Abstract
Background:
Sunitinib is approved worldwide for treatment of advanced pancreatic neuroendocrine tumours (pNET), but no validated markers exist to predict response. This analysis explored biomarkers associated with sunitinib activity and clinical benefit in patients with pNET and carcinoid tumours in a phase II study.
Methods:
Plasma was assessed for vascular endothelial growth factor (VEGF)-A, soluble VEGF receptor (sVEGFR)-2, sVEGFR-3, interleukin (IL)-8 (n=105), and stromal cell-derived factor (SDF)-1α (n=28). Pre-treatment levels were compared between tumour types and correlated with response, progression-free (PFS), and overall survival (OS). Changes in circulating myelomonocytic and endothelial cells were also analysed.
Results:
Stromal cell-derived factor-1α and sVEGFR-2 levels were higher in pNET than in carcinoid (P=0.003 and 0.041, respectively). High (above-median) baseline SDF-1α was associated with worse PFS, OS, and response in pNET, and high sVEGFR-2 with longer OS (P⩽0.05). For carcinoid, high IL-8, sVEGFR-3, and SDF-1α were associated with shorter PFS and OS, and high IL-8 and SDF-1α with worse response (P⩽0.05). Among circulating cell types, monocytes showed the largest on-treatment decrease, particularly CD14+ monocytes co-expressing VEGFR-1 or CXCR4.
Conclusions:
Interleukin-8, sVEGFR-3, and SDF-1α were identified as predictors of sunitinib clinical outcome. Putative pro-tumorigenic CXCR4+ and VEGFR-1+ monocytes represent novel candidate markers and biologically relevant targets explaining the activity of sunitinib.
Keywords: carcinoid, pancreatic neuroendocrine tumours, circulating biomarkers, cellular responses to anticancer drugs, pro-tumorigenic myeloid cells
Carcinoids are low- to intermediate-grade neuroendocrine tumours (NETs) arising outside the pancreas, whereas pancreatic NETs (pNETs) typically behave more aggressively and respond more frequently to cytotoxic chemotherapy (Pape et al, 2008; Oberg, 2009; Auernhammer and Goke, 2011). The widely heterogeneous clinical behaviour correlates with histology and grade of differentiation, proliferation rate, site of origin, and the presence, site, and extent of metastases (Panzuto et al, 2005, 2011; Pape et al, 2008; Bosman, 2010). Although surgery is potentially curative in patients with early disease, patients with advanced stages have limited therapeutic options (Auernhammer and Goke, 2011; Kulke et al, 2011).
Agents targeting the vascular endothelial growth factor (VEGF) signalling pathway are in clinical testing for patients with advanced NETs. These tumours, particularly pNET, are highly vascularized and express VEGF-A (henceforth referred to as VEGF) and its receptors VEGFR-2 and VEGFR-3 (Hansel et al, 2003), suggesting that VEGF activation may promote NET growth. In addition, implicated in NET pathogenesis are the platelet-derived growth factors (PDGFs; Fjallskog et al, 2003) and stem cell factor (SCF) (Zhang et al, 2009). VEGF-targeted agents appear to be more effective in pNET than in carcinoid, much similar to chemotherapy. Results from a randomized phase III trial (Raymond et al, 2011) led to the international approval of the multitargeted tyrosine kinase inhibitor sunitinib (SUTENT, Pfizer Inc., New York, NY, USA), which inhibits VEGFR-1, -2, and -3, PDGFR-α and -β, and SCF receptor (KIT), for unresectable, locally advanced or metastatic pNET.
Owing to the heterogeneity in clinical behaviour and treatment response of NETs, to optimize treatment doses and combinations and to discern mechanisms underlying resistance, biomarkers are critically needed. No marker however has yet been fully validated for any of these purposes, but circulating candidates for sunitinib have been identified in different tumour types (Gerger et al, 2011). Our goal was therefore to identify blood markers associated with prognosis and/or sunitinib efficacy and biological activity in patients with advanced pNET and carcinoid tumours, using specimens from a phase II study (Kulke et al, 2008). We decided to include VEGF, soluble (s)VEGFR-2, sVEGFR-3, interleukin (IL)-8, stromal cell-derived factor (SDF)-1, and circulating endothelial cells (CECs) in this analysis because of their implication in angiogenesis and sunitinib's activity, together with the results of prior correlative studies, suggesting prognostic value for circulating VEGF and IL-8 in NETs (Pavel et al, 2005; Deprimo et al, 2007; Norden-Zfoni et al, 2007; Abdel-Rahman, 2014). As specific types of myeloid cells are pro-tumorigenic and may mediate anti-VEGF treatment resistance, we decided to include them as well (Norden-Zfoni et al, 2007; Bergers and Hanahan, 2008; Du et al, 2008).
Materials and Methods
Patients and treatment
The design and main results of the phase II study (NCT00056693) are reported elsewhere (Kulke et al, 2008). Briefly, patients with histologic evidence of pNET or carcinoid tumours, who were not candidates for curative surgery were treated with repeated self-administered 6-week cycles of oral sunitinib (50 mg per day for 4 weeks, followed by 2 weeks off treatment). Patients were observed for response, survival, and adverse events, and treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent.
Sample collection and analytical methods
All patients provided written Institutional Review Board-approved informed consent to collect blood samples for biomarker analysis. Specimens for soluble protein assessment were collected on days 1, 14 (C1D14), and 28 (C1D28) of cycle 1, and days 1 and 28 of cycles 2−4 (C2D1 refers to cycle 2 day 1; online only). Vascular endothelial growth factor, IL-8, sVEGFR-2, and sVEGFR-3 concentrations were measured by enzyme-linked immunosorbent assay (ELISA). Stromal cell-derived factor-1α, a small cytokine modulated by sunitinib (Ebos et al, 2007), was measured in a subset of 28 patients with corresponding peripheral blood mononuclear cell fraction specimens available (Supplementary Table S1). Validated ELISA kits or kit components (R&D Systems, Minneapolis, MN, USA) were used to measure the proteins in heparin plasma.
EDTA plasma samples for combined trough levels of sunitinib and its active metabolite SU12662 were obtained before dosing on days 1, 14, and 28 of cycles 1, 2, and 3, respectively. All assays were run under Good Laboratory Practice conditions, using previously described liquid chromatography/mass spectrometry methodology (Deprimo et al, 2007).
White blood cell (WBC) counts were obtained from automated differentials. Circulating endothelial cells and myelomonocytic cell subsets were quantified by eight-colour flow cytometry using a FACSCanto analyser (BD Biosciences, San Jose, CA, USA). Peripheral blood mononuclear cells were isolated and stained as previously described, with modifications (Supplementary Methods, Supplementary Figure S1, and Supplementary Table S2; Bergers and Hanahan, 2008; Mancuso et al, 2009). To minimize inter-assay variability, baseline and subsequent follow-up specimens from individual patients were thawed and analysed in a single batch.
Statistical analyses
Wilcoxon signed-rank and rank-sum tests were used to compare changes in soluble protein levels and cell populations. Clinical benefit response (CBR) was defined as a complete or partial response (by Response Evaluation Criteria in Solid Tumors (RECIST)) or stable disease for ⩾6 months. The Spearman correlation coefficient was used to assess the relationship between changes in cellular and plasma markers, and tumour burden. Log-rank test was used to analyse progression-free survival (PFS) and overall survival (OS). Cox proportional hazards models were fitted to assess the association between clinical outcomes and protein biomarkers. Owing to the exploratory nature of these studies, screening candidate angiogenic markers for usefulness as biomarkers in advanced NETs, no correction for multiple testing was performed. All statistical analyses were completed using SAS 9.1 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Baseline VEGF, sVEGFR-2, sVEGFR-3, IL-8, and SDF-1α
Of the 107 patients receiving sunitinib, 105 (66 pNET and 39 carcinoid) had data evaluable for soluble biomarkers, with the exception of SDF-1α (n=28). The characteristics of the study population were shown in the clinical report (Kulke et al, 2008). At baseline, median SDF-1α and sVEGFR-2 concentrations were significantly higher in pNET compared with carcinoid (1235.7 vs 414.8 pg ml−1, P=0.003, and 9562 pg ml−1 vs 8722 pg ml−1, P=0.041, respectively; Table 1). Patients with pNET also showed a trend towards higher VEGF levels. Baseline sVEGFR-3 and IL-8 did not differ between tumour types. No correlation was found between the concentrations of four of the soluble factors and the tumour burden (estimated using RECIST's sum of longest diameters (SLD)), using a regression model (ρ=0.0059 for VEGF, ρ=0.0005 for sVEGFR-2, ρ=0.0166 for sVEGFR-3, and ρ=0.0297 for IL-8; all P>0.05).
Table 1. Baseline levels of soluble proteins and ratios of C1D28 to baseline levels during sunitinib treatment.
| Biomarker | All patients, median (range) | N | P-value | Carcinoid tumour, median (range) | N | pNET, median (range) | N | P-valuea |
|---|---|---|---|---|---|---|---|---|
| VEGF Baseline, pg ml−1 | 38.6 (8.2–408.5) | 101 | 30.1 (9.2–138.9) | 37 | 43.4 (8.2–408.5) | 64 | 0.069 | |
| VEGF C1D28:C1D1 | 3.4 | 100 | <0.0001 | 3.6 | 35 | 3.2 | 65 | 0.599 |
| sVEGFR-2 Baseline, pg ml−1 | 9120 (3251–15 689) | 104 | 8722 (3251–12 776) | 39 | 9562 (3894–15 689) | 65 | 0.041 | |
| sVEGFR-2 C1D28:C1D1 | 0.7 | 102 | <0.0001 | 0.8 | 37 | 0.6 | 65 | 0.105 |
| sVEGFR-3 Baseline, pg ml−1 | 100 850 (30 700–2 569 000) | 104 | 96 800 (43 500–2 569 000) | 39 | 100 800 (30 700–168 400) | 65 | 0.817 | |
| sVEGFR-3 C1D28:C1D1 | 0.6 | 98 | <0.0001 | 0.6 | 34 | 0.6 | 64 | 0.429 |
| IL-8 Baseline, pg ml−1 | 16.9 (3.3–763.1) | 104 | 15.0 (3.3–763.1) | 39 | 17.6 (6.1–130.3) | 65 | 0.302 | |
| IL-8 C1D28:C1D1 | 1.8 | 99 | 0.008 | 1.4 | 36 | 1.8 | 66 | 0.528 |
| SDF-1α Baseline, pg ml−1 | 583.4 (209.9–2218.8) | 28 | 414.8 (209.9–2218.8) | 14 | 1235.7 (285.7–2049.8) | 14 | 0.003 | |
| SDF-1α C1D28:C1D1 | 1.2 | 21 | 0.027 | 1.8 | 10 | 1.1 | 11 | 0.053 |
Abbreviations: C(N)D(N)=cycle number, day number; IL-8=interleukin 8; pNET=pancreatic neuroendocrine tumour; SDF-1α=stromal cell-derived factor 1α; sVEGFR-2=soluble VEGF receptor 2; sVEGFR-3=soluble VEGF receptor 3; VEGF=vascular endothelial growth factor.
P-values <0.05 are underlined and shown in bold.
Wilcoxon rank-sum test comparing carcinoid with pNET.
Changes in soluble protein biomarkers during treatment and correlation with drug exposure
At C1D28, sunitinib treatment was associated with significant increases from baseline in VEGF, IL-8, and SDF-1α, and decreases in sVEGFR-2 and sVEGFR-3 (Table 1 and Supplementary Figure S2), with no difference between tumour types (Table 1). Very mild to moderate but significant correlations between plasma trough drug levels (Cmin) by C1D28 for sunitinib and changes in VEGF (ρ=0.30, P<0.001), sVEGFR-3 (ρ=0.23, P<0.001), and IL-8 (ρ=0.09, P=0.014) were found in patients with pNET, but not for sVEGFR-2 (ρ=0.05, P=0.067). In patients with carcinoid tumours, only sVEGFR-3 concentrations (ρ=0.33, P<0.001) correlated with drug level. However, no correlation was observed for VEGF (ρ=0.06), sVEGFR-2 (ρ=0.02) or IL-8 (ρ=0.10).
Relationship between protein biomarkers and outcomes
Pancreatic NET
No significant associations were found between soluble protein levels and CBR or PFS (Table 2). However, above-median (‘high') baseline sVEGFR-2 levels predicted for longer OS (P=0.01, hazard ratio (HR): 0.22, 95% confidence interval (95% CI): 0.06–0.78).
Table 2. Correlation between plasma markers and outcomes by tumour type.
|
Carcinoid tumour |
pNET |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
PFS |
OS |
PFS |
OS |
|||||||||
| Biomarker | Median (range) | N | P-values | HR (95% CI) | P-values | HR (95% CI) | Median (range) | N | P-values | HR (95% CI) | P-values | HR (95% CI) |
| VEGF Baseline, pg ml−1 | 30.1 (9.2–138.9) | 37 | 0.479 | 0.715 (0.280–1.826) | 0.86 | 0.325 (0.085–1.257) | 43.4 (8.2–408.5) | 64 | 0.237 | 1.533 (0.751–3.130) | 0.215 | 2.004 (0.653–6.146) |
| sVEGFR-2 Baseline, pg ml−1 | 8722 (3251–12 776) | 39 | 0.191 | 1.87 (0.72–4.88) | 0.698 | 0.797 (0.251–2.524) | 9562 (3894–15 689) | 65 | 0.206 | 0.639 (0.317–1.290) | 0.010 | 0.2152 (0.059–0.780) |
| sVEGFR-3 Baseline, pg ml−1 | 96800 (43 500–2 569 000) | 39 | 0.006 | 3.91 (1.38–11.10) | 0.047 | 3.227 (0.957–10.88) | 100 800 (30 700–168 400) | 65 | 0.091 | 0.549 (0.271–1.113) | 0.893 | 0.929 (0.319–2.706) |
| IL-8 Baseline, pg ml−1 | 15.0 (3.3–763.1) | 39 | 0.045 | 2.58 (0.985–6.780) | 0.014 | 5.507 (1.198–25.32) | 17.6 (6.1–130.3) | 65 | 0.135 | 1.700 (0.839–3.446) | 0.137 | 2.351 (0.737–7.503) |
Abbreviations: CI=confidence interval; HR=hazard ratio; IL-8=interleukin 8; OS=overall survival; PFS=progression-free survival; pNET=pancreatic neuroendocrine tumour; sVEGFR-2= soluble VEGF receptor 2; sVEGFR-3=soluble VEGF receptor 3; VEGF=vascular endothelial growth factor.
P-values <0.05 are underlined and shown in bold.
Carcinoid tumours
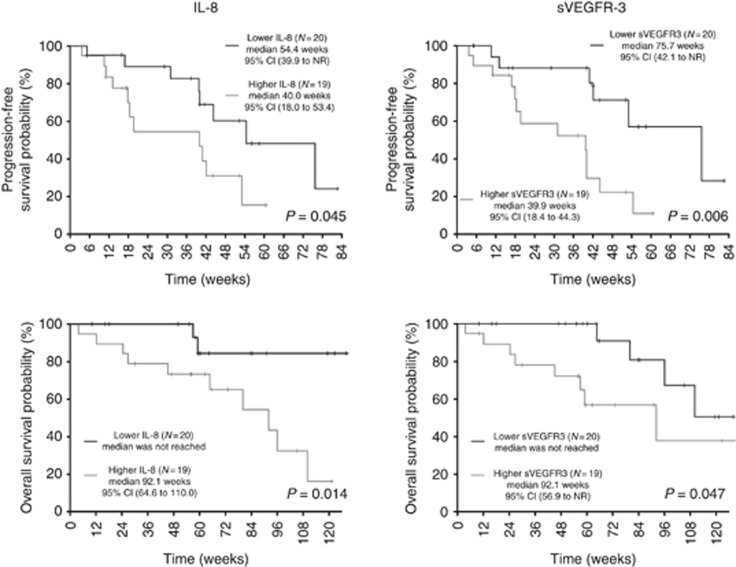
Only baseline IL-8 levels were lower in patients with CBR (11.3 vs 30.8 pg ml−1; P=0.005). High sVEGFR-3 and IL-8 levels correlated with shorter PFS (P=0.006, HR for progression/death: 3.91, 95% CI: 1.38–11.10 and P=0.045, HR: 2.58, 95% CI: 0.98–6.78, respectively) and shorter OS (P=0.047, HR for death: 3.23, 95% CI: 0.96–10.88 and P=0.014, HR: 5.51, 95% CI: 1.20–25.32, respectively; Table 2 and Figure 1).
Figure 1.
Kaplan–Meier estimates of PFS and OS for IL-8 and sVEGFR3 in carcinoids.
Stromal cell-derived factor-1α
Owing to the limited number of samples (n=28; 14 pNET and 14 carcinoid), the relationships between SDF-1α and outcomes were investigated in all available patients as a group. In this cohort, the two disease groups (Supplementary Table S1) did not differ in terms of PFS (P=0.06) or OS (P=0.48). Progression or death occurred in 22 patients. Using fitted Cox models for PFS and OS that included SDF-1α concentrations and disease group as covariates, high SDF-1α predicted for an increased risk of progression or death (PFS: P=0.005, HR: 3.59, 95% CI: 1.47–8.76 and OS: P=0.02, HR: 2.34, 95% CI: 1.16–4.72). Patients achieving CBR had lower SDF-1α concentrations (median 418 (range 210–1760) vs median 1069 ([range 229–2219) pg ml−1; P=0.04).
Changes in WBC and monocyte subsets during sunitinib treatment
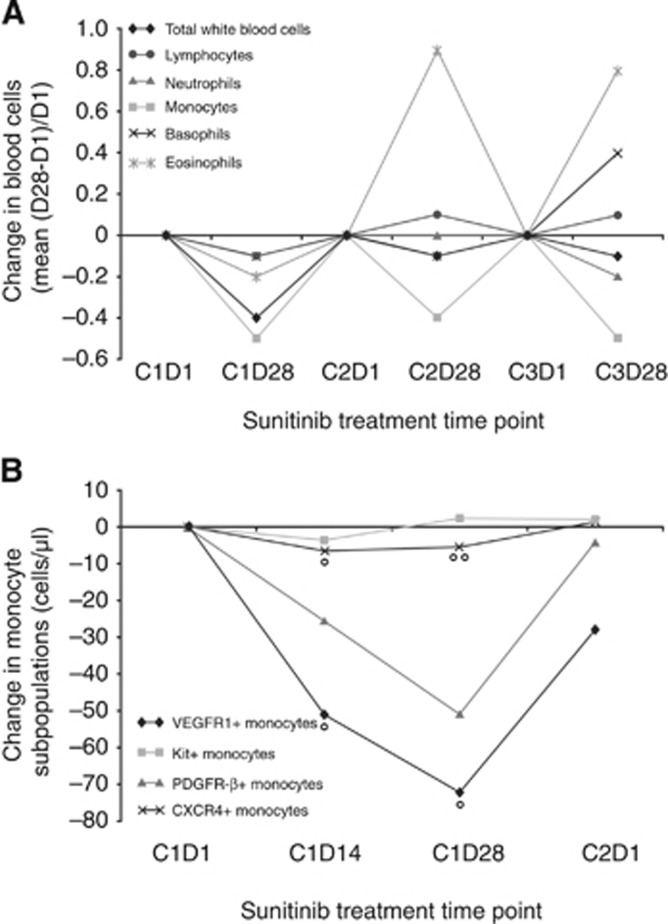
All WBC types, except lymphocytes and basophils, showed highly significant changes during the first three cycles of sunitinib, most notably a decrease in monocytes (greater than for any of the other WBC types, with the exception of that for neutrophils on C1D28; Wilcoxon signed-rank test; Figure 2A, and Supplementary Tables S3 and S4). The decrease in monocyte count correlated negatively with the plasma trough drug levels (Cmin) by C1D28 (ρ=–0.22, P=0.03).
Figure 2.
Change in WBC types and monocyte subsets. (A) Change in WBC types during the first three cycles of sunitinib treatment. (B) Change in monocyte subsets during the first cycle. °P<0.05. °°P<0.1.
Peripheral blood mononuclear cell specimens from 17 patients were assessed by flow cytometry at baseline, C1D14, C1D28, and C2D1. On sunitinib, CD14+ monocyte subpopulations decreased, in particular those bearing VEGFR-1 or CXCR4 (median change from C1D1 to C1D14, –50 cells per μl and –5 cells per μl, P=0.04 and P=0.03, respectively; median change from C1D1 to C1D28, –54 cells per μl and –3 cells per μl, P=0.02 and P=0.07, respectively; Wilcoxon signed rank-sum test; Figure 2B and Supplementary Table S5).
Changes in CECs
During the first sunitinib cycle, mature CECs decreased from baseline in the 17 available specimens (median change –3 cells per μl and –4 cells per μl, P=0.037 and P=0.045 for D14 and D28, respectively). There were no significant changes in circulating endothelial progenitor (CEP) cell levels during cycle 1 (not shown) or in CEC subtypes at day 42 relative to baseline (after 2 weeks off therapy).
Exploratory correlations between changes in cellular and plasma markers
Change from baseline to C1D28 in monocyte count showed moderately positive correlations with sVEGFR-3 change in all patients (ρ=0.32, P=0.002, particularly in those with carcinoid (ρ=0.37, P=0.04)), and with sVEGFR-2 changes in patients with pNET (ρ=0.31, P=0.01; Table 3).
Table 3. Correlation between change from baseline to C1D28 in monocyte count and plasma markers.
|
Correlation with change in monocytes, expressed as Spearman correlation coefficient (ρ)/P-value | |||
|---|---|---|---|
| Carcinoid tumour (n=37) | pNET (n=62) | All patients (N=99) | |
| Change in VEGF | −0.25/0.16 | −0.13/0.33 | −0.17/0.10 |
| Change in sVEGFR-2 | 0.130/0.46 | 0.31/0.01 | 0.19/0.07 |
| Change in sVEGFR-3 | 0.37/0.04 | 0.22/0.10 | 0.32/0.002 |
| Change in IL-8 | −0.06/0.75 | −0.10/0.45 | −0.07/0.53 |
Abbreviations: IL-8=interleukin 8; pNET, pancreatic neuroendocrine tumour; sVEGFR-2=soluble VEGF receptor 2; sVEGFR-3=soluble VEGF receptor 3; VEGF=vascular endothelial growth factor. Correlation coefficients with P-values ⩽0.05 are shown in bold.
Positive correlations were observed between changes in IL-8 and CXCR4-expressing monocytes and CEP (ρ=0.58, P=0.03 and ρ=0.71, P=0.006, respectively), suggesting that IL-8 may contribute to mobilization and/or tumour recruitment of these cells. Negative correlations were found between changes in sVEGFR-2 and sVEGFR-3, and mature CEC (ρ=–0.62, P=0.03 and ρ=–0.58, P=0.04, respectively; Table 4), adding to their pharmacodynamic value.
Table 4. Correlation between changes from baseline to C1D28 in soluble protein and cellular biomarkers (monocytes and CEC).
| VEGF | sVEGFR-2 | sVEGFR-3 | IL-8 | SDF-1α | |
|---|---|---|---|---|---|
|
VEGFR-1+ monocytes | |||||
| Spearman correlation coefficient | –0.25275 | –0.23956 | 0.51648 | –0.07253 | 0.21818 |
| P-value | 0.4048 | 0.4094 | 0.0586 | 0.8054 | 0.5192 |
| Sample size | 13 | 14 | 14 | 14 | 11 |
|
CXCR4+ monocytes | |||||
| Spearman correlation coefficient | 0.09341 | –0.35824 | 0.01538 | 0.57802 | 0.31818 |
| P-value | 0.7615 | 0.2085 | 0.9584 | 0.0304 | 0.3403 |
| Sample size | 13 | 14 | 14 | 14 | 11 |
|
CEP | |||||
| Spearman correlation coefficient | –0.01408 | 0.01657 | 0.35360 | 0.71272 | –0.30277 |
| P-value | 0.9653 | 0.9571 | 0.2359 | 0.0063 | 0.3655 |
| Sample size | 12 | 13 | 13 | 13 | 11 |
|
CEC | |||||
| Spearman correlation coefficient | 0.49650 | –0.61538 | –0.57692 | –0.05495 | 0.57273 |
| P-value | 0.1006 | 0.0252 | 0.0390 | 0.8585 | 0.0655 |
| Sample size | 12 | 13 | 13 | 13 | 11 |
Abbreviations: CEC=circulating endothelial cell; CEP=circulating endothelial progenitor cell; SDF-1α=stromal cell-derived factor 1α; VEGFR-1+=vascular endothelial growth factor receptor. Correlation coefficients with P-values ⩽0.05 are shown in bold.
Discussion
Molecular classifiers are urgently needed to better assess prognosis and optimize drug selection in NET. This analysis of circulating protein and cellular biomarkers in sunitinib-treated patients with pNET and carcinoid identified differences between tumour types and new candidate markers associated with outcomes, offering fresh insights into the biological activity of sunitinib and its mechanism of action.
Pre-treatment concentrations of SDF-1α and sVEGFR-2 were higher in pNET compared with carcinoid tumours, supporting the notion that pNET malignancies are generally more angiogenic (Panzuto et al, 2011). In contrast to what has been observed in other tumour types (at least for IL-8 and in animal tumour models for sVEGFR-2; Ebos et al, 2008; Sanmamed et al, 2014), we found no correlation between the levels of any of the five soluble biomarkers evaluated and tumour burden (as assessed by the SLD). Changes in VEGF, sVEGFR-2, sVEGFR-3, SDF-1α, and IL-8 during the first cycle of sunitinib treatment were independent of tumour type and were similar in range to those in patients with renal cell and hepatocellular carcinomas and GIST (Deprimo et al, 2007; Norden-Zfoni et al, 2007; Harmon et al, 2011). Most of these changes are related to the effects of the drug on host rather than tumour cells (Ebos et al, 2007; Lindauer et al, 2010).
Sunitinib exerts antitumour and antiangiogenic effects via tyrosine kinase inhibition on signalling cascades that regulate tumour growth, survival, and angiogenesis. Although the biologic targets of sunitinib are known and clinically validated, the cellular and molecular determinants of clinical benefit are still unclear. In our study, high pre-treatment sVEGFR-2 levels were associated with longer OS in patients with pNET, the first such report to the best of our knowledge. Other groups however have shown high levels of sVEGFR-2 to predict for PFS benefit from anti-VEGF treatment in different cancer types, including metastatic NETs (Cameron et al, 2013; Grande et al, 2013; Miles et al, 2013; Cremolini et al, 2014). VEGFR-2 plasma levels are thought to be modulated by VEGF and possibly serve as a surrogate biomarker for VEGF-dependent tumour growth (Ebos et al, 2008). In patients with carcinoids, low pre-treatment IL-8 levels predicted for CBR, longer PFS, and longer OS, indicating that IL-8 is a candidate marker of prognosis and/or sunitinib treatment benefit in these patients. Indeed, IL-8 has been reported as possibly prognostic in a smaller study that included carcinoid, pNET, and poorly differentiated neuroendocrine carcinomas (Pavel et al, 2005), perhaps through its effects favouring angiogenesis (directly on endothelial cells and by attracting myeloid cells to the tumour) and malignant cell proliferation and migration. High IL-8 expression has also been linked to prognosis (Tran et al, 2012) and sunitinib resistance in renal cell carcinoma (Huang et al, 2010). The relative importance of IL-8 in the biology of carcinoids however remains an open question. Regarding sVEGFR-3, we observed that low baseline levels also predicted for longer PFS and OS in carcinoid tumours. VEGFR-3, which is restricted in expression to the mucosal lymphatics of the intestines (not in blood capillaries; Partanen et al, 2000), may be an important sunitinib target in this tumour type through its role in lymphangiogenesis (Alitalo and Detmar, 2012).
We found low SDF-1α concentrations associated with CBR and longer PFS and OS in a pooled subgroup of patients including both carcinoid and pNET. Consistently, high expression of SDF-1 in pNET cells in tissue was previously linked to variables representing tumour growth and metastasis (Takahashi et al, 2007), while the expression of its receptor CXCR4 has been implicated in the metastatic potential of ileal carcinoid tumours (Arvidsson et al, 2010). Our findings suggest that the SDF-1α/CXCR4 pathway and the relative value of IL-8, sVEGFR-2, and sVEGFR-3 should be further investigated in well-differentiated NET in comparison with other emerging markers of prognosis such as chromogranin A and neuron-specific enolase (Yao et al, 2011; Chou and Chang Gung, 2013).
Among WBC types, sunitinib led to a distinct decrease in monocytes, specifically CD14+ monocyte subpopulations bearing VEGFR-1 and CXCR4, suggesting that these cells may be useful markers of sunitinib activity and response. The rationale for evaluating monocyte subsets was threefold. First, they express molecular targets for sunitinib: we previously showed that the majority of VEGFR-1-expressing WBCs in peripheral blood are CD14+ monocytes (Norden-Zfoni et al, 2007), which can co-express other targets such as PDGFR, KIT, and CD115. Second, myelomonocytic cells under different designations facilitate angiogenesis, tumour progression, and metastasis (Bergers and Hanahan, 2008; Du et al, 2008). Third, they have been implicated in tumour refractoriness to anti-VEGF therapy in animal models (Bergers and Hanahan, 2008). Together, our results suggest that the specific targeting of VEGFR-1 and CXCR4 monocytes may be a novel and important contributor to the effectiveness of sunitinib and perhaps other VEGFR-targeted agents in NET and other cancer types. Compared with CECs, the evaluation of myelomonocytic cell subpopulations in clinical specimens is more robust. CECs are extremely rare (1–2 orders of magnitude less common than VEGFR-1 monocytes in this study) and difficult to reliably quantify. Future studies should be considered in larger, more homogeneous patient sets to better assess correlations with prognosis and/or treatment outcomes.
As all patients in our study received sunitinib, we cannot determine whether the associations between biologic and clinical endpoints were treatment related. Moreover, we did not use corrections for multiple testing, because our goal was to be inclusive and not miss any candidate biomarker in the limited sample set. Our findings regarding IL-8 and sVEGFR-3 in carcinoid tumours, sVEGFR-2 in NETs, and SDF-1α in both should therefore be viewed as exploratory, and will require further evaluation and confirmation in future studies and clinical trials. The effects of sunitinib on VEGFR-1- and CXCR4-expressing myeloid cells may be mechanistically important to explain clinical effects in specific cancer types.
Acknowledgments
We thank Dr James C Yao for valuable insights. This study was sponsored by Pfizer Inc. Editorial assistance was provided by Susanne Gilbert and Emily Seidman at ACUMED (New York, NY, USA) and was funded by Pfizer Inc.
Employment or leadership position: LT and XH, Pfizer; WC, MindPiece Partners, prior employee of Pfizer; SEDP, prior employee of Pfizer. Consultant or advisory role: MHK, Novartis, Pfizer, Lexicon; CSH, Pfizer (was an employee of Atrium Staffing (New York, NY, USA) and a paid contractor of Pfizer during the development of this manuscript and the analysis and interpretation of the data); JVH, Pfizer. Stock ownership: LT, XH, WC, Pfizer; Charles S. Harmon, Pfizer (family member). Honoraria: JVH, Pfizer. Research funding: AJZ, WC and JVH, Pfizer; MHK, Novartis. Expert testimony: MHK, Pfizer. Other remuneration: None. MK, H-KW, H-JL, NJM, EL, and XW declare no conflicts of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology (ASCO), June 3–7, 2011, Chicago, IL, USA
Supplementary Material
References
- Abdel-Rahman O. Vascular endothelial growth factor (VEGF) pathway and neuroendocrine neoplasms (NENs): prognostic and therapeutic considerations. Tumour Biol. 2014;35 (11:10615–10625. doi: 10.1007/s13277-014-2612-7. [DOI] [PubMed] [Google Scholar]
- Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31 (42:4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- Arvidsson Y, Bergstrom A, Arvidsson L, Kristiansson E, Ahlman H, Nilsson O. Hypoxia stimulates CXCR4 signalling in ileal carcinoids. Endocr Relat Cancer. 2010;17 (2:303–316. doi: 10.1677/ERC-09-0085. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Goke B. Therapeutic strategies for advanced neuroendocrine carcinomas of jejunum/ileum and pancreatic origin. Gut. 2011;60 (7:1009–1021. doi: 10.1136/gut.2009.204453. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8 (8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman FT.World Health Organization. International Agency for Research on Cancer 2010WHO Classification of Tumours of the Digestive System4th ednInternational Agency for Research on Cancer: Lyon [Google Scholar]
- Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili-Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14 (10:933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- Chou W-C, Chang Gung L. Baseline and dynamic plasma chromogranin A (CgA) level to predict clinical outcome and tumor response in Asian patients with gastroenteropancreatic neuroendocrine tumor (GEP-NET) J Clin Oncol. 2013;31 (suppl:abstr e15151. [Google Scholar]
- Cremolini C, Loupakis F, Bocci G, Falcone A. Biomarkers and response to bevacizumab—letter. Clin Cancer Res. 2014;20 (4:1056–1057. doi: 10.1158/1078-0432.CCR-13-2763. [DOI] [PubMed] [Google Scholar]
- Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13 (3:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, Xu P, Mutsaers AJ, Dumont DJ, Kerbel RS. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68 (2:521–529. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104 (43:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjallskog ML, Lejonklou MH, Oberg KE, Eriksson BK, Janson ET. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9 (4:1469–1473. [PubMed] [Google Scholar]
- Gerger A, LaBonte M, Lenz HJ. Molecular predictors of response to antiangiogenesis therapies. Cancer J. 2011;17 (2:134–141. doi: 10.1097/PPO.0b013e318212db3c. [DOI] [PubMed] [Google Scholar]
- Grande E, Casanovas O, Earl J, Castellano DE, Garcia-Carbonero R, Teule A, Duran I, Fuster J, Sevilla I, Escudero P, Sastre J, Garcia-Donas J, Ortega L, Diez JJ, Rivas AS, Capdevila J.2013sVEGFR2 and circulating tumor cells to predict for the efficacy of pazopanib in neuroendocrine tumors (NETs): PAZONET subgroup analysis J Clin Oncol 31(15Abstract #4140. [Google Scholar]
- Hansel DE, Rahman A, Hermans J, de Krijger RR, Ashfaq R, Yeo CJ, Cameron JL, Maitra A. Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Mod Pathol. 2003;16 (7:652–659. doi: 10.1097/01.MP.0000077416.68489.50. [DOI] [PubMed] [Google Scholar]
- Harmon CS, DePrimo SE, Raymond E, Cheng AL, Boucher E, Douillard JY, Lim HY, Kim JS, Lechuga MJ, Lanzalone S, Lin X, Faivre S. Mechanism-related circulating proteins as biomarkers for clinical outcome in patients with unresectable hepatocellular carcinoma receiving sunitinib. J Transl Med. 2011;9:120. doi: 10.1186/1479-5876-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70 (3:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26 (20:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, Ellis LM, Benedetti JK, Bergsland EK, Hobday TJ, Van Cutsem E, Pingpank J, Oberg K, Cohen SJ, Posner MC, Yao JC. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29 (7:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer A, Di Gion P, Kanefendt F, Tomalik-Scharte D, Kinzig M, Rodamer M, Dodos F, Sorgel F, Fuhr U, Jaehde U. Pharmacokinetic/pharmacodynamic modeling of biomarker response to sunitinib in healthy volunteers. Clin Pharmacol Ther. 2010;87 (5:601–608. doi: 10.1038/clpt.2010.20. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Antoniotti P, Quarna J, Calleri A, Rabascio C, Tacchetti C, Braidotti P, Wu HK, Zurita AJ, Saronni L, Cheng JB, Shalinsky DR, Heymach JV, Bertolini F. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15 (1:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- Miles DW, de Haas SL, Dirix LY, Romieu G, Chan A, Pivot X, Tomczak P, Provencher L, Cortes J, Delmar PR, Scherer SJ. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer. 2013;108 (5:1052–1060. doi: 10.1038/bjc.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13 (9:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- Oberg K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors) Curr Opin Endocrinol Diabetes Obes. 2009;16 (1:72–78. doi: 10.1097/med.0b013e328320d845. [DOI] [PubMed] [Google Scholar]
- Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, Delle Fave G, Falconi M. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29 (17:2372–2377. doi: 10.1200/JCO.2010.33.0688. [DOI] [PubMed] [Google Scholar]
- Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, Di Fonzo M, Tornatore V, Milione M, Angeletti S, Cattaruzza MS, Ziparo V, Bordi C, Pederzoli P, Delle Fave G. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12 (4:1083–1092. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- Pape UF, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, Willich SN, Wiedenmann B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15 (4:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14 (13:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- Pavel ME, Hassler G, Baum U, Hahn EG, Lohmann T, Schuppan D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol (Oxf) 2005;62 (4:434–443. doi: 10.1111/j.1365-2265.2005.02238.x. [DOI] [PubMed] [Google Scholar]
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Horsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364 (6:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martin-Algarra S, Perez G, Landazuri SF, Gonzalez A, Gross S, Rodriguez I, Munoz-Calleja C, Rodriguez-Ruiz M, Sangro B, Lopez-Picazo JM, Rizzo M, Mazzolini G, Pascual JI, Andueza MP, Perez-Gracia JL, Melero I. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20 (22:5697–5707. doi: 10.1158/1078-0432.CCR-13-3203. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Akishima-Fukasawa Y, Kobayashi N, Sano T, Kosuge T, Nimura Y, Kanai Y, Hiraoka N. Prognostic value of tumor architecture, tumor-associated vascular characteristics, and expression of angiogenic molecules in pancreatic endocrine tumors. Clin Cancer Res. 2007;13 (1:187–196. doi: 10.1158/1078-0432.CCR-06-1408. [DOI] [PubMed] [Google Scholar]
- Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM, Figlin RA, Hutson TE, Sternberg CN, Amado RG, Pandite LN, Heymach JV. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13 (8:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- Yao JC, Pavel M, Phan AT, Kulke MH, Hoosen S, St Peter J, Cherfi A, Oberg KE. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96 (12:3741–3749. doi: 10.1210/jc.2011-0666. [DOI] [PubMed] [Google Scholar]
- Zhang L, Smyrk TC, Oliveira AM, Lohse CM, Zhang S, Johnson MR, Lloyd RV. KIT is an independent prognostic marker for pancreatic endocrine tumors: a finding derived from analysis of islet cell differentiation markers. Am J Surg Pathol. 2009;33 (10:1562–1569. doi: 10.1097/PAS.0b013e3181ac675b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.