Abstract
This study examines how heme biosynthesis modulation with δ-aminolevulinic acid (ALA) potentially functions to prevent 21-day hypoxia (10% oxygen)-induced pulmonary hypertension in mice and the effects of 24-h organoid culture with bovine pulmonary arteries (BPA) with the hypoxia and pulmonary hypertension mediator endothelin-1 (ET-1), with a focus on changes in superoxide and regulation of micro-RNA 204 (miR204) expression by src kinase phosphorylation of signal transducer and activator of transcription-3 (STAT3). The treatment of mice with ALA attenuated pulmonary hypertension (assessed through echo Doppler flow of the pulmonary valve, and direct measurements of right ventricular systolic pressure and right ventricular hypertrophy), increases in pulmonary arterial superoxide (detected by lucigenin), and decreases in lung miR204 and mitochondrial superoxide dismutase (SOD2) expression. ALA treatment of BPA attenuated ET-1-induced increases in mitochondrial superoxide (detected by MitoSox), STAT3 phosphorylation, and decreases in miR204 and SOD2 expression. Because ALA increases BPA protoporphyrin IX (a stimulator of guanylate cyclase) and cGMP-mediated protein kinase G (PKG) activity, the effects of the PKG activator 8-bromo-cGMP were examined and found to also attenuate the ET-1-induced increase in superoxide. ET-1 increased superoxide production and the detection of protoporphyrin IX fluorescence, suggesting oxidant conditions might impair heme biosynthesis by ferrochelatase. However, chronic hypoxia actually increased ferrochelatase activity in mouse pulmonary arteries. Thus, a reversal of factors increasing mitochondrial superoxide and oxidant effects that potentially influence remodeling signaling related to miR204 expression and perhaps iron availability needed for the biosynthesis of heme by the ferrochelatase reaction could be factors in the beneficial actions of ALA in pulmonary hypertension.
Keywords: endothelin, ferrochelatase, guanylate cyclase, micro-RNA 204, superoxide
chronic hypoxia is an important factor in the development of pulmonary hypertension in diseases such as chronic obstructive pulmonary disease (COPD), sleep apnea, and mountain sickness (10, 20). It is thought to promote pulmonary hypertension development through persistent pulmonary vasoconstriction and vascular remodeling (31). There is substantial evidence for increases in the generation of reactive oxygen species from sources including Nox oxidases and mitochondria having important roles in the development and/or progression of pulmonary hypertension caused by chronic hypoxia and other stimuli of this disease process (7, 15, 18, 27, 30). Pulmonary arterial generation of nitric oxide (NO) is normally thought to oppose this process of vasoconstriction and remodeling through promoting cGMP signaling (12). Regulation of soluble guanylate cyclase (sGC) by NO is one of the systems impaired by pulmonary hypertension as a result of processes including decreased biosynthesis of NO, increased inactivation of NO by superoxide, and oxidation of the Fe2+ heme of sGC that binds NO (11, 12, 23, 37). Oxidation of the sGC heme in pulmonary hypertension appears to enhance the pulmonary vasodilator activity of direct activators of sGC which bind its heme site (11, 37). The iron-free precursor of heme, protoporphyrin IX (PPIX), can activate sGC by directly binding its heme site (40), and treatment of pulmonary arteries with δ-aminolevulinic acid (ALA) promotes sGC activation as a result of the accumulation of PPIX (29). Because drugs such as Riociguat, which stimulate sGC, or Sildenafil, which inhibits the removal of cGMP by phosphodiesterase, are beneficial in treating pulmonary hypertension (6, 25, 37), this study examined aspects of how treatment of mice with ALA could protect against several oxidant processes associated with pulmonary hypertension development.
The src-mediated phosphorylation of signal transducer and activator of transcription-3 (STAT3) is a key cell growth-regulating pathway that participates in the pulmonary arterial smooth muscle proproliferative and anti-apoptotic remodeling seen in models of pulmonary hypertension (13, 34). Decreasing micro-RNA 204 (miR204) in pulmonary arterial smooth muscle as a result of increased STAT3 phosphorylation appears to be a major coordinating event in this regulatory process (13). Based on oxidant-promoting pulmonary hypertension mediators such as endothelin-1 (ET-1) activating src-STAT3-miR204 signaling in a manner inhibited by dehydroepiandrosterone (13, 34), an agent that has recently been shown to promote PKG activation in pulmonary arteries (33), this study focused on examining the hypothesis that PKG signaling promoted by ALA could attenuate the oxidant activation of this pathway.
Analysis of red blood cells from pulmonary hypertensive patients has recently (14) detected evidence for an impairment of iron availability for the generation of heme from PPIX by mitochondrial ferrochelatase (FECH). Pulmonary hypertension is also associated with a deficiency in the mitochondrial Mn-containing superoxide dismutase (SOD2) enzyme, suggesting increased mitochondrial matrix superoxide levels are an important factor in pulmonary hypertension (3). Because mice deficient in hematopoietic stem cell SOD2 have been shown to have decreased bone marrow FECH activity and impaired erythrocyte maturation (9), it was hypothesized that pulmonary arteries might also show evidence for inhibition of the insertion of Fe2+ heme into PPIX by FECH under conditions promoting pulmonary hypertension. Oxidant conditions present during pulmonary hypertension development could potentially favor a therapeutic benefit of ALA promoting PPIX-mediated sGC stimulation under conditions where the normal beneficial effects of sGC regulation by endogenous NO generation are lost. Because ET-1 is one of the main mediators of the effects of chronic hypoxia on the pulmonary vasculature contributing to the development of chronic hypoxia-induced pulmonary hypertension (17, 27), this study examines how modulation of pulmonary artery heme biosynthesis from ALA could be a factor in the initial effects of ET-1 on endothelium-denuded bovine pulmonary arteries under a 24-h organoid culture system and in the prolonged therapeutic effects of treatment with 50 mg·kg−1·day−1 ALA during the 21-day period of exposure to hypoxia on aspects of oxidant signaling associated with miR204 regulation and pulmonary hypertension development. Initial probing studies are also included to detect if the oxidant conditions associated with pulmonary hypertension show evidence of a disruption of vascular heme biosynthesis in pulmonary arteries that could enable PPIX accumulation.
METHODS
Conditions for exposure of mice to chronic hypoxia.
All protocols were approved by the Institutional Animal Care and Use Committee at New York Medical College. Male C57BL/6J mice (8–10 wk) were purchased from Jackson Laboratories. Mice were either exposed for 21 days to normoxic (21% O2) or hypoxic (10% O2) conditions using a hypoxic in vivo cabinet (model 30) and an oxygen controller (COY Laboratory Products) during which some of the mice were treated with 6 mM ALA (50 mg·kg−1·day−1) in the drinking water, which was changed every 2 days, when cages were cleaned. After 21 days, pulmonary arteries, lungs, and hearts were isolated from each mouse after they were anesthetized via an intraperitoneal injection of pentobarbital sodium (50 mg/kg).
Right heart catheterization.
Mice were anesthetized with a continuous isoflurane-oxygen mixture. A fluid-filled polyethylene catheter with heparinized saline was placed in the right jugular vein and advanced into the right ventricle. After reaching a heart rate of ∼500 beats/min via adjusting the flow of isoflurane to the mouse and waiting for 5 min until the recordings were stabilized, pressure measurements were then acquired over the next 5 min, and pressures representing the average of this period were recorded. Heart rate and right ventricular systolic pressure (RVSP) were recorded and analyzed using a Kent scientific transducer and a Powerlab data acquisition system.
Doppler echocardiographic measurements.
Transthoracic echocardiography was performed on the mice that were under light anesthesia, through a constant flow of isoflurane using an adaptation of previously described methods (38). A mechanical transducer centered on 30 MHz (Vevo 770; Visualsonics, Toronto, Ontario, Canada) was used to obtain an aortic B-mode image of the heart. The pulsed-wave Doppler sampler was positioned on the pulmonary valve leaflets and aligned with the direction of the flow for obtaining a pulsed-wave Doppler recording of the pulmonary blood flow. The pulmonary blood flow parameters measured included: 1) pulmonary acceleration time (PAT), the time from the onset of the pulmonary flow to peak velocity by pulsed-wave Doppler recordings; 2) ejection time (ET), the time from the onset to the end of the systolic flow; 3) the PAT-to-ET ratio, an index of pulmonary arterial pressure that corresponds inversely with the severity of the pulmonary hypertension. PAT/ET ratios are calibrated based on measurements of RVSP; and 4) velocity-time integral (VTI), the integral of the area under the captured image of Doppler flow of the pulmonary valve's velocity-time wave was calculated using a mechanical transducer centered on 30 MHz (Vevo 770; Visualsonics) for each experimental group under a constant flow of isoflurane. Heart rate was recorded by electrocardiograph electrodes located on the platform of the echocardiograph, and it was adjusted with isoflurane to a constant rate of ∼500 beats/min as described for right heart catheterization.
Detection of right ventricular hypertrophy.
Hearts were dissected to obtain the right ventricle and the left ventricle + septum, and the percent ratio of these tissues was used to detect changes in ventricular hypertrophy. While mice exposed to hypoxia had lower body weights, treatment with ALA did not appear to alter body weight in either the 21-day normoxia or hypoxia groups.
Detection of changes in superoxide by chemiluminescence.
Uniform rings of mouse pulmonary arteries and BPA were placed in plastic scintillation minivials containing 5 μM lucigenin in 1 ml of Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4). The chemiluminescence from superoxide was measured by a liquid scintillation counter (LS6000IC; Beckman Instruments, San Diego, CA) with a single active photomultiplier tube in a dark room using previously described methods (1). Background chemiluminescence in absence of the tissue was subtracted from subsequent measurements made in the presence of the pulmonary artery tissue. At the end the tissue was weighed. The counts were normalized to the weight of each tissue to give the readings in counts per minute per gram of tissue.
Western blot analysis.
Lung slices and bovine pulmonary artery segments were flash-frozen in liquid nitrogen, crushed, and homogenized in lysis buffer cocktail [50 mM Tris·HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, protease inhibitor cocktail (Sigma), and phosphatase inhibitor cocktail (Sigma)]. A modified Bradford protein assay was performed, and loading samples were prepared in electrophoresis sample buffer and separated using a 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes. The membranes were blocked for 1 h with TBS-Tween 20–5% milk and incubated overnight with the primary antibodies. Antibodies for phospho-STAT3 and STAT3 (79 kDa) were obtained from Cell Signaling. Antibodies for SOD2 (25 kDa) were obtained from Abcam. The β-actin (42 kDa) antibody was obtained from Sigma Chemicals. Membranes were exposed to a secondary horseradish peroxidase-linked antibody and stained using materials in an ECL kit (Amersham). The membranes were exposed to X-OMAT autoradiography paper (Kodak). Protein loading was normalized to β-actin. Bands were analyzed by UN-SCAN-IT gel 6.1 software.
Quantitative real-time PCR measurements of miR204.
Lung slices and bovine pulmonary artery segments were flash-frozen in liquid nitrogen and crushed, and total RNA was extracted and quantified for reverse transcription. Reverse transcription and real-time PCR were performed using a miScript Starter kit (Qiagen). The results measuring miR204 were normalized to a primer that targets snRNA RNU6B (RNU6-2) (13).
Organoid culture.
Organoid cultures were performed for inducing ET-1 signaling cascade in BPA by exposing them to 10 nM ET-1 under conditions previously described for studies using ALA to modulate sGC (29). Branches of BPA were isolated from fresh slaughterhouse-derived calf lungs transported in chilled buffered saline. The second- and third-order branches were isolated and de-endothelialized by gentle rubbing. BPA rings were cut at a width of 2–3 mm. These rings were incubated for 24 h at 37°C in DMEM containing 10% fetal bovine serum, antibiotics (penicillin, streptomycin, and fungizone) in 5% carbon dioxide in air. The BPA rings were incubated in the following drugs: ALA (100 μM), 8-bromo-cGMP (100 μM), and gp91ds-tat (50 μM) in the presence or absence of ET-1 (10 nM), as indicated in results. After the incubation period, the rings were flash-frozen in liquid nitrogen and stored in a −80°C freezer to be processed for Western blot analysis, quantitative real-time PCR (qRT-PCR), and chemiluminescence measurements of superoxide.
Detection of changes in PPIX.
Changes in endogenous PPIX in BPA segments were measured using a BIOTEK fluorescent microplate reader (model FLx800i), as previously described (29). BPA segments of approximately equal sizes ∼4 mm diameter and length were placed at the bottom of ∼6-mm-diameter wells of a 96-well microplate with 200 μl of HEPES-buffered Krebs solution (pH 7.4). The fluorescence was measured from the bottom surface of the plate. The excitation wavelength of 528 ± 20 nm and emission wavelength of 620 ± 40 nm were employed to detect alterations in PPIX in organ-cultured BPA segments. Data were reported in arbitrary fluorescence units measured, after subtraction of the low levels of background fluorescence observed in the absence of BPA segments.
Ferrochelatase activity assay.
Pulmonary arteries from approximately two mice were pulverized in liquid nitrogen, incubated on ice for 45 min with 50 μl of buffer (0.25 M Tris·HCl buffer, pH 8.2, containing 1% Triton X-100 and 1.75 mM of palmitic acid), and then sonicated on ice for 10 s. After centrifugation, the supernatant was assayed for protein content by the Bradford method, and protein assay was performed. The amount of FECH activity present after 60 min incubation was assayed by the accumulation of Zn-PPIX from 67 μM PPIX and 42 μM zinc acetate, using 20 μg protein in a final volume of 30 μl, as previously described (39). A dimethyl sulfoxide-methanol (30:70) solution was used to stop the FECH reaction. HPLC measurement of the amount of ZnPPIX formation was used to determine the ferrochelatase activity in each sample. Before the start of the experiment, increasing concentrations of ZnPPIX were loaded into the column to generate a standard curve for ZnPPIX. We employed an Agilent 1100 HPLC system using a normal-phase Phenomenex column (Luna 5μ Silica-2 100A, 250 × 4.6 mm) with acetone-methanol-water-formic acid (560:240:200:2) and 1 ml/min, as the mobile phase. ZnPPIX was detected based on the amount of fluorescence observed employing excitation and emission wavelengths of 415 and 580 nm, respectively.
Detection of changes in mitochondrial and extramitochondrial superoxide.
HPLC measurement of the superoxide-specific hydroxylated products of MitoSox and dihydroethidium were employed for quantifying changes in mitochondrial matrix and extramitochondrial matrix superoxide, using previously described methods (41). Before the start of the experiment, increasing concentrations of Mito-2-hydroxyethidium or 2-hydroxyethidium were loaded into the column to generate a standard curve. Tissues were incubated with 5 μM MitoSox or dihydroethidium, respectively, for 1 h in the dark (19) under conditions described in results, to measure mitochondrial and extramitochondrial superoxide, respectively. They were washed several times with Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4) and then frozen with liquid nitrogen. Tissues were pulverized in the presence of liquid nitrogen, dissolved in a solution of 1:1 acetonitrile-water (HPLC grade). After sonication and centrifugation, the supernatant was used for HPLC analysis of the superoxide-specific hydroxylated product of MitoSox (Mito-2-hydroxyethidium) or of dihydroethidine (2-hydroxyethidium) using an HPLC system with a Jasco FP-1520 fluorescence detector and a Beckman ultrasphere reverse column (C18) (5μ, 250 × 4.6 mm), and pellets obtained from centrifugation were used for a Bradford protein assay.
Statistical analysis.
Student's t-tests were used to assess significance of changes in experimental groups examined compared with the control for that group. ANOVA using Bonferroni's post hoc tests are used to determine significance between multiple experimental groups. Values were represented as means ± SE, and P < 0.05 was used to determine statistical significance.
RESULTS
Effects of 21-day ALA treatment on chronic hypoxia-induced pulmonary hypertension as measured by right heart catheterization.
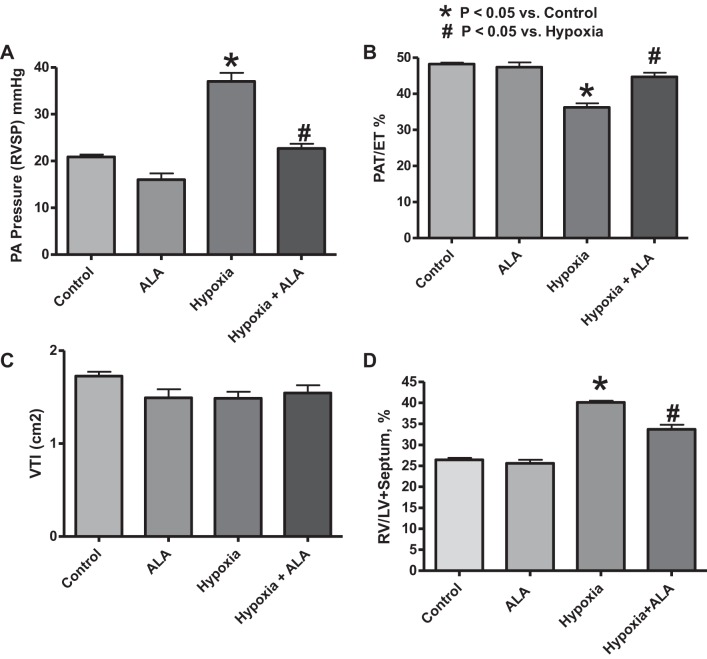
To measure the effect of ALA treatment on an indicator of pulmonary arterial pressure (PAP) under both normoxic and chronic hypoxic conditions, RVSP was determined through right heart catheterization (Fig. 1A) under conditions where changes in heart rate were minimized by adjusting the delivery of isoflurane to keep the heart rate at ∼500 beats/min. Twenty one days of exposure to 10% oxygen caused the increase in the RVSP, indicating the detection of increased pulmonary arterial pressure potentially caused by elevated pulmonary vascular resistance. ALA treatment attenuated the effects of chronic hypoxia on pulmonary arterial pressure, demonstrating a protective role played by ALA in attenuating the pathogenesis of chronic hypoxia-induced pulmonary hypertension. ALA may also have a modest lowering effect on RVSP in the control mice exposed to normoxia for 21 days, because this effect was significant when analyzed by a Student's t-test.
Fig. 1.
Effects of treating mice with δ-aminolevulinic acid (ALA) for 21 days in the absence and presence of hypoxia on indicators of chronic hypoxia-induced pulmonary hypertension. A: right ventricle systolic pressure (RVSP) measured through right heart catheterization at the end of 21 days of treatment (n ≥ 5/group). B: pulmonary arterial pressure (PAP) monitored at the end of the 21-day treatment through assessing the pulmonary valve Doppler flow time intervals [pulmonary acceleration time/%ejection time (PAT/%ET)]. Note that a decrease in PAT/%ET is consistent with an increase in PAP (n = 10). C: velocity-time integral (VTI) of the area under the measured pulmonary artery Doppler flow. D: right ventricle normalized to left ventricle (including septum) reported as %weight ratios (n = 12). *P < 0.05 vs. control and #P < 0.05 vs. hypoxia (ANOVA). Note that the modest effect of ALA on lowering RVSP in the normoxic control mice was significant by a t-test analysis.
Effects of ALA treatment on chronic hypoxia-induced pulmonary hypertension measured by Doppler flow echocardiography.
To measure the effect of ALA treatment on the pulmonary arterial pressure under both normoxic and chronic hypoxic conditions, pulmonary valve Doppler flow acceleration time (PAT) was measured as a function of the ET at the end of the treatment (Fig. 1B), under conditions similar to those described for right heart catheterization, where heart rate was maintained constant at ∼500 beats/min. Twenty one days of exposure to 10% oxygen caused the lowering of the PAT/ET ratio, indicating increased pulmonary arterial pressure caused by elevated pulmonary vascular resistance (38). ALA treatment during the 21-day period of exposure to hypoxia attenuated the effects of chronic hypoxia on pulmonary arterial pressure (Fig. 1B). In contrast to the direct measurements of RVSP, evaluation of PAT/ET ratios did not detect any effect of ALA in the control mice exposed to normoxia for 21 days. The absence of changes in VTI (Fig. 1C) and maintenance of relatively constant heart rates across experimental groups suggest that cardiac output may have been similar under all the conditions examined, which is consistent with changes in the PAT/ET ratio (or PAP) potentially originating primarily from changes in pulmonary vascular resistance.
Effects of ALA treatment on chronic hypoxia-induced right ventricle hypertrophy.
To assess the effects of ALA treatment on right ventricular hypertrophy, right ventricle weights were normalized to left ventricle weight (including septum) as percent ratios. A significant increase in right ventricle mass in the 21-day hypoxia group was observed, which was attenuated in the 21-day hypoxia-treated with ALA group, indicating that the ALA treatment showed an antihypertrophic effect under chronic hypoxia (Fig. 1D).
Effects of 21-day treatment with ALA on the detection of superoxide by lucigenin in pulmonary arteries isolated from mice exposed to chronic hypoxia.
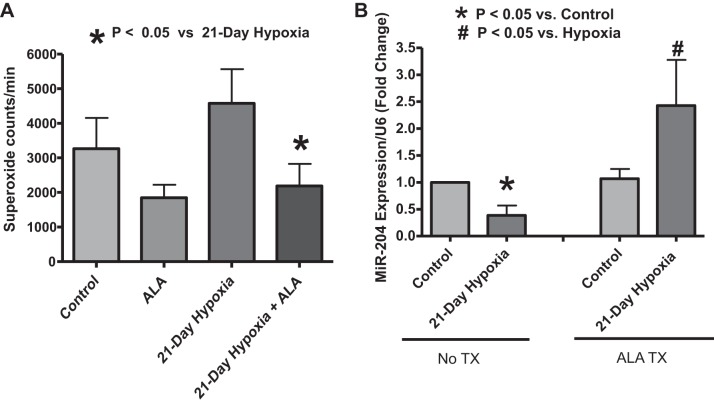
To assess the effect of long-term ALA treatment on pulmonary artery superoxide generation, segments of the intralobar pulmonary artery of mice from the four experimental groups (21-day normoxia control, ALA treated, 21-day hypoxia exposed, and 21-day hypoxia exposed and ALA treated) were subjected to measurements of the chemiluminescence detected in the presence of 5 μM lucigenin. The ALA treatment appeared to be decreasing the detection of superoxide compared with the untreated control in pulmonary arteries from the 21-day normoxia-exposed mice, but this change was not statistically significant. Pulmonary artery segments from mice exposed to 21-day hypoxia showed an apparent elevation in superoxide production, and 21-day ALA treatment significantly decreased the detection of superoxide in pulmonary artery segments isolated from the mice exposed to hypoxia. Thus, ALA has a distinct lowering effect on levels of superoxide detected in pulmonary arteries obtained from mice exposed to 21 days of hypoxia (Fig. 2A).
Fig. 2.
Effects of treating mice with ALA for 21 days in the absence and presence of chronic hypoxia on superoxide generation in isolated pulmonary arteries detected by 5 μM lucigenin chemiluminescence (A) and lung micro-RNA 204 (miR204) expression as measured through quantitative real-time polymerase chain reaction (qRT-PCR) (B). *P < 0.05 vs. hypoxia (t-test, n = 5) (A). *P < 0.05 vs. normoxia control and #P < 0.05 vs. hypoxia (t-test, n = 5) (B).
Effects of 21-day treatment of mice with ALA on the decrease in lung miR204 expression caused by exposure to chronic hypoxia.
To assess the role of ALA treatment on the expression of the micro-RNA (miR204), qRT-PCR was used to measure its expression in mice lung lysates. These measurements were made in lung tissue because it has previously been demonstrated that changes in lung miR204 levels originate from its downregulation in the pulmonary arteries associated with STAT3 regulation of vascular remodeling (13). ALA treatment prevented the drop in miR204 caused by chronic hypoxia (Fig. 2B). Thus, ALA can potentially have an antimitogenic role in pulmonary vasculature by modulating miR204 expression.
Effects of treatments of BPA with combinations of ET-1 and ALA after 24-h organ culture on the detection of superoxide by lucigenin.
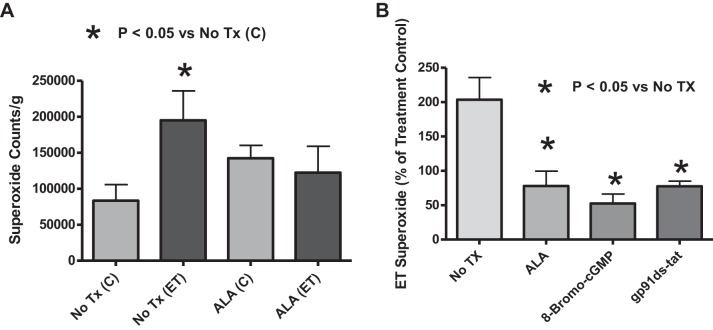
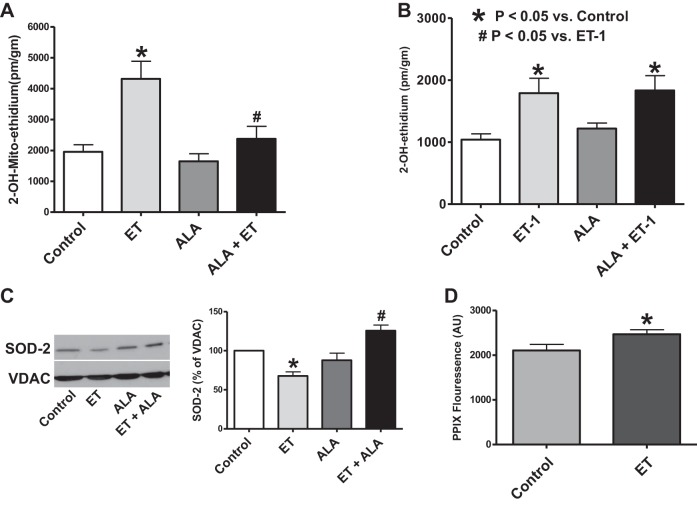
The data in Fig. 3A demonstrate that ET-1 increased superoxide detected by 5 μM lucigenin in endothelium-denuded organoid-cultured BPA compared with BPA organoid cultured for 24 h in the absence of ET-1. BPA segments treated with ALA under organoid-cultured conditions showed an attenuation of the ET-1-mediated generation of superoxide (Fig. 3A).
Fig. 3.
Effects of ALA, an activator of protein kinase G (PKG) and an inhibitor of NADPH oxidase-2 (Nox2), on endothelin (ET)-1-induced increases in superoxide production in 24-h organoid-cultured endothelium-removed bovine pulmonary artery (BPA) segments. A: effects of the absence and presence of ALA during organoid culture on superoxide measured through the chemiluminescence of 5 μM lucigenin (counts/g) in the presence and absence of 10 nM ET (n = 10). B: comparison of the influence of ALA, 8-bromo-cGMP, and gp91ds-tat in 24-h organoid culture on lucigenin-detectible superoxide in the presence of ET, reported as a percent of the non-ET-treated BPA ring for each experimental group of BPA segments that are nontreated, ALA treated, 8-bromo-cGMP treated, and gp91ds-tat treated for 24 h in organoid culture (n = 7).
Effects of the presence of ALA, 8-bromo-cGMP, and gp91ds-tat during 24-h organoid culture of BPA on the increase in superoxide caused by ET-1.
Detection of changes in chemiluminescence of 5 μM lucigenin was used to compare the role of ALA treatment of BPA segments with that of 8-bromo-cGMP (a direct PKG activator), and gp91ds-tat (an NADPH oxidase-2 or NOX2 inhibitor) on ET-1-induced generation of superoxide, in HEPES-buffered Krebs buffer solution (pH = 7.4). Changes in superoxide were analyzed based on ET-1-exposed superoxide (counts/g) as a percent of the control for the same treatment (nontreated, ALA treated, 8-bromo-cGMP treated, and gp91ds-tat treated). The direct PKG activator, 8-bromo-cGMP, showed a decrease in ET-1-induced superoxide generation that was similar to ALA treatment, suggesting PKG signaling can attenuate the stimulation of superoxide production by ET-1. gp91ds-tat, an inhibitor of NADPH oxidase-2 (Nox2), also showed a similar inhibition of the superoxide-stimulating effects of ET-1, suggesting Nox2 has a key role in the ET-1 stimulation of superoxide generation (Fig. 3B).
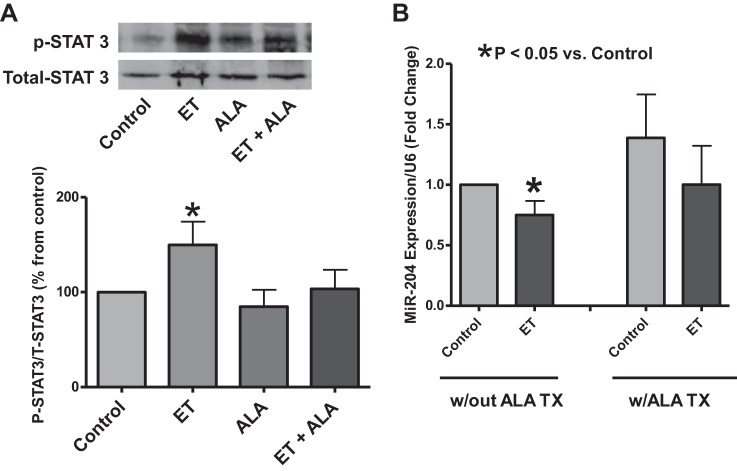
Effects of the presence of ALA during 24-h organoid culture of BPA on the increase in STAT3 phosphorylation caused by ET-1.
Western blot analysis of the phosphorylation of STAT3 at the tyrosine-705 residue was used to assess the effects of ALA on activation of STAT3 by ET-1 in the endothelium-denuded BPA segments. ET-1 increased STAT3 phosphorylation in the 24-h-cultured BPA rings. This was attenuated by ALA treatment, suggesting that ALA may have an inhibitory effect on vascular remodeling promoted through by STAT3 activation (Fig. 4A).
Fig. 4.
Effects of ALA on ET-1-induced increases in phosphorylation of signal transducer and activator of transcription (STAT-3) and decreases in miR204 expression observed in 24-h organoid-cultured BPA segments. A: STAT3 activation as measured through the phosphorylation of tyrosine-705 residue (79 kDa) via Western blot analysis of the endothelium-removed BPA segments in both the nontreated (Control) and ALA-treated segments with the presence or absence of 10 nM ET-1 in 24-h organoid culture (n = 6, ANOVA). B: miR204 expression measured through qRT-PCR in endothelium-removed BPA 24-h organoid cultured with or without the presence of ET-1 in the absence and presence of exposure to ALA (n = 8, t-test). *P < 0.05 vs. Control.
Effects of the presence of ALA during 24-h organoid culture of BPA on the decrease in miR204 expression caused by ET-1.
The expression of miR204 detected by qRT-PCR in 24-h organ-cultured endothelium-denuded BPA was decreased by the presence of ET-1 (Fig. 4B). This effect of ET-1 was not observed in BPA organoid cultured in the presence of ALA, suggesting that that ALA treatment in 24-h organ culture attenuated the ET-1-mediated decrease in miR204 expression.
Effects of organoid culture of BPA with ET-1 mitochondrial and extramitochondrial superoxide, and SOD2 expression in the absence and presence of ALA.
To evaluate the source of ET-1-induced superoxide generation, BPA segments were organ cultured for 24 h with 10 nM ET-1 and then exposed to 5 μM of MitoSox or dihydroethidine to detect changes in mitochondrial matrix or extramitochondrial matrix superoxide levels. The superoxide-specific hydroxylated products of MitoSox or dihydroethidine were then extracted from BPA and quantified by HPLC, normalized to BPA protein. The data in Fig. 5A indicate that ET-1 can cause an increase in mitochondrial matrix superoxide, and this ET-1-elicited increase does not appear to occur in BPA exposed to ALA under the 24-h organoid-cultured conditions examined. Based on the data in Fig. 5B, ET-1 also increases superoxide in the extramitochondrial regions. In contrast, cotreatment with ALA did not appear to alter basal or ET-1-elevated levels of extramitochondrial superoxide. Organoid culture of BPA with ET-1 for 24 h also decreased the expression of SOD2, and the presence of ALA during exposure to ET-1 prevented this decrease in SOD2 expression (Fig. 5C). Thus, whereas ET-1 increases superoxide in both the extramitochondrial and mitochondrial matrix regions, ALA appears to only have a prominent effect in attenuating mitochondrial matrix superoxide under conditions where it also prevents the ET-1-elicited decrease in SOD2.
Fig. 5.
Effects of ALA on ET-1-elicited increases on mitochondrial (A) and extramitochondrial (B) superoxide and decreases in mitochondrial superoxide dismutase (SOD) 2 (C) in 24-h organoid-cultured BPA. D: effects of ET on protoporphyrin (PPIX) accumulation in 24-h organoid-cultured BPA segments. Changes in BPA mitochondrial (n = 10, A) and extramitochondrial (n = 8, B) superoxide generation were quantified in BPA segments using HPLC measurements of the superoxide-specific oxidation product of 5 μM MitoSox and dihydroethidine, respectively, that accumulated during a 1-h incubation after a 24-h organoid culture with 10 nM ET-1 (*P < 0.05 vs. control; #P < 0.05 vs. ET). C: changes in lung SOD2 (25 kDa) expression were detected by Western analysis (n = 5) after 24-h organoid culture of BPA with or without 10 nM ET in the presence and absence of ALA (*P < 0.05 vs. control; #P < 0.05 vs. ET). D: BPA segments were exposed to 24-h organ culture without or with 10 nM ET-1, after which they were subjected to surface fluorescence measurement of PPIX (n = 10). *P < 0.05 vs. Control.
Effects of organoid culture of BPA with ET-1 on the detection of increases in PPIX fluorescence.
Because ET-1 increased mitochondrial matrix superoxide, measurements were made to examine if this was associated with an increase in PPIX accumulation detected by an increase in its surface fluorescence from BPA (29). The data in Fig. 5D indicate that BPA segments exposed to 24-h organ culture with ET-1 showed a small, but significant, increase in fluorescence in the spectral region used to detect changes in PPIX, suggesting that ET-1 might impair the function of FECH.
Effects of chronic hypoxia in the absence and presence of treatment with ALA on mouse lung mitochondrial matrix SOD2 expression.
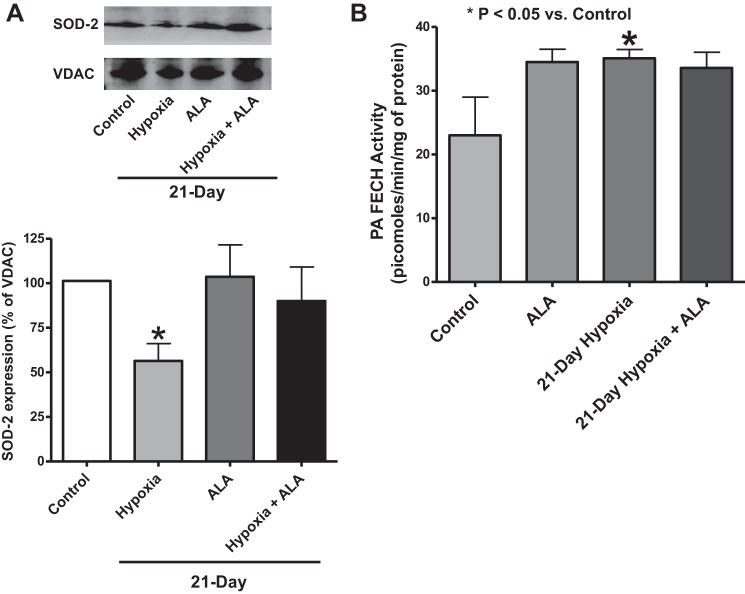
The effects of chronic hypoxia with and without ALA treatment on SOD2 expression were studied by Western blot analysis of lung tissue lysates obtained from each experimental group (control, ALA treated, 21-day hypoxia, 21-day hypoxia + ALA). SOD2 expression was lowered in mice exposed to 21 days of chronic hypoxia exposure. Mice treated with ALA during this 21-day period did not show a detectible decrease in SOD2 (Fig. 6A). Thus, ALA treatment appears to prevent the decrease in SOD2 expression caused by chronic hypoxia.
Fig. 6.
Effects of treating mice with ALA for 21 days in the absence and presence of hypoxia on measurements of changes in mouse lung mitochondrial SOD2 expression (A) and pulmonary artery ferrochelatase activity (B). Changes in lung SOD2 (25 kDa) expression were detected by Western analysis (n = 7) (A), and ferrochelatase (FECH) activity in homogenates of isolated pulmonary arteries was detected by the conversion of PPIX to ZnPPIX, followed by HPLC analysis (n = 3–5/group from 5–8 mice) (B). *P < 0.05 vs. control (t-test).
Effects of chronic hypoxia in the absence and presence of treatment with ALA on pulmonary artery FECH activity.
To examine the effects of chronic hypoxia exposure and ALA on FECH activity, pulmonary arteries were isolated from mice exposed to the four experimental conditions studied, and these tissues were used for measurements of FECH activity, by a subsequent HPLC analysis. Chronic hypoxia was observed to cause a significant increase in FECH activity that was not observed in the presence of treatment with ALA (Fig. 6B).
DISCUSSION
This study identified protective properties originating from the modulation of heme biosynthesis through ALA administration in attenuating the progression of chronic hypoxia-induced pulmonary hypertension in vivo. These protective effects were demonstrated through detection of evidence for an attenuated pulmonary arterial pressure increase detected by both RVSP and echocardiography Doppler flow analysis across the pulmonary valve, and by a decrease in right ventricle hypertrophy. Although an oral or intravenous dose of 60 mg/kg ALA has been observed to cause an initial lowering of pulmonary arterial systolic pressure in humans with congestive heart failure for several hours (21), providing 50 mg·kg−1·day−1 ALA in drinking water did not appear to have a major prolonged lowering effect on indicators of pulmonary artery pressure under normoxia conditions (Fig. 1, A and B) or to alter the hypoxic pulmonary vasoconstriction response seen in vivo or in pulmonary arteries isolated from mice treated with ALA (32). The ALA treatment also decreased superoxide in pulmonary arteries isolated from mice exposed to chronic hypoxia, and it prevented detection of a loss of miR204 caused by chronic hypoxia.
The 24-h organoid culture model of ET-1-promoting STAT3 phosphorylation-associated decreases in miR204 employed in this study provided evidence that ALA could be attenuating remodeling signaling processes associated with pulmonary hypertension development. Organ culture of BPA with ET-1 with or without ALA showed that ALA was able to lower ET-1-induced elevation in superoxide that was detected by lucigenin chemiluminescence. In addition, a known PKG activator (8-bromo-cGMP) and a NADPH oxidase-2 inhibitor (gp91ds-tat) also lowered sources of superoxide detected by lucigenin. ALA was able to lower the ET-1-induced rise in STAT3 activation in 24-h organ-cultured BPA segments and to prevent the ET-1-elicited lowering of miR204. The organoid culture conditions used for studying ALA were selected based on a previous study optimizing the accumulation of PPIX and its activation of sGC based on vasodilator-stimulated phosphoprotein phosphorylation by PKG (29). In this previous study, it was demonstrated that the ALA treatment decreased contraction to serotonin (29), a mediator that has been shown to promote a superoxide-mediated increased level of contraction in pulmonary arteries isolated from mice exposed to 21 days of hypoxia (27). Thus, ALA may function through a cGMP-mediated PKG activation mechanism that attenuates ET-1-elicited stimulation of superoxide generation, potentially contributing to activating src-mediated phosphorylation of STAT3.
Studies examining the sources of superoxide stimulated by ET-1 using more quantitative HPLC methods detected evidence for increases in superoxide originating from both mitochondria and extramitochondrial sources. Although these individual sources of superoxide may activate each other (16, 36), evidence for this type of an interaction was not readily detected because ALA showed evidence of major differences in its effects on these subcellular sources of superoxide in the ET-1 organoid culture BPA model studied. Whereas ALA appeared to eliminate the increase in mitochondrial matrix superoxide detected with MitoSox, it did not have a detectible inhibitory effect on the extramitochondrial matrix sources of superoxide detected by DHE. In addition, ALA prevented the depletion of the mitochondrial matrix scavenger of superoxide, SOD-2. Thus, based on these in vitro studies in BPA, ALA may have a more dominant effect on inhibiting increases in mitochondrial superoxide.
The detection by MitoSox of increased mitochondrial superoxide and increased PPIX fluorescence from BPA organoid cultured with ET-1 suggested that these conditions could promote an inhibition of FECH activity by a process such as superoxide disrupting its iron-sulfur center (9). The loss of mitochondrial SOD2 expression in chronic hypoxia (Fig. 6A) and in other models of pulmonary hypertension (3) suggested an inhibition of FECH activity might be detected in pulmonary arteries isolated from animals with pulmonary hypertension because mice with a genetic deficiency in SOD2 show evidence of a superoxide-mediated inhibition of FECH activity (9). However, investigation of the effects of chronic hypoxia in mice on pulmonary arteries isolated from these animals detected an actual increase in FECH activity that was not seen in pulmonary arteries from mice treated with ALA together with hypoxia (Fig. 6B). These observations are consistent with other studies showing that, under hypoxia, hypoxia-inducible factor-1α (HIF-1α) binding to hypoxia response elements in the ferrochelatase gene promoter is likely to be upregulating FECH expression and activity (28, 35). The rate-limiting step in heme biosynthesis, δ-aminolevulinic acid synthase, has also been reported to be upregulated under hypoxia in erythroid cells, but this increase does not appear to be mediated by HIF-1α (22). We hypothesize that this overall induction of the heme biosynthesis pathway by hypoxia could be part of an adaptation mechanism in which the pulmonary vasculature responds to hypoxia-induced stress by upregulating the heme biosynthesis pathway to meet the heme requirements for the assembly of hemo-proteins, including cytochrome c oxidase (complex IV in the mitochondrial electron transport system) and perhaps sGC. However, oxidant conditions may have a more dominant effect on decreasing the availability of Fe2+ for heme biosynthesis from PPIX by FECH. The evidence for a systemic iron deficiency in pulmonary hypertension associated with iron availability limitations in red blood cell heme biosynthesis (14) supports the possibility of a similar deficiency in the pulmonary vasculature. Thus, alterations in vascular heme biosynthesis could be an important factor in influencing the progression of pulmonary hypertension development.
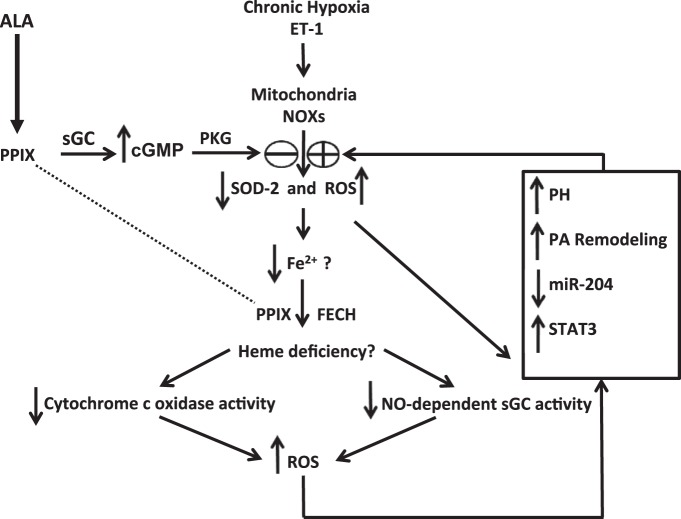
ALA can be acting to lower mitochondrial superoxide in a manner that modulates both the biosynthesis of heme and its influence on the progression of pulmonary hypertension in a number of ways. Initially, ALA may function via increasing cGMP as a pulmonary vasodilator (21, 29), which also attenuates increases in superoxide caused by ET-1 (Fig. 3A) and perhaps other pulmonary hypertension mediators. ALA also prevents the depletion of mitochondrial matrix-localized SOD2 (Figs. 5C and 6A), which appears to normally be downregulated by epigenetic mechanisms in pulmonary hypertension (3). By providing the limiting biosynthetic precursor for heme biosynthesis, treatment with ALA will increase the availability of PPIX for heme generation by FECH. This could provide heme needed for the maintenance of mitochondrial complex IV (cytochrome c oxidase) (4), a component of the electron transport chain reported to be depleted in pulmonary hypertension and associated with increased mitochondrial superoxide generation (7). Restoring heme in complex IV could prevent the decrease in its activity, thus attenuating a process that could normally increase mitochondrial superoxide production (5). Under oxidative stress, ferrous iron is prone to oxidation to the ferric state, leading to ferrous iron deficiency, and, ultimately, a heme deficiency (24). Thus, as ALA lowers the oxidant effects of increased mitochondrial superoxide production, it may further restore heme biosynthesis and reverse the effects of any deficiency in heme. Increased mitochondrial superoxide elevation is believed to be one of the triggers for vascular remodeling that takes place in chronic hypoxia-induced pulmonary hypertension (8). Whereas ALA provides PPIX used for the production of heme needed for NO stimulation of sGC, it will also increase PPIX to levels that can also elevate cGMP and activate PKG (29, 32), and this mechanism may be favored when heme biosynthesis by FECH is disrupted. Both of these processes stimulating PKG could function during prolonged treatment with ALA to attenuate the persistent vasoconstriction and vascular remodeling elicited by chronic exposure to hypoxia and perhaps other factors promoting pulmonary hypertension through processes hypothesized in the model shown in Fig. 7.
Fig. 7.
Hypothesized processes potentially contributing to the ability of ALA to attenuate the development of hypoxia-induced pulmonary hypertension. The model begins with the initial effects of ALA on attenuating increases in mitochondrial superoxide and decreases in SOD2, which could potentially occur through PPIX stimulating cGMP signaling and by decreased mitochondrial superoxide. Decreased mitochondrial reactive oxygen species (ROS) could then help restore the biosynthesis of heme. A combination of decreased ROS and increased cGMP generation by soluble guanylate cyclase (sGC) could function to prevent increased vascular reactivity and pulmonary arterial smooth muscle remodeling processes that contribute to the progression of pulmonary hypertension development.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-031069, P01-HL-043023, R01-HL-066331, and R01-HL-115124.
DISCLOSURES
Michael S Wolin is the inventor on a patent held by New York Medical College for promoting protoporphyrin IX generation as a therapy for improving smooth muscle relaxation.
AUTHOR CONTRIBUTIONS
Author contributions: R.A., D.S., and M.S.W. conception and design of research; R.A., D.P., M.R.K., and D.S. performed experiments; R.A., M.R.K., D.S., and M.S.W. analyzed data; R.A., D.S., and M.S.W. interpreted results of experiments; R.A., D.P., and M.S.W. prepared figures; R.A., D.S., and M.S.W. drafted manuscript; R.A., D.P., M.R.K., D.S., and M.S.W. edited and revised manuscript; R.A., D.P., M.R.K., D.S., and M.S.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the assistance of Ghezal Froogh in conducting some of the measurements of superoxide by HPLC.
Portions of this study were presented at the 2013 Experimental Biology Meeting in Boston, MA (2).
REFERENCES
- 1.Ahmad M, Zhao X, Kelly MR, Kandhi S, Perez O, Abraham NG, Wolin MS. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecSOD. Am J Physiol Heart Circ Physiol 297: H1453–H1461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhawaj R, Wolin MS. Aminolevulinic acid attenuates increases in pulmonary arterial superoxide elicited by organoid culture with endothelin-1 and exposure of mice to chronic hypoxia through mechanisms potentially involving protoporphyrin IX-elicited stimulation of soluble guanylate cyclase and cGMP protein kinase signaling (Abstract). FASEB J 27: lb701, 2013. [Google Scholar]
- 3.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 121: 2661–2671, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atamna H, Liu J, Ames BN. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts. Relevance to aging. J Biol Chem 276: 48410–48416, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bansal S, Biswas G, Avadhani NG. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol 2: 273–283, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, Sastry BKS, Pulido T, Layton GR, Serdarevic-Pehar M, Wessel DL. Randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 125: 324–334, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 104: 11418–11423, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Case AJ, Madsen JM, Motto DG, Meyerholz DK, Domann FE. Manganese superoxide dismutase depletion in murine hematopoietic stem cells perturbs iron homeostasis, globin switching, and epigenetic control in erythrocyte precursor cells. Free Rad Biol Med 56: 17–27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 32: 1371–1385, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Chester M, Seedorf G, Tourneux P, Gien J, Tseng N, Grover T, Wright J, Stasch JP, Abman SH. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatalpulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 301: L755–L764, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol 27: 1877–1885, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decker I, Ghosh S, Comhair SA, Farha S, Tang WH, Park M, Wang S, Lichtin AE, Erzurum SC. High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci 4: 253–258, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res 2: 90–101, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frazziano G, Champion HC, Pagano PJ. NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. Am J Physiol Heart Circ Physiol 302: H2166–H2177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Q, Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 295: H978–H989, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupte SA, Wolin MS. Relationships between vascular oxygen sensing mechanisms and hypertensive disease processes. Hypertension 60: 269–275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman MA, Webber J, Fromm D, Kessel D. Hemodynamic effects of 5-aminolevulinic acid in humans. J Photochem Photobiol B 43: 61–65, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Hofer T, Wenger RH, Kramer MF, Ferreira GC, Gassmann M. Hypoxic up-regulation of erythroid 5-aminolevulinate synthase. Blood 101: 348–350, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Jernigan NL, Resta TC. Chronic hypoxia attenuates cGMP-dependent pulmonary vasodilation. Am J Physiol Lung Cell Mol Physiol 282: L1366–L1375, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Rad Biol Med 65: 1174–1194, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Lasker GF, Maley JH, Pankey EA, Kadowitz PJ. Targeting soluble guanylate cyclase for the treatment of pulmonary hypertension. Expert Rev Respir Med 5: 153–161, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol 77: 1451–1459, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Liu YL, Ang SO, Weigent DA, Prchal JT, Bloomer JR. Regulation of ferrochelatase gene expression by hypoxia. Life Sci 75: 2035–2043, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Mingone CJ, Gupte SA, Chow JL, Ahmad M, Abraham NG, Wolin MS. Protoporphyrin IX generation from delta-aminolevulinic acid elicits pulmonary artery relaxation and soluble guanylate cyclase activation. Am J Physiol Lung Cell Mol Physiol 291: L337–L344, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel D, Alhawaj R, Wolin MS. Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide. Am J Physiol Regul Integr Comp Physiol 307: R426–R433, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am J Physiol Lung Cell Mol Physiol 306: L383–L391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in Pulmonary Arterial Hypertension. Am J Physiol Heart Circ Physiol 301: H1798–H1809, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Qiao A, Khechaduri A, Kannan Mutharasan R, Wu R, Nagpal V, Ardehali H. MicroRNA-210 decreases heme levels by targeting ferrochelatase in cardiomyocytes (Abstract). J Am Heart Assoc 2: e000121, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, Halpern EF, Bloch KD, Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging 3: 157–163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Hillegersberg R, Van den Berg JW, Kort WJ, Terpstra OT, Wilson JH. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology 103: 647–651, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Wolin MS, Wood KS, Ignarro LJ. Guanylate cyclase from bovine lung. A kinetic analysis of the regulation of the purified soluble enzyme by protoporphyrin IX, heme, and nitrosyl-heme. J Biol Chem 257: 13312–13320, 1982. [PubMed] [Google Scholar]
- 41.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Rad Biol Med 46: 329–338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]