Abstract
Influenza infection results in considerable pulmonary pathology, a significant component of which is mediated by CD8+ T cell effector functions. To isolate the specific contribution of CD8+ T cells to lung immunopathology, we utilized a nonviral murine model in which alveolar epithelial cells express an influenza antigen and injury is initiated by adoptive transfer of influenza-specific CD8+ T cells. We report that IFN-γ production by adoptively transferred influenza-specific CD8+ T cells is a significant contributor to acute lung injury following influenza antigen recognition, in isolation from its impact on viral clearance. CD8+ T cell production of IFN-γ enhanced lung epithelial cell expression of chemokines and the subsequent recruitment of inflammatory cells into the airways. Surprisingly, Stat1 deficiency in the adoptive-transfer recipients exacerbated the lung injury that was mediated by the transferred influenza-specific CD8+ T cells but was still dependent on IFN-γ production by these cells. Loss of Stat1 resulted in sustained activation of Stat3 signaling, dysregulated chemokine expression, and increased infiltration of the airways by inflammatory cells. Taken together, these data identify important roles for IFN-γ signaling and Stat1-independent IFN-γ signaling in regulating CD8+ T cell-mediated acute lung injury. This is the first study to demonstrate an anti-inflammatory effect of Stat1 on CD8+ T cell-mediated lung immunopathology without the complication of differences in viral load.
Keywords: CD8+ T cell, interferon-γ, immunopathology, Stat1, acute lung injury
significant lung injury frequently accompanies influenza viral infection, which is mediated by the direct effects of the virus and is a result of host immune responses. While CD8+ T cells are important for influenza viral clearance (11), it is believed that these cells can contribute to this injury, as dysregulated CD8+ T cell responses are associated with enhanced pulmonary pathology during influenza infection in mice and humans (5, 17). However, it is difficult to separate the direct effects of the viral infection on lung pathology from the effects of the CD8+ T cell effector functions, such as cytotoxicity and production of the proinflammatory cytokines IFN-γ and TNF-α. Using a transgenic mouse model of influenza infection in which influenza hemagglutinin (HA) is expressed by lung epithelial cells (6), we have identified critical mechanisms by which CD8+ T cells mediate influenza immunopathology in the absence of replicating virus. We previously demonstrated that, following alveolar antigen recognition, TNF-α production by HA-specific CD8+ T cells induced lung epithelial cell chemokine expression, which resulted in extensive airway inflammation and lethal lung injury (4, 32).
While the role of TNF-α production by CD8+ T cells in mediating influenza immunopathology is well characterized, the role of IFN-γ production by CD8+ T cells in acute lung injury during influenza infection is equivocal. IFN-γ production by influenza-specific CD8+ T cells has been shown to mitigate the extent of lung injury during infection (31), and IFN-γ expression is important for a protective recall response against secondary challenge with influenza virus (2). The protective effects of IFN-γ during infection may be due in part to direct stimulatory effects of IFN-γ on CD8+ T cell responses (30). In contrast, antibody neutralization of IFN-γ during influenza infection has been shown to reduce the extent of influenza-specific CD8+ T cell-mediated injury and the magnitude of inflammatory cell infiltration into the lungs (1, 18). Moreover, IFN-γ production by virus-specific CD8+ T cells has been implicated in mediating immunopathology during respiratory syncytial viral infection (19), and IFN-γ is thought to have an important role in the development of lung injury during severe acute respiratory disease (12). Importantly, IFN-γ deficiency does not appear to impair influenza viral clearance (10), suggesting that IFN-γ may primarily function to dampen or exacerbate immunopathology during influenza infection.
The biological effects of IFN-γ are mainly mediated by rapid activation of the Jak/Stat1 pathway and the subsequent changes in gene expression (21). The role of Stat1 in regulating IFN-γ-mediated immunopathology during influenza infection is unclear, as Stat1 deficiency during influenza infection results in the inability of the host to control viral replication and systemic spread of the virus (8). IFN-γ production by CD8+ T cells has been shown to activate Stat1 in neurons and mediate loss of dendrites and synapses (14). However, Stat1 deficiency has been shown to exacerbate pathology in a model of central nervous system disease (29), suggesting that Stat1 may mediate anti-inflammatory effects, in addition to its obvious antiviral role. The increased pathology in the absence of Stat1 may be due in part to Stat1-independent IFN-γ signaling (9), as loss of Stat1 results in enhanced and sustained IFN-γ-induced Stat3 activation (23). Overexpression of Stat3 in lung epithelial cells results in enhanced inflammation during tumorigenesis (15), while loss of lung epithelial cell Stat3 expression attenuates allergic airway inflammation (26). The balance of Stat1 and Stat3 activation has been proposed to have profound effects on cytokine gene expression and inflammation (24), suggesting that Stat1-independent IFN-γ signaling may result in alternative activation of Stat3 and increased pulmonary inflammation.
In this study we investigated the roles of Stat1-dependent and -independent IFN-γ signaling in CD8+ T cell-mediated influenza immunopathology in the absence of replicating virus. Using an adoptive transfer model, in which wild-type (WT) or IFN-γ-deficient HA-specific CD8+ T cells were transferred into WT or Stat1-deficient HA-transgenic mice, we identified important roles for Stat1-dependent and -independent IFN-γ signaling in mediating the extent of CD8+ T cell influenza immunopathology.
METHODS
Mice.
BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice lacking IFN-γ [gene knockout (GKO)] were kindly provided by Dr. Thomas Braciale. Mice lacking Stat1 (Stat1−/−) were kindly provided by Dr. Joan Durbin. HA-transgenic mice expressing the influenza HA of A/Japan/57 virus in the lung are described elsewhere (4). To generate HA-transgenic mice lacking Stat1, HA-transgenic mice were bred with Stat1−/− mice. All animal studies were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee at Geisel School of Medicine at Dartmouth University.
CD8+ T cell generation and adoptive transfer.
WT and GKO CD8+ T cells specific for HA210–219 (a Kd-restricted epitope of A/Japan/57 HA; hereafter referred to as HA210) were generated as previously described (4) and restimulated in vitro with HA210–219 peptide-pulsed, irradiated syngeneic splenocytes to maintain bulk cultures of HA210-specific CD8+ T cells. HA210-specific CD8+ T cells were adoptively transferred into HA-transgenic mice by tail vein injection. In some experiments, CD8+ T cells were labeled with carboxyfluorescein succinimidyl ester prior to transfer to monitor T cell trafficking to the lung. Morbidity (as measured by weight loss) was monitored daily, and loss of ≥20% of the initial body weight was used as an end point in accordance with our Institutional Animal Care and Use Committee-approved protocol.
Bronchoalveolar lavage and tissue preparation.
At the appropriate time points, lungs were lavaged in situ as previously described (4). The recovered fluid was centrifuged, and the supernatant was analyzed for cytokines and chemokines by Millipore mouse 32 Plex Luminex assay performed by DartLab (Lebanon, NH). Albumin concentrations in the bronchoalveolar lavage fluid (BALF) were determined by ELISA (Bethyl Laboratories, Montgomery, TX). Cells were counted on a hemocytometer with Trypan blue exclusion to determine the total number of viable cells, and cell populations were enumerated using the PROTOCOL Hema 3 stain set (Fisher, Houston, TX). For assessment of lung histology, lungs were inflated with 0.5% low-melting-point agarose in PBS and fixed in formalin, and sectioned slices were stained with hematoxylin-eosin. For primary lung type II cell gene and protein expression, cells were purified as described previously (16), and Western blot analysis was performed.
Mouse lung epithelial cell culture.
Mouse lung epithelial (MLE) cells were pulsed with HA210–219 peptide and cultured with HA210-specific CD8+ T cells. Alternatively, MLE cells were cultured with mouse recombinant TNF-α and IFN-γ (Biolegend, San Diego, CA). ELISA was used to analyze cytokine and chemokine production. Gene expression was analyzed as previously described (22).
Statistics.
Statistical analysis was performed with GraphPad Prism (GraphPad Software, La Jolla, CA). Two-tailed unpaired t-test with 95% confidence interval was used to analyze differences between groups, while two-way ANOVA with 95% confidence interval was used to analyze differences between groups over time.
RESULTS AND DISCUSSION
CD8+ T cell expression of IFN-γ enhances lung epithelial cell chemokine expression.
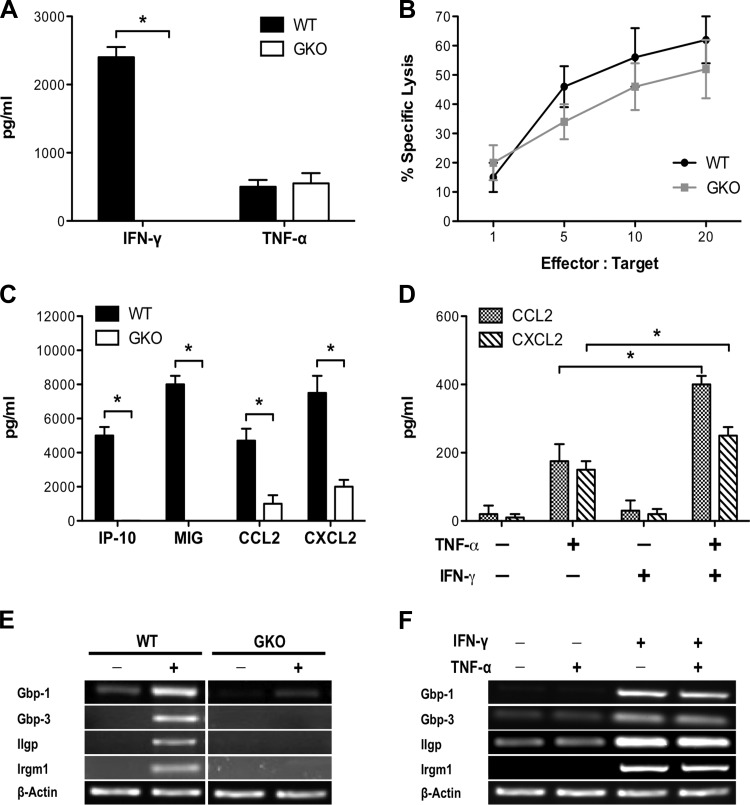
First, using an in vitro system that reflects the interaction between CD8+ T cells and lung epithelial cells in vivo, we sought to characterize the effects of IFN-γ production by CD8+ T cells on lung epithelial cell function. MLE cells were pulsed with HA210–219 peptide and cultured with HA210-specific WT or GKO CD8+ T cells. As expected, there was no detectable production of IFN-γ by activated GKO CD8+ T cells, in contrast to WT cells (Fig. 1A). However, GKO CD8+ T cells were not impaired in other examined effector functions, including TNF-α production (Fig. 1A) and cytotoxicity (Fig. 1B). Loss of CD8+ T cell production of IFN-γ abrogated MLE production of IFN-inducible chemokines, IFN-γ-inducible protein-10 (IP-10) and monokine induced by IFN-γ (MIG), and resulted in reduced production of CCL2 and CXCL2 (Fig. 1C). IFN-γ and TNF-α appeared to act synergistically to induce MLE expression of CCL2 and CXCL2, as treatment of MLE cells with TNF-α and IFN-γ increased production of CCL2 and CXCL2 compared with treatment with TNF-α alone (Fig. 1D). Furthermore, IFN-γ, but not TNF-α, was required for the induction of guanylate-binding protein and GTPase gene expression in MLE cells (Fig. 1, D and E). GTPases are localized to the endoplasmic reticulum and Golgi compartments and have been shown to have important roles in chemokine secretion (25, 27), suggesting that CD8+ T cell-produced IFN-γ functions synergistically with TNF-α to enhance lung epithelial cell chemokine release.
Fig. 1.
IFN-γ production by CD8+ T cells enhances lung epithelial chemokine expression. A: IFN-γ and TNF-α production by HA210-specific wild-type (WT) or gene knockout (GKO) CD8+ T cells cultured with peptide-pulsed mouse lung epithelial (MLE) cells. B: specific lysis of peptide-pulsed target cells by HA210-specific WT or GKO CD8+ T cells. C: chemokine production by peptide-pulsed MLE cells cultured with HA210-specific WT or GKO CD8+ T cells. IP-10, IFN-γ-inducible protein-10; MIG, monokine induced by IFN-γ. D: CCL2 and CXCL2 production by MLE cells treated with IFN-γ and TNF-α. E: expression of IFN-inducible genes in MLE cells cultured with HA210-specific WT or GKO CD8+ T cells. Gbp, guanylate-binding protein; Iigp, IFN-inducible GTPase; Irgm, IFN-inducible protein 1. F: expression of IFN-inducible genes in MLE cells treated with IFN-γ and TNF-α. Values are means ± SD of ≥3 independent experiments, with conditions conducted in triplicate. *P < 0.05.
CD8+ T cell expression of IFN-γ enhances acute lung injury.
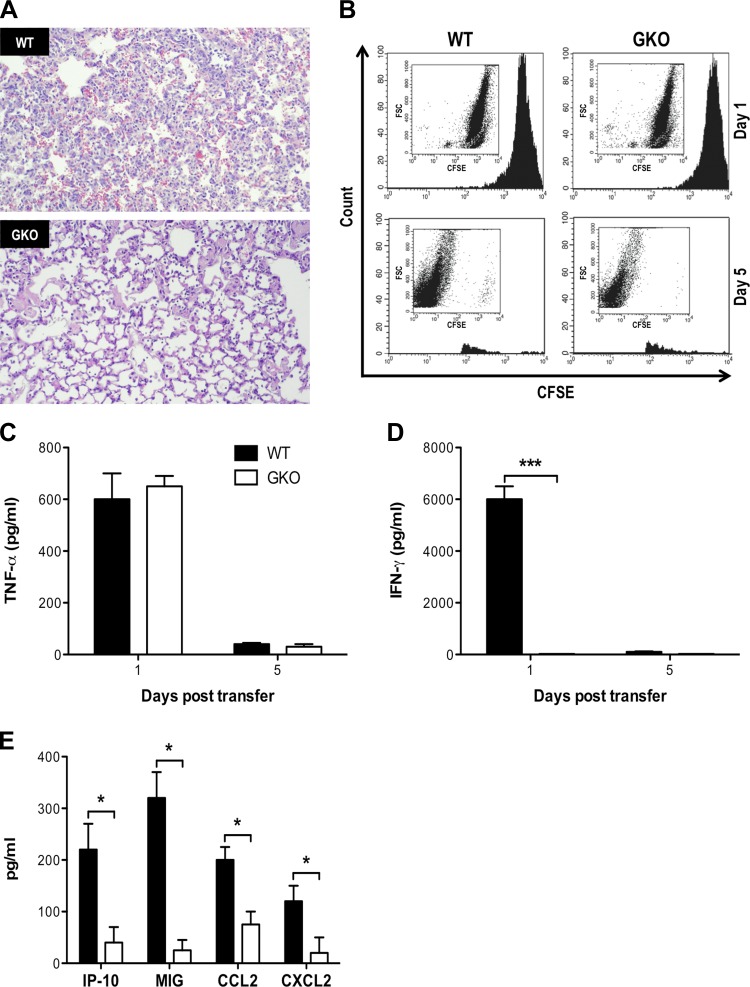
Next, we examined the role of IFN-γ production by influenza-specific CD8+ T cells in the development of pulmonary pathology in a CD8+ T cell-mediated model of acute lung injury. We adoptively transferred HA210-specific WT or GKO CD8+ T cells into HA-transgenic mice. We observed a reduction in the total number of inflammatory cells infiltrating the airways and lung parenchyma, with attenuated alveolar damage on histology in GKO recipients, indicating that IFN-γ production by the transferred CD8+ T cells was critical for the full extent of pulmonary pathology (Fig. 2A). GKO recipients also exhibited reduced weight loss morbidity and increased oxygen saturation capability compared with WT recipients (data not shown). To confirm that reduced CD8+ T cell trafficking to the lung in GKO recipients was not responsible for the attenuated injury that occurred in these mice, we labeled HA210-specific CD8+ T cells with carboxyfluorescein succinimidyl ester prior to transfer. We observed similar numbers of WT and GKO HA210-specific CD8+ T cells in the lung 24 h after transfer, suggesting that these cells had similar ability to traffic to the lung (Fig. 2B). Moreover, there were similar levels of TNF-α in the airways of WT and GKO recipients 24 h after transfer, suggesting that these cells had similar capacity to recognize alveolar antigen and mediate effector activities (Fig. 2C). As expected, IFN-γ was not detected in GKO recipients, indicating that the transferred CD8+ T cells were the primary source of IFN-γ (Fig. 2D). We previously showed that expression of chemokines by alveolar epithelial cells is critical for CD8+ T cell-mediated acute lung injury (4, 33), and we observed significantly reduced levels of IP-10, MIG, CCL2, and CXCL2 in GKO recipients compared with WT recipients (Fig. 2E). Preliminary data also demonstrated greater induction of guanylate-binding protein and GTPases in lung epithelial cells in WT than GKO recipients (data not shown), indicating that CD8+ T cell production of IFN-γ increased chemokine release by lung epithelial cells, resulting in enhanced recruitment of inflammatory cells and the subsequent exacerbated pulmonary pathology.
Fig. 2.
IFN-γ production following alveolar antigen recognition by influenza-specific CD8+ T cells exacerbates lung injury. A: representative hematoxylin-eosin-stained whole lung histology 5 days after transfer of 5 × 106 HA210-specific WT (top) or GKO (bottom) CD8+ T cells into hemagglutinin (HA)-transgenic mice. B: HA210-specific CD8+ T cells were labeled with carboxyfluorescein succinimidyl ester prior to transfer; on days 1 and 5, whole lungs were digested, and the number of carboxyfluorescein succinimidyl ester-labeled cells was determined by flow cytometry. C and D: bronchoalveolar lavage (BAL) fluid (BALF) concentrations of IFN-γ and TNF-α 1 and 5 days after CD8+ T cell transfer. E: chemokine expression in the airways 24 h after CD8+ T cell transfer. Values are means ± SD of 3 independent experiments with 3–4 mice per group. *P < 0.05, ***P < 0.005.
Stat1 deficiency enhances CD8+ T cell-mediated acute lung injury independent of its antiviral activities.
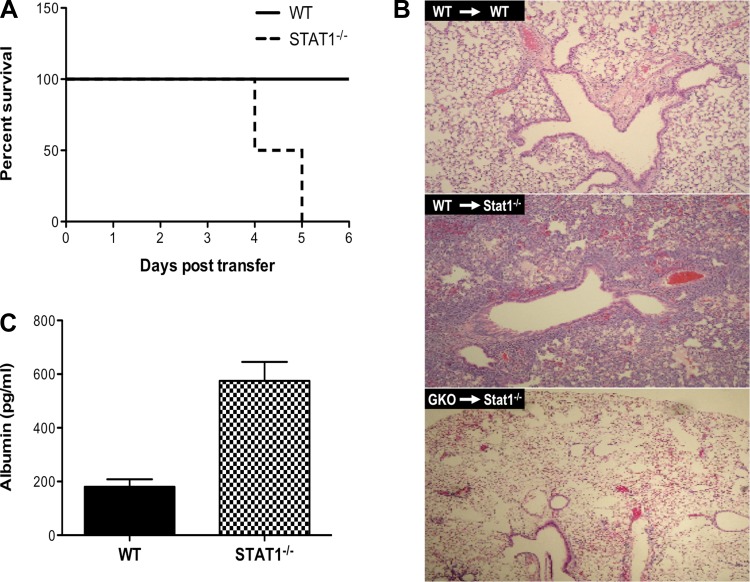
Since the biological effects of IFN-γ are primarily mediated by the Stat1 pathway (21) and we observed reduced Stat1 gene expression in GKO recipients compared with WT recipients (data not shown), we generated HA-transgenic mice that lacked Stat1 to examine whether Stat1-dependent IFN-γ signaling was required to mediate the full extent of CD8+ T cell-mediated lung injury. Because of the specific constraints of studying the specific impact of Stat1 deficiency in a viral infection (in which control of viral replication is severely impaired and it is impossible to control for antigen load), this model enabled us to demonstrate the specific impact of Stat1 deficiency on CD8+ T cell-mediated pulmonary immunopathology. To our surprise, we found that Stat1 deficiency resulted in enhanced morbidity and eventual death of all animals in the experiment at an otherwise nonlethal dose (in Stat1-sufficient animals) of transferred HA210-specific WT CD8+ T cells (Fig. 3A). Transfer of as few as 106 HA210-specific CD8+ T cells was sufficient to induce death in Stat1−/− HA-transgenic mice, whereas a CD8+ T cell transfer of ≥10 times more cells (107) was required for comparable inflammatory influx and lung injury in WT HA-transgenic mice (6). Stat1−/− HA-transgenic mice exhibited enhanced infiltration of the airways and lung interstitium, with increased disruption of the alveolar air space, and this was dependent on IFN-γ production by the transferred CD8+ T cells, as lung injury was abrogated in Stat1−/− HA-transgenic recipients of GKO CD8+ T cells (Fig. 3B). Unlike viral infection, CD8+ T cell recognition of nonviral antigen expressed by alveolar epithelial cells does not result in type I IFN expression, further indicating that CD8+ T cell expression of IFN-γ is absolutely required for this process. Consistent with the enhanced lung injury observed on histology, levels of albumin, a marker of vascular leakage, in the airways were elevated (Fig. 3C) and peripheral oxygen saturation was reduced (data not shown) in Stat1−/− HA-transgenic mice compared with WT HA-transgenic mice. These data indicate that Stat1 has an important role in regulating the extent of immunopathology, in addition to its obvious role in antiviral immunity, and underscore a critical proinflammatory role for Stat1-independent IFN-γ signaling.
Fig. 3.
Stat1 deficiency exacerbates CD8+ T cell-mediated lung injury. A: survival of WT and Stat1−/− HA-transgenic mice after adoptive transfer of 2.5 × 106 HA210-specific WT CD8+ T cells. B: representative hematoxylin-eosin-stained whole lung histology 5 days after transfer of 2.5 × 106 HA210-specific WT CD8+ T cells into WT (top) or Stat1−/− (middle) HA-transgenic mice or transfer of 2.5 × 106 HA210-specific GKO CD8+ T cells into Stat1−/− HA-transgenic mice (bottom). C: BALF concentration of albumin 48 h after CD8+ T cell transfer. Values are means ± SD of 3 independent experiments with 2–4 mice per group.
Stat1 deficiency enhances airway inflammatory responses.
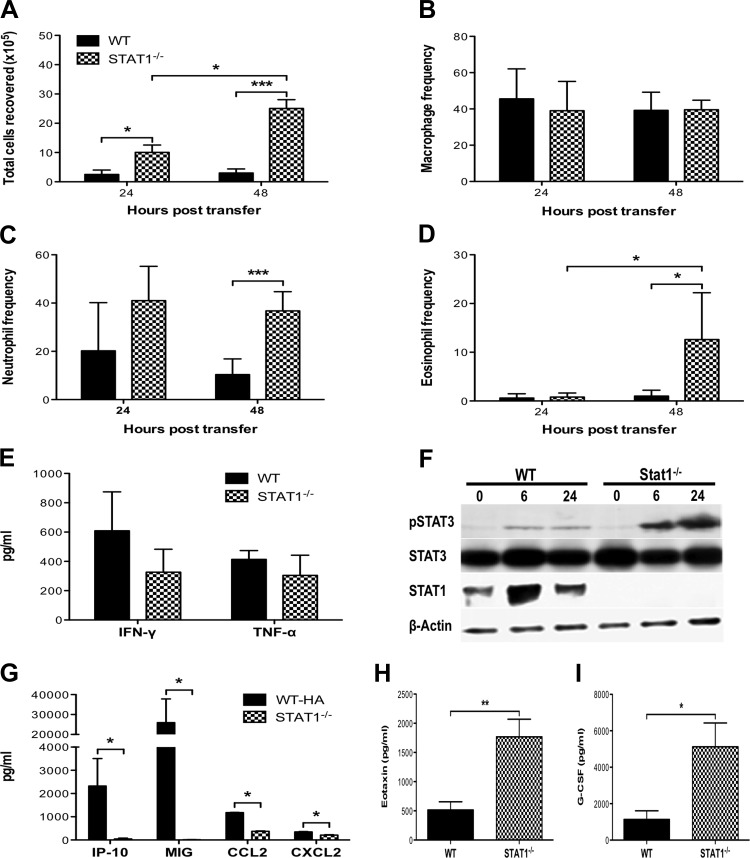
Next we sought to characterize the inflammatory responses that contributed to the enhanced morbidity and mortality in Stat1−/− HA-transgenic mice. The total number of cells recovered from the airways of Stat1−/− HA-transgenic mice increased 24 and 48 h after transfer of CD8+ T cells (Fig. 4A). While there were no changes in the frequency of macrophages, there were increases in the frequency of neutrophils and eosinophils in the airways of Stat1−/− HA-transgenic mice compared with WT HA-transgenic mice (Fig. 4, B–D), and there were significant increases in the absolute number of macrophages, neutrophils, and eosinophils in Stat1−/− HA-transgenic mice compared with WT HA-transgenic mice (data not shown). We also observed an increase in matrix metalloproteinase 9 gene expression in the lungs of Stat1−/− HA-transgenic mice (data not shown), consistent with the enhanced neutrophil response in these mice, as expression of matrix metalloproteinase 9 by neutrophils has been shown to be important for neutrophil migration into the respiratory tract and the extent of lung injury during influenza infection (3). To elucidate the mechanisms contributing to the enhanced cellular responses in Stat1−/− HA-transgenic mice, we examined TNF-α and IFN-γ production by the transferred CD8+ T cells. There were no significant differences in the total levels of TNF-α and IFN-γ between Stat1−/− HA-transgenic and WT HA-transgenic mice (Fig. 4E), suggesting that HA210-specific CD8+ T cells were similarly capable of trafficking to the lungs, recognizing antigen, and being activated to express effector activities in WT and Stat1−/− HA-transgenic mice. Taken together, these data demonstrate that, following the induction of similar effector responses by the transferred CD8+ T cells, loss of Stat1 in the recipient mice results in an increase in airway inflammatory responses, which contributes to the enhanced pulmonary pathology.
Fig. 4.
Dysregulated inflammatory responses in Stat1−/− mice. A: total number of viable cells recovered from the BAL 24 and 48 h after transfer of 2.5 × 106 HA210-specific WT CD8+ T cells into WT and Stat1−/− HA-transgenic mice. B–D: frequencies of macrophages (B), neutrophils (C), and eosinophils (D) in the BAL 24 and 48 h after CD8+ T cell transfer. E: BALF concentrations of IFN-γ and TNF-α 24 h after CD8+ T cell transfer. F: phosphorylated Stat3 levels in lung type II cells isolated from WT or Stat1−/− HA-transgenic mice 0, 6, and 24 h after CD8+ T cell transfer. G: BALF concentrations of IP-10, MIG, CCL2, and CXCL2 24 h after CD8+ T cell transfer. H and I: BALF concentrations of granulocyte-colony stimulating factor (H) and eotaxin (I) 24 h after CD8+ T cell transfer. Values are means ± SD of 2–3 independent experiments with 3–5 mice per group. *P < 0.05, ***P < 0.005.
Stat1 deficiency results in sustained Stat3 activation in lung epithelial cells and altered chemokine expression.
As we previously described a critical role for lung epithelial cells in mediating lung injury following CD8+ T cell transfer (33), we next examined lung epithelial cell responses in Stat1−/− HA-transgenic mice. Stat1-dependent genes, such as suppressor of cytokine signaling 1, were ablated in the lung epithelial cells in Stat1−/− HA-transgenic mice following CD8+ T cell transfer (data not shown). Interestingly, in the absence of Stat1, we observed enhanced and prolonged Stat3 signaling in lung epithelial cells recovered from Stat1−/− HA-transgenic mice compared with WT HA-transgenic mice (Fig. 4F). Enhanced phosphorylation and sustained activation of Stat3 in lung epithelial cells of Stat1−/− HA-transgenic mice were evident 6 h after CD8+ T cell transfer, indicating that Stat3 activation in the Stat1−/− HA-transgenic mice was likely mediated by IFN-γ produced by the transferred CD8+ T cells (within 5–6 h after transfer). This is consistent with previous studies that have shown that IFN-γ activates Stat3 rapidly and in a sustained manner in Stat1−/− mouse embryonic fibroblasts (20, 23). As IFN-γ is absolutely required for CD8+ T cell-mediated lung injury in Stat1−/− HA-transgenic mice and Stat3 has been implicated in mediating airway inflammation (15, 26), it is likely that alternative activation of Stat3 by IFN-γ contributes to the dysregulated inflammatory responses in Stat1−/− HA-transgenic mice. Lung epithelial cell production of IP-10 and MIG was abrogated in Stat1−/− HA-transgenic mice, indicating that IFN-γ signaling through Stat1 was required for expression of these chemokines (Fig. 4G). CCL2 and CXCL2 expression was also reduced in Stat1−/− HA-transgenic mice (Fig. 4G). In contrast, levels of eotaxin were significantly increased in the airways of Stat1−/− HA-transgenic mice (Fig. 4I), consistent with the enhanced eosinophil response in these mice. Eotaxin release by airway smooth muscle cells has been shown to be dependent on Stat3 activation (7), and loss of lung epithelial cell Stat3 expression attenuates eosinophil airway infiltration during asthma (26), indicating that the enhanced eotaxin expression in Stat1−/− HA-transgenic mice may be due in part to the dysregulated Stat3 signaling in lung epithelial cells. We also observed increased levels of granulocyte-colony stimulating factor in the airways of Stat1−/− HA-transgenic mice (Fig. 4H), which may have contributed to the enhanced neutrophil mobilization observed in these mice. Thus it is likely that alternative activation of Stat3 regulates lung epithelial inflammatory gene expression in Stat1−/− HA-transgenic mice, resulting in severe lung injury.
Taken together, our data indicate that Stat1-dependent and -independent IFN-γ signaling mechanisms mediate CD8+ T cell-mediated immunopathology following influenza alveolar antigen recognition, in the absence of the complicating factor of replicating virus. While direct comparison of the impact of IFN-γ and Stat1 deficiencies on pulmonary pathology was complicated by the fact that different doses of CD8+ T cells were used in the two different models, our data show that 1) in the presence of Stat1, IFN-γ and TNF-α produced by effector CD8+ T cells act synergistically to increase lung epithelial cell release of chemokines and enhance airway inflammatory cell infiltration and 2) in the absence of Stat1, IFN-γ signaling results in sustained Stat3 activation, increased recruitment of neutrophils and eosinophils to the airways, and lethal pulmonary pathology. While Stat3 binding to the IFN-γ receptor compensates for the deficiency of Stat1 binding, the net result is enhanced immunopathology. Interestingly, influenza virus is capable of inhibiting IFN-γ-induced Stat1 activation (28), but it is unknown whether the Stat3 pathway is also affected. It is tempting to speculate that viral interference with IFN-γ-induced Stat1 activation may skew the response toward enhanced and prolonged Stat3 activation and exacerbated pulmonary pathology. In contrast, it is also possible that type I IFN signaling in lung epithelial cells during influenza infection may trigger Stat1 activation, limiting alternative Stat3 activation and abrogating injury early after infection. However, the kinetics of type I IFN expression and the CD8+ T cell response to the virus may still permit the mechanisms described here to exacerbate pulmonary pathology, as type I IFN expression peaks shortly after infection (days 2–3) (13), while the peak CD8+ T cell response occurs later (days 8–10). Therefore, the presence of early Stat1 activation by type I IFN may not be sufficient to prevent the Stat1-dependent or -independent lung injury mediated by CD8+ T cell expression of IFN-γ following influenza infection. It has not been previously possible to isolate the specific impact of Stat1 deficiency on the immunopathology associated with immune-mediated viral clearance, since Stat1−/− mice are exquisitely permissive to viral replication. This study is the first to specifically demonstrate that Stat1 deficiency has an important impact on T cell-mediated immunopathology, independent of the impact on antiviral activities. Additionally, given our observations that IFN-γ expression by influenza-specific CD8+ T cells largely serves a proinflammatory role, independent of any antiviral functions, and that IFN-γ expression by T cells is dispensable for influenza viral clearance, IFN-γ may be a novel target to limit the extent of immunopathology during acute influenza infection.
GRANTS
This work was supported by National Institutes of Health Grants R01 AI-047226 (J. E. Durbin), T32 AI-007363 (M. P. DeBerge), R01 HL-070816 (R. I. Enelow), and U19 AI-083024 (R. I. Enelow). The authors gratefully acknowledge the generous support of the Brody Idiopathic Pulmonary Fibrosis Research Fund and the Ira Jerome Brody '44 Memorial Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.V.R., M.P.D., J.E.D., and R.I.E. developed the concept and designed the research; C.V.R., M.P.D., and C.S.A. performed the experiments; C.V.R., M.P.D., A.K., C.S.A., and R.I.E. analyzed the data; C.V.R., M.P.D., C.S.A., J.E.D., and R.I.E. interpreted the results of the experiments; C.V.R., M.P.D., and C.S.A. prepared the figures; C.V.R. and M.P.D. drafted the manuscript; C.V.R., M.P.D., A.K., J.E.D., and R.I.E. edited and revised the manuscript; C.V.R., M.P.D., A.K., C.S.A., J.E.D., and R.I.E. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the assistance of DartLab: The Immunoassay and Flow Cytometry Shared Resource at the Geisel School of Medicine at Dartmouth University.
REFERENCES
- 1.Baumgarth N, Kelso A. In vivo blockade of γ-interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol 70: 4411–4418, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bot A, Bot S, Bona CA. Protective role of γ-interferon during the recall response to influenza virus. J Virol 72: 6637–6645, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog 8: e1002641, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBerge MP, Ely KH, Cheng GS, Enelow RI. ADAM17-mediated processing of TNF-α expressed by antiviral effector CD8+ T cells is required for severe T-cell-mediated lung injury. PLos One 8: e79340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBerge MP, Ely KH, Enelow RI. Soluble, but not transmembrane, TNF-α is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology. J Immunol 192: 5839–5851, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enelow RI, Mohammed AZ, Stoler MH, Liu AN, Young JS, Lou YH, Braciale TJ. Structural and functional consequences of alveolar cell recognition by CD8+ T lymphocytes in experimental lung disease. J Clin Invest 102: 1653–1661, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faffe DS, Flynt L, Mellema M, Moore PE, Silverman ES, Subramaniam V, Jones MR, Mizgerd JP, Whitehead T, Imrich A, Panettieri RA Jr, Shore SA. Oncostatin M causes eotaxin-1 release from airway smooth muscle: synergy with IL-4 and IL-13. J Allergy Clin Immunol 115: 514–520, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Sastre A, Durbin RK, Zheng H, Palese P, Gertner R, Levy DE, Durbin JE. The role of interferon in influenza virus tissue tropism. J Virol 72: 8550–8558, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil MP, Bohn E, O'Guin AK, Ramana CV, Levine B, Stark GR, Virgin HW, Schreiber RD. Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci USA 98: 6680–6685, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon-γ gene. J Exp Med 178: 1725–1732, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamada H, Bassity E, Flies A, Strutt TM, Garcia-Hernandez ML, McKinstry KK, Zou T, Swain SL, Dutton RW. Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J Immunol 190: 296–306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-γ-related cytokine storm in SARS patients. J Med Virol 75: 185–194, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS Jr, Bakaletz LO, Durbin RK, Flano E, Durbin JE. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J Virol 81: 9790–9800, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreutzfeldt M, Bergthaler A, Fernandez M, Bruck W, Steinbach K, Vorm M, Coras R, Blumcke I, Bonilla WV, Fleige A, Forman R, Muller W, Becher B, Misgeld T, Kerschensteiner M, Pinschewer DD, Merkler D. Neuroprotective intervention by interferon-γ blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J Exp Med 210: 2087–2103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res 67: 8494–8503, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Liu AN, Mohammed AZ, Rice WR, Fiedeldey DT, Liebermann JS, Whitsett JA, Braciale TJ, Enelow RI. Perforin-independent CD8+ T-cell-mediated cytotoxicity of alveolar epithelial cells is preferentially mediated by tumor necrosis factor-α: relative insensitivity to Fas ligand. Am J Respir Cell Mol Biol 20: 849–858, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, Alves VA, Malheiros DM, Auler JO Jr, Ferreira AF, Borsato MR, Bezerra SM, Gutierrez PS, Caldini ET, Pasqualucci CA, Dolhnikoff M, Saldiva PH. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 181: 72–79, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med 188: 223–232, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8+ T cells during infection with respiratory syncytial virus are mediated by IFN-γ. Eur J Immunol 32: 2117–2123, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-γ. J Biol Chem 279: 41679–41685, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Ramana CV, Chatterjee-Kishore M, Nguyen H, Stark GR. Complex roles of Stat1 in regulating gene expression. Oncogene 19: 2619–2627, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Ramana CV, Cheng GS, Kumar A, Kwon HJ, Enelow RI. Role of alveolar epithelial early growth response-1 (Egr-1) in CD8+ T cell-mediated lung injury. Mol Immunol 47: 623–631, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramana CV, Kumar A, Enelow R. Stat1-independent induction of SOCS-3 by interferon-γ is mediated by sustained activation of Stat3 in mouse embryonic fibroblasts. Biochem Biophys Res Commun 327: 727–733, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of interferon and gp130 receptor signalling. Semin Cell Dev Biol 19: 351–359, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Shenoy AR, Kim BH, Choi HP, Matsuzawa T, Tiwari S, MacMicking JD. Emerging themes in IFN-γ-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiology 212: 771–784, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol 178: 6191–6199, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Stow JL, Low PC, Offenhauser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology 214: 601–612, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Uetani K, Hiroi M, Meguro T, Ogawa H, Kamisako T, Ohmori Y, Erzurum SC. Influenza A virus abrogates IFN-γ response in respiratory epithelial cells by disruption of the Jak/Stat pathway. Eur J Immunol 38: 1559–1573, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Schreiber RD, Campbell IL. STAT1 deficiency unexpectedly and markedly exacerbates the pathophysiological actions of IFN-α in the central nervous system. Proc Natl Acad Sci USA 99: 16209–16214, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmire JK, Tan JT, Whitton JL. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med 201: 1053–1059, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-γ by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol 158: 119–130, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-α by antiviral CD8+ T cells. J Immunol 173: 721–725, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Zhao MQ, Stoler MH, Liu AN, Wei B, Soguero C, Hahn YS, Enelow RI. Alveolar epithelial cell chemokine expression triggered by antigen-specific cytolytic CD8+ T cell recognition. J Clin Invest 106: R49–R58, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]