Abstract
Increasing amounts of evidence strongly suggests that dysregulation of ubiquitin-proteasome system is closely associated with cancer pathogenesis. Speckle-type POZ protein (SPOP) is an adapter protein of the CUL3-based E3 ubiquitin ligase complexes. It selectively recruits substrates for their ubiquitination and subsequent degradation. Recently, several exome-sequencing studies of endometrial cancer revealed high frequency somatic mutations in SPOP (5.7–10%). However, how SPOP mutations contribute to endometrial cancer remains unknown. Here, we identified estrogen receptor-α (ERα), a major endometrial cancer promoter, as a substrate for the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex. SPOP specifically recognizes multiple Ser/Thr (S/T)-rich degrons located in the AF2 domain of ERα, and triggers ERα degradation via the ubiquitin-proteasome pathway. SPOP depletion by siRNAs promotes endometrial cells growth. Strikingly, endometrial cancer-associated mutants of SPOP are defective in regulating ERα degradation and ubiquitination. Furthermore, we found that SPOP participates in estrogen-induced ERα degradation and transactivation. Our study revealed novel molecular mechanisms underlying the regulation of ERα protein homeostasis in physiological and pathological conditions, and provided insights in understanding the relationship between SPOP mutations and the development of endometrial cancer.
Endometrial cancer is the most common gynecologic malignancy that arises from the endometrium, or lining, of the uterus. Endometrial cancer causes ~74 000 deaths annually among women worldwide.1 Most patients present with low-grade, early-stage disease. However, patients with more aggressive, high-grade tumors that spread beyond the uterus will usually progress within 1 year.2 For effective cancer prevention and treatment, it is necessary to identify genetic alterations that initiate endometrial cancer and contribute to its progression. Recently, significant progress has been made in identifying the genetic alterations in endometrial cancer using array-based technologies and next-generation sequencing.3, 4, 5, 6 Mapping the genomic landscape of endometrial cancer has produced comprehensive molecular classification of these tumors, which may ultimately serve to improve the diagnosis and treatment of patients with endometrial cancer.7 Among these investigations, speckle-type POZ protein (SPOP) was identified as one of the most frequently altered genes by somatic point mutations in endometrial cancers through large-scale exome-sequencing approaches.3, 4, 5, 6 Nonetheless, how SPOP mutations contribute to the pathogenesis and progression of endometrial cancer remains unknown.
SPOP is an adaptor protein of the CUL3-RBX1 E3 ubiquitin ligase complex. It selectively recruits substrates via its N-terminal MATH domain, whereas its BTB domain mediates dimerization and interaction with CUL3.8 SPOP has been linked to the ubiquitination of several substrates in both Drosophila and human cells, including the steroid receptor coactivator SRC-3, death domain-associated protein Daxx, the phosphatase Puc, the transcriptional regulator Ci/Gli, and several others.9, 10, 11, 12, 13 All endometrial cancer-associated SPOP mutations identified so far affect evolutionarily conserved residues in the MATH domain, suggesting that the mutations may alter the interaction of SPOP with its substrates.3, 4, 5, 6 In addition to endometrial cancer, SPOP is also mutated in 4.6 to 14.4% of patients with prostate cancer across different ethnic and demographic backgrounds.14 Importantly, mutual exclusivity of SPOP mutation with ETS family gene rearrangement, as well as a high association with CHD1 deletion reinforces SPOP mutation as defining a distinct molecular subclass of prostate cancer.14, 15
Estrogen receptor-α (ERα), encoded by ESR1 gene, is a nuclear transcriptional factor that mediates estrogen-stimulated cell proliferation in hormone-responsive cancers, such as breast, endometrial and ovarian cancers.16 The ERα protein is highly overexpressed in breast, endometrial, and ovarian cancers and is among the first known targets for molecular therapy in any cancers.16 After binding to estrogen, ERα dimerizes and translocates into the nucleus, where it recruits co-activators or co-repressors, as well as chromatin-remodeling factors, to estrogen response elements on target gene promoters to activate or repress transcription.17 ERα is a member of the sex steroid receptors family that ligand-dependently regulates the functions of the sexual organs. Other sex steroid receptors include the androgen receptor (AR), the estrogen receptor-β, and the progesterone receptor. All sex steroid receptors are built with similar modular structure, including a DNA-binding domain, a hinge region with a nuclear location signal, and a ligand-binding domain.18 Recently, we reported that SPOP influences the AR stability and inhibits AR-mediated gene transcription and prostate cancer cell growth.19 Importantly, prostate cancer-associated mutants of SPOP cannot bind to AR and fail to promote AR ubiquitination and degradation, implying the importance of this pathway in the resistance to antiandrogen therapy of prostate cancer.19 On the basis of that SPOP regulates AR abundance, as well as similar domain architecture between ERα and AR, we investigated the possible role of SPOP in controlling ERα protein stability.
In this study, we demonstrated that SPOP forms a functional CUL3-SPOP-RBX1 E3 ubiquitin ligase complex which targets ERα for ubiquitination and proteasomal degradation in endometrial cancer cells. Moreover, this effect is abrogated by the endometrial cancer-associated SPOP mutations. Our results provide a functional insight into the molecular mechanism of endometrial cancer pathway involved with SPOP mutations.
Results
SPOP interacts with ERα in cells
It was previously reported that SPOP regulates AR stability.19 Because ERα is the most extensively studied biomarker and the best predictor for response to endocrine therapy in patients with endometrial cancer, we explored the possibility that SPOP regulates ERα protein stability via a similar mechanism as that of AR stability. Accordingly, we first examined whether SPOP interacts with ERα in cells. To do this, FLAG-HA (FH)-SPOP and Myc-ERα constructs were co-expressed in 293T cells. Cell lysates were subsequently prepared for co-immunoprecipitation (co-IP) with anti-FLAG antibody. As shown in Figure 1a, Myc-ERα was immunoprecipitated by FH-SPOP, suggesting an interaction between these two proteins. Similar results were also obtained in the reciprocal co-IP experiment in which FH-ERα was able to immunoprecipiate Myc-SPOP (Figure 1b). Next, we decided to extend our analysis by investigating whether endogenous SPOP and ERα can interact with each other. In this case, we chose Ishikawa cells, an ERα-positive human endometrial adenocarcinoma cell line, for subsequent study. Sanger sequencing of SPOP exon6/7 of Ishikawa cells revealed no mutation in MATH domain (data not shown). Immunoprecipitation using anti-ERα antibody was performed using cell lysates prepared from Ishikawa cells. As shown in Figure 1c, endogenous SPOP was efficiently immunoprecipitated by ERα, suggesting these two proteins can also interact with each other at endogenous levels.
Figure 1.
SPOP interacts with ERα in cells. (a,b) Ectopically expressed SPOP and ERα interact with each other. The 293T cells were co-transfected with FLAG-HA (FH)-SPOP and Myc-ERα constructs. After 24 h, cell lysates were prepared for co-IP with anti-FLAG antibody and WB analyzes. (b) co-IP assay was performed between ectopically expressed FH-ERα and Myc-SPOP. (c) Endogenous SPOP and ERα proteins interact with each other in Ishikawa cells. After being treated with 20 μM MG132 for 4 h, cell lysates were prepared for co-IP with anti-ERα antibody and WB analyzes with indicated antibodies. (d) Schematic representation of SPOP deletion mutants. Binding capacity of SPOP to ERα is indicated with the symbol. (e) ERα binds to the MATH domain of SPOP. The 293T cells were co-transfected with FH-ERα and Myc-SPOP-WT or deletion mutants (ΔMATH, ΔBTB) constructs. After 24 h, cell lysates were prepared for co-IP assay with anti-FLAG antibody and WB analyzes. (f) Schematic representation of MATH domain deletion mutants of SPOP. Binding capacity of SPOP to ERα is indicated with the symbol. (g) The integrity MATH domain of SPOP is crtical for ERα binding. The 293T cells were co-transfected with FH-ERα and Myc-SPOP-WT or deletion mutants (D1–D8) constructs. After 24 h, cell lysates were prepared for co-IP assay with anti-Myc antibody and WB analyzes
SPOP contains two structural domains: a substrate-binding MATH domain at the N-terminus and a CUL3-binding BTB domain at the C-terminus.8 To determine which domain may mediate its interaction with ERα, we generated two deletion mutants of SPOP (SPOP-ΔBTB and ΔMATH), corresponding to the deletion of these two domains, respectively (Figure 1d). co-IP assay was performed to test the binding affinity of the full length SPOP (SPOP-WT) and the two deletion mutants with overexpressed ERα in 293T cells. As shown in Figure 1e, while SPOP-WT and SPOP-ΔBTB interacted efficiently with ERα, the interaction between SPOP-ΔMATH and ERα was totally abolished. To further map the ERα-binding sites in the MATH domain of SPOP, we constructed a series of small deletion mutants of SPOP in MATH domain (SPOP-D1–D8), then we compared the binding affinity of SPOP-WT and mutants (D1–D8) with ERα. As shown in Figures 1f and g, all SPOP mutants (D1–D8) completely lost ERα binding capacity, suggesting that the integrity of MATH domain was critical for binding to ERα efficiently. Therefore, this result suggests that the MATH domain is responsible for the interaction between SPOP and ERα. Taken together, our findings demonstrate that SPOP binds ERα in cells through the MATH domain.
ERα is a bona fide substrate of the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex
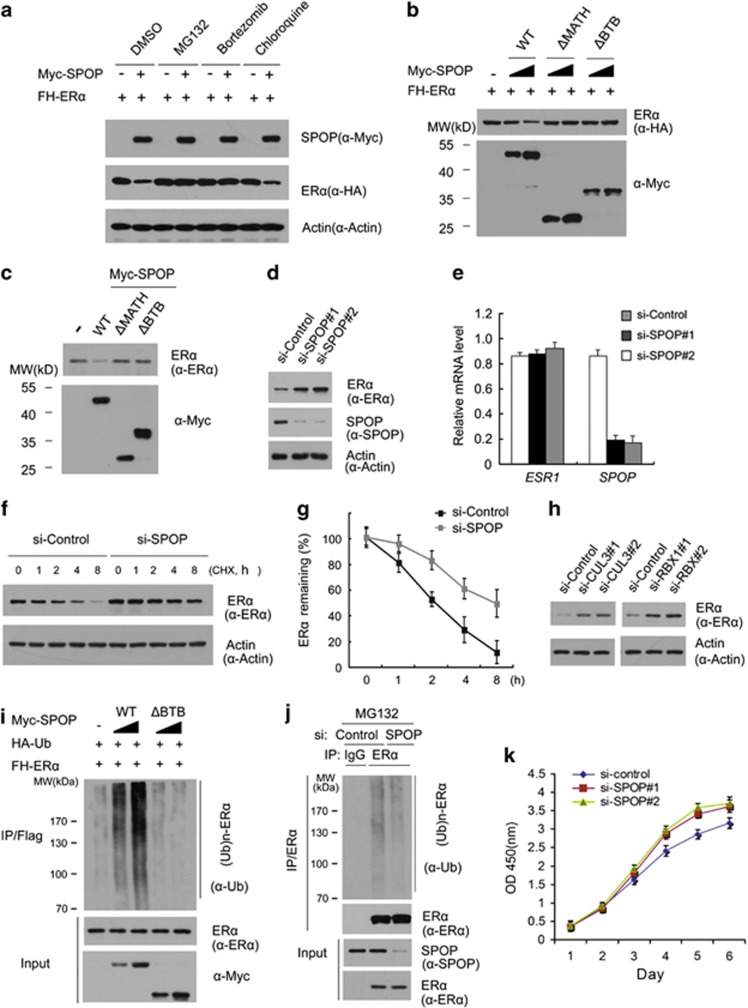
Then we explored whether the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex can promote the ubiquitination and degradation of ERα. As shown in Figure 2a, expression of SPOP decreased the ectopically co-expressed ERα protein level in 293T cells in a dose-dependent manner. This effect was completely blocked when cells were treated with the proteasome inhibitors MG132 or Bortezomib (Figure 2a). In contrast, lysosome inhibitor chloroquine could not block SPOP-mediated ERα degradation. These results indicated that SPOP downregulates ERα protein via the proteasomal and not the lysosomal degradation pathway. Moreover, SPOP-WT, but not the SPOP-ΔBTB or SPOP-ΔMATH mutant, promoted ERα degradation (Figure 2b), indicating that the BTB and MATH domains are both required for SPOP-mediated ERα degradation. Next, we examined the effect of SPOP on the degradation of endogenous ERα. Similarly, as shown in Figure 2c, overexpression of SPOP-WT, but not the SPOP-ΔBTB or SPOP-ΔMATH mutant in Ishikawa cells resulted in a moderate decrease in the protein level of endogenous ERα. Moreover, knockdown of endogenous SPOP using two gene-specific siRNAs increased ERα protein levels in Ishikawa cells (Figure 2d). To exclude the possibility that ERα protein elevation resulted from transcriptional upregulation, we performed qRT-PCR to measure the mRNA levels of SPOP and ERα in SPOP-depleted Ishikawa cells. In contrast to the significant decrease of SPOP mRNA levels, the mRNA levels of ESR1 gene (encoding ERα) in SPOP-depleted Ishikawa cells stayed at a level similar to that of the control cells (Figure 2e), indicating that the effect of SPOP on ERα protein levels is not mediated through the upregulation of ERα mRNA levels. In addition, knockdown of SPOP remarkably prolonged the half-life of endogenous ERα protein in Ishikawa cells (Figures 2f and g), further suggesting that SPOP regulates ERα protein at the post-translational level.
Figure 2.
The SPOP-CUL3-RBX1 ubiquitin ligase complex targets ERα for ubiquitination and degradation. (a) SPOP regulates ERα protein levels through the proteasome pathway. The 293T cells were transfected with FH-ERα in combination with or without the Myc-SPOP constructs. After 24 h, cells were treated with MG132 (20 μM), Bortezomib (200 nM), chloroquine (100 mM), or DMSO for 4 h before cell lysates were prepared for WB analyzes. Actin, a loading control. (b) The BTB and MATH domains in SPOP are essential for SPOP-mediated degradation of ERα. FH-ERα and different amounts of Myc-SPOP-WT or deletion mutants (ΔMATH, ΔBTB) constructs were transfected into 293T cells. After 24 h, cell lysates were prepared for WB analyzes. (c) SPOP regulates endogenous ERα protein levels. Ishikawa cells were transfected with Myc-SPOP-WT, or deletion mutants (ΔMATH, ΔBTB) constructs. After 24 h, cell lysates were prepared for WB analyzes. (d) Knockdown of SPOP increases endogenous ERα protein levels. Ishikawa cells were transfected with control or two SPOP-specific siRNAs. After 48 h, cell lysates were prepared for WB analyzes. (e) Quantitative RT-PCR measurement of the mRNA levels of SPOP and ESR1 in SPOP-knockdown Ishikawa cells. The mRNA level of GAPDH was used for normalization. The mean values (S.D.) of three independent experiments are shown. (f,g) Knockdown of SPOP prolongs ERα protein half-life. Ishikawa cells were transfected with control or SPOP-specific siRNA. After 48 h, cells were chased with 30 μM cycloheximide (CHX). At the indicated time points, cell lysates were prepared for WB analyzes. (f) At each time point, the intensity of ERα was first normalized to the intensity of Actin (loading control) and then to the value of the 0-h time point (g). The mean values (S.D.) of three independent experiments are shown. (h) Knockdown of RBX1 or CUL3 increases endogenous ERα protein levels. Ishikawa cells were transfected with control siRNA or siRNAs for RBX1 or CUL3. After 48 h, cell lysates were prepared for WB analyzes. (i) SPOP promotes ERα polyubiquitination in vivo. FH-ERα, HA-Ub, and Myc-SPOP-WT or ΔBTB mutant constructs were co-transfected into 293T cells. After 24 h, cells were treated with 20 μM MG132 for 4 h. ERα proteins were immunoprecipitated with anti-FLAG antibody and resolved by SDS/PAGE. The ubiquitinated forms of ERα were analyzed by WB with anti-Ub antibody. (j) Knockdown of SPOP decreases ubiquitination of endogenous ERα. Ishiwaka cells were transfected control or SPOP-specific siRNA. After 48 h, cells were treated with 20 μM MG132 for 4 h and then the same procedure was performed as i. (k) Knockdown of SPOP promotes Ishikawa cells growth. Ishikawa cells were transfected with control or two SPOP-specific siRNAs. After 48 h, the cell growth was measured by CCK-8 assay at indicated days. The mean values (S.D.) of three independent experiments are shown
Next we sought to determine whether other subunits of the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex are also required for ERα degradation. We respectively knocked down RBX1 or CUL3 using two gene-specific siRNAs and examined the changes in ERα protein level in Ishikawa cells. As shown in Figure 2h, knockdown of either RBX1 or CUL3 resulted in a significant increase in ERα protein levels, an effect similar to SPOP knockdown. These results suggest that other subunits of ubiquitin ligase complex are also required for the degradation of ERα.
To further determine whether ERα was degraded through SPOP-mediated polyubiquitination, HA-Ubiquitin and FH-ERα constructs were co-expressed in 293T cells with different doses of SPOP-WT or SPOP-ΔBTB mutant. As shown in Figure 2i, ERα protein was robustly polyubiquitinated by the co-expressed SPOP-WT in a dose-dependent manner. In contrast, little or no ERα polyubiquitination was observed in SPOP-ΔBTB expressing cells (Figure 2i). Accordingly, knockdown of endogenous SPOP in Ishikawa cells decreased the polyubiquitination of endogenous ERα (Figure 2j). ERα is a key pro-oncogenic transcriptional factor in endometrial cancer. It activates target genes that promote cell proliferation or decrease apoptosis. As SPOP regulates ERα protein abundance in Ishikawa cells, we measured the cells growth of SPOP-depleted Ishikawa cells. As shown in Figure 2k, knockdown of SPOP by two gene-specific siRNAs promote Ishikawa cells growth when compared with control knockdown cells. Similar effects were observed in other two endometrial cell lines, RL95-2 (ERα positive), and KLE cells (ERα negative; Supplementary Figures S1A, 1B). Sanger sequencing of SPOP exon6/7 of RL95-2 and KLE cells revealed no mutation in MATH domain (data not shown). The results that SPOP knockdown promote the cells growth of ERα negative endometrial cells suggested SPOP may regulate other oncoproteins in endometrial cells in addition to ERα.
Taken together, these data demonstrate that the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex regulates ERα stability through ubiquitin-dependent proteasomal degradation pathway in endometrial cancer cell lines. It also suggested SPOP may regulate endometrial cells growth in an ERα-dependent and independent manner.
Multiple Ser/Thr (S/T)-rich motifs in ERα are required for SPOP-mediated ERα degradation
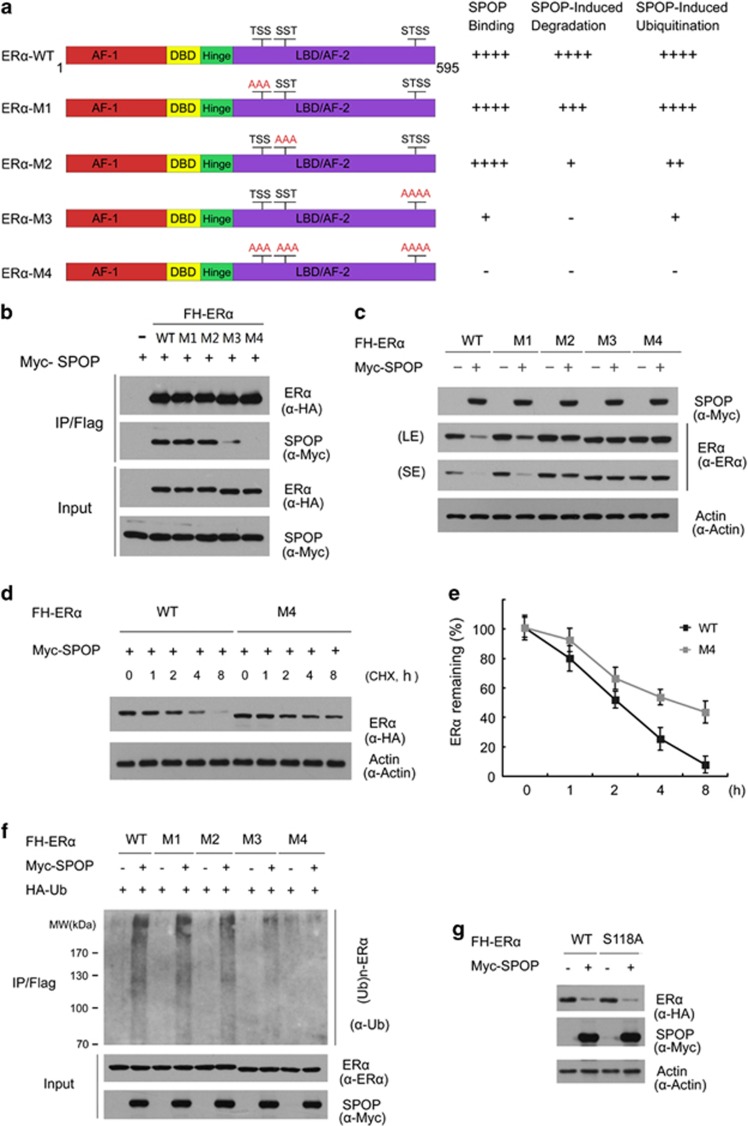
Previous study has reported that an optimal SPOP-binding motif contains 3–4 contiguous Ser/Thr (S/T) residues.11 Similar S/T-rich motifs are present in known SPOP-binding proteins, such as Puc, MacroH2A, Ci/Gli, SRC-3, and AR. Therefore, we examined the protein sequence of ERα and found three S/T-rich motifs located in the ligand-binding domain/AF2 domain resembling the SPOP-binding pattern (Figure 3a). In order to examine whether these S/T-rich motifs are actually required for SPOP-ERα interaction, we generated three ERα mutants (M1, M2, and M3) in which the Ser/Thr residues in each motif were mutated to Ala (A). An ERα-M4 mutant was also generated in which all 10 Ser/Thr residues in 3 motifs were mutated to Ala (A; Figure 3a). The 293T cells were co-transfected with SPOP and wild-type ERα or one of these mutants. Co-IP assays demonstrated that SPOP was co-immunoprecipitated by ERα-WT, M1, or M2 mutant at similar levels, whereas the interaction was significantly reduced between SPOP and ERα-M3 mutant. Moreover, the interaction was completely abolished between SPOP and ERα-M4 mutant (Figure 3b). Thus, these results suggested that the S/T-rich motifs of ERα were essential for SPOP binding. Each S/T-rich motif may partially contribute to the SPOP-binding capacity, but the third S/T-rich motif appeared most critical for the ERα-SPOP interaction.
Figure 3.
The S/T-rich motifs in ERα are degrons recognized by SPOP. (a) Schematic representation of wild-type ERα protein with the upper contiguous Ser/Thr residues indicating the S/T-rich motifs in its amino-acid sequence. The ERα point mutants (M1, M2, M3, and M4) were constructed starting from the FH-ERα-WT vector are schematically reported below the wild-type protein. On the right of each schematic protein is summarized its SPOP-binding capacity, sensitivity to SPOP-induced degradation or ubiquitination. (b) The S/T-rich motifs in ERα are required for its binding to SPOP. The 293T cells were transfected with the indicated constructs. After 24 h, cell lysates were prepared for co-IP assay with anti-FLAG antibody and WB analyzes. (c) The S/T-rich motifs in ERα are required for SPOP-mediated ERα degradation. The 293T cells were transfected with the indicated constructs. After 24 h, cell lysates were prepared for WB analyzes. SE, short exposure; LE, long exposure. (d, e) Mutation of the S/T-rich motifs prolongs the half-life of ERα. ERα-WT or M4 mutant was transfected into 293T cells. After 24 h, cells were treated with 30 μM CHX. At the indicated time points, cell lysates were prepared for WB analyzes (d). At each time point, the intensity of ERα was first normalized to the intensity of Actin and then to the value of the 0-h time point (e). (f )The S/T-rich motifs are required for SPOP-mediated ERα polyubiquitination. The 293T cells were transfected with the indicated constructs. After 24 h, cells were treated with 20 μM MG132 for 4 h and the cell lysates were prepared for co-IP assay with anti-FLAG antibody and WB analyzes. The mean values (S.D.) of three independent experiments are shown. (g) Ser118 of ERα is not required for SPOP-mediated ERα degradation. The 293T cells were transfected with FH-ERα or S118A mutant in combination with or without Myc-SPOP constructs. After 24 h, cell lysates were prepared for WB analyzes
Next, we examined whether the S/T-rich motifs are required for SPOP-mediated ERα degradation. As shown in Figure 3c, mutation of the first S/T-rich motif (ERα-M1) mildly reduced SPOP-mediated ERα degradation when compared with ERα-WT, whereas mutation of the second S/T-rich motif (ERα-M2) severely reduced SPOP-mediated ERα degradation. In addition, mutation of the third or all S/T-rich motifs (ERα-M3 or M4) completely abrogated SPOP-mediated ERα degradation (Figure 3c). These results suggested that the S/T-rich motifs are important in SPOP-mediated ERα degradation. To further explore whether the S/T-rich motifs are also important for ERα turnover, we measured the half-lives of the ERα-WT or ERα-M4 mutant in 293T cells. As shown in Figures 3d and e, ERα-M4 mutant exhibited a significantly prolonged half-life than that of ERα-WT. To further determine the importance of these motifs as degrons, ERα-WT or mutants was co-transfected with or without SPOP in 293T cells. In vivo ubiquitination assay demonstrated that mutation of all S/T-rich motifs (ERα-M4) rather than any single motif (ERα-M1, M2, or M3) totally abolished SPOP-mediated ERα ubiquitination (Figure 3f).
ERα ubiquitination can be mediated by multiple E3 ubiquitin ligases in response to changing cellular conditions.16 E6-AP is the most extensively studied E3 ligase for ERα, and its loss attenuates 17β-estradiol (E2)-induced ERα degradation.20 Previous studies reported E6-AP binding to ERα in a Ser118-dependent manner.20 However, our results showed that SPOP could efficiently target ERα-S118A for degradation to the same extent as ERα-WT (Figure 3g), suggesting the Ser118 is dispensable for SPOP-mediated ERα degradation. Taken together, these observations demonstrate that the three S/T-rich motifs functions as ERα degrons, which are essential for SPOP binding and subsequent degradation of ERα, and the third S/T-rich motif is most crucial for this function.
Endometrial cancer-associated mutants of SPOP are defective in promoting ERα degradation and ubiquitination
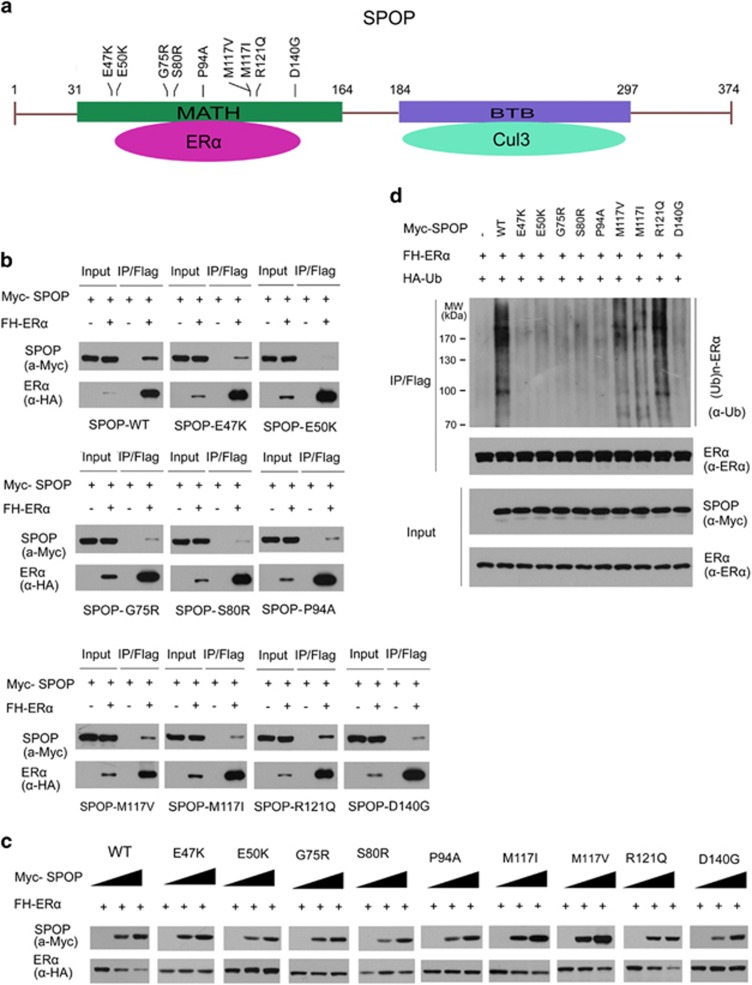
SPOP was mutated in 5.7–10% of patients with endometrial cancer across multiple independent cohorts (Table 1). Moreover, SPOP mutations were detected in three major histological subtypes of endometrial cancer (endometrioid, clear cell and serous). Notably, all the SPOP mutations found in endometrial cancer far-exclusively occur in the MATH domain which is responsible for ERα binding (Figure 4a). Therefore, we propose that endometrial cancer-associated SPOP mutations may cause dysfunction in regulating ERα protein level. To test this, nine Myc-tagged endometrial cancer-associated mutants of SPOP were generated according to four large-scale exome-sequencing studies (Table 1), including E47K, E50K, G75R, S80R, P94A, M117V, M117I, R121Q, and D140G. We initially examined their interactions with ERα by co-IP assay. As shown in Figure 4b, mutations of the residues at MATH domain substantially decreased the capacity of SPOP to interact with ERα in vivo, suggesting the endometrial cancer-associated SPOP mutants are defective in binding to ERα. We then examined the capacities of endometrial cancer-associated SPOP mutants in promoting ERα degradation. As shown in Figure 4c, a group of SPOP mutants (SPOP-M117V, M117I, and R121Q) displayed reduced capacity to promote ERα degradation when compared with SPOP-WT, whereas other groups did not alter ERα protein level (SPOP-E47K, E50K, G75R, P94A, and D140G) or increased ERα protein level (SPOP-S80R), suggesting that endometrial cancer-associated SPOP mutants may differentially regulate ERα stability. Furthermore, in vivo ubiquitination assays indicated that some SPOP mutants (SPOP-E47K, E50K, G75R, S80R, and D140G) lost the capacity to promote ERα polyubiquitination, whereas SPOP-M117V, M117I, and R121Q mutants could generate partially polyubiquitinated form of ERα (Figure 4d). Taken together, our findings suggest that endometrial cancer-associated mutants of SPOP are defective in promoting ERα degradation and ubiquitination.
Table 1. The somatic mutations of SPOP identified in exome sequencing of endometrial cancers.
| Amino-acid change | Mutation types | Histological subtypes | ratios | Reference | |
|---|---|---|---|---|---|
| 1 | G75R | Missense | Serous | 10% (1/10) | Kuhn E et al.6 |
| 2 | M117V D140G | Missense | Serous | 5.9% (2/34) | Zhao S et al.5 |
| 3 | E47K S80R P94A M117I R121Q | Missense | Serous Clear cell | 8% (4/52) 9% (2/23) | Le Gallo M et al.7 |
| 4 | E50K M117I R121Q D140G | Missense | Endometrioid Serous | 5.7% (10/175) 7% (3/43) | Kandoth C et al.4 |
Figure 4.
Endometrial cancer-associated mutants of SPOP are defective in promoting ERα degradation and ubiquitination. (a) Distribution of the point mutations on the SPOP gene found in endometrial cancer samples. These mutations are exclusively located in the N-terminal MATH domain of SPOP. (b) Endometrial cancer-associated mutants of SPOP are defective in promoting ERα degradation. 293T cells were transfected with FH-ERα and wild-type or mutated SPOP constructs as indicated. After 24 h, cell lysates were prepared for WB analyzes. (c) Endometrial cancer-associated mutants of SPOP are defective in interacting with ERα. The 293T cells were transfected with the indicated constructs. After 24 h, cell lysates were prepared for co-IP assay with anti-FLAG antibody and WB analyzes. (d) Endometrial cancer-associated mutants of SPOP are defective in promoting ERα ubiquitination. The 293T cells were transfected with the indicated constructs. After 24 h, cells were treated with 20 μM MG132 for 4 h and cell lysates were prepared for immunoprecipitation and WB analyzes
We then explored the impact of SPOP mutations on endometrial cell proliferation. First, we established Ishikawa cell lines that were stably transfected with control constructs, SPOP-WT or SPOP mutants, respectively. SPOP expression levels were determined by WB analyzes. As shown in Supplementary Figure S2A, FH-SPOP-WT or mutants were stably expressed in Ishikawa cells. We found stable overexpression of SPOP-WT in Ishikawa cells resulted in a marked decrease in the protein level of endogenous ERα, whereas stable overexpression of SPOP mutants did not alter the endogenous ERα protein level (Supplementary Figure S2A). Next, we measured the cells growth of Ishikawa stable cell lines using CCK-8 assay. As shown in Supplementary Figure S1B, stable overexpression of SPOP-WT, but not the SPOP mutants, resulted in a retardation of Ishikawa cell growth. Interestingly, a subset of SPOP mutants (S80R, M117V, M117I, and R121Q, D140G) accelerated Ishikawa cells growth when compared with control cells, suggesting a possible gain-of-function dominant-negative effect of these SPOP mutants on the Ishikawa cells growth (Supplementary Figure S2B). Thus, our findings suggest that wild-type SPOP can suppress endometrial cell proliferation, and this suppression is abrogated by endometrial cancer-associated SPOP mutations.
SPOP participated in estrogen-induced ERα degradation
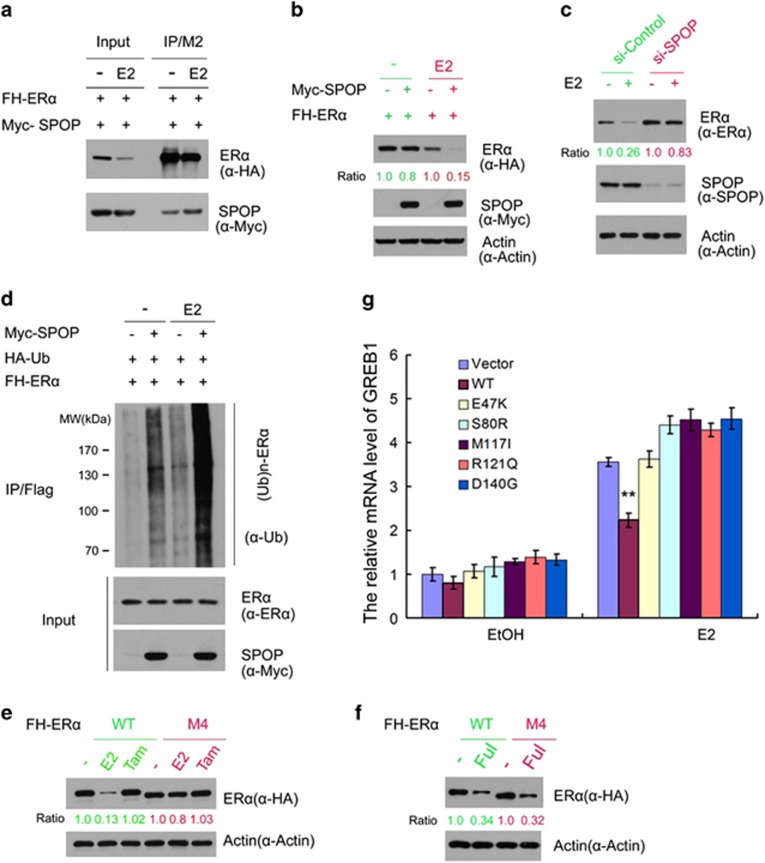
ERα is rapidly ubiquitinated and degraded through the ubiquitin-proteasome pathway after estrogen binding.21 However, the molecular mechanism by which estrogen downregulates ERα protein is not fully understood. To determine whether SPOP has a role in this process, we first examined whether estrogen treatment can affect SPOP-ERα interaction. FH-ERα and Myc-SPOP were overexpressed in 293T cells, and then the cells were treated with hormone 17β-estradiol (E2) or vehicle ethanol (EtOH) before harvesting. co-IP assay was performed to test the impact of estrogen treatment on SPOP-ERα interaction. As shown in Figure 5a, E2 treatment leads to a significant downregulation of overexpressed ERα. However, more SPOP was co-immunoprecipitated by ERα, suggesting that estrogen treatment enhances the interaction between ERα and SPOP. We further demonstrated that SPOP-induced downregulation of ERα protein was markedly enhanced by E2 treatment (Figure 5b). Furthermore, we demonstrated that knockdown of endogenous SPOP largely diminished E2-induced downregulation of endogenous ERα protein in Ishikawa cells (Figure 5c). Consistent with these findings, E2 treatment resulted an increase of SPOP-mediated ERα ubiquitination (Figure 5d). Finally, we demonstrated that stable overexpression of SPOP-WT but not the SPOP mutants diminished E2–dependent transactivation of ERα target gene GREB1 in Ishikawa cells (Figure 5e). Similar effects were observed in other two ERα target genes, Cyclin D1 and ABCA3 (Supplementary Figure S3A and 3B). We also demonstrated that SPOP-WT and mutants differentially regulated the protein levels of GREB1 and Cyclin D1 (Supplementary Figure S3C).
Figure 5.
Estrogen potentiates SPOP-mediated degradation of ERα. (a) Estrogen enhances the SPOP-ERα interaction. FH-ERα and Myc-SPOP constructs were co-transfected into 293T cells. After 24 h, cells were treated with the vehicle ethanol (EtOH,−) or 10 nM 17β-estradiol (E2) for 4 h before cell lysates were prepared for co-IP and WB analyzes. (b) Estrogen enhances SPOP-mediated ERα degradation. The 293T cells were transfected with the indicated constructs. A small amount of Myc-SPOP constructs was used in transfection. After 24 h, cells were treated with the vehicle ethanol (EtOH) or 10 nM 17β-estradiol (E2) for 4 h before cells lysates were prepared for WB analyzes. The density of ERα was determined by normalizing to actin (loading control) first and then to the normalized value in mock-treated cells. (c) Knockdown of SPOP attenuates estrogen-induced degradation of ERα. Ishikawa cells were transfected with control or SPOP-specific siRNA. After 48 h, cells were then treated with the vehicle ethanol (EtOH,−) or 10 nM 17β-estradiol (E2) for 4 h before cell lysates were prepared for WB analyzes. (d) Estrogen potentiates SPOP-induced polyubiquitination of ERα. The 293T cells were transfected with the indicated constructs. After 24 h, cells were treated with the vehicle ethanol (EtOH,−) or 10 nM 17β-estradiol (E2). Cells were then treated with MG132 for 4 h before cell lysates were prepared for IP and WB analyzes. (e) Ishikawa cells lines that stably transfected with control, SPOP-WT or SPOP mutants constructs were treated with 10 nM 17β-estradiol (E2) for 24 h. The mRNA level of ERα target gene GREB1 was measured by qRT-PCR. The mRNA level of GAPDH was used for normalization. The mean values (S.D.) of three independent experiments are shown. **indicates statistical significance (**P<0.01). (f, g) Differential effects of estrogen on the protein level of ERα-WT and the SPOP degradation-resistant mutant (ERα-M4). The 293T cells were transfected with FH- ERα-WT or M4 mutant construct. After 24 h, cells were treated with vehicle ethanol (EtOH,−), 10 nM 17β-estradiol (E2), 10 nM Tamoxifen (Tam), and 10 nM Fulvestrant (Ful) for 4 h before cell lysates were prepared for WB analyzes
We then examined whether the estrogenic effect on SPOP regulation of ERα degradation is mediated through the S/T-rich motifs. As shown in Figure 5f, E2 but not the Tamoxifen treatment significantly decreased the protein level of ERα-WT. However, this effect was largely diminished with the ERα-M4 mutant, which can't bind SPOP (Figure 5f). The anti-estrogen drug fulvestrant causes immobilization of ERα in the nuclear matrix accompanied by rapid degradation by the ubiquitin-proteasome pathway.22, 23 We also examined whether SPOP is involved in fulvestrant-induced ERα degradation. As shown in Figure 5g, ERα-M4 mutant remained susceptible to degradation induced by fulvestrant, suggesting fulvestrant-induced ERα Degradation occurs through SPOP-independent mechanisms. Taken together, these data suggest that SPOP has an important role in estrogen-induced ERα degradation and transactivation in endometrial cancer cells.
Discussion
Recurrent SPOP mutations in endometrial cancer have been confirmed by four independent genome-wide studies (Table 1). Although frequent mutations of SPOP in endometrial cancer have been identified, the functional impact of these mutations remains unknown. In this study, we demonstrated that ERα is a bona fide substrate for the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex (Figure 6). SPOP recognizes the Ser/Thr-rich degrons in the AF2 domain of ERα, and promotes ERα ubiquitination and proteasomal degradation. Endometrial cancer-associated mutants of SPOP are defective in promoting ERα degradation and ubiquitination. Moreover, SPOP participates in estrogen-induced ERα degradation. Taken together, these findings provide new insights in our understanding of the physiological and pathophysiological significance of SPOP in regard to the development of endometrial cancer.
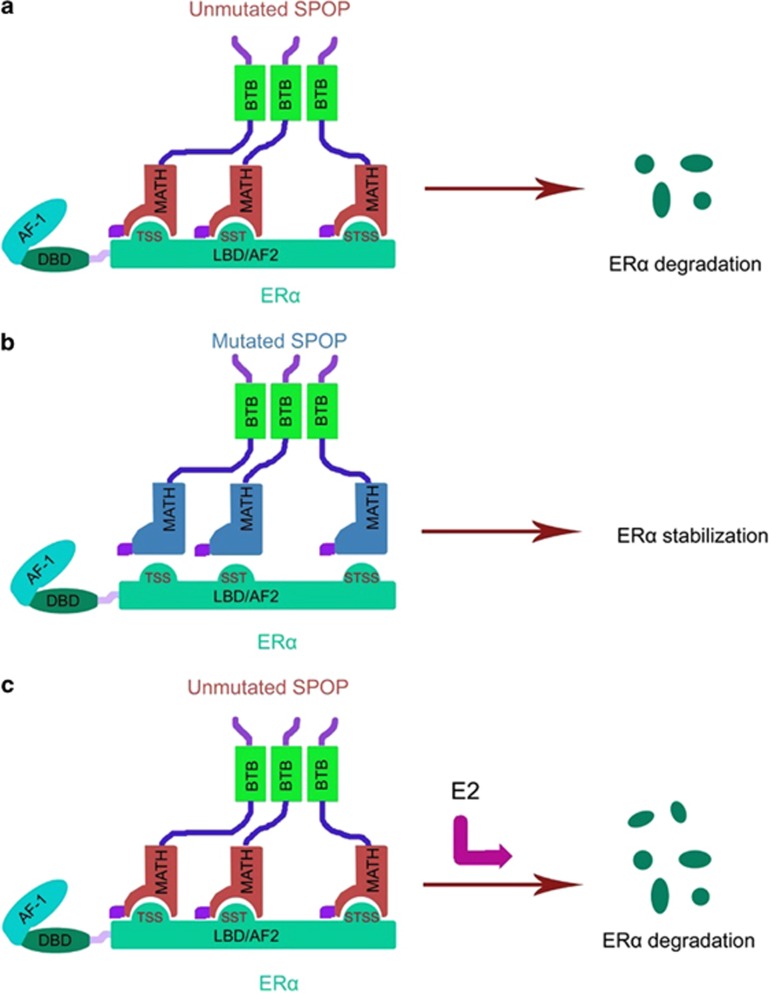
Figure 6.
Models depicting SPOP-mediated ERα degradation in physiological and pathological conditions in endometrial cancer. (a) Unmutated SPOP promotes degradation of wild-type ERα. (b) Endometrial cancer-associated mutants of SPOP are defective in promoting ERα ubiquitination and degradation. (c) Estrogen potentiates SPOP-mediated ERα degradation
Mining of the cancer exome-sequencing data deposited in COSMIC (Catalouge of Somatic Mutations in Cancer) database (http://www.sanger.ac.uk/cosmic) revealed that SPOP mutations are common in endometrial and prostate cancers, but rare in cancers of other tissue types. The copy number analyzes of amplification, loss of heterozygosity (LOH), and deletion in 24 cancer types revealed LOH at high percentages in the SPOP locus, suggesting genomic loss of the SPOP locus occurs frequently in human cancers.9 Thus, multiple mechanisms such as somatic mutations, LOH, and epigenetic silencing might be utilized by different cancer types to inactivate SPOP. Another unexpected finding is that SPOP mutational spectra are entirely different between endometrial and prostate cancer. The endometrial cancer-associated SPOP mutants were not observed in previous prostate cancer data. Reciprocally, prostate cancer-associated SPOP mutants were not observed in endometrial cancer data. An in-depth understanding of this difference is still lacking. A plausible explanation is that the SPOP mutants from different cancers might show differential effect on the regulation of certain substrates. Further investigation is needed to determine which substrates and related signaling pathways were dysregulated in SPOP mutated endometrial or prostate cancer.
Through detailed mutation analysis, we identified three S/T-rich motifs located in the AF2 domain of ERα. Our results suggested that SPOP-mediated degradation and ubiquitination of ERα may depend on cooperatively among multiple S/T-rich motifs. Notably, multiple S/T-rich degrons are also found in several other SPOP substrates, such as Puc and Ci. For example, Puc contains one optimal and two suboptimal degrons.24 The results of in vitro ubiquitination with Puc mutants indicated that the relative contributions of S/T-rich motifs of Puc are proportional to their abilities to bind SPOP.24 A similar situation was observed for SPOP-ERα interaction (Figures 3b and f). Moreover, recent studies reported that SPOP can form large oligomers.25 SPOP oligomerization can serve to enhance SPOP-substrate avidity through the presentation of multiple substrate-binding MATH domains. Reciprocally, SPOP-substrate avidity would further enhanced by multiple S/T-rich motifs within a single substrate, a property especially common among the various SPOP substrates.25 So it is possible that oligomeric SPOP engages multiple S/T-rich motifs of ERα for ubiquitination and degradation.
Another important finding of our study is that estrogen induces ERα degradation by facilitating SPOP-ERα interaction. Although the detailed molecular basis for this remains unknown, one possible explanation is that, when estrogen bind to ERα, ERα undergoes conformation changes, thereby affecting the binding of the S/T-rich motifs to SPOP and subsequent ERα degradation. Interestingly, we found that ERα-M3 mutant protein showed a retarded electrophoretic motility compared with ERα-WT, whereas such a mobility shift was not observed in ERα-M1 or M2 mutant (Figure 3c), suggesting the third S/T-rich motif might be important for ERα conformation. This result is consistent with the finding that only ERα-M3 mutant is totally resistant to SPOP-mediated ERα degradation (Figure 3c). Our studies also demonstrated that SPOP knockdown largely diminished but not abolished estrogen-induced ERα degradation (Figure 5c). Previous studies reported that E6-AP knockdown also attenuates estrogen-induced ERα degradation,20 suggesting multiple E3 ubiquitin ligases may participate in this process. Unlike estrogen, the anti-estrogen drug fulvestrant induces a distinct conformational change in the ERα and inhibits receptor homo-dimerization, nuclear localization, and enhances rapid degradation of the ERα.26 This process is driven by a SPOP-independent manner because ERα-M4 mutant protein that cannot bind SPOP remained susceptible to fulvestrant-induced degradation (Figure 5f).
For future studies, it will be useful to generate mice models of conditional endometrial-specific SPOP knockout or knockin to further characterize the phenotype of SPOP mutations in vivo, and determine whether ERα pathway is dysregulated in SPOP mutated endometrial cancer.
Materials and Methods
Cell culture, treatments, and transfection
Ishikawa, RL95-2, KLE and 293T cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The 293T cells were maintained in DMEM medium with 10% FBS, whereas Ishikawa, RL95-2, and KLE cells were maintained in DMEM/F12 medium with 10% FBS. Cells were transiently transfected using Lipofectamine RNAiMAX (for siRNA transfection) or 3000 (for plasmids transfection) (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. In experiments involving treatment with 17β-estradiol (E2), Tamoxifen, fulvestrant, or vehicle ethanol (EtOH), cells were placed in phenol-red-free medium with 10% dextran-coated, charcoal-stripped FBS for 48 h prior to treatment with hormone or vehicle.
Expression constructs
The HA-SPOP vectors were kindly provided by Dr. Masatoshi Hagiwara (Tokyo Medical and Dental University, Japan) and subcloned into pCIN4-FLAG-HA and pCMV-Myc expression vectors. The ERα cDNA was purchased from Genechem (Shanghai, China), and subcloned into pCIN4-FLAG-HA and pCMV-Myc expression vectors. ERα mutants and SPOP mutants were generated by QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA).
RNA interference
Non-specific control siRNA and siRNAs for human SPOP, CUL3, and RBX1 were purchased from GenePharma (Shanghai, China). siRNA transfection of cells was performed following the manufacturer's instructions. The siRNA oligos sequences for SPOP are: si-SPOP1#1: 5′-GGAUGAUGUAAAUGAGCAA-3′. si-SPOP#2: 5′- GGACAGCGACTCTGAATCT-3′. The sequence of negative control is: si-Control: 5′-ACAGACUUCGGAGUACCUG-3′.
Antibodies
The following antibodies were used: SPOP (ab137537; Abcam, Cambridge, UK), GREB1 (ab72999; Abcam), Cyclin D1 (ab134175; Abcam), ERα (8644; Cell Signaling, Beverly, MA, USA), ubiquitin (6652-1; Epitomics, Burlingame, CA, USA), Myc (9E10; Sigma Aldrich, St. Louis, MO, USA), FLAG (M2; Sigma), actin (AC-74; Sigma) and HA (MM5-101R; Millipore, Darmstadt, Germany).
SPOP exon6/7 sequencing
The sanger sequencing strategy to detect SPOP exon6/7 sequence was performed as previously described.14 Genomic DNA from cell samples was extracted using the QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany) following the manufacturer's instruction. PCR amplification and sequence of exon6/7 of SPOP was performed with the primers listed in supplementary Table 1.
Immunoprecipitation
To immunoprecipitate the ectopically expressed FLAG-tagged proteins, transfected cells were lysed 24 h post-transfection in BC100 buffer. The whole-cell lysates were immunoprecipitated with the monoclonal anti-FLAG antibody-conjugated M2 agarose beads (Sigma) at 4 °C overnight. After three washes with FLAG lysis buffer, followed by two washes with BC100 buffer, the bound proteins were eluted using FLAG-Peptide (Sigma)/BC100 for 3 h at 4 °C. The eluted material was resolved by SDS-PAGE. To immunoprecipitate the endogenous proteins, cells were lysed with 1 × cell lysis buffer (Cell Signaling), and the lysate was centrifuged. The supernatant was precleared with protein A/G beads (Sigma) and incubated with indicated antibody overnight. Thereafter, protein A/G beads were applied, all at 4 °C. After 2 h of incubation, pellets were washed five times with lysis buffer and resuspended in sample buffer and analyzed by SDS-PAGE.
Western blot
Cell lysates or immunoprecipitates were subjected to SDS-PAGE and proteins were transferred to nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). The membranes were blocked in Tris-buffered saline (pH 7.4) containing 5% non-fat milk and 0.1% Tween-20, washed twice in TBS containing 0.1% Tween-20, and incubated with primary antibody for 2 h and followed by secondary antibody for 1 h at room temperature. The proteins of interest were visualized using ECL chemiluminescence system (Santa Cruz, Santa Cruz, CA, USA).
Quantitative RT-PCR
Total RNA was isolated from transiently transfected cells using the TRIzol reagent (Tiangen, Shanghai, China), and cDNA was reversed-transcribed using the Superscript RT kit (TOYOBO, Osaka, Japan), according to the manufacturer's instructions. PCR amplification was performed using the SYBR Green PCR master mix Kit (TOYOBO). All quantization were normalized to the level of endogenous control GAPDH.
Cell proliferation assay
Cell proliferation rate was determined using Cell Counting Kit-8 (CCK-8) according to the manufacturer's protocol (Dojindo Laboratories, Kumamoto, Japan). Briefly, The Ishikawa cells were seeded onto 96-well plates at a density of 2000 cells per well. During a 2–7 day culture periods, 10 μl of the CCK-8 solution was added to cell culture and incubated for 2 h. The resulting color was assayed at 450 nm using a microplate absorbance reader (Bio-Rad, Hercules, CA, USA). Each assay was carried out in triplicate.
Acknowledgments
We thank Dr. Masatoshi Hagiwara for providing the HA-SPOP constructs. This work was supported by the National Natural Science Foundation of China (30872947 to PZ, 81472567 to LY, and 81171964 to CJ).
Glossary
- ERα
estrogen receptor-α
- SPOP
Speckle-type POZ protein
- Co-IP
co-immunoprecipitation
- WB
Western blot
- E2
17β-estradiol
- AR
androgen Receptor
- EtOH
ethanol
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by J Chipuk
Supplementary Material
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2005;97:755–763. doi: 10.1016/j.ygyno.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Le Gallo M, O'Hara AJ, Rudd ML, Urick ME, Hansen NF, O'Neil NJ, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci USA. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E, Wu RC, Guan B, Wu G, Zhang J, Wang Y, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst. 2012;104:1503–1513. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gallo M, Bell DW. The emerging genomic landscape of endometrial cancer. Clin Chem. 2014;60:98–110. doi: 10.1373/clinchem.2013.205740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschik P, Sumara I, Lechner E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications. Embo J. 2013;32:2307–2320. doi: 10.1038/emboj.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ao J, Fu J, Lee DF, Xu J, Lonard D, et al. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene. 2011;30:4350–4364. doi: 10.1038/onc.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, et al. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem. 2006;281:12664–12672. doi: 10.1074/jbc.M600204200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi Q, Chen Y, Yue T, Li S, Wang B, et al. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci USA. 2009;106:21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ghanim M, Xue L, Brown CD, Iossifov I, Angeletti C, et al. Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science. 2009;323:1218–1222. doi: 10.1126/science.1157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Munoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, et al. Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci USA. 2005;102:7635–7640. doi: 10.1073/pnas.0408918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner M, Lee DJ, O'Reilly C, Park K, MacDonald TY, Khani F, et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia. 2014;16:14–20. doi: 10.1593/neo.131704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Slingerland JM. Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat Rev Cancer. 2014;14:26–38. doi: 10.1038/nrc3622. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R, Fuqua SA, Shou J.Estrogen receptor: current understanding of its activation and modulation Clin Cancer Res 200174338s–4342s.Discussion 4411s–4412s. [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- An J, Wang C, Deng Y, Yu L, Huang H. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell Rep. 2014;6:657–669. doi: 10.1016/j.celrep.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari P, Schalper KA, Solodin NM, Ellison-Zelski SJ, Ping LK, Rimm DL, et al. Pin1 modulates ERalpha levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene. 2014;33:1438–1447. doi: 10.1038/onc.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA. 1999;96:1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci USA. 1992;89:4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M, et al. Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol Cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington WJ, Khan MQ, Bueler SA, Rubinstein JL, Chakrabartty A, Prive GG. Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure. 2012;20:1141–1153. doi: 10.1016/j.str.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Hurvitz SA, Pietras RJ. Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008;113:2385–2397. doi: 10.1002/cncr.23875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.