Abstract
Apolipoprotein A-V (apoA-V), a liver-synthesized apolipoprotein discovered in 2001, strongly modulates fasting plasma triglycerides (TG). Little is reported on the effect of apoA-V on postprandial plasma TG, an independent predictor for atherosclerosis. Overexpressing apoA-V in mice suppresses postprandial TG, but mechanisms focus on increased lipolysis or clearance of remnant particles. Unknown is whether apoA-V suppresses the absorption of dietary lipids by the gut. This study examines how apoA-V deficiency affects the steady-state absorption and lymphatic transport of dietary lipids in chow-fed mice. Using apoA-V knockout (KO, n = 8) and wild-type (WT, n = 8) lymph fistula mice, we analyzed the uptake and lymphatic transport of lipids during a continuous infusion of an emulsion containing [3H]triolein and [14C]cholesterol. ApoA-V KO mice showed a twofold increase in 3H (P < 0.001) and a threefold increase in 14C (P < 0.001) transport into the lymph compared with WT. The increased lymphatic transport was accompanied by a twofold reduction (P < 0.05) in mucosal 3H, suggesting that apoA-V KO mice more rapidly secreted [3H]TG out of the mucosa into the lymph. ApoA-V KO mice also produced chylomicrons more rapidly than WT (P < 0.05), as measured by the transit time of [14C]oleic acid from the intestinal lumen to lymph. Interestingly, apoA-V KO mice produced a steadily increasing number of chylomicron particles over time, as measured by lymphatic apoB output. The data suggest that apoA-V suppresses the production of chylomicrons, playing a previously unknown role in lipid metabolism that may contribute to the postprandial hypertriglyceridemia associated with apoA-V deficiency.
Keywords: absorption, intestine
after dietary fat intake, the increase of plasma triglycerides (TG) is a normal metabolic consequence characterized by a transient accumulation of TG-rich lipoproteins comprised of intestinally derived chylomicrons and liver-derived very low density lipoprotein (VLDL). Hydrolysis of their TG cores releases free fatty acids to be taken up by muscle and adipose tissues, generating remnant particles that are efficiently removed from the circulation by the liver. Aberrations in lipoprotein metabolism result in elevated plasma TG and a prolonged accumulation of remnant particles that are atherogenic (16, 76, 24) and constitute a major risk factor for cardiovascular disease (51, 43).
Apolipoproteins play crucial roles in the regulation of lipoprotein metabolism by acting as structural proteins, as cofactors for enzymes involved in lipolysis, or as ligands for receptor-mediated clearance of lipoproteins (35). Apolipoprotein A-V (apoA-V) was discovered in 2001 to be expressed exclusively in the liver and located ∼30 kb proximal to the APOAI/CIII/AIV gene cluster (49, 69), a region well-known for regulating lipid metabolism (19). Early studies using genetically engineered mouse models revealed apoA-V to be a potent modulator of plasma TG. Knocking out apoA-V in mice resulted in a fourfold increase in plasma TG while overexpressing human apoA-V in mice reduced plasma TG by threefold compared with WT mice (49). Structural analysis showed that apoA-V is very hydrophobic and predominately lipid-bound (3, 72), associating with plasma high-density lipoprotein, VLDL, and chylomicrons (47). What potentially made apoA-V elusive in its discovery was its low plasma concentration, a range of 114–258 ng/ml in normolipemic individuals (26, 47, 59, 64). The interesting question is how can an apolipoprotein of such low plasma concentration affect plasma TG so dramatically?
Over the years, diverse functions have been proposed for apoA-V. ApoA-V may facilitate lipolysis by interacting with lipoprotein lipase (LPL) and heparin sulfate proteoglycans (38) or with an endothelial cell surface protein, glycosylphosphatidylinositol high-density lipoprotein-binding protein 1 (4). ApoA-V may also interact with members of the low-density lipoprotein-receptor family to enhance receptor-mediated particle uptake (13, 20, 45, 46). However, its low plasma concentration indicates that apoA-V exists on a subfraction of plasma lipoproteins, ∼1 apoA-V molecule for every 24 VLDL particles (38), suggesting that apoA-V must be recycled for efficient lipolysis or clearance of particles to occur. Some studies suggest that apoA-V exerts its effects intracellularly rather than extracellularly, by interacting with lipid droplets in hepatocytes (8, 17, 62, 63). In the hepatocyte, and also in the enterocyte although the mechanisms are nuanced, TG accumulates as cytosolic lipid droplets on the endoplasmic reticulum (ER) membrane before being incorporated into lipoproteins for secretion (see Ref. 41). Hence apoA-V is proposed to facilitate the intrahepatic accumulation of lipid (8, 64), which may reduce the production rate of VLDL (8, 49), although this effect was not seen in other studies (15, 20, 38).
In 2001, apoA-V was also discovered to be a novel factor significantly upregulated during early liver regeneration (70). Originally, the authors thought apoA-V stimulated lipid uptake by the liver for regeneration of cell membranes. However, they observed that apoA-V expression and plasma levels peaked at 6 h after partial hepatectomy, while cell mitosis began 20 h later. ApoA-V was speculated to inhibit lipid flux to protect the reduced-size liver against a possible lipid overload, thereby behaving like a hepatic signal protein. It was apparent that, since its discovery, apoA-V was thought as an atypical apolipoprotein with its low plasma concentration and significant ability to modulate plasma TG through a variety of proposed mechanisms.
While mechanisms of apoA-V have been focused on the liver or plasma, no studies have investigated its physiological role in the gut. Only a few studies have looked at effects of apoA-V on plasma TG after a fatty meal. High levels of apoA-V in transgenic mice protected against postprandial lipemia after an intragastric dose of sunflower oil (15). Adenoviral gene transfer of apoA-V to mice also dose-dependently reduced postprandial TG response (49). Authors of these studies concluded that, since apoA-V was only expressed in the liver, the effects were unlikely a result from reduced absorption but rather increased clearance of lipoproteins. However, what has not been investigated thus far is the critical question: does apoA-V play a role in the absorption of dietary lipids by the gut?
The present study was designed to examine the effects of apoA-V deficiency on the steady-state absorption and lymphatic transport of dietary lipids in chow-fed mice. Based on previous observations of the effects of apoA-V on postprandial TG levels (15, 49), and its association with lipid droplets and potential facilitation of TG accumulation in the ER (8, 62, 63, 64), we hypothesized that apoA-V deficiency would increase the transport of dietary TG across the enterocyte and its subsequent secretion as chylomicrons. To test this, we employed the conscious lymph fistula model to directly examine the transport of dietary lipids from the intestinal lumen to the lymph in apoA-V knockout (KO) and wild-type (WT) mice, providing insight into whether a lack of apoA-V affected uptake, intracellular metabolism, or the secretion of lipid into lymph. Our studies show that apoA-V deficiency in mice significantly increases the absorption and lymphatic transport of dietary lipids into lymph, providing compelling evidence for a role of apoA-V in inhibiting lipid absorption and thereby acting as a hepatic signal to modulate the production of chylomicrons by the gut.
MATERIALS AND METHODS
Animals.
ApoA-V KO mice used in this study were backcrossed to a C57BL6/J background for at least eight generations, and their genotypes were confirmed by PCR. ApoA-V KO and age-matched WT control mice were maintained on standard rodent chow (LM-485 Mouse/Rat Sterilizable Diet; Harlan Laboratories, Madison, WI) under a normal 12:12-h light-dark cycle at the University of Cincinnati Laboratory Animal Medical Services. Only 3- to 4-mo-old males were used in the study. All procedures performed were approved by the University of Cincinnati Internal Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Lymph and duodenal cannulation.
Before surgery, mice were fasted overnight with free access to water. The following morning, the animals were anesthetized with ketamine (80 mg/kg) and xylazine (20 mg/kg), and their intestinal lymph ducts were cannulated with polyvinylchloride (PVC) tubing (0.20 mm ID; 0.50 mm OD), with slight modifications to the procedures originally described (9). Instead of being secured with a suture string, the cannula was secured with a drop of tissue glue. A surgical incision was made on the fundus of the stomach, and a second PVC tubing (0.5 mm ID, 0.8 mm OD) was introduced through the fundus of the stomach into the duodenum. The tubing was secured by a purse-string and then with a drop of cryanoacrylate glue. After surgery, the animals were allowed to recover for at least 24 h in Bollman restraining cages that were maintained at a warm temperature of 30°C. Despite the restraints, the animals still had considerable freedom to move forward and backward and laterally. During recovery, the animals received a continuous infusion of a 5% saline-glucose solution at a constant rate of 0.3 ml/h to compensate for fluid and electrolyte loss due to lymphatic drainage.
Lipid infusion and lymph collection.
A lipid emulsion was prepared by dissolving triolein {labeled with [9,10-3H(N)]triolein}, cholesterol {labeled with [1,2-14C(N)]cholesterol}, and egg phosphatidylcholine in chloroform, which was then evaporated under nitrogen gas. The chloroform-free mixture was emulsified with 19 mM sodium taurocholate (NaTC) in phosphate-buffered saline (PBS) at pH 6.4. The emulsion was pulse sonicated until the emulsion appeared homogeneous. Homogeneity was verified by determining radioactivity in samples taken from the top, middle, and bottom of the emulsion. Emulsions were considered homogeneous if the counts did not vary >1%. The morning after surgery, the overnight saline-glucose solution was replaced with the lipid emulsion described above. Each mouse received a continuous intraduodenal infusion of the lipid emulsion for 6 h at a rate of 0.3 ml/h. A continuous infusion enables measurement of transport rates at the steady state (5). The hourly infusate contained 4 μmol triolein labeled with 1 μCi [3H]triolein, 0.78 μmol cholesterol labeled with 0.1 μCi [14C]cholesterol, 0.78 μmol egg phosphatidylcholine, and 5.7 μmol NaTC in PBS. Lymph samples were collected hourly in microcentrifuge tubes on ice. Because the animals are conscious during lipid infusion, our experiments lacked the complications from anesthesia, which could affect the production of chylomicrons (56).
Collection of intestinal tissues and luminal contents.
At the end of the 6-h study, mice were anesthetized in preparation for tissue and organ removal. The small intestine was carefully removed with its mucosal tissue left intact and cut into four equal-length segments through three separate suture ties. The segments were labeled M1–M4 [M1, (duodenum), M2 and M3 (two equal length segments of the jejunum), and M4 (ileum)]. The content from each segment was emptied into a tube and followed by three washes of 10 mM of NaTC solution. Luminal contents from the stomach, small intestine, and colon were collected from three rinses of 10 mM NaTC. The mucosal tissue of the small intestine was left intact and homogenized using a Polytron homogenizer. Samples from the hourly lymph, luminal washings, and intestinal tissues were taken for determination of radioactivity by scintillation spectroscopy. Lipids recovered from the intestinal lumen and mucosa at the end of the study allowed for analysis of steady-state accumulation of lipids that were not secreted (1).
Absorptive and lymphatic transport index calculations.
To determine the efficiency of the enterocyte in absorbing and transporting lipids into the lymph, absorptive and lymphatic transport indexes were computed as previously described (7). The absorptive index represents the percentage of infused lipid taken up by the enterocyte and is calculated from the following equation: absorptive index = 100% − (%total dose recovered in the lumen). The lymphatic transport index represents the percentage of absorbed lipid secreted into the lymph. It determines the ability of the small intestine to transport the absorbed lipid into the lymph independent of the amount of lipid absorbed. The transport index is calculated from the equation: lymphatic transport index = (%infused dose recovered in lymph)/(absorptive index) × 100.
Chylomicron appearance time.
Chylomicron appearance time was determined as previously described (67, 68). Mice were given a continuous intraduodenal infusion of the same lipid emulsion as described above at a rate of 0.3 ml/h. At the 4th h of lipid infusion when steady-state lymphatic TG transport was reached, the infusion pump was stopped, and a bolus dose of [14C]oleic acid (0.5 μCi in 100 μl oleic acid) was injected intraduodenally. After injection, the infusion was resumed to maintain steady-state lymphatic TG transport, and lymph samples were collected every minute for 45 min in small glass scintillation vials. Radioactivity was determined by scintillation counting. The first appearance of radioactivity (in min) represented the time for radioactive oleic acid to be taken up by the enterocyte, resynthesized as TG, packaged, and secreted as chylomicrons in the lymph. It is very important to assess the cellular packaging and release of chylomicrons at the steady state of lymphatic output, since chylomicron production rates can depend on factors such as lymph flow or interstitial fluid hydration as previously studied (67, 68).
Measurement of lymphatic outputs of apoB and apoA-IV.
The lymphatic outputs of apoB and apoA-IV were measured by Western blotting. Samples equivalent to 3 s of lymph flow were resolved on 4–15% polyacrylamide gradient gels and transferred to polyvinylidene difluoride membranes. Following transfer, membranes were blocked with 5% nonfat milk in 0.1% Tween 20 in Tris-buffered saline (TBS) for 1 h. Membranes were incubated with antibodies against apoB and apoA-IV (1:5,000) overnight. After being rinsed with TBS plus 0.1% Tween 20, membranes were incubated with peroxidase-conjugated anti-goat antibody at 1:10,000 for 30 min, and signal was developed with Immobilon Western Chemiluminescent HRP substrate (EMD Millipore, Billerica, MA). Protein bands were quantified by densitometric analysis using Total Lab Quant Analysis Software (Fotodyne, Hartland, WI).
Statistical analysis.
The data shown are mean values ± SE. To compare groups throughout the 6-h infusion period, two-way repeated-measures ANOVA with Tukey posttest analysis were used. For comparison of data that have two independent variables, two-way ANOVA was used. A t-test was used for comparisons of only two groups. Statistical analyses were performed using GraphPad Prism version 6. Data were considered significant if P < 0.05.
RESULTS
Lymphatic transport of lipids.
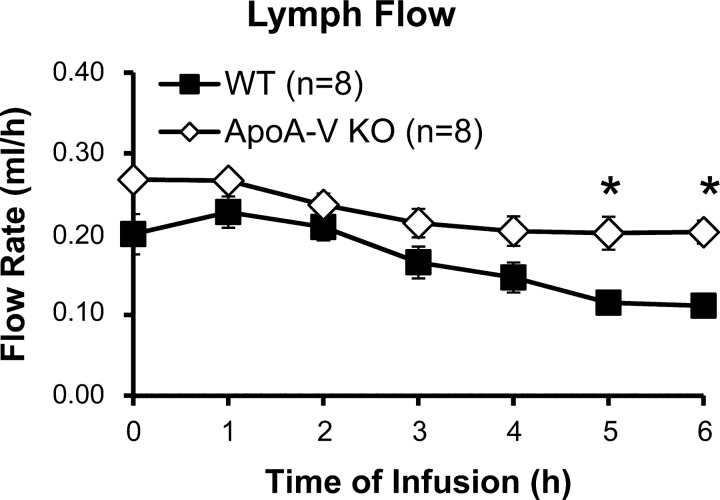
Lymph flow was maintained at 0.2–0.3 ml/h by setting the intraduodenal infusion rate at 0.3 ml/h (Fig. 1). There were no significant differences between the two groups except at the 5th and 6th h, where the lymph flow of the WT mice decreased to 0.11–0.12 ml/h. It should be noted that this decrease occurred after steady state has been achieved and does not affect our analyses of lymphatic output of lipid (68).
Fig. 1.
Lymph flow was maintained at 0.2–0.3 ml/h in apolipoprotein A-V (apoA-V) knockout (KO) and wild-type (WT) mice. Mesenteric lymph flow was measured hourly for 6 h. *P < 0.05. Values are means ± SE.
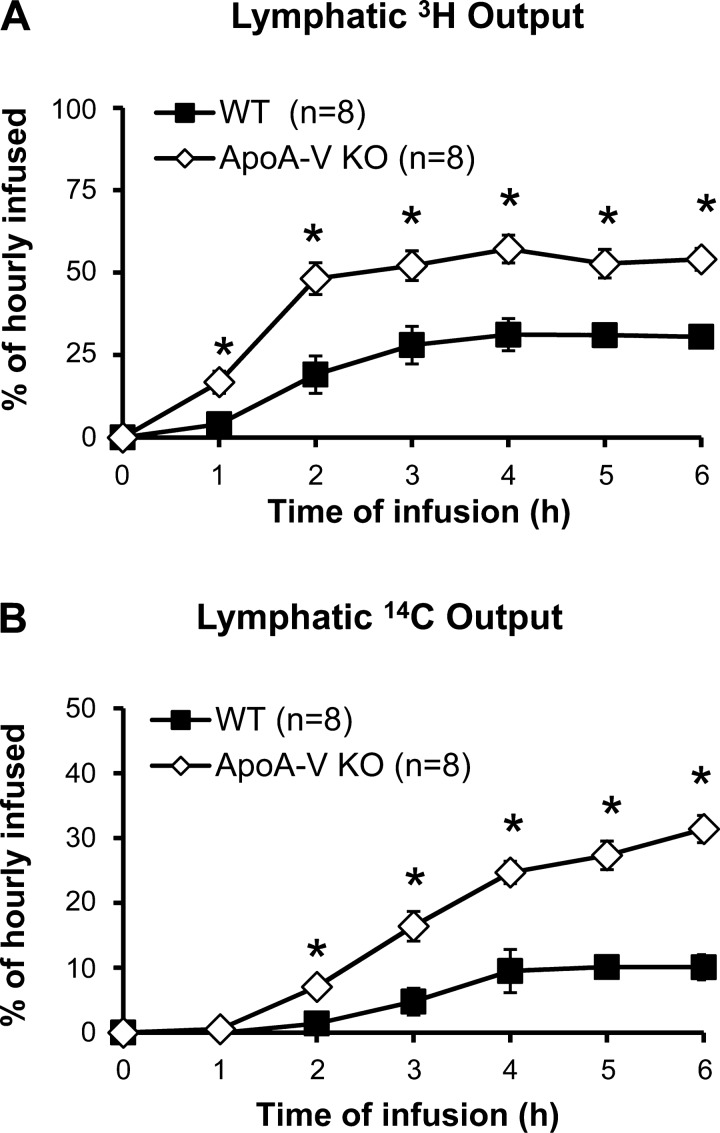
The amount of TG transported into the lymph per hour was measured by 3H and expressed as a percentage of the hourly infused dose (Fig. 2A). Interestingly, apoA-V KO mice showed substantially higher lymphatic 3H transport at the steady state (52.9 ± 3.3%) than WT controls (30.2 ± 1.5%), with differences seen as early as 1 h after the start of lipid infusion. Steady state was achieved within 3–4 h for both groups of animals.
Fig. 2.
ApoA-V KO mice show increased triglyceride (TG) and cholesterol transport into the lymph compared with WT. Lymphatic outputs of 3H (A) and 14C (B) were expressed as percentages of the hourly dose. Lymph fistula mice were intraduodenally infused with a lipid emulsion containing [3H]TG and [14C]cholesterol for 6 h. *P < 0.05. Values are means ± SE.
The amount of cholesterol transported into the lymph was measured by 14C and expressed as a percentage of the hourly infused dose (Fig. 2B). As with 3H, the lymphatic transport of 14C was also significantly higher in apoA-V KO (29.4 ± 2.9%) than in WT mice (9.9 ± 4.3%) at the 6th h, with differences evident within 2 h after the start of lipid infusion. With lymphatic transports of both 3H and 14C higher in apoA-V KO mice, the data suggest that chylomicron production is significantly higher in these mice, revealing a novel role for apoA-V in regulating intestinal lipid secretion under physiological conditions.
Distribution of lipid along segments of the small intestine.
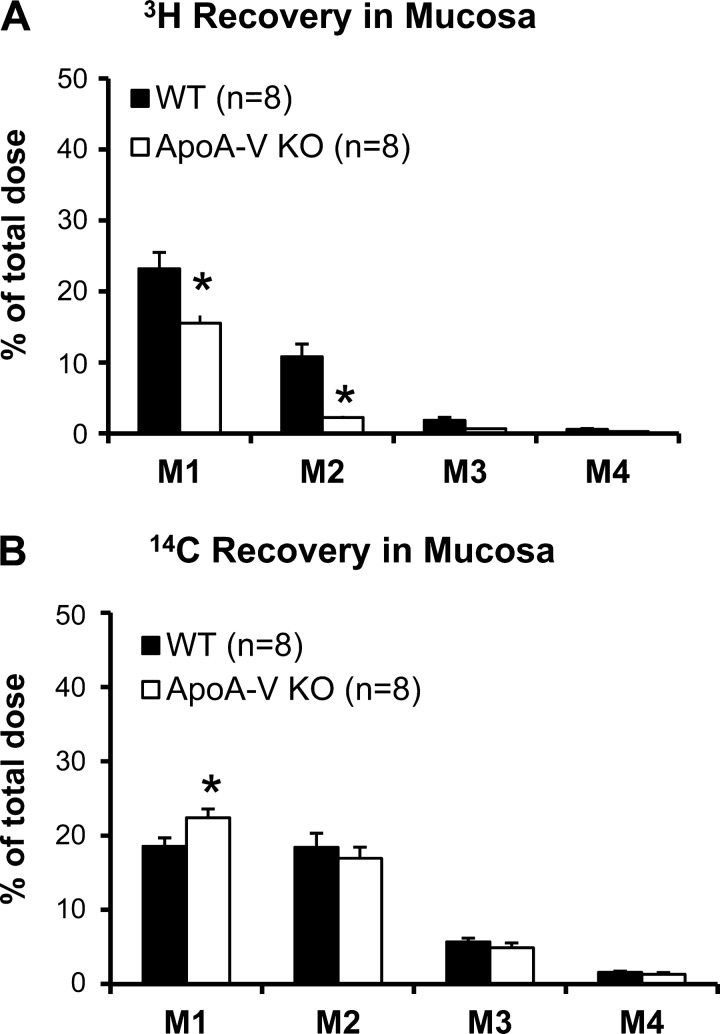
With increased lymphatic transport of lipid in the apoA-V KO mice, we wondered if the mucosa accumulation and distribution of lipids were different in these animals. Figure 3 illustrates the distribution of 3H and 14C accumulated along four equal-length segments of the small intestine, M1–M4 (proximal to distal) at the end of the 6-h infusion period. The pattern of accumulation of 3H and 14C for both groups of animals showed a decrease from M1 to M4, confirming that the primary sites of lipid absorption were mainly located at the proximal half of the intestine (44). ApoA-V KO mice had significantly less 3H in the whole mucosa than WT (P = 0.0004), with the difference being significant in M1 (P = 0.0025) and M2 (P = 0.0003) (Fig. 3A). The data are consistent with our notion that A-V KO mice are more efficient in packaging the absorbed TG into chylomicrons for secretion.
Fig. 3.
Distribution of 3H (A) and 14C (B) along the small intestine divided into 4 segments of equal length, from proximal to distal: M1 (duodenum), M2 and M3 (jejunum), and M4 (ileum). *P < 0.05. Values are means ± SE.
This trend was not seen with cholesterol recovery in the mucosa. Although apoA-V KO mice had slightly more 14C recovered from M1 (P = 0.0359), they had a similar distribution of 14C recovered from the rest of the segments compared with WT (Fig. 3B). Taken together, the data suggests that apoA-V affects the handling of dietary TG and cholesterol in the intestinal mucosa differently.
Recovery of radiolabeled lipid at the end of 6 h continuous infusion.
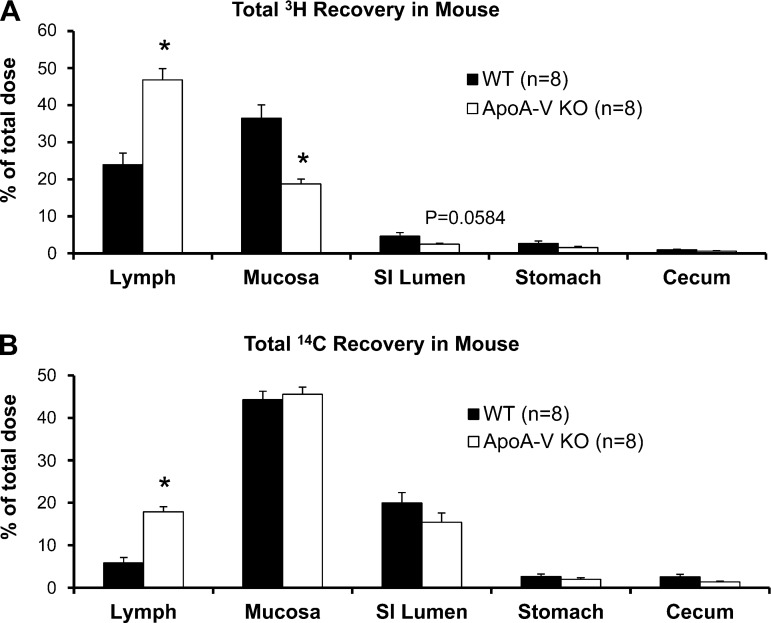
By accounting for the total amount of lipid from the lymph and from the intestinal mucosa and lumen at the end of the study, we were able to analyze how a lack of apoA-V affected steady-state transport of lipids from lumen to lymph. We also collected luminal contents from the stomach and colon to account for lipids that may have refluxed or have been excreted. Figure 4 illustrates the total amount of dietary TG and cholesterol remaining in the lymph, intestinal mucosa, intestinal lumen, stomach, and colon after 6 h of infusion. ApoA-V KO mice showed significantly increased 3H in lymph (P = 0.0001) with a concomitant reduction in 3H recovered from the intestinal mucosa (P = 0.0004) and lumen (although insignificant, P = 0.0584) compared with WT (Fig. 4A). apoA-V KO mice also had significantly increased 3H recovered from the lymph (P = 0.0001), with a possible slight reduction in recovery from the lumen (P = 0.08) compared with WT (Fig. 4B). Total 3H recovery was no different between the groups (WT 68.72 ± 3.01 vs. KO 70.25 ± 3.56%). Although total recovery was <100%, the value obtained is consistent with a number of previous studies showing incomplete absorption of long-chain TG into the lymph (10, 75), possibly due to a small percentage of transport to the portal vein (25, 37) or oxidation.
Fig. 4.
Total recovery of 3H (A) and 14C (B) at the end of the 6-h lipid infusion. Lymph refers to the total radiolabeled lipid collected from the lymph from the 6-h infusion period. Mucosa refers to radioactivity recovered after homogenization of the small intestine. Lumen refers to luminal contents from the small intestine that were washed and collected at the end of infusion. Very low amounts of TG were recovered from the colon and stomach, indicating little excretion and reflux, respectively. *P < 0.05. Values are means ± SE.
Unlike 3H recovery, there was no difference in the amount of 14C recovered from the mucosa of the two groups of mice, probably as a result of the mixing of labeled cholesterol with unlabeled cholesterol in the various pools within the mucosa. Very little amounts of 3H (Fig. 4A) and 14C (Fig. 4B) were recovered from the stomach, indicating that the infused lipids refluxed very minimally back into the stomach. Also, very little 3H and 14C were recovered from the cecum, showing that the infused lipids were minimally excreted during our 6-h study. Overall, the data suggest that the apoA-V KO mice were more efficient in taking up the TG and cholesterol presented in the intestinal lumen and secreting them in the lymph.
To numerically represent the efficiency of the intestine to take up lipid and to transport the absorbed lipid into the lymph, two indexes were calculated and shown in Table 1. Both the absorptive index and lymph transport index for 3H were higher in apoA-V KO mice than in WT, suggesting more efficient uptake and secretion of dietary TG. The lymph transport index for 14C was significantly higher in apoA-V KO mice, indicating more efficient secretion of dietary cholesterol into the lymph. These indexes illustrate apoA-V deficiency results in more efficient chylomicron secretion, independent of lipid uptake.
Table 1.
Absorptive and lymphatic transport index
| TG |
Cholesterol |
|||
|---|---|---|---|---|
| Absorptive Index | Lymphatic Transport Index | Absorptive Index | Lymphatic Transport Index | |
| WT | 91.75 ± 1.54 | 25.81 ± 3.56 | 74.80 ± 2.82 | 7.62 ± 1.51 |
| apoA-V KO | 95.36 ± 0.32* | 49.08 ± 3.08* | 81.23 ± 1.99 | 22.00 ± 1.30* |
Values are means ± SE.
TG, triglyceride, WT, wild type; apoA-V, apolipoprotein A-V; KO, knockout.
P < 0.05.
Chylomicron appearance time.
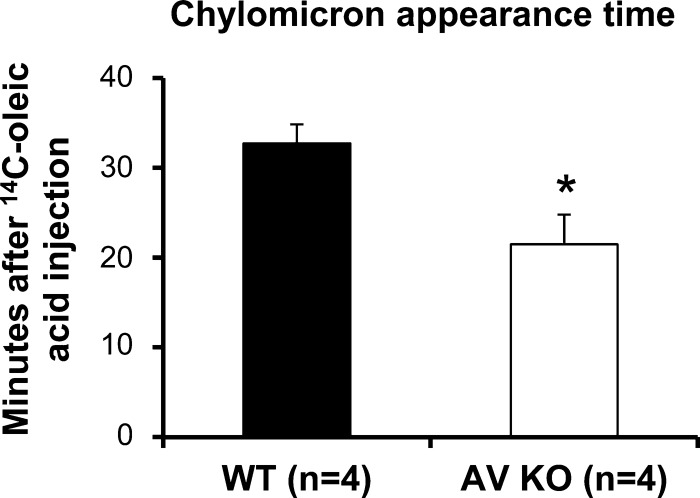
As an alternative measure of chylomicron secretion efficiency, we measured the chylomicron appearance time, which is the time for [14C]oleic acid placed in the intestinal lumen to appear in the lymph as 14C chylomicrons during steady-state transport. It represents the total time required for lipid uptake, resynthesis into TG, assembly into chylomicrons, and secretion into the lymphatics. Figure 5 shows the chylomicron appearance time is significantly (P < 0.05) shorter in apoA-V KO mice (21.5 ± 3.3 min) than in WT mice (32.8 ± 2.1 min), further demonstrating that apoA-V KO mice are faster at generating chylomicrons.
Fig. 5.
ApoA-V KO mice have a shorter chylomicron appearance time (min) after [14C]oleic acid injection in the intestinal lumen. apoA-V KO and WT mice were infused intraduodenally with a lipid emulsion containing TG. At the 4th h of lipid infusion when steady-state lymphatic TG transport was reached, [14C]oleic acid was introduced into the lumen, and lymph samples were collected every minute afterward for 60 min. *P < 0.05. Values are means ± SE.
Lymphatic output of apoB and -A-IV.
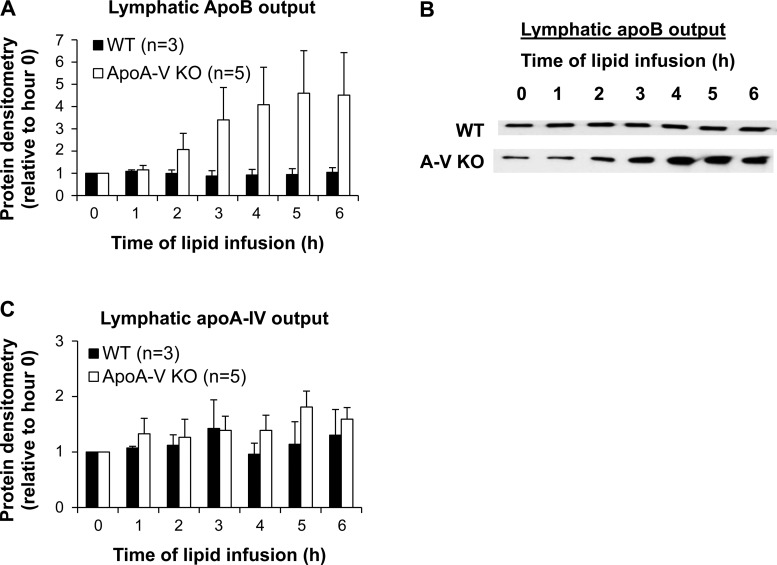
Because the data thus far suggest that chylomicron production is enhanced in apoA-V KO mice, we measured the lymphatic output of apoB, which represented the number of chylomicrons produced over time, since only one apoB protein is assumed to be one chylomicron particle (2). While WT mice had a constant lymphatic output of apoB as expected, apoA-V KO mice showed a steady increase in the lymphatic output of apoB (Fig. 6, A and B), suggesting a greater number of chylomicron particles produced over time. Because apoA-IV is presumed to facilitate the assembly of chylomicrons (73), we also measured the lymphatic output of apoA-IV. There was no difference between the two groups in the lymphatic output of apoA-IV relative to fasting (Fig. 6C) although both groups displayed an ∼1.5-fold increase over 6 h that is comparable to previous studies (22, 28). This suggests that apoA-V does not affect the lipid-stimulated secretion of apoA-IV by the intestine. Overall, these data suggest that the increased lymphatic output of dietary TG in apoA-V KO mice is a result of increased production of chylomicron particles.
Fig. 6.
ApoA-V KO mice have increased intestinal apoB secretion into the lymph. A: lymph samples were analyzed for apoB by Western blot. Protein concentration was quantified by densitometric analysis and normalized to hour 0 (fasting). B: a representative blot is shown for lymphatic apoB output. C: lymph samples were analyzed for apoA-IV by Western blot.
DISCUSSION
While apoA-V is well-demonstrated to profoundly regulate fasting plasma TG in both mice (20, 49, 15, 49, 61, 70, 64) and humans (1, 14, 23, 32, 21), little is known about the contribution of apoA-V to lipid absorption and postprandial TG metabolism. Results from apoA-V transgenic mice (15) and adenovirus-mediated gene transfer experiments to mice (49) suggest that apoA-V suppresses the surge in plasma TG after a lipid meal. However, because apoA-V is only synthesized in the liver (49, 69), mechanistic studies have been focused on enhanced lipolysis (18, 38, 64) or clearance of plasma lipoproteins (20, 38) and not on actions in the gut. The present studies demonstrate a novel role for apoA-V in the absorption of dietary lipids and secretion of chylomicrons by the gut. We show that apoA-V deficiency in mice enhances the transport rate of dietary lipids across the enterocyte, which is accompanied by their rapid secretion from the mucosa into the lymph. This is the first report of apoA-V mediating physiological actions in the intestine.
Postprandial hypertriglyceridemia is an independent risk factor for coronary artery disease (see Ref. 30). The surge in postprandial TG-rich lipoproteins, both intestinally derived chylomicrons and hepatic-derived VLDL, are thought to have deleterious effects on arterial vessel walls (53, 36) and thus contribute to atherosclerosis (34, 71, 76). Increased postprandial plasma TG is also an inherent feature of diabetic dyslipidemia (12, 33), but the underlying mechanisms remain unclear. Since nearly all dietary lipids enter the bloodstream through the intestine, understanding the contribution of intestine to postprandial hypertriglyceridemia is of high clinical and therapeutic importance.
The lymph cannulation method allows us to examine chylomicrons before entering the circulation, which dissociates chylomicron production from clearance. A previous study by Merkel et al. (38) reported that the intestinal secretion of dietary TG was not different in apoA-V transgenic mice compared with WT, although the authors elegantly demonstrated that apoA-V enhanced LPL-mediated hydrolysis of lipoproteins in the plasma. The lipids were administered by intragastric gavage, which is commonly used to measure total absorption but cannot determine its absorptive ability due to variability of stomach emptying (1, 5). Also, studies performed with a bolus dose of lipid cannot give information on the absorption rate of lipid at the steady state, which is an important question when evaluating the contribution of chylomicron delivery to the postprandial TG surge. Furthermore, in the study by Merkel et al., it is possible that a superphysiological level of apoA-V cannot alter the absorption of TG, hence the lack of difference seen in apoA-V transgenic mice. Therefore, to directly address the hypothesis that apoA-V plays an important role in regulating the production rate of chylomicrons, we employed the conscious lymph fistula model under a continuous infusion setting in apoA-V-deficient mice and WT controls.
The results of this study demonstrate that apoA-V deficiency enhances the efficiency of the small intestine to absorb and secrete dietary lipid into the lymph. We show that the transport of [3H]TG from lumen to lymph is accelerated in apoA-V KO mice, as reflected by an increase in the lymphatic transport rate of 3H (Fig. 2A), a decrease in the amount of [3H]TG retained in the intestinal mucosa, and a trend for decrease in the intestinal lumen (Fig. 4A). Further quantitative analyses show that apoA-V KO mice have a greater ability to take up [3H]TG and secrete TG into the lymph (Table 1). This effect is seen specifically in the proximal sections of the small intestine (Fig. 3A). Because more TG is transported as chylomicrons in the apoA-V KO mice, more cholesterol is presumed to be transported out of the small intestine. We find that the transport of 14C from lumen to lymph is indeed enhanced in apoA-V KO mice (Figs. 2B and 4B), indicating the effect of apoA-V deficiency was not specific to TG. However, there is no difference in 14C retained in the intestinal mucosa (Fig. 3B), which could be explained by the large intracellular cholesterol pool diluting the exogenous labeled cholesterol.
These results suggest a role for apoA-V in the lipidation of primordial chylomicron particles, which is supported by studies in the hepatocytes where apoA-V is postulated to stabilize lipid droplets (8, 62, 63). It is possible that apoA-V may gain access to enterocyte lipid droplets and, in doing so, serve to modulate TG secretion, and that apoA-V deficiency could cause more rapid movement of intracellular TG into primordial chylomicrons and subsequently enhances chylomicron production. Indeed, when chylomicron appearance time was measured, we found that apoA-V KO mice took less time to produce chylomicrons (Fig. 5) than WT mice, which further strengthens the argument that apoA-V plays an important role in suppressing chylomicron production.
While some reports (15, 20, 38), plus studies in our lab (data not shown), have found no effect of apoA-V on hepatic VLDL secretion, there is a body of evidence that shows that apoA-V KO mice have impaired removal of lipoprotein remnants (15, 20). Our finding that apoA-V deficiency resulted in greater chylomicron particle secretion (Fig. 6, A and B) provides an additional mechanism by which a lack of apoA-V contributes to postprandial lipemia. An increased rate of chylomicron production may result in a greater flux of chylomicron particles to the remnant pool in the plasma. Because apoA-V-deficient mice were previously shown to have reduced LPL activity (20), the rapid influx of chylomicrons might tax the capacity for hydrolysis of TG-rich lipoproteins (chylomicrons plus VLDL) and thereby reduce their removal rate.
Applied to the human population, only a few studies have examined the association of apoA-V polymorphisms with postprandial plasma TG although a clear association of apoA-V polymorphisms and elevated fasting plasma TG has been documented in a wide range of ethnic groups (29, 32, 42, 50, 57). These studies observed an inverse relationship between plasma apoA-V and postprandial TG in patients after a lipid meal. For example, the C allele of the T-1131C polymorphism was found to be associated with hypertriglyceridemia and higher postprandial TG in nonobese Korean (27) and Caucasian (39) populations. Additionally, healthy young males with the APOA5*2 haplotype (−1131C>T) or APOA5*3 haplotype (c.56C>G) also showed higher postprandial response after a fat load test (40). However, the mechanisms underlying these effects were not further explored and were thought to be caused by slower TG hydrolysis and removal of remnant lipoproteins based on evidence in mouse models. Our studies direct attention to a major contribution by the gut in the delivery of chylomicron particles in the postprandial state, signifying the importance of dietary considerations or gut-targeted therapeutics in patients with apoA-V polymorphisms. Our findings are particularly important because they predict higher levels of postprandial apoB48, and significant evidence links increased apoB48 with adverse cardiovascular events (see Ref. 66) and the presence of apoB48 in atherosclerotic plagues (48, 54).
In summary, we have demonstrated for the first time that apoA-V deficiency in mice significantly increases the production rate of chylomicrons by the gut and that this effect seems to come from more efficient shuttling of dietary TG out of the mucosa and into the lymph. Our findings suggest that apoA-V does not behave as a typical classical apolipoprotein but rather a signal protein, communicating between the liver and the gut. This suggestion was first put forth by Van der Vliet and colleagues in 2001, who speculated that apoA-V may act as a signal released by the liver during regeneration to protect itself against a lipid overload (70). In a normal physiological condition, apoA-V may be released by the liver to reduce lipid flux from the gut. Interestingly, cases of apoA-V deficiency in humans do demonstrate a higher postprandial TG excursion (27, 31, 40). By extension, the postprandial hypertriglyceridemia seen in these patient populations may be attributed to an increased rate of delivery of dietary lipids to the bloodstream.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-092138 and DK-059630 (University of Cincinnati MMPC) to P. Tso and American Heart Association Grant 13PRE17140013 to L. Zhang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.S.Z. and P.T. conception and design of research; L.S.Z., M.X., and Q.Y. performed experiments; L.S.Z. analyzed data; L.S.Z. interpreted results of experiments; L.S.Z. prepared figures; L.S.Z. drafted manuscript; L.S.Z., R.O.R., P.N.H., and P.T. edited and revised manuscript; L.S.Z., M.X., Q.Y., R.O.R., P.N.H., and P.T. approved final version of manuscript.
REFERENCES
- 1.Aberdeen V, Shepherd PA, Simmonds WJ. Concurrent measurement, in unanaesthetized rats, of intestinal transport and fat absorption from the lumen. Exp Physiol 45: 265–274, 1960. [DOI] [PubMed] [Google Scholar]
- 2.Albers JJ, Kennedy H, Marcovina SM. Evidence that Lp[a] contains one molecule of apo[a] and one molecule of apoB: evaluation of amino acid analysis data. J Lipid Res 37: 192–196, 1996. [PubMed] [Google Scholar]
- 3.Beckstead JA, Oda MN, Martin DD, Forte TM, Bielicki JK, Berger T, Luty R, Kay CM, Ryan RO. Structure-function studies of human apolipoprotein A-V: a regulator of plasma lipid homeostasis. Biochemistry 42: 9416–9423, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Beigneux AP, Davies B, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 5: 279–291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett S, Simmonds WJ. Absorptive capacity and intestinal motility in unanaesthetized rats during intraduodenal infusion of fat. Exp Physiol 47: 32–38, 1961. [DOI] [PubMed] [Google Scholar]
- 6.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290: 1771–1775, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Bergstedt SE, Hayashi H, Kritchevsky D, Tso P. A comparison of absorption of glycerol tristearate and glycerol trioleate by rat small intestine. Am J Physiol Gastrointest Liver Physiol 259: G386–G393, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Blade AM, Fabritius MA, Hou L, Weinberg RB, Shelness GS. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. J Lipid Res 52: 237–244, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33: 1349–1352, 1948. [PubMed] [Google Scholar]
- 10.Borgstrom B. On the mechanism of the intestinal fat absorption. The effect of bile diversion on fat absorption in the rat. Acta Physiol Scand 28: 278–286, 1953. [DOI] [PubMed] [Google Scholar]
- 11.Calandra S, Oliva CP, Tarugi P, Bertolini S. APOA5 and triglyceride metabolism, lesson from human APOA5 deficiency. Curr Opin Lipidol 17: 122–127, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Chen YD, Swami S, Skowronski S, Coulston A, Reaven GM. Differences in postprandial lipemia between patients with normal glucose tolerance and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 76: 172–177, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Dichlberger A, Cogburn LA, Nimpf J, Schneider WJ. Avian apolipoprotein A-V binds to LDL receptor gene family members. J Lipid Res 48: 1451–1456, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Evans D, Buchwald A, Beil FU. The single nucleotide polymorphism -1131T>C in the apolipoprotein A5 (APOA5) gene is associated with elevated triglycerides in patients with hyperlipidemia. J Mol Med 81: 645–654, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Fruchart-Najib J, Bauge E, Loredan-Stefan N, Pham T, Thomas B, Rommens C, Majd Z, Brewer B, Pennacchio LA, Fruchart JC. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun 319: 397–404, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb 16: 145–154, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Forte TM, Ryan RO. Influence of apolipoprotein A-V on hepatocyte lipid droplet formation. Biochem Biophys Res Commun 427: 361–365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales JC, Gordts PL, Foley EM, Esko JD. Apolipoproteins E and AV mediate lipoprotein clearance by hepatic proteoglycans. J Clin Invest 123: 2742–2751, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenendijk M, Cantor RM, de Bruin TW, Dallinga-Thie GM. The apoAI-CIII-AIV gene cluster. Atherosclerosis 157: 1–11, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Grosskopf I, Baroukh N, Lee SJ, Kamari Y, Harats D, Rubin EM, Pennacchio LA, Cooper AD. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler Thromb Vasc Biol 25: 2445–2447, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Havasi V, Szolnoki Z, Talian G, Bene J, Komlosi K, Maasz A, Somogyvari F, Kondacs A, Szabo M, Fodor L, Bodor A, Melegh B. Apolipoprotein A5 Gene Promoter Region T-1131C Polymorphism associates with elevated circulating triglyceride levels and confers susceptibility for development of ischemic stroke. J Mol Neurosci 29: 177–183, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black B, Tso P. Transport of lipid and apolipoprotein A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31: 1613–1625, 1990. [PubMed] [Google Scholar]
- 23.Henneman P, Schaap FG, Havekes LM, Rensen PC, Frants RR, van Tol A, Hattori H, Smelt AH, van Dijk KW. Plasma apoAV levels are markedly elevated in severe hypertriglyceridemia and positively correlated with the APOA5 S19W polymorphism. Atherosclerosis 193: 129–134, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Hodis HN. Triglyceride-rich lipoprotein remnant particles and risk of atherosclerosis. Circulation 99: 2852–2854, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Hyun SA, Vahouny GV, Treadwell CR. Portal absorption of fatty acids in lymph- and portal vein-cannulated rats. Biochim Biophys Acta 137: 296–305, 1967. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara M, Kujiraoka T, Iwasaki T, Nagano M, Takano M, Ishii Tsuji M, Ide H, Miller IP, Miller NE, Hattori H. A sandwich enzyme-linked immunosorbent assay for human plasma apolipoprotein A-V concentration. J Lipid Res 46: 2015–2022, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Jang Y, Kim JY, Kim OY, Lee JE, Cho H, Ordovas JM, Lee JH. The -1131TC polymorphism in the apolipoprotein A5 gene is associated with postprandial hypertriacylglycerolemia; elevated small, dense LDL concentrations, and oxidative stress in nonobese Korean men. Am J Clin Nutr 80: 832–840, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Kalogeris TJ, Fukagawa K, Tso P. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J Lipid Res 35: 1141–1151, 1994. [PubMed] [Google Scholar]
- 29.Kao J, Wen H, Chien K, Hsu H, Li S. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum Mol Genet 12: 2533–2539, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Karpe F, Steiner G, Uffelman K, Olivercrona T, Hamsten A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis 106: 83–97, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Kohan AB, Howles PN, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr Pro Mouse Biol 2: 219–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai C, Tai E, Tan CE, Cutter J, Chew SK, Zhu Y, Adiconis X, Ordovas JM. The APOA5 locus is a strong determinant of plasma triglyceride concentrations across ethnic groups in Singapore. J Lipid Res 44: 2365–2373, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Lewis GF, O'Meara NM, Soltys PA, Blackman JD, Iverius PH, Pugh WL, Getz GS, Polonsky KS. Fasting hypertriglyceridemia in noninsulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoproteins abnormalities. J Clin Endocrinol Metab 72: 934–944, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Miranda L, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences in postprandial lipid metabolism. Brit J Nutr 98: 458–473, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Mahley RW, Innerarity TL, Rall SC, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lip Res 25: 1277–1294, 1984. [PubMed] [Google Scholar]
- 36.Mamo JC, Proctor SD, Smith D. Retention of chylomicron remnants by arterial tissue; importance of an efficient clearance mechanisms from plasma. Atherosclerosis 141: S63–S69, 1998. [DOI] [PubMed] [Google Scholar]
- 37.McDonald GB, Saunders DR, Weidman M, Fisher L. Portal venous transport of long-chain fatty acids absorbed from rat intestine. Am J Phyiol Gastrointest Liver Physiol 239: G141–G150, 1980. [DOI] [PubMed] [Google Scholar]
- 38.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem 280: 21553–21560, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Moreno R, Perez-Jimenez F, Marin C, Moreno JA, Gomez P, Bellido C, Perez-Martinez P, Jimenez-Gomez Y, Fuentes FJ, Lopez-Miranda J. A single nucleotide polymorphism of the apolipoprotein A-V gene -1131T>C modulates postprandial lipoprotein metabolism. Atherosclerosis 189: 163–168, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Luna R, Perez-Jimenez F, Marin C, Perez-Martinez P, Gomez P, Jimenez-Gomez Y, Delgado-Lista J, Moreno JA, Tanaka T, Ordovas JM, Lopez-Miranda J. Two independent apolipoprotein A5 haplotypes modulate postprandial lipoprotein metabolism in a healthy caucasian population. J Clin Endocrinol Metab 92: 2280–2285, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40: 325–438, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Nabika T, Nasreen S, Kobayashi S, Masuda J. The genetic effect of the apoprotein AV gene on the serum triglyceride level in Japanese. Atherosclerosis 165: 201–204, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Kugiyama K. Triglycerides and remnant particles as risk factors for coronary artery disease. Curr Atheroscler Rep 8: 107–110, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131: 1197–1207, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsson SK, Christensen S, Raarup MK, Ryan RO, Nielson MS, Olivecrona G. Endocytosis of apolipoprotein AV by members of the low density lipoprotein receptor and the VPS10p domain receptor families. J Biol Chem 283: 25920–25927, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson SK, Lookene A, Beckstead JA, Gliemann G, Ryan RO, Olivecrona G. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry 46: 3896–3904, 2007. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem 51: 351–359, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Pal S, Semorine K, Watts GF, Mamo J. Identification of lipoproteins of intestinal origin in human atherosclerotic plaque. Clin Chem Lab Med 41: 792–795, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 294: 169–173, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet 11: 3031–3038, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Phillips NR, Waters D, Havel RJ. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation 88: 2762–2770, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Plosch T, Kosters A, Groen AK, Kuipers F. The ABC of hepatic and intestinal cholesterol transport. Hepatology 170: 465–482, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Proctor SD, Mamo JC. Arterial fatty lesions have increased uptake of chylomicron remnants but not low-density lipoproteins. Coron Artery Dis 7: 239–245, 1996. [PubMed] [Google Scholar]
- 54.Proctor SD, Mamo JC. Intimal retention of cholesterol derived from apolipoprotein B100- and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscl Thromb Vasc Biol 23: 1595–1600, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Qu S, Perdomo G, Su D, D'Souza FM, Shachter NS, Dong HH. Effects of apoA-V on HDL and VLDL metabolism in APOC3 transgenic mice. J Lipid Res 48: 1476–1487, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redgrave TG. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest 49: 465–471, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribalta J, Figuera L, Fernandez-Ballart J, Vilella E, Cabezas MC, Masana L, Joven J. Newly identified apolipoprotein AV gene predisposes to high plasma triglycerides in familial combined hyperlipidemia. Clin Chem 48: 1597–1600, 2002. [PubMed] [Google Scholar]
- 58.Schaap FG, Rensen PC, Voshol PJ, Vrins C, van der Vliet HN, Chamuleau RA, Havekes LM, Groen AK, van Dijk KW. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J Bio Chem 279: 27941–27947, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Schaap FG, Nierman MC, Berbee JF, Hattori H, Talmud PJ, Vaessen SF, Rensen PC, Chamuleau RA, Kuivenhoven JA, Groen AK. Evidence for a complex relationship between apoA-V and apoC-III in patients with severe hypertriglyceridemia. J Lipid Res 47: 2333–2339, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Sewell RB, Mao SJ, Kawamoto T, LaRusso NF. Apolipoproteins of high, low, and very low density lipoproteins in human bile. J Lipid Res 24: 391–401, 1983. [PubMed] [Google Scholar]
- 61.Sharma V, Beckstead JA, Simonsen JB, Nelbach L, Watson G, Forte TM, Ryan RO. Gene transfer of apolipoprotein A-V improves the hypertriglyceridemic phenotype of apoa5 (−/−) mice. Arterioscler Thromb Vasc Biol 33: 474–480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shu X, Chan J, Ryan RO, Forte TM. ApoA-V association with intracellular lipid droplets. J Lipid Res 48: 1445–1450, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Shu X, Ryan RO, Forte TM. Intracellular lipid droplets targeting by apolipoprotein A-V requires the carboxyl-terminal segment. J Lipid Res 49: 1670–1676, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu X, Nelbach L, Ryan RO, Forte TM. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim Biophys Acta 1801: 605–608, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talmud PJ, Cooper JA, Hattori H, Miller IP, Miller GJ, Humphries SE. The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia 49: 2337–2340, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Tomkin GH, Owens D. The chylomicron: relationship to atherosclerosis. Int J Vasc Med 2012: 784536, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tso P, Barrowman JA, Granger DN. Importance of interstitial matrix hydration in intestinal chylomicron transport. Am J Physiol Gastrointest Liver Physiol 250: G497–G500, 1986. [DOI] [PubMed] [Google Scholar]
- 68.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol Gastrointest Liver Physiol 249: G21–G28, 1985. [DOI] [PubMed] [Google Scholar]
- 69.Van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem 276: 44512–44520, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Van der Vliet HN, Schaap FG, Levels JH, Ottenhoff R, Looije N, Wesseling JG, Groen AK, Chamuleau RA. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem Biophys Res Commun 295: 1156–1159, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Watts GF, Mamo JC, Redgrave TG. Postprandial dyslipidaemia in a nutshell: food for thought. Aust N Z J Med 28: 816–823, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Weinberg RB, Cook VR, Beckstead JA, Martin DD, Gallagher JW, Shelness GS, Ryan RO. Structure and Interfacial Properties of Human Apolipoprotein A-V. J Biol Chem 278: 34438–34444, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Weinberg RB, Gallagher JW, Fabritius MA, Shelness GS. ApoA-IV modulates the secretory trafficking of apoB and the size of triglyceride-rich lipoproteins. J Lip Res 53: 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White D, Bennett A, Billett M, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylcerol transfer protein. Br J Nutr 80: 219–229, 1998. [PubMed] [Google Scholar]
- 75.Wu AL, Clark SB, Holt PR. Transmucosal triglyceride transport rates in proximal and distal rat intestine in vivo. J Lip Res 16: 251–257, 1975. [PubMed] [Google Scholar]
- 76.Zilversmit DB. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem 41: 153–158, 1995. [PubMed] [Google Scholar]