Abstract
Recent studies suggest that sex of the animal and T cell impact ANG II hypertension in Rag−/− mice, with females being protected relative to males. This study tested the hypothesis that ANG II results in greater increases in proinflammatory T cells and cytokines in males than in females. Male and female Sprague-Dawley (SD) rats, aged 12 wk, were treated with vehicle or ANG II (200 ng·kg−1·min−1) for 2 wk. Renal CD4+ T cells and Tregs were comparable between vehicle-treated males and females, although males expressed more Th17 and IL-17+ T cells and fewer IL-10+ T cells than females. ANG II resulted in greater increases in CD4+ T cells, Th17 cells, and IL-17+ cells in males; Tregs increased only in females. We previously showed that ANG (1–7) antagonizes ANG II-induced increases in blood pressure in females and ANG (1–7) has been suggested to be anti-inflammatory. Renal ANG (1–7) levels were greater in female SD at baseline and following ANG II infusion. Additional rats were treated with ANG II plus the ANG (1–7)-mas receptor antagonist A-779 (48 μg·kg−1·h−1) to test the hypothesis that greater ANG (1–7) in females results in more Tregs relative to males. Inhibition of ANG (1–7) did not alter renal T cells in either sex. In conclusion, ANG II induces a sex-specific effect on the renal T cell profile. Males have greater increases in proinflammatory T cells, and females have greater increases in anti-inflammatory Tregs; however, sex differences in the renal T cell profile are not mediated by ANG (1–7).
Keywords: adaptive immune system, sex differences, Sprague-Dawley, ANG (1–7), gender, cytokines
all of the early studies establishing a role for T cells in ANG II hypertension were performed exclusively in males (8, 9, 13); however, two recent studies have greatly expanded our understanding of the impact of sex on T cells during ANG II hypertension. Male Rag−/− mice, which lack functional B and T cells, have greater renal T cell infiltration than female mice regardless of ANG II infusion, although only males exhibit an increase in proinflammatory cytokines following ANG II treatment (22). In a separate study, adoptive transfer of T cells from wild-type male mice to male Rag−/− mice before ANG II infusion resulted in different circulating, splenic, and perivascular adipose tissue T cell profiles than if the donor T cells were from a female (14). The impact of ANG II on the T cell profile in female Rag−/− was not studied. Regardless, both of these studies support the hypothesis that females exhibit less proinflammatory T cell activation in response to ANG II infusion relative to males.
The majority of studies examining T cells in hypertension have focused on total CD3+ T cells, although CD3+CD4+ T cells further differentiate into proinflammatory T helper 17 (Th17) cells or immune-suppressive regulatory T cells (Tregs). Th17 cells have been implicated in contributing to ANG II hypertension (19), while Tregs attenuate ANG II hypertension in male experimental animal models (2). We previously published that there is a sex difference in the renal T cell profile in hypertensive rats. Kidneys of female spontaneously hypertensive rats (SHR) have more Tregs and kidneys from males have more Th17 cells (4, 36, 37). Our current study is focused on the kidney since the kidney is critical in the long-term control of blood pressure (BP) and renal immune cell infiltration contributes to increases in BP in experimental hypertension (7, 24).
There are numerous sex differences in the renin-angiotensin system (RAS) and in functional responses to ANG II infusion (6, 41). The molecular mechanism(s) accounting for sex differences in response to ANG II are still being investigated; however, a differential balance in the expression and activation of the classical and nonclassical RAS has been implicated. In particular, our group and others have shown that ANG (1–7) antagonizes ANG II-induced increases in BP and renal injury in female SHR and mice relative to males (34, 40). While the impact of ANG (1–7) on T cells in hypertension in either sex remains unknown, ANG (1–7) stimulates anti-inflammatory pathways of the innate immune system (11, 12, 15, 21, 32). Overexpression of ANG (1–7) increases the anti-inflammatory cytokine IL-10 and decreases proinflammatory cytokines in male Sprague-Dawley (SD) rats (30). Moreover, male mice lacking angiotensin-converting enzyme 2 (ACE2), a key enzyme for the synthesis of ANG (1–7), have increased renal CD3+ T cells (18). These studies support a potential role for ANG (1–7) to also antagonize ANG II-induced increases in inflammation.
The goal of the current study was to determine the effect of ANG II hypertension on renal T cells in males and females. We hypothesized that ANG II would result in greater increases in proinflammatory T cells and cytokines in males, while females would exhibit greater increases in immune-suppressive Tregs. Additional studies further tested the hypothesis that greater ANG (1–7) in females would promote a more anti-inflammatory profile in females relative to males.
MATERIALS AND METHODS
Animals.
Male and female SD rats, aged 12 wk, were used in this study (Harlan Laboratories, Indianapolis, IN). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Georgia Regents University Institutional Animal Care and Use Committee. Rats were housed in temperature- and humidity-controlled, light-cycled quarters and maintained on standard rat chow (Harlan Teklad).
In the first study, male and female SD rats (n = 6/group) were anesthetized with isoflurane (1.5%) and randomized to receive either subcutaneous osmotic minipumps (Alzet, Cupertino, CA) to deliver ANG II (200 ng·kg−1·min−1 for 14 days; Phoenix, Burlingame, CA) or vehicle control. Additional male and female SD rats (n = 5–6) were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) at 10 wk of age as previously described (34). Rats were allowed 1 wk to recover before being placed on receivers for the measurement of baseline BP for an additional week before ANG II infusion was initiated. All rats were placed in metabolic cages before treatment was initiated and at the end of the 14-day treatment period to facilitate 24-h urine collection. Urinary protein excretion was determined by Bradford Assay (Bio-Rad, Hercules, CA).
In the second study, the contribution of ANG (1–7) to ANG II-mediated changes in the renal T cell profile was determined by randomizing male and female SD rats (n = 6/group) to receive osmotic mini-pumps to deliver either ANG II alone or in combination with the ANG (1–7)-mas receptor antagonist d-alanine-[ANG-(1–7)] (A-779; 48 μg·kg−1·h−1; Bachem, Torrance, CA).
For all experiments, rats were anesthetized with ketamine/xylazine following 2 wk of ANG II infusion (48 and 6.4 mg/kg, respectively, ip; Phoenix Pharmaceuticals, St. Joseph, MO) and a terminal blood sample was taken in the presence of heparin (Hospira, Lake Forest, IL) via aortic puncture. Kidneys were isolated and placed in ice-cold PBS and one kidney was immediately subjected to flow cytometric analysis.
Analytical flow cytometry.
Single cell suspensions of whole kidneys were prepared as previously described (37). Analytical flow cytometry was performed to define the T cell profile using both surface and intracellular markers as previously described to determine the numbers of CD3+ and CD4+ T cells, Tregs (CD3+/CD4+/Foxp3+), or Th17 cells (CD3+/CD4+/ROR-γ) as well as the percent of total T cells that express the proinflammatory cytokine IL-17 or the anti-inflammatory cytokine IL-10 (ROR-γ antibody from R&D Systems, Minneapolis, MN; all other antibodies from BD Biosciences, San Diego, CA) (1, 37). Antibody specificity was confirmed using isotype controls. Samples were double-stained with control IgG and cell markers and were used to assess any spillover signal of fluorochromes. Proper compensation was set to ensure that the median fluorescence intensities of negative and positive cells were identical and then was used to gate the population. Gating excluded dead cells and debris using forward and side scatter plots.
Statistical analysis.
All data are presented as means ± SE. BP and protein excretion data within each sex were analyzed using repeated-measures ANOVA and between-sex comparisons were made using a Student's t-test. Flow cytometry data in 1) vehicle control and ANG II-treated rats and 2) ANG II and A-779-treated rats were compared using two-way ANOVA. Factor 1 was effect of sex and factor 2 was effect of treatment. For all comparisons, differences were considered statistically significant with P < 0.05. Analyses were performed using GraphPad Prism Version 5.0 software (GraphPad Software, La Jolla, CA) and SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
ANG II significantly increases BP and protein excretion in male and female SD rats.
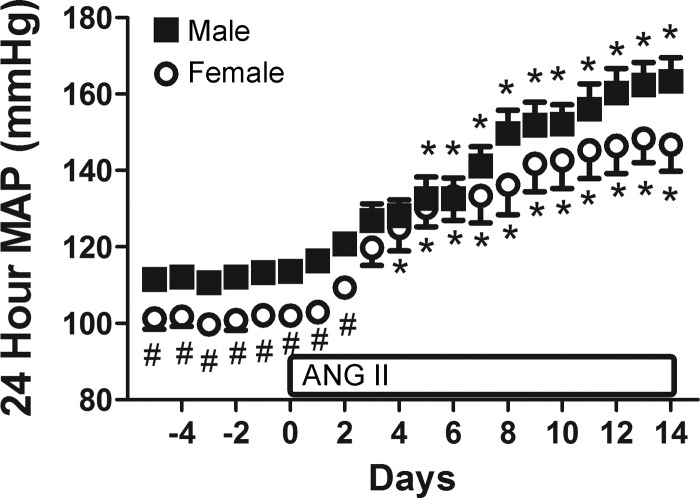
Consistent with previous reports in the literature (27), baseline BP when measured by radiotelemetry was significantly greater in males compared with females (P = 0.008; Fig. 1). ANG II resulted in a significant increase in BP in both males and females (P < 0.001) and the degree of increase was comparable between sexes (percent increase: 30 vs. 29%, respectively, ns). Similarly, urinary protein excretion was greater in male SD rats at baseline than in females (6 ± 1 vs. 1 ± 1 mg/day, respectively; effect of sex: P < 0.001). ANG II significantly increased protein excretion in both male (48 ± 1 mg/day) and female SD rats (9 ± 2 mg/day; effect of treatment: P < 0.001); however, protein excretion remained greater in males (effect of interaction: P < 0.001).
Fig. 1.
Effect of ANG II treatment on 24-h mean arterial pressure (MAP) as measured by telemetry in male and female Sprague-Dawley (SD) rats. ANG II infusion was initiated on day 0. #P < 0.05 vs. blood pressure (BP) in males. *P < 0.05 vs. same-sex baseline BP; n = 4–6.
ANG II increases the number of T cells in the kidney of SD rats in a sex-dependent manner.
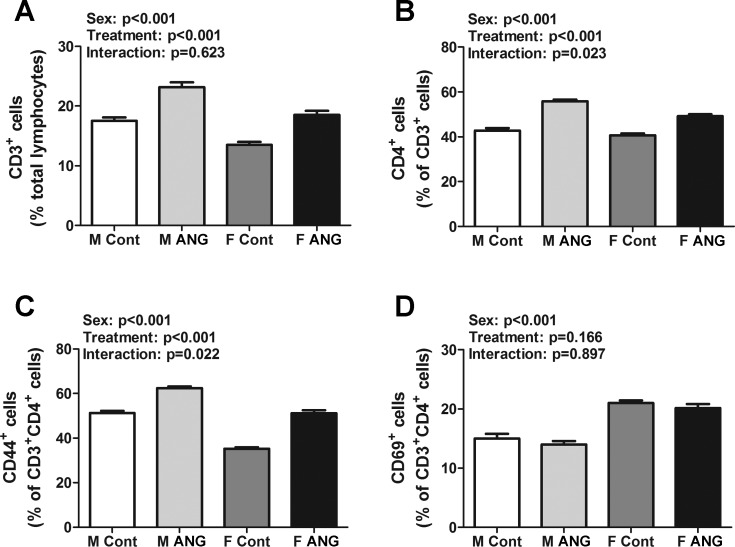
The renal T cell profile was measured by flow cytometric analysis in vehicle control male and female SD rats and following chronic ANG II infusion. Vehicle-treated male SD rats had more renal CD3+ T cells than females (effect of sex: P < 0.001; Fig. 2A). ANG II infusion increased the number of CD3+ T cells in both sexes to a similar degree, although males maintained more CD3+ T cells than females (effect of treatment: P < 0.001, interaction P = 0.623). In contrast, the number of CD4+ T cells was comparable in kidneys of vehicle control male and female SD rats (P = 0.2; Fig. 2B). ANG II infusion also increased the number of CD4+ T cells in both males and females. However, the increase in CD4+ T cells was greater in males (effect of treatment: P < 0.0001, effect of sex: P < 0.001, interaction P = 0.023).
Fig. 2.
T cell profiles in kidneys of vehicle control (Cont) and ANG II-treated (ANG) male (M) and female (F) SD rats. A: percent CD3+ T cells expressed as a percent of total renal lymphocytes. B: percent of CD4+ T cells expressed as a percent of total CD3+ T cells. C and D: percent of CD44+ and CD69+ T cells expressed as a percent of total CD3+CD4+ T cells; n = 6.
T cell activation was further measured via analysis of the early activation T cell markers CD44 and CD69. Vehicle control male SD rats had more CD44+ T cells in their kidneys compared with females, while vehicle control female SD rats had a greater number of CD69+ T cells (Fig. 2, C and D, respectively; effect of sex: P < 0.001 for both). ANG II infusion increased the number of CD44+ T cells in both sexes, yet males maintained more CD44+ T cells than females (effect of treatment: P < 0.001, interaction P = 0.022). ANG II infusion did not alter the number of CD69+ T cells in either sex (effect of treatment: P = 0.166, interaction P = 0.897).
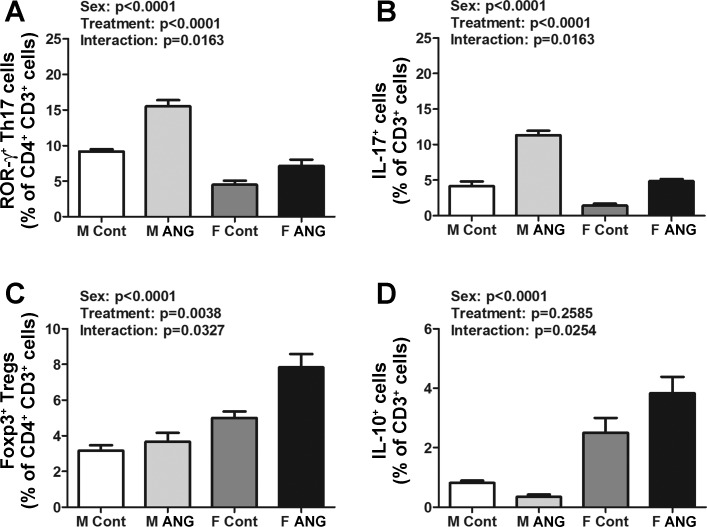
Following ANG II infusion male SD rats have more renal Th17 cells than females, and females have more renal Tregs than males.
The renal T cell profile in male and female SD rats was further characterized by measuring proinflammatory Th17 cells and IL-17+ T cells and anti-inflammatory Tregs and IL-10+ T cells. The number of Th17 cells was significantly greater in kidneys of vehicle control males compared with females (effect of sex: P < 0.001; Fig. 3A). ANG II infusion only increased the number of Th17 cells in males (effect of treatment: P < 0.001, interaction P = 0.016). Consistent with these data, vehicle control male SD rats also had a greater number of IL-17+ T cells in their kidneys compared with females (effect of sex: P < 0.001; Fig. 3B). ANG II infusion increased the number of IL-17+ T cells in both males and females, although the increase was greater in males (effect of treatment: P < 0.001, interaction P = 0.001).
Fig. 3.
T cell profiles in kidneys of vehicle control (Cont) and ANG II-treated (ANG) M and F SD rats. A: percent of ROR-gamma+ Th17 cells expressed as a percent of CD3+CD4+ T cells. B: percent of IL-17+ cells expressed as a percent of total CD3+ T cells. C: percent of Foxp3+ Tregs expressed as a percent of CD3+CD4+ T cells. D: percent of IL-10+ cells expressed as a percent of total CD3+ T cells; n = 6.
In contrast, vehicle control female SD rats had more renal Tregs than males (effect of sex: P < 0.001; Fig. 3C). ANG II infusion increased the numbers of Tregs exclusively in the kidneys of female SD rats (effect of treatment: P = 0.004, interaction P = 0.033). Female SD rats also had more renal IL-10+ T cells compared with males (effect of sex: P < 0.001; Fig. 3D). Although ANG II infusion did not significantly alter the number of IL-10+ T cells in the kidneys of either sex (effect of treatment: P = 0.259), there was a significant interaction term (interaction P = 0.025) consistent with the observed decrease in IL-10+ T cells in males and an increase in IL-10+ T cells in females.
Female SD rats have higher renal cortical ANG (1–7) concentrations than male SD rats.
Renal cortical ANG II and ANG (1–7) peptide levels were measured in vehicle control and ANG II-treated male and female SD rats (Table 1). Renal cortical ANG II levels were comparable between vehicle control male and female rats (effect of sex: P = 0.421). ANG II infusion increased ANG II peptide levels to a similar degree in both sexes (effect of treatment: P < 0.001; interaction P = 0.756). In contrast, renal cortical ANG (1–7) levels were less in vehicle control males compared with females (effect of sex: P < 0.001), although ANG II infusion did not alter the concentration of ANG (1–7) in either sex (effect of treatment: P = 0.413; interaction P = 0.878).
Table 1.
Renal cortical angiotensin peptide expression in male and female vehicle and ANG II-infused SD
| Renal Cortical ANG II Peptide, pg/g cortex |
Renal Cortical ANG (1–7) Peptide, pg/g cortex |
|||
|---|---|---|---|---|
| Control | ANG II | Control | ANG II | |
| Male | 213 ± 31 | 915 ± 185* | 174 ± 19 | 209 ± 21 |
| Female | 149 ± 31 | 773 ± 125* | 364 ± 34† | 388 ± 35† |
Data are means ± SE.
Significant difference from same-sex controls.
Significant difference from males, n = 5–14. SD, Sprague-Dawley.
ANG (1–7) does not modulate the impact of ANG II on the renal immune profile of female SD rats.
Since female SD rats exhibited greater renal concentrations of ANG (1–7) than males and ANG (1–7) antagonizes many of the effects of ANG II, additional studies determined the contribution of ANG (1–7)/mas receptor activation to sex differences in the renal immune profile in male and female SD following ANG II infusion. A-779 did not significantly alter ANG II-induced alterations in renal T cells of either sex (Table 2).
Table 2.
Impact of ANG (1-7)-mas receptor blockade on ANG II-induced alterations in renal T cells in male and female SD
| Male |
Female |
|||
|---|---|---|---|---|
| ANG II | ANG II + A-779 | ANG II | ANG II + A-779 | |
| CD3+, % total lymphocytes | 19.4 ± 0.5 | 19.8 ± 0.9 | 16.6 ± 0.2* | 14.5 ± 0.5† |
| CD4+, % CD3+ cells | 45.6 ± 0.7 | 43.5 ± 0.9 | 42.6 ± 1.0* | 41.3 ± 1.0 |
| CD44+, % CD3+CD4+ cells | 50.8 ± 1.0 | 53.5 ± 1.3 | 35.8 ± 1.0* | 38.5 ± 1.6† |
| CD69+, % CD3+CD4+ cells | 10.2 ± 0.4 | 10.0 ± 0.4 | 19.4 ± 1.1* | 20.3 ± 0.5† |
| Th17 cells, % CD3+CD4+ cells | 9.2 ± 0.4 | 10.5 ± 0.7 | 5.8 ± 0.5* | 6.3 ± 0.8† |
| Treg, % CD3+CD4+ cell | 4.6 ± 0.2 | 3.5 ± 0.3 | 7.8 ± 0.7* | 8.5 ± 0.9† |
| IL-17, % CD3+ cells | 6.0 ± 0.3 | 5.5 ± 0.9 | 2.6 ± 0.2* | 3.8 ± 0.3† |
| IL-10, % CD3+ cells | 1.4 ± 0.5 | 1.5 ± 0.3 | 4.6 ± 0.5* | 3.8 ± 0.5† |
Data are means ± SE.
Significant difference from ANG II-treated male ± SD.
Significant difference from ANG II + A-779 treatment; n = 5.
DISCUSSION
While there is growing support for a critical role for T cells in hypertension, the vast majority of these studies have been conducted exclusively using male experimental animals, despite the fact that half of the hypertensive population is female. It was recently published that while T cells exacerbate ANG II hypertension in male Rag−/− mice, adoptive transfer of T cells from a male donor does not alter BP responses to ANG II in female Rag−/− mice and adoptive transfer of T cells from a female donor mitigates ANG II hypertension in male Rag−/− mice (14, 22). These studies led us to hypothesize that ANG II differentially impacts the T cell profile in males and females and that females maintain a less proinflammatory immune profile. The novel findings of the current study are 1) ANG II results in greater increases in the number of renal proinflammatory Th17 and IL-17+ T cells in males than in females, 2) ANG II results in greater increases in the number of renal anti-inflammatory Tregs and IL-10+ T cells in females than in males, and 3) ANG (1–7) does not drive these sex differences in the renal T cell profile during ANG II infusion. ANG II and the RAS are critical in the regulation of BP and salt and water homeostasis (5) and there are well-established sex differences in both the expression level of the major components of the RAS and in functional responses to RAS activation (6, 33). Our studies suggest that these sex differences extend to T cell activation and sex differences in ANG II activation of T cells may underlie sex differences in other physiological responses to ANG II infusion (38).
Although numerous studies have reported sex differences in BP responses to ANG II infusion, ANG II resulted in comparable increases in BP in male and female SD rats in the current study. We propose that this is related to the dose of ANG II used. While male SD rats consistently have larger increases in BP to ANG II than females at a dose of 400 ng·kg−1·min−1 or greater (25, 26, 35), consistent with our findings, there was not a sex difference in the BP response to ANG II in SD rats when dosed at 100 ng·kg−1·min−1 (20). These findings lead us to propose that there is a threshold dose of ANG II in normotensive animals required to elicit a sex difference in BP. Alternatively, we treated the animals with a lower dose of ANG II; therefore, waiting additional time may have uncovered a sex difference in the BP responses. Indeed, the telemetry data suggest that BP in the females has plateaued at 2 wk while BP in males appears to still be increasing.
The impact of ANG II on the renal T cell profile was sex-specific. Male SD rats exhibited greater increases in the number of renal Th17 cells and Th17 cells have been implicated in ANG II-induced increases in BP (19). In contrast, females exhibited greater increases in the number of renal Tregs, and Tregs mitigate ANG II-induced increases in BP (2). Therefore, despite sex differences in T cells that have been shown to regulate BP, there was no difference in BP. While the current study did not assess the relative contribution of individual T cell subtypes to ANG II hypertension in male and female SD rats, sex differences in ANG II hypertension were abolished in Rag−/− mice compared with wild-type mice (14, 22), implicating T cells in sex differences in BP responses to ANG II. Alternatively, these data may be interpreted to suggest that large numbers of T cells are required to establish sex differences in ANG II hypertension. Rag−/− mice are deficient in T cells and the relatively low dose of ANG II in the current study may have been insufficient to activate enough T cells to differentially impact BP. Moreover, studies that have shown an impact of Tregs on BP have employed adoptive transfer of only Tregs, resulting in an enriched population of those specific cells. Few studies have directly examined the impact of T cells on BP in rats, although we showed that preventing age-related increases in BP in male and female SHR does not alter renal CD3+, CD4+, CD8+ T cells or Th17 cell vs. same-sex vehicle controls (36). More studies are needed to define the impact of individual T cell subtypes in males and females.
The current study focused on T cell subtypes that have been implicated in BP control and ANG II infusion exacerbated the numbers of these cells present in the kidney in both sexes. However, we did not fully define all of the T cells present in the kidney or assess additional immune cells such as monocytes, macrophages, and dendritic cells. These could be additional important considerations regarding the role of the immune system following ANG II infusion. It would be of interest to know whether ANG II infusion also results in sex-specific changes in the types of T cells and other immune cells infiltrating the kidney compared with vehicle control rats.
Although BP responses to ANG II were comparable between the sexes, male SD rats exhibited greater increases in protein excretion in response to ANG II than females, suggesting that deleterious renal effects of ANG II are beginning to be expressed in males despite comparable BP. ANG II infusion increases T cells in kidneys of male 129/SvEv mice (8), male Wistar rats (29), male and female SHR (34) and T cells contribute to end-organ damage (23, 28). Therefore, the sex difference in the renal T cell profile may contribute to sex differences in proteinuria following ANG II infusion. It has been shown that even a single injection of Tregs attenuates ANG II-induced cardiac damage in male NMRI mice (a colony of inbred mice generated at the US Naval Medical Research Institute) independent of changes in BP (17), suggesting that even moderate increases in Tregs may offer protection against ANG II-induced tissue damage. Therefore, it is tempting to speculate that the increase in Tregs in female SD rats with ANG II infusion attenuates ANG II-induced increases in proteinuria. Future studies will be designed to test this hypothesis.
Similar to Th17 cells and Tregs, IL-17 contributes to increases in BP and males had more IL-17+ T cells regardless of ANG II infusion, while IL-10 mitigates ANG II-induced increases in BP and female SD rats had more renal IL-10+ cells (16, 19). Sex differences in renal Tregs, Th17 cells, IL-17+, and IL-10+ T cells in the current study are consistent with our previous publications in kidneys of SHR (4, 36, 37). It should be noted that while male SD exhibited greater increases in the absolute number of IL-17+ T cells, females had a greater percent increase in IL-17+ T cells than males (240 vs. 172%) underscoring the fact that ANG II does increase the proinflammatory immune profile of both sexes. In contrast to our results, male Rag−/− mice had more splenic and renal Tregs than females and ANG II infusion did not alter Tregs in either sex. However, donor T cells for both male and female Rag−/− mice were from wild-type male mice, and Ji et al. (14, 22) reported that sex of the T cell impacts the response to ANG II. Indeed, while there were no sex differences in renal Tregs when measured by immunohistochemical analysis depending on sex of the T cell donor in male Rag−/− mice, renal Foxp3 mRNA levels were greater in kidneys from male Rag−/− mice if donor T cells were from a female (14). Therefore, it remains possible that donor T cells from a female wild-type mouse to female Rag−/− mice would potentiate the increase in Tregs to a larger extent than in males. The full immune profile, including all cytokines and antigen presenting cells, is an important determinant of T cell differentiation and activation and the “male” immune environment may not be conducive to stimulating Treg differentiation to the same degree as in females. In the current study, CD44+ and CD69+ T cells were measured as early activation markers of T cells. ANG II resulted in greater increases in CD44+ in males following ANG II infusion, suggesting greater T cell activation in males which would be consistent with greater numbers of effector T cells. In support of this notion, only male Rag−/− mice exhibit increases in the proinflammatory cytokines TNF-α, MCP-1, and IL-2 following ANG II infusion (22). Regardless, these data suggest that ANG II induced T cells in a sex-specific manner, where females were able to limit the proinflammatory effects of ANG II on T cells.
To begin to assess the mechanism mediating sex differences in renal T cells following ANG II infusion, ANG (1–7) was measured in the renal cortex of male and female SD rats and consistent with our previous reports in Wistar-Kyoto rats (3) and SHR (34), female SD rats have greater ANG (1–7) concentrations than males. While ANG II infusion did not alter ANG (1–7) in either sex, females maintained higher levels of ANG (1–7) than males and we previously showed that greater ANG (1–7) in female SHR antagonizes ANG II-induced increases in BP and renal injury (34). Additional studies tested the hypothesis that greater ANG (1–7) in females will also antagonize ANG II induction of proinflammatory cells. However, blocking ANG (1–7) during ANG II infusion did not alter the T cell profile in either sex. In contrast to our findings, overexpression of ANG (1–7) in monocrotaline-treated male SD rats stimulates IL-10 and attenuates proinflammatory cytokine release (30). Furthermore, male mice lacking ACE2, a key enzyme for the formation of ANG (1–7), exhibit an increase in total T cells infiltrating the renal tubulointerstitium (18), suggesting a role for ANG (1–7) to impact T cells. The finding that blocking ANG (1–7) during ANG II infusion did not alter T cells may be related to the fact that while ANG (1–7) levels were not significantly altered, ANG II levels increased. It is possible that the increase in ANG II had a greater impact on the T cell profile than blocking ANG (1–7). Alternatively, a limitation of the current study is that we did not measure BP responses to ANG II during A-779 infusion and there are no selective downstream targets of mas receptor activation that can be measured to assess the effectiveness of the blockade in the current study. Therefore, despite using the same dose and route of A-779 that we previously showed exacerbate ANG II hypertension exclusively in female SHR (34), we cannot conclusively state that ANG (1–7) was not involved. Future studies will infuse ANG (1–7) to more directly assess the impact on the T cell profile.
Perspectives
Women are more likely than men to develop inflammatory and immunological disorders (39), and there is growing awareness that certain autoimmune diseases are associated with an increased risk of cardiovascular disease (10, 31). However, we still know very little about the role of the immune system in essential hypertension in females. Our novel findings indicate that ANG II infusion mediates a sex-specific effect on the T cell profile and our studies build on recent work establishing that females are able to limit the prohypertensive effects of T cells from males in response to ANG II hypertension, but T cells from females have a less proinflammatory and prohypertensive phenotype than T cells from males (14, 22). Despite these recent advances, many questions remain regarding the molecular mechanisms responsible for these sex differences and how T cell infiltration in multiple organs impacts overall cardiovascular health. Based on the key role now thought to be played by T cells in BP control, better understanding of how females limit proinflammatory responses may be critical in improving cardiovascular health for both sexes.
GRANTS
The authors acknowledge funding from the National Institutes of Health (1R01 HL-093271-01A1 to J. C. Sullivan) and the American Heart Association (predoctoral fellowships to M. A. Zimmerman and A. J. Tipton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.Z., A.J.T., and J.C.S. conception and design of research; M.A.Z., B.B., and A.J.T. performed experiments; M.A.Z., B.B., A.J.T., P.M.O., and J.C.S. analyzed data; M.A.Z., B.B., A.J.T., P.M.O., and J.C.S. interpreted results of experiments; M.A.Z. and J.C.S. prepared figures; M.A.Z. and J.C.S. drafted manuscript; M.A.Z., B.B., A.J.T., P.M.O., and J.C.S. edited and revised manuscript; M.A.Z., B.B., A.J.T., and P.M.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the excellent technical assistance of Vanessa Kemp, Erica Ralph, and G. Ryan Crislip.
REFERENCES
- 1.Baban B, Chandler PR, Johnson BA 3rd, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. Physiologic control of IDO competence in splenic dendritic cells. J Immunol 187: 2329–2335, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia K, Zimmerman MA, Sullivan JC. Sex differences in angiotensin-converting enzyme modulation of Ang (1–7) levels in normotensive WKY rats. Am J Hypertens 26: 591–598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol 305: R701–R710, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des 18: 963–970, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1–7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 4: 201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 124: 2341–2347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298: R1089–R1097, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowson CS, Liao KP, Davis JM 3rd, Solomon DH, Matteson EL, Knutson KL, Hlatky MA, Gabriel SE. Rheumatoid arthritis and cardiovascular disease. Am Heart J 166: 622–628, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, Bretas TL, Bader M, de Sousa LP, da Silva TA, dos Santos RA, Simoes e Silva AC, Teixeira MM. Anti-inflammatory effects of the activation of the angiotensin-(1–7) receptor, MAS, in experimental models of arthritis. J Immunol 185: 5569–5576, 2010. [DOI] [PubMed] [Google Scholar]
- 12.El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1–7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kappaB-dependent pathways. Br J Pharmacol 166: 1964–1976, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64: 573–582, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin HY, Song B, Oudit GY, Davidge ST, Yu HM, Jiang YY, Gao PJ, Zhu DL, Ning G, Kassiri Z, Penninger JM, Zhong JC. ACE2 deficiency enhances angiotensin II-mediated aortic profilin-1 expression, inflammation and peroxynitrite production. PLos One 7: e38502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 31: 2534–2542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119: 2904–2912, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Huang XR, Chen HY, Penninger JM, Lan HY. Loss of angiotensin-converting enzyme 2 enhances TGF-beta/Smad-mediated renal fibrosis and NF-kappaB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest 92: 650–661, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matrai M, Hetthessy J, Nadasy GL, Monos E, Szekacs B, Varbiro S. Sex differences in the biomechanics and contractility of intramural coronary arteries in angiotensin II-induced hypertension. Gend Med 9: 548–556, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Moore ED, Kooshki M, Metheny-Barlow LJ, Gallagher PE, Robbins ME. Angiotensin-(1–7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP kinase signaling. Free Radic Biol Med 65: 1060–1068, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. The role of T cells in the pathogenesis of primary hypertension. Nephrol Dial Transplant 27, Suppl 4: iv2–5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension 62: 226–230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Sampson AK, Widdop RE, Denton KM. Sex differences in circadian blood pressure variations in response to chronic angiotensin II infusion in rats. Clin Exp Pharmacol Physiol 35: 391–395, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 51: 1170–1176, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol 29: 543–548, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Diez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 182: 1065–1072, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev 9: 15–19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sukumaran V, Veeraveedu PT, Gurusamy N, Lakshmanan AP, Yamaguchi K, Ma M, Suzuki K, Nagata M, Takagi R, Kodama M, Watanabe K. Olmesartan attenuates the development of heart failure after experimental autoimmune myocarditis in rats through the modulation of ANG 1–7 mas receptor. Mol Cell Endocrinol 351: 208–219, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol 83: 413–422, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tipton AJ, Sullivan JC. Sex differences in blood pressure control: are T lymphocytes the missing link? Hypertension 64: 237–239, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol 2: 777–780, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol 307: H191–H198, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman MA, Sullivan JC. Hypertension: what's sex got to do with it? Physiology (Bethesda) 28: 234–244, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]