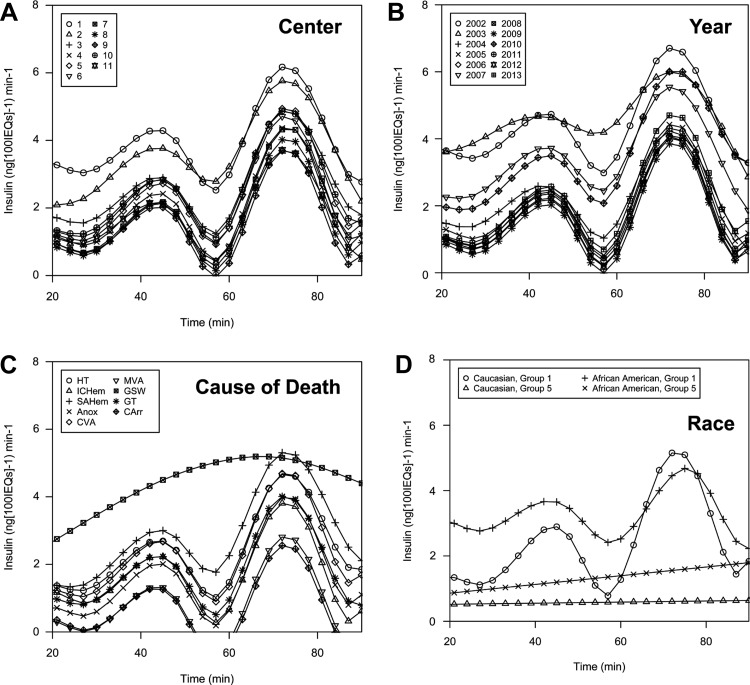
To graphically represent the effects of significant attributes uncovered in our univariate analyses, we fit splines (curves) of the raw combined perifusion data for all preparations using nonlinear mixed effect models, which produce representative curves separated by different attributes of interest, such as Center, Year, and Cause of Death. Splines are defined by the full complement of in vitro data points, but they have smoothed shapes that ease visual interpretation and reduce degrees of freedom. Differences in secretion correlated with Center (Fig. 5A) and Year (Fig. 5B), in that two centers had a higher Baseline and PeakMax values than the majority. However, Centers had similar average curve shapes. Baseline and PeakMax values generally decreased with Year, but Fold changes increased, as observed in our linear regression analyses (Fig. 4, D–F). Cause of Death (Fig. 5C) is the single attribute that affected Baseline, Peak1Max, and Peak2Max values as well as the shape of the curve (such as the width of Peak2 and the consistency of the Baseline), by way of fitted spline modeling. Cardiac arrest (CArr) and motor vehicle accident (MVA) were associated with lower Baseline, Peak1Max, and Peak2Max than other Causes of Death, and subarachnoid hemorrhage with the highest. It remained unclear whether Cause of Death biologically impacts islets or is associated with an attribute that does. The relationship between Race and response Group (Fig. 5D) revealed that, within Group 1 preparations, African American preparations had smaller average Fold changes than Caucasian preparations and that Baseline of Group 5 preparations was higher if from African American donors. Race did not significantly influence any individual in vitro measures but was clearly related to the response Group and the shape of the curve in both Group 1 and Group 5 islet preparations. Collectively, these analyses both confirm our polytomous regression results that Race impacted likelihood of Group 1 vs. Group 5 response type and revealed the influence of Cause of Death on in vitro response (on Baseline, PeakMax values, and curve shape).
Gene expression differences between Group 1 and Group 5 islets.
To investigate the reason for functional differences between Group 1 and Group 5 preparations, we compared expression of key islet-enriched genes in preparations matched for Age, Sex, and BMI (Fig. 6, A and B). Transcript levels of GLUT2 (SLC2A2), glucokinase (GCK), and MafA (MAFA) were significantly lower in Group 5 islets (Fig. 6C), but insulin was similar (Fig. 6C), highlighting alterations in glucose sensing rather than in insulin production. Notably, there was no difference in the expression of apoptosis markers Chop, Bid, and Bad (Fig. 6D), indicating that a lack of response to stimuli is not simply due to apoptosis or β-cell death. We assessed insulin content from 30 human islet preparations (Fig. 6E) to address whether insulin content differed among the response groups,. The values and amount of variation appear similar among the five groups, with the two values for Group 5 (unresponsive) preparations calling within the range of values for Group 1 preparations. We were unable to perform an ANOVA due to the low frequency of Groups 2–5 preparations in the 30 preparations analyzed.
DISCUSSION
Research using human islets is providing new insight into human islet biology and diabetes. The fact that human islet preparations for research are isolated at multiple centers from donors with varying characteristics presents a challenge to understanding, interpreting, and integrating research findings that arise from multiple laboratories using these islets, as little is known about the variation among preparations. We report the first comprehensive, standardized assessment of human islet function by using preparations from multiple isolation centers. We used islet perifusion to assess human islet health and function, because it is more informative than static incubation, by allowing measurement of a sequential and temporal response to multiple secretagogues. We assessed insulin secretion from 202 human islet preparations from 15 centers over an 11-year period, and examined whether the variation among islet preparations related to biological differences or variability in islet isolation procedures.
We noted five recurring insulin secretion patterns (groups), defined by the degree and nature of responsiveness to two stimuli (16.7 mM glucose with or without IBMX). The five insulin secretion patterns (groups) suggest differences in the underlying biology of the preparations. For example, Group 2 preparations differed fromthose of Group 1 (ideal responders) by having a lower Peak2Max than Peak1Max, potentially indicating that insulin stores were depleted after stimulation with 16.7 mM glucose or that elevation of cAMP via the phosphodiesterase inhibitor IBMX is not a contributory mechanism for insulin secretion in these islets. Group 3 preparations lacked a Peak1 but maintained a modest Peak2, which suggests that cAMP may be the sole signaling mechanism for stimulated insulin secretion in these islets. Group 4 preparations had inconsistent basal secretion, evidencing unregulated release. Last, Group 5 preparations lacked both peaks, but these islets were not apoptotic, meaning that, because they may have normal insulin content, they may be able to recover functionality. The expression of genes encoding proteins in the glucose-sensing pathway is reduced in Group 5 islets, suggesting that these preparations are unhealthy in other ways, which requires further investigation. Although the retrospective nature of our study did not permit comparison of protein levels of these glucose-sensing genes between Group 1 and Group 5 islets, we note that, in prior work (10, 14), mRNA levels of human islet transcription factors correlated well with their respective protein levels.
Interestingly, neither islet attributes nor information from the islet isolation center predicted the likelihood of a preparation being in a particular response group. However, within Group 1 (highly responsive) human islet preparations specifically, both Center and Year did influence individual measures of insulin secretion (Baseline, Fold 1, and Fold 2). Overall, the pattern of insulin secretion in these preparations was remarkably similar among centers and across the years studied.
To limit the potential interrelatedness of Center and Year as variables, we controlled for either Center or Year and examined the effect of donor and islet attributes on Baseline, Fold 1, and Fold 2. In these controlled analyses, Cause of Death, Purity, and BMI influenced individual measures of insulin secretion (Fold 2, Baseline, and Fold 2, respectively). Each of these variables influenced only one of the three measures, which may simply highlight that basal, glucose-stimulated, and cAMP-mediated insulin secretion work via distinct mechanisms, or it may suggest that no attribute is potent enough to impact all three measures.
The influence of Center on individual measures of in vitro function may be partly procedural, or it may reflect the donor pool seen by that center (e.g., perhaps one center receives more organs from donors dying in motor vehicle accidents). Year of isolation is of interest for both procedural and practical reasons: not only because an influence of Year could stem from changes in personnel at isolation centers, standards of practice, or adherence to protocol among centers, but also because practical aspects of isolation have changed with time, such as changes in the lot number or provider of digestive enzymes. Our linear regression and spline modeling results indicate that in vitro insulin secretion from human islet preparations has improved over the years studied.
The assessment of 12 transplanted preparations with Group 1 response profiles demonstrated a poor correlation between in vitro and in vivo stimulation-induced changes in insulin secretion (Fig. 4G). It has previously been suggested that in vitro stimulated insulin secretion does not well predict in vivo graft function (33, 34), although those studies did not use perifusion as the in vitro assay. Conversely, a comparison of multiple quality control assessment methods, which did not include perifusion, found that only static islet stimulation identified preparations as being “good” or “poor,” based on their in vivo function (16). A limitation of our analysis is the lack of in vivo data from Group 5 preparations, because we deemed these not suitable for transplantation. A study directly comparing the relationship between in vitro and in vivo function of Group 1 and Group 5 preparations is needed to further address whether perifusion data can be useful for predicting in vivo function.
We used perifusion to assess in vitro islet function because it integrates β-cell function with high temporal resolution and allows sequential responses to multiple secretagogues. Static incubation is widely used to assess glucose-stimulated insulin secretion. However, our analyses (Fig. 4, H and I) indicate that stimulation of insulin secretion in static islet culture does not correlate with stimulated insulin secretion via perifusion. Other approaches used to assess islet health have included glucose-induced changes in oxygen consumption rate (7, 31, 34, 35), glucose-induced preproinsulin mRNA expression (30), and mitochondrial integrity (16). Given that these approaches, including islet perifusion, are not widely available and preexperimental human islet assessment is critical, a new approach for islet distribution programs is necessary. Perhaps every islet preparation should be perifused or assessed by the islet isolation center and this functional information provided to investigators receiving the islets for research.
We noted a significant relationship between human islet responsiveness and Cause of Death, observed by spline modeling, but this interpretation is complicated by interrelated variables. Despite the fact that Cause of Death is reported by the institution where the organ was procured, using nationally standardized phrases, some causes of death can have multiple appropriate definitions, such as “anoxia” encompassing multiple types of hemorrhage or “motor vehicle accident” causing “head trauma.” The mechanism by which Cause of Death influences islet response remains unclear, but it is known that events immediately preceding death can impact islet health, such as oxidative stress impairing islet function and islets from brain-dead donor rats being functionally inferior (both in vitro and in vivo) (8, 11). However, the fact that our data suggest various types of anoxic events affect islet function differently suggests that Cause of Death is acting as a surrogate for more than one variable.
The implications of our findings for human islet research are both encouraging and cautionary. The majority of islet preparations from each center and year (with the exception of Center 15 and Year 2004) have a responsive profile (Group 1). However, dysfunctional islet preparations are being shipped from all centers and are being used in studies where islet responsiveness is assumed. The information currently provided to researchers is insufficient to predict the functional profile of a human islet preparation.
The insulin secretion profiles of islet preparations should guide the way investigators represent collected data. For example, if two of six human islet preparations in a study had a pattern like in Groups 2–5, it might confound interpretation to combine gene expression data. In a study with only three or four human islet preparations, there would be an even greater impact of including a dysfunctional preparation, which would be statistically likely. Likewise, in a study examining the contribution of cAMP-mediated insulin release, Group 2 preparations should perhaps be treated separately from Group 1 preparations. Thus, combining data from islet preparations with different health and functional statuses may confound interpretation, leading to inappropriate conclusions. It is advisable that researchers perform a preexperimental functional assessment to select appropriate islet preparations for experimental purposes.
GRANTS
This work was supported by grants from the Department of Veterans Affairs, the National Institute of Diabetes and Digestive and Kidney Diseases (DK-68854, DK-66636, DK-69603, DK-63439, DK-62641, DK-72473, DK-89572, DK-89538, DK-68764, DK-92758, DK-97829, DK-94199, DK-104211), JDRF, and the Vanderbilt Diabetes Research and Training Center (DK-20593).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.S.K., J.H., W.N., M.B., and A.C.P. conception and design of research; N.S.K., G.P., J.H., C.D., M.B., and W.S.B. analyzed data; N.S.K., G.P., J.H., M.B., W.S.B., and A.C.P. interpreted results of experiments; N.S.K., G.P., C.D., and W.S.B. prepared figures; N.S.K. and W.S.B. drafted manuscript; N.S.K., G.P., J.H., C.D., R.A., W.N., M.B., W.S.B., and A.C.P. edited and revised manuscript; G.P., J.H., C.D., C.T., R.A., A.S., W.N., and M.B. performed experiments; A.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
A very special thanks to Barbara Olack, Quality Assurance Administrator, Integrated Islet Distribution Program, for critical insight into ICR/IIDP protocols, data reporting, and history.
REFERENCES
- 1.Andrali SS, Virostko J, Henske J, Vanderford NL, Vinet L, Özcan S, Lamprianou S, Dai C, Radhika A, Baldwin RM, Ansari MS, Hefti F, Skovronsky D, Kung HF, Herrera PL, Peterson TE, Meda P, Powers AC. Multimodal image coregistration and inducible selective cell ablation to evaluate imaging ligands. Proc Natl Acad Sci USA 108: 20719–20724, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avrahami D, Changhong L, Yu M, Jiao Y, Zhang J, Naji A, Ziaie S, Glaser B, Kaestner KH. Targeting the cell cycle inhibitor p57Kip2 promotes adult human β cell replication. J Clin Invest 124: 670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentsi-Barnes K, Doyle ME, Abad D, Kandeel F, Al-Abdullah IH. Detailed protocol for evaluation of dynamic perifusion of human islets to assess β-cell function. Islets 3: 284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest 123: 1275–1284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, Powers AC. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab 19: 498–511, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53: 1087–1097, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald P. A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor Biol Med Model 8: 20, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Thompson JA, Chaudry IH, Chaudry IH, Eckhoff DE, Eckhoff DE. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 52: 2935–2942, 2003. [DOI] [PubMed] [Google Scholar]
- 9.D'Aleo V, Del Guerra S, Gualtierotti G, Filipponi F, Boggi U, De Simone P, Vistoli F, Del Prato S, Marchetti P, Lupi R. Functional and survival analysis of isolated human islets. Transplant Proc 42: 2250–2251, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55: 707–718, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckhoff DE, Eckstein C, Smyth CA, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Anthony Thompson J, Chaudry IH, Contreras JL. Enhanced isolated pancreatic islet recovery and functionality in rats by 17beta-estradiol treatment of brain death donors. Surgery 136: 336–345, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Srinivas H, Scott DK, Stewart AF. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human-cell replication: a revised model of human-cell G1/S control. Diabetes 62: 2460–2470, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerling IC, Kotb M, Fraga D, Sabek O, Gaber AO. No correlation between in vitro and in vivo function of human islets. Transplant Proc 30: 587–588, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 123: 3305–3316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism 63: 218–225, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson MS, Park EE, Sears ML, Greenwood KK, Danobeitia JS, Hullett DA, Fernandez LA. A simplified approach to human islet quality assessment. Transplantation 89: 1178–1188, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huggett JF, Foy CA, Benes V, Emslie K, Garson JA, Haynes R, Hellemans J, Kubista M, Mueller RD, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT, Bustin SA. The digital MIQE guidelines: minimum information for publication of quantitative digital PCR experiments. Clin Chem 59: 892–902, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes 63: 1283–1288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaddis JS, Danobeitia JS, Niland JC, Stiller T, Fernandez LA. Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. Am J Transplant 10: 646–656, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaddis JS, Hanson MS, Cravens J, Qian D, Olack B, Antler M, Papas KK, Iglesias I, Barbaro B, Fernandez L, Powers AC, Niland JC. Standardized transportation of human islets: an islet cell resource center study of more than 2,000 shipments. Cell Transplant 22: 1101–1111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaddis JS, Olack BJ, Sowinski J, Cravens J, Contreras JL, Niland JC. Human pancreatic islets and diabetes research. JAMA 301: 1580–1587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kameswaran V, Bramswig NC, McKenna LB, Penn M, Schug J, Hand NJ, Chen Y, Choi I, Vourekas A, Won KJ, Liu C, Vivek K, Naji A, Friedman JR, Kaestner KH. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab 19: 135–145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondegowda NG, Mozar A, Chin C, Otero A, Garcia-Ocana A, Vasavada RC. Lactogens protect rodent and human β-cells against glucolipotoxicity-induced cell death through Janus kinase□2 (JAK2)/signal transducer and activator of transcription-5 (STAT5) signalling. Diabetologia 55: 1721–1732, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61: 2205–2213, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in β-cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 109: E524–E532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitt HE, Cyphert TJ, Pascoe JL, Hollern DA, Abraham N, Lundell RJ, Rosa T, Romano LC, Zou B, O'Donnell CP, Stewart AF, Garcia-Ocana A, Alonso LCL. Glucose stimulates human β-cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 54: 572–582, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezza T, Kulkarni RN. The regulation of pre- and post-maturational plasticity of mammalian islet cell mass. Diabetologia 57: 1291–1303, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Oh E, Kalwat MA, Kim MJ, Verhage M, Thurmond DC. Munc18–1 regulates first-phase insulin release by promoting granule docking to multiple syntaxin isoforms. J Biol Chem 287: 25821–25833, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh E, Stull ND, Mirmira RG, Thurmond DC. Syntaxin 4 up-regulation increases efficiency of insulin release in pancreatic islets from humans with and without type 2 diabetes mellitus. J Clin Endocrinol Metab 99: E866–E870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omori K, Mitsuhashi M, Todorov I, Rawson J, Shiang KD, Kandeel F, Mullen Y. Microassay for glucose-induced preproinsulin mRNA expression to assess islet functional potency for islet transplantation. Transplantation 89: 146–154, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE, Wildey GM, Pisania A, Weir GC, Hering BJ. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant 7: 707–713, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakuma Y, Ricordi C, Miki A, Yamamoto T, Pileggi A, Khan A, Alejandro R, Inverardi L, Ichii H. Factors that affect human islet isolation. Transplant Proc 40: 343–345, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Street CN, Lakey JRT, Shapiro AMJ, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, Korbutt GS. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes 53: 3107–3114, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Sweet IR, Gilbert M, Jensen R, Sabek O, Fraga DW, Gaber AO, Reems J. Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality. Transplantation 80: 1003–1011, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Sweet IR, Gilbert M, Scott S, Todorov I, Jensen R, Nair I, Al-Abdullah I, Rawson J, Kandeel F, Ferreri K. Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. Am J Transplant 8: 183–192, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Vernier S, Chiu A, Schober J, Weber T, Nguyen P, Luer M, McPherson T, Wanda PE, Marshall CA, Rohatgi N, McDaniel ML, Greenberg AS, Kwon G. β-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets 4: 379–392, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Danielson KK, Ropski A, Harvat T, Barbaro B, Paushter D, Qi M, Oberholzer J. Systematic analysis of donor and isolation factor's impact on human islet yield and size distribution. Cell Transplant 22: 2323–2333, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]