Abstract
The vascular response to local skin cooling is dependent in part on a cold-induced translocation of α2C-receptors and an increased α-adrenoreceptor function. To discover whether β-adrenergic function might contribute, we examined whether β-receptor sensitivity to the β-agonist isoproterenol was affected by local skin temperature. In seven healthy volunteers, skin blood flow was measured from the forearm by laser-Doppler flowmetry and blood pressure was measured by finger photoplethysmography. Data were expressed as cutaneous vascular conductance (CVC; laser-Doppler flux/mean arterial blood pressure). Pharmacological agents were administered via intradermal microdialysis. We prepared four skin sites: one site was maintained at a thermoneutral temperature of 34°C (32 ± 10%CVCmax) one site was heated to 39°C (38 ± 11%CVCmax); and two sites were cooled, one to 29°C (22 ± 7%CVCmax) and the other 24°C (16 ± 4%CVCmax). After 20 min at these temperatures to allow stabilization of skin blood flow, isoproterenol was perfused in concentrations of 10, 30, 100, and 300 μM. Each concentration was perfused for 15 min. Relative to the CVC responses to isoproterenol at the thermoneutral skin temperature (34°C) (+21 ± 10%max), low skin temperatures reduced (at 29°C) (+17 ± 6%max) or abolished (at 24°C) (+1 ± 5%max) the vasodilator response, and warm (39°C) skin temperatures enhanced the vasodilator response (+40 ± 9%max) to isoproterenol. These data indicate that β-adrenergic function was influenced by local skin temperature. This finding raises the possibility that a part of the vasoconstrictor response to direct skin cooling could include reduced background β-receptor mediated vasodilation.
Keywords: local cooling, local heating, β-adrenergic receptors, noradrenaline
a constant, normal body temperature is the result of a regulated balance between heat production and heat loss. Controlling skin blood flow manipulates the rate of heat transfer from the core to the surface of the body and its elimination to the environment. Adjustments in perfusion of the skin are largely in response to changes in core, whole body skin, and local skin temperature. The reflex portion of the fluctuations in skin perfusion in response to reductions in whole body skin temperature are achieved primarily through the sympathetic noradrenergic system, relying principally on skin sympathetic nerve activity causing the release of norepinephrine and neuropeptide Y (24, 34–36). Local changes in skin temperature from direct cooling of the skin also stimulate a cutaneous vasoconstriction via both local stimulation of norepinephrine release and inhibition of a nitric oxide-based system (17, 22, 46).
Crandall et al. (9) demonstrated the presence of functional β-receptors in the cutaneous vasculature of humans. Although these receptors played no significant role in mediating reflex cutaneous vasodilation [a cholinergic cotransmitter mechanism (25)], Stephens et al. (34) found that addition of the β-receptor antagonist propranolol revealed a greater reflex cutaneous vasoconstriction to whole body cooling than under conditions of α-receptor antagonism only. This latter finding suggests some inclusion of β-receptors in the net vascular response (i.e., their presence buffers the α-mediated vasoconstriction). Furthermore, Stephens et al. (34) reported that administration of norepinephrine under conditions of α-receptor antagonism frequently resulted in vasodilation, a phenomenon that was blocked by the inclusion of propranolol. This finding suggests a potential for β-receptor function to be an important background factor in cutaneous vascular control.
Previously, we demonstrated that the cutaneous vasoconstrictor response to direct local skin cooling can be abolished under conditions of combined blockade of transmitter release from sympathetic noradrenergic nerve endings and nitric oxide synthase inhibition (17). In vitro and in vivo data suggest that the adrenergic portion of the vascular response to reduced local skin temperature is due primarily to increased α2C-receptor expression. Although that portion of the vasoconstrictor response to reduced tissue temperature is mediated by norepinephrine, data from in vitro and isolated systems data clearly demonstrate that synthesis and release of norepinephrine is impaired by cooling (4, 10, 42). The adrenergic response to local skin cooling has been shown to be dependent on a cold-induced translocation of α2C-receptors from the Golgi apparatus to the plasma membrane via Rho kinase (2, 3, 7, 8, 20), rather than on an increase in norepinephrine release. This finding was demonstrated in the cutaneous circulation of humans by Thompson-Torgerson et al. (38), who reported a marked reduction of the cutaneous vasoconstrictor response to local cooling under conditions of Rho kinase inhibition.
It is not known whether a concomitant change occurs in β-receptor function during local skin cooling. Could local tissue cooling also enhance β-receptor function? Contrary to that supposition, we have unpublished observations that β-receptor sensitivity might actually be reduced by local skin cooling. We found that the concentration of the β-receptor agonist isoproterenol required to elicit a given degree of vasodilation was greater at cooler temperatures relative to warmer temperatures. If local cooling reduces β-receptor-mediated vasodilation, that mechanism could contribute to the vasoconstrictor response to cooling. Consequently, we sought to resolve the question of whether β-receptor-mediated cutaneous vasodilation was positively or negatively affected by changes in local skin temperature.
METHODS
Participants.
The Institutional Review Board of The University of Texas Health Science Center at San Antonio approved this study. All participants were fully informed of the methods and risks before written and verbal consent was obtained. All seven participants (five men and two women, age 28 ± 3 yr) were healthy nonsmokers, nonobese (body mass index 23 ± 2), and not taking any medications. Participants refrained from consuming alcoholic or caffeinated beverages for at least 12 h prior to the study. For women participants, the phase of the menstrual cycle was recorded but not controlled for in these experiments. Previous studies have reported that the vasoconstrictor responses to local cooling are unaffected by female reproductive hormones (6).
Instrumentation.
Each participant had four microdialysis probes placed intradermally on the ventral aspect of the left forearm. As described previously (9, 23), these custom-built microdialysis probes consisted of 2 cm of cellulose microdialysis tubing (ID 200 μm, 18 kDa nominal molecular weight cutoff) attached at each end to polyimide tubing, leaving 1 cm of membrane for drug exchange. Before implantation, the area of forearm skin selected for probe insertion was temporarily anesthetized by the application of an ice pack for 5 min (13). A 22-gauge needle was introduced aseptically for ∼2.5 cm into the skin before exiting. The microdialysis probe and the connecting tubing were introduced into the skin via the lumen of the needle; the needle was then removed leaving the probe in place. All probes were placed in this manner, and ∼1.5 h was allowed for the effects of the insertion trauma to subside. The probes were placed ∼3 cm apart.
Measurements.
All measurements were performed with participants in the supine position. Skin blood flow was measured from the ventral aspect of the forearm by laser-Doppler flowmetry (Moor Instruments, Axminster, UK) and expressed as laser-Doppler flow (21, 29). Laser-Doppler flow measures are exclusive to the skin and are not contaminated by underlying skeletal muscle blood flow (32). Local temperature control was achieved with custom-designed metal Peltier cooling and heating probe holders (1, 14–16, 22, 46). These probe holders were able to control the surface temperature within 0.1°C over an area of 6.3 cm2 with the exception of a small aperture (0.28 cm2) in the center of the holder to enable placement of the laser-Doppler probe. A thermocouple between the skin surface and the probe holder enabled local skin temperature assessment and feedback control. Blood pressure was recorded noninvasively and continuously by the Peňáz method (30) from the middle finger of the experimental arm (Finapres; Ohmeda, Madison, WI). Mean arterial pressure was obtained from the electrical integration of the continuous blood pressure signal. Cutaneous vascular conductance (CVC) was calculated as the ratio of laser-Doppler flow to mean arterial pressure (in arbitrary units). Whole-body skin temperature was recorded via the weighted mean from six thermocouples placed on the body surface and controlled by the use of water-perfused suits (37). The suit covered the entire body surface apart from the head, hands, feet, and forearm used for blood flow measurements. This arrangement allowed independent control of local skin temperature and whole body skin temperature. Local and whole body skin temperatures were initially maintained at 34°C (thermoneutral).
Chemicals.
Sodium nitroprusside and isoproterenol (Sigma Aldrich, St. Louis, MO) were dissolved in sterile saline immediately prior to use. Isoproterenol was prepared in four concentrations (10, 30, 100, and 300 μM). Sodium nitroprusside was prepared at 58 mM. Solutions were sterilized using a 0.2-μm syringe filter (Acrodisc; Pall, Port Washington, NY).
Protocols.
Protocols were designed to test whether local temperature affects the vasodilator response to the β-agonist isoproterenol (Fig. 1). Following 15 min of baseline measures at a local temperature of 34°C, one site was heated to 39°C, and two sites were cooled, one to 29°C and the other 24°C, while the final site remained at the thermoneutral temperature of 34°C. After 20 min at these temperatures to allow stabilization of skin blood flow, isoproterenol was perfused in increasing concentrations of 10, 30, 100, and 300 μM to each site. Each concentration was perfused for 15 min. All sites were then returned to a saline infusion for 15 min and local skin temperature was returned to 34°C. Sodium nitroprusside (58 mM) was then administered and local temperature was increased to 42°C to attain maximal CVC (26, 27).
Fig. 1.
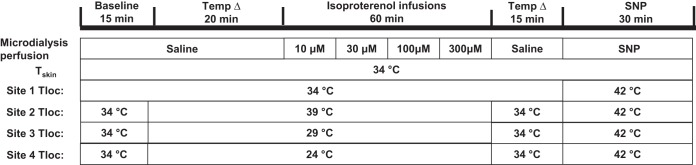
Outline of protocol. Four sites were prepared with microdialysis probes local skin heater/cooler laser-Doppler probe holders, and laser-Doppler probes. Local skin temperature (Tloc) and whole-body skin temperature (Tskin) were initially maintained at 34°C. All probes were perfused with saline for 35 min. After 15 min of baseline data collection, Tloc was increased to 39°C at one site and reduced at two sites to 29°C and 24°C, respectively. Perfusate for all sites was then infused every 15 min to 10, 30, 100, and 300 μM isoproterenol. Following these infusions, perfusate was changed to saline and Tloc changed to 34°C at all sites for 15 min. Finally, Tloc was increased to 42°C and 58 mM of sodium nitroprusside (SNP) was infused at all sites to obtain maximal cutaneous vascular conductance.
Data and statistical analysis.
Data for CVC were analyzed for the final 5 min of each section (see Fig. 1). CVC data were expressed as a percentage of maximal as determined by the administration of sodium nitroprusside and local skin warming to 42°C. Statistical analysis was by repeated-measures ANOVA with a Bonferroni post hoc test. Statistical significance was assumed when P < 0.05. Power analysis indicated that a minimum of six participants would be required for a P < 0.05 with 90% power (nQuery; Statistical Solutions, Cork, Ireland). Data are presented as means ± SD.
RESULTS
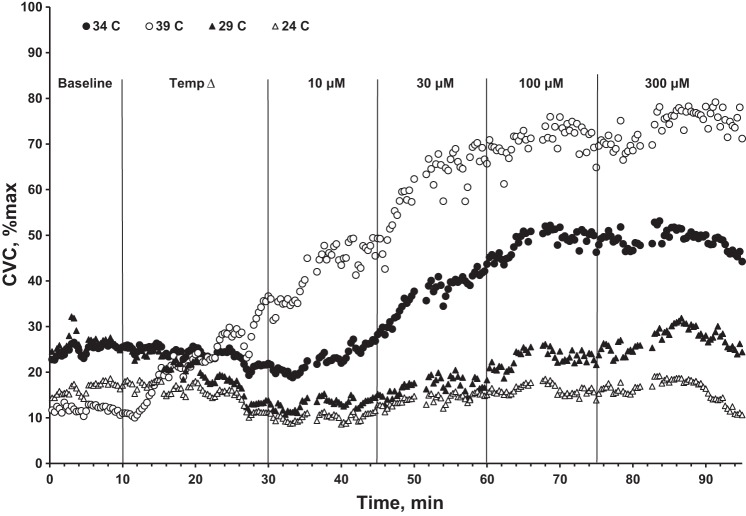
Figure 2 shows representative results from one participant in the protocol outlined in Fig. 1. Observe the distinct differences in the responses to isoproterenol among sites, in particular how the magnitude of the CVC response is reduced at lower local temperatures. Furthermore, note the slight vasoconstriction toward the end of the administration of 300 μM isoproterenol; this was observed in all participants and is most likely due to isoproterenol saturation of β-adrenergic receptors and having an action via α-adrenergic receptors.
Fig. 2.
Cutaneous vascular conductance (CVC) responses normalized to maximal (%max) from a representative participant. Note: compared with the response at the thermoneutral skin site (34°C, ●) the markedly reduced vasodilator responses to increasing concentrations of isoproterenol at the skin sites cooled to 29°C (▲) and 24°C (△); in contrast, at the warmed skin site (39°C, ○) the vasodilation to increasing concentrations of isoproterenol is augmented.
During the local temperature adjustment phase, CVC (group averages) at the warm site increased from 25 ± 8%max to 38 ± 11%max, and CVC decreased at the cool and cold sites from 34 ± 5%max to 22 ± 7%max and 26 ± 2%max to 16 ± 4%max, respectively (all P < 0.05). CVC at the thermoneutral control site (34°C) did not change during this period (32 ± 7%max to 32 ± 10%max; P > 0.05). CVC generally reached a steady state during the 20-min period following the temperature changes. This was always the case for cooled sites. However, at the warmed sites, a steady-state CVC was less clear (see Fig. 2). Nevertheless, this inconsistency was small and did not affect our fundamental conclusions.
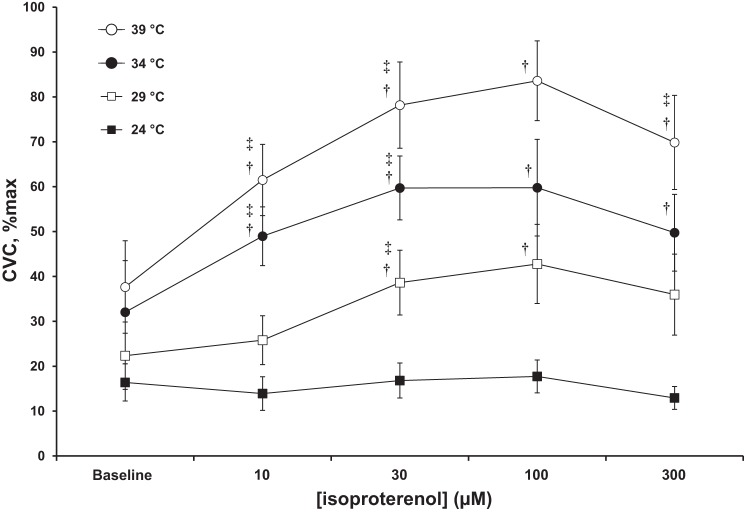
There were main effects for both local temperature and isoproterenol treatment (both P < 0.05). There was also statistically significant interaction between local temperature and treatment (P < 0.05). Figure 3 shows the responses in CVC normalized to maximum averaged from the seven participants. In response to the administration of 10 μM isoproterenol, CVC at the thermoneutral and warm skin sites increased to 49 ± 8%max and 61 ± 6%max, respectively (both P < 0.05). At the cool skin site (29°C), CVC averaged 22 ± 7%max before and 25 ± 5%max during perfusion with 10 μM isoproterenol. CVC at the cold site (24°C) was 16 ± 4%max before and 14 ± 4%max during perfusion with isoproterenol. Neither of these latter responses was statistically significant (P > 0.05).
Fig. 3.
CVC responses normalized to maximal for all seven participants. Lower local skin temperature either reduced (at 29°C) or abolished (at 24°C) the increase in CVC in response to isoproterenol infusions compared with that at the thermoneutral (34°C) skin site. In contrast, increasing the local skin temperature to 39°C markedly increased the vasodilator response to isoproterenol. The highest concentration of isoproterenol elicited vasoconstriction rather than vasodilation at all skin temperatures. Control values are the steady-state levels following the change in local temperature. Data are means ± SD. †P < 0.05 compared with baseline; ‡P < 0.05 compared with prior phase.
Isoproterenol at 30 μM elicited an increase in CVC from 49 ± 8%max to 78 ± 7%max at the warm (39°C) sites, and from 25 ± 5%max to 39 ± 7%max at the cool (29°C) skin sites (both P < 0.05). CVC at the thermoneutral (34°C) site changed from 10 ± 3% max to 59 ± 10%max (P = 0.06) in response to 30 μM isoproterenol. Finally, CVC at the cold skin sites (24°C) remained statistically unchanged (P > 0.05) at 14 ± 4%max before and 17 ± 4%max during 30 μM isoproterenol perfusion.
Perfusion of 100 μM isoproterenol elicited no further changes of statistical significance in CVC at any of the skin sites relative to the levels observed with 30 μM isoproterenol (all P > 0.05). Interestingly, in response to 300 μM isoproterenol, CVC decreased (P < 0.05) at the warm (70 ± 10%max) and thermoneutral (49 ± 8%max) sites relative to the responses achieved from perfusion of 100 μM isoproterenol. CVC at the cool and cold sites was statistically unchanged (P > 0.05), although there was a slight reduction.
DISCUSSION
The major finding of this study was that local skin temperature is an important determinant of the CVC response to the infusion of isoproterenol, suggesting that β-adrenergic function is influenced by local skin temperature. Relative to the CVC responses achieved at the thermoneutral skin temperature (34°C), low skin temperatures reduced (at 29°C) or abolished (at 24°C) the vasodilator response to isoproterenol, whereas warm (39°C) skin temperatures enhanced the vasodilator response. However, whether these effects are via changes in β-receptor number or receptor sensitivity remains an open question.
Crandall et al. (9) reported the existence of functional β-adrenergic receptors in human cutaneous circulation. Data consistent with a role for β-adrenergic receptors in cutaneous vascular control comes from studies by Stephens et al. (34), who found that β-adrenergic receptor blockade enhanced the vasoconstrictor responses to whole-body cooling (a reflex vasoconstriction) and to directly applied norepinephrine. Accepting the fact that local skin cooling involves vasoconstrictor effects of norepinephrine release (17, 22, 31, 46), the above observations fit with a working model in which locally released norepinephrine also affects β-adrenergic receptors. Hence, the possibility is raised that β-adrenergic function contributes to the vasoconstriction stimulated via direct tissue cooling.
We have unpublished observations that the concentration of the β-receptor agonist isoproterenol required to elicit a given degree of vasodilation was greater at cooler local skin temperatures relative to that required at warmer skin temperatures. Because cutaneous vasoconstriction in response to local cooling appears to be dependent in part on changes in α2C-adrenergic receptor expression via a Rho kinase system (2, 7, 20, 38), we thought it important to resolve the question of whether β-receptor sensitivity was similarly affected by changes in local skin temperature. We found this to be the case, with cooling reducing and warming enhancing the vascular responses to the β-agonist isoproterenol. These data suggest marked changes in the sensitivity of β-receptor function in response to changes in local skin temperature. Herman et al. (12) reported that in frogs, blood pressure responses to isoproterenol infusion were greater at warm ambient temperature compared with cool temperature, indicating β-receptor function was diminished under cool conditions (12), similar to the vascular responses we found in human cutaneous circulation.
Temperature effects on β-receptor responsiveness has been reported in other tissues. Examining isolated canine saphenous veins, Vanhoutte and Shepherd (40) reported that at 29°C, isoproterenol had no vascular effect; by contrast, at 37°C isoproterenol elicited a marked venodilation. In conjunction with electrical stimulation, the authors concluded that under conditions of lower temperatures, higher doses of isoproterenol were required for maximal β-adrenergic stimulation and that increasing the concentration of isoproterenol may result in a partial α-receptor stimulation. Additionally, Venugopalan et al. (43) reported that cooling reduced β-receptor relaxation to isoproterenol in guinea pig pulmonary tissues. These data and conclusions are congruent with our current human skin data that β-receptor actions are temperature sensitive. It is worth noting, however, that in earlier in vivo experiments using canine cutaneous veins (45), no significant effect of perfusate temperature on the dilator response to isoproterenol infusions was demonstrated.
At the highest concentration of isoproterenol, we observed a vasoconstriction at all skin temperatures. Vanhoutte and Shepherd (40) previously demonstrated α-adrenergic actions in response to high concentrations of isoproterenol. Indeed, they observed that these α-adrenergic actions were augmented when preparations were cool (27°C). As previously stated, recent work demonstrated that cooling causes an increase in α-receptor expression (2), which might explain why cooling augmented the isoproterenol-induced α-adrenergic stimulation. In the present study, the vasoconstrictor effects of isoproterenol were most apparent at the higher skin temperature (Fig. 3); this may be a product of the degree of constriction already developed by the vascular smooth muscle (i.e., the size of the constrictions caused by 300 μM isoproterenol appear greater due to the increased prior levels of smooth muscle relaxation). Further work examining the effects of local temperature on isoproterenol-mediated vasoconstriction might consider increasing the level of smooth muscle relaxation prior to administration to augment the effects.
Any role of modified β-receptor function in the cutaneous vasoconstrictor response to local skin cooling is dependent on both the temperature-induced changes in receptor sensitivity to norepinephrine and to the rate of norepinephrine release at the vasoconstrictor nerve terminals (4, 11, 33, 39). At neutral tissue temperatures in the region of 34°C, the level of cutaneous vasoconstrictor nerve activity is low, approaching zero as indicated by the lack of effect of blockade of transmitter release from adrenergic nerves (14). Any perturbation that increased norepinephrine release would support cutaneous vasoconstriction and a simultaneous reduction in β-receptor function would enhance vasoconstriction. This will take on greater importance in situations in which the extant level of vasoconstrictor activity and norepinephrine release at neutral temperatures are affected by age (19) or disease (5, 18).
The current study does not allow us to completely define the mechanism(s) for the temperature-induced alteration of the cutaneous vasodilator response to isoproterenol. Whether the observed differences in vasodilation to isoproterenol at differing local skin temperatures are due to changes in β-receptor number, sensitivity, or the affinity of isoproterenol to β-receptors has yet to be determined. Additionally, the vasoconstriction elicited by high concentrations of isoproterenol may not be due only to isoproterenol binding to α-receptors, because stimulation of β2-receptors has been reported to potentiate the release of catecholamines, which may be acting on α-receptors to produce vasoconstriction (33, 41, 44). Additionally, differences in baseline CVC can affect the degree of response to a vasoactive substance (14). Indeed, normalization of the data to baseline tends to bring the data to similar response patterns, with the important exception that, at the coolest local temperature, there was a complete abolition of the vasodilator response to isoproterenol. That cannot be explained on the basis of the baseline level of CVC against which the β-adrenergic agonist was delivered. Nevertheless, differences in baseline may be one of the contributors to the differences in response among temperatures. Furthermore, it has been shown that epinephrine elicits a different response than isoproterenol in human cardiac tissue (28); consequently, it is important to note that endogenous epinephrine and norepinephrine could produce different responses compared with those observed with (synthetic) isoproterenol.
In summary, we observed that cooling of the skin either markedly reduced (at 29°C) or abolished (at 24°C) the vasodilator response, whereas warming the skin (to 39°C) enhanced the vasodilator response to the β-adrenergic agonist isoproterenol. These data strongly suggest that β-adrenergic function is altered by local skin temperature, which raises the distinct possibility that β-adrenergic receptors may contribute to the vascular responses to changes in local skin temperature.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01 HL-059166 to J.M. Johnson and R01 HL-065599 to D.L. Kellogg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.J.H. and J.M.J. conception and design of research; G.J.H. and J.M.J. performed experiments; G.J.H. and J.M.J. analyzed data; G.J.H., D.L.K., and J.M.J. interpreted results of experiments; G.J.H. and J.M.J. prepared figures; G.J.H. and J.M.J. drafted manuscript; G.J.H., D.L.K., and J.M.J. edited and revised manuscript; J.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study participants for their time and effort.
REFERENCES
- 1.Alvarez GE, Zhao K, Kosiba WA, Johnson JM. Relative roles of local and reflex components in cutaneous vasoconstriction during skin cooling in humans. J Appl Physiol 100: 2083–2088, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res 94: 1367–1374, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Boels PJ, Verbeuren TJ, Vanhoutte PM. Moderate cooling depresses the accumulation and the release of newly synthesized catecholamines in isolated canine saphenous veins. Experientia 41: 1374–1377, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Bruning RS, Kenney WL, Alexander LM. Altered skin flowmotion in hypertensive humans. Microvasc Res 97: 81–87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol 87: 1719–1723, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 278: H1075–H1083, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Chotani MA, Mitra S, Su BY, Flavahan S, Eid AH, Clark KR, Montague CR, Paris H, Handy DE, Flavahan NA. Regulation of α2-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 286: H59–H67, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Crandall CG, Etzel RA, Johnson JM. Evidence of functional beta-adrenoceptors in the cutaneous vasculature. Am J Physiol Heart Circ Physiol 273: H1038–H1043, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Flavahan NA. The role of vascular alpha-2-adrenoceptors as cutaneous thermosensors. News Physiol Sci 6: 251–255, 1991. [Google Scholar]
- 11.Flavahan NA, Lindblad LE, Verbeuren TJ, Shepherd JT, Vanhoutte PM. Cooling and alpha 1- and alpha 2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol Heart Circ Physiol 249: H950–H955, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Herman CA, Robleto DO, Mata PL, Heller RS. Cardiovascular responses to catecholamines at 12 degrees C in the American bullfrog (Rana catesbeiana). J Exp Zool 240: 17–23, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Hodges GJ, Chiu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol 106: 1112–1118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293: H3187–H3192, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol 296: H51–H56, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol 574: 849–857, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol 109: 1538–1544, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol 60: 1195–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JM. The cutaneous circulation. In: Laser-Doppler Blood Flowmetry, edited by Shepherd AP, Öberg PÅ. Boston: Kluwer Academic Publishers, 1990, p. 121–139. [Google Scholar]
- 22.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288: H1573–H1579, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Kellogg DL Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DL Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellogg DL Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol 107: 1438–1444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leenen FH, Coletta E, Fourney A, White R. Aging and cardiac responses to epinephrine in humans: role of neuronal uptake. Am J Physiol Heart Circ Physiol 288: H2498–H2503, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Öberg PA. Laser-Doppler flowmetry. Crit Rev Biomed Eng 18: 125–163, 1990. [PubMed] [Google Scholar]
- 30.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Pérgola PE, Kellogg DL Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol 265: H785–H792, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Saumet JL, Kellogg DL Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol 65: 478–481, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd JT, Vanhoutte PM. Local modulation of adrenergic neurotransmission in blood vessels. J Cardiovasc Pharmacol 7, Suppl 3: S167–S178, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280: H1496–H1504, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Stephens DP, Bennett LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol 282: H264–H272, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1404–H1409, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol 292: H1700–H1705, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Vanhoutte PM, Cooke JP, Lindblad LE, Shepherd JT, Flavahan NA. Modulation of postjunctional alpha-adrenergic responsiveness by local changes in temperature. Clin Sci (Lond) 68, Suppl 10: 121S–123S, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Vanhoutte PM, Shepherd JT. Effect of cooling on beta-receptor mechanisms in isolated cutaneous veins of the dog. Microvasc Res 2: 454–461, 1970. [DOI] [PubMed] [Google Scholar]
- 41.Vanhoutte PM, Shepherd JT. Muscarinic and beta-adrenergic prejunctional modulation of adrenergic neurotransmission in the blood vessel wall. Gen Pharmacol 14: 35–37, 1983. [DOI] [PubMed] [Google Scholar]
- 42.Vanhoutte PM, Verbeuren TJ. Depression by local cooling of 3H-norepinephrine release evoked by nerve stimulation in cutaneous veins. Blood Vessels 13: 92–99, 1976. [DOI] [PubMed] [Google Scholar]
- 43.Venugopalan CS, Jenkins HJ, Drazen JM. Effect of temperature on beta receptor responsiveness in guinea pig pulmonary tissues. Res Commun Chem Pathol Pharmacol 53: 275–288, 1986. [PubMed] [Google Scholar]
- 44.Verbeuren TJ, Lorenz RR, Aarhus LL, Shepherd JT, Vanhoutte PM. Prejunctional beta-adrenoceptors in human and canine saphenous veins. J Auton Nerv Syst 8: 261–271, 1983. [DOI] [PubMed] [Google Scholar]
- 45.Webb-Peploe MM, Shepherd JT. Beta-receptor mechanisms in the superficial limb veins of the dog. J Clin Invest 48: 1328–1335, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J Appl Physiol 100: 42–50, 2006. [DOI] [PubMed] [Google Scholar]