Abstract
Acclimatization to high-altitude, long-term hypoxia (LTH) reportedly alters cerebral artery contraction-relaxation responses associated with changes in K+ channel activity. We hypothesized that to maintain oxygenation during LTH, basilar arteries (BA) in the ovine adult and near-term fetus would show increased large-conductance Ca2+ activated potassium (BK) channel activity. We measured BK channel activity, expression, and cell surface distribution by use of patch-clamp electrophysiology, flow cytometry, and confocal microscopy, respectively, in myocytes from normoxic control and LTH adult and near-term fetus BA. Electrophysiological data showed that BK channels in LTH myocytes exhibited 1) lowered Ca2+ set points, 2) left-shifted activation voltages, and 3) longer dwell times. BK channels in LTH myocytes also appeared to be more dephosphorylated. These differences collectively make LTH BK channels more sensitive to activation. Studies using flow cytometry showed that the LTH fetus exhibited increased BK β1 subunit surface expression. In addition, in both fetal groups confocal microscopy revealed increased BK channel clustering and colocalization to myocyte lipid rafts. We conclude that increased BK channel activity in LTH BA occurred in association with increased channel affinity for Ca2+ and left-shifted voltage activation. Increased cerebrovascular BK channel activity may be a mechanism by which LTH adult and near-term fetal sheep can acclimatize to long-term high altitude hypoxia. Our findings suggest that increasing BK channel activity in cerebral myocytes may be a therapeutic target to ameliorate the adverse effects of high altitude in adults or of intrauterine hypoxia in the fetus.
Keywords: development, Ca2+ signaling, high altitude
the human brain represents 2% of total body weight, yet, for its proper function, requires 15% of cardiac output and consumes 20% of total oxygen (O2) and 25% of total glucose. Because of its high metabolic activity and O2 requirement, the brain is particularly vulnerable to hypoxia. During hypoxia, autoregulation of cerebral blood flow (CBF) is a crucial mechanism for meeting the metabolic needs of the brain. In response to acute, short-term hypoxia, the cerebral vasculature of adults dilates to increase CBF (22). This response, however, may develop into cerebral edema and other central neurological disorders. For example, exposure to high altitude increases CBF and can lead to mountain sickness (24, 64) and high altitude cerebral edema (16). A similar increase in CBF occurs in the ovine near-term fetus in response to short-term hypoxia (29). When subjected to hypoxia, infants in utero exhibit increased CBF, which can cause intraventricular and germinal matrix hemorrhage (15). Such complications result in neurologic sequelae such as cerebral palsy, mental retardation, epilepsy (11, 43, 61, 65), and other health-related issues (12). Consistent with reported CBF changes to short-term hypoxia are relaxation responses of ex vivo cerebral vessels to short-term hypoxic challenges. Ex vivo cerebral vascular relaxation responses to short-term hypoxia suggest that changes are mediated, at least partially, by activation of smooth muscle BK (large-conductance, Ca2+-activated K+) channels, which are well known to modulate vascular tone and promote vasorelaxation (3, 7, 13, 28).
In contrast with the role of BK channels in mediating cerebrovascular response to short-term hypoxia, their role in acclimatization to long-term hypoxia (LTH) is less well known. During high-altitude LTH, the CBF in adult humans (23, 60) and sheep (34, 63) returns to normal following a period of transitional increased blood flow, as compiled by Brugniaux et al. (8). The ovine near-term fetus diverts an increased fraction of total cardiac output to the brain, when subjected to hypoxia during gestation. This implies that the cerebral vasculature is more dilated than the systemic vasculature (37, 38). Increased vessel dilation was also the major factor contributing to increased CBF and maintenance of oxygenation in human volunteers taken to high altitude or subjected to acute hypoxia at sea level (66). Because increased blood flow correlates directly with increased vessel diameter in mid-cerebral arteries in humans, as reviewed by Ainslie and Subudhi (1), increased basilar artery blood flow with acute hypoxia in mountaineers (23) likely also correlates with increased basilar diameter. Such functional correlations suggest hypoxia-induced increases in BK channel activity in the LTH adult and near-term fetal cerebral arteries. However, ex vivo studies with ovine mid-cerebral arteries from LTH adult and near-term fetuses yielded conflicting results and failed to convincingly confirm this prediction (14, 36). Because of the apparent disparity between in vivo CBF and ex vivo vascular responsiveness, the role of K+ channels in mediating LTH acclimatization begs further clarification.
In the present study, we hypothesized that cerebrovascular acclimatization to the demands of LTH involves increased BK channel activity compared with normoxic controls. Using patch-clamp electrophysiological and immunohistochemical techniques, we show that BK channel activities are significantly increased in basilar artery smooth muscle of ovine LTH-acclimatized adult and near-term fetuses. These acclimatization changes occur, however, by apparently different mechanisms.
METHODS
Experimental animals.
All surgical and experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and “The Guiding Principles in the Care and use of Animals” approved by the Council of the American Physiological Society and approved by the Animal Care and Use Committee of Loma Linda University. Nonpregnant and time-dated pregnant ewes of mixed Western breed were divided between normoxic control (n = 8) and LTH (n = 8) groups. All pregnant and nonpregnant ewes were obtained from the Nebeker Ranch (Lancaster, CA; elevation 720 m) where they were maintained at near sea level (normoxia) until 30 days gestation. At this time, some of the pregnant and nonpregnant ewes were transported to the Barcroft Laboratory, White Mountain Research Station (Bishop, CA) (3,801 m; maternal arterial PO2: 60 ± 3 Torr; fetal arterial PO2: 19 ± 2 Torr) for the final 110 days of gestation and/or acclimatization for nonpregnant ewes. At this time, the ewes were transported to Loma Linda University (a 6- to 7-h trip) for study. After arrival at Loma Linda University Medical Center Animal Research Facility (elevation: 346 m), LTH ewes were surgically implanted with arterial and tracheal catheters. The adult PO2 was maintained at ∼60 Torr by adjusting humidified nitrogen gas flow through the tracheal catheter for several hours to days, as previously described (25), until the animal was euthanized for surgery. Normoxic control pregnant and nonpregnant ewes were maintained near sea level throughout gestation.
At the time of study, ewes were sedated with thiopental sodium (10 mg/kg iv), and, following intubation, anesthesia was maintained with inhalation of 1% isoflurane in O2 throughout surgery. Following delivery of the fetus by hysterotomy, the fetuses (male and female in ∼1:1 ratio) ewes were euthanized with an overdose of Euthacol (pentobarbital sodium, 100 mg/kg) and phenytoin sodium (10 mg kg) (Virbao, Ft. Worth, TX). Female, nonpregnant adult and mixed-sex, near-term fetal brains were removed and placed in iced saline, and the basilar arteries were rapidly dissected out. All experiments were performed in normoxic conditions, and observed changes were assumed to be due to the effects of LTH.
Artery and cell isolation.
Arteries were selected from the same anatomic segments of adult and fetal basilar arteries to approximate segments of similar function and embryonic origin. Consequently, the adult and fetal arteries were of different diameter (∼300 µm vs. 200 µm, respectively). To determine the extent to which arteries of different size within age groups have the same current densities, we sampled current densities from proximal and distal segments of both adult and fetal basilar arteries. We observed no significant differences in current densities within age groups for arteries of different diameter. As described previously, basilar arterial smooth muscle cells were enzymatically dissociated and isolated (31).
Whole cell electrophysiological recordings.
Vascular smooth muscle cells adhering to precleaned glass cover slips were mounted in a perfusion chamber containing cell isolation solution for 15 min on the stage of an inverted microscope (Axiovert 35M; Carl Zeiss Instruments, Jena, Germany). The cell isolation solution was exchanged for the bathing medium. Normoxic controls and LTH smooth myocytes showed characteristic elongated shapes with axial ratios of about 10:1 for adult and 5:1 for the fetus.
Positive outward currents were measured in conventional and perforated-patch (20, 30) whole-cell voltage-clamp configurations using an Axopatch 200B amplifier with Clampex 8 (Axon Instruments, Foster City, CA). Currents were filtered at 1 kHz, using an Axopatch 200B internal 4-pole low-pass Bessel filter, and digitized at 2 kHz. For perforated-patch recordings, patch pipettes were back-filled with pipette solution containing amphotericin B (see Reagents and solutions). Resting membrane potentials were obtained from perforated-patch cells before voltage-clamp recording. Due to differences in adult and fetal cell size, as well as within age groups, whole-cell currents were normalized with cell capacitance to obtain current density. An agar salt-bridge was used to minimize the solution junction potential differences.
Single-channel recordings.
Single-channel currents were recorded from inside-out membrane patches (17) of isolated arterial myocytes. Patch pipettes were fabricated using a programmable Flaming-Brown pipette puller and standardized fire polishing procedures. Because the patch pipettes so produced had similar tip resistances (≈15 MΩ), the area of contact with each membrane was also similar (56). Currents were filtered at 2 KHz and digitized at 10 KHz. The number of channels present in any given excised patch (N) was estimated from all-points histograms. Channel activity, NPo, was calculated by using Equ. 1.
Equation 1 is where i is the number of open channels (0 is the number for closed state), and Ai is the area associated with each channel state, as determined from curve-fit individual peak areas. The single-channel open probability (Po) was then calculated from NPo/N. The values for N were obtained by using high [Ca2+] and/or depolarization to ascertain that less than three coincidental open events occurred during long recordings (>20 s) at a Po higher than 0.8. Preparations with more than three channels present were discarded.
Dwell-time analysis.
Single channel currents were analyzed with QuB software from SUNY (Buffalo NY; http://www.qub.buffalo.edu). For idealization, half-amplitude threshold analysis was used. Kinetic modeling of the idealized intervals used the maximum interval likelihood method. Dwell-time data were plotted with a logarithmic time x-axis and a square-root y-axis for the number of events in each bin. Bin density was 50 bins per decade.
Flow cytometry.
Freshly dissociated basilar myocytes were filtered in polystyrene round-bottom tubes fitted with cell-strainer caps (100-μm nylon mesh; Fisher Scientific, Chino, CA). Filtration separated the dispersed cells from larger debris and undigested arteries. The filtrate was centrifuged at 600 g for 10 min at 4°C. The supernatant was discarded and the pellet resuspended in 300 μl PBS (in mM) and 137 NaCl, 2.7 KCl, 10 Na2HPO4, and 2 KH2PO4 (pH 7.4). To block nonspecific binding, 15 μl of 1% intravenous immunoglobulin (1% solution of human serum IgG) in PBS was added to 100 μl of cell suspension and incubated at 4°C for 15 min. After blocking, 1 μl of rabbit primary antibody to BKβ1 (1 mg/ml; Cat. No. ab 587; Abcam, Cambridge, MA) and 1 μl of phycoerythrin-conjugated goat anti-rabbit IgG (secondary antibody Cat. No. 20303; Imgenex, San Diego, CA) were added for 15 min on ice. Cells were washed in 1 ml of PBS and centrifuged at 200 g for 5 min at 4°C. The supernatant was aspirated and the pellet was resuspended in 200 μl of 1% paraformaldehyde in PBS and stored at 4°C. Fixed cells were analyzed within three days. For each experiment, two controls were included consisting of untreated, fixed cells and fixed cells treated with secondary antibody only. For cytometric analysis, a FACsCalibur flow cytometer (BD Biosciences, Billerica, MA) equipped with the Cellquest Program was used.
Flow cytometric data were analyzed using FlowJo software (Tree Star, Ashland, OR), and image profiles were displayed as relative cell number against the log of fluorescence intensity. Histograms from representative experiments were expressed as geometric means ± SE. Dead cells and debris were excluded (gated out) according to their forward and vertical scattering pattern. To provide sufficient numbers of cells for experiments, cells from two animals were pooled. For comparisons between adult and fetal groups, independent “t” tests were used. P values of <0.05 were considered to be statistically significant.
Confocal microscopy protocol and analysis.
Fresh basilar arteries excised from anesthetized, nonpregnant adult and near-term fetal sheep were flash frozen with liquid nitrogen in OCT compound (Sakura Biotech, Torrance, CA) and stored at −80°C. Frozen sections (10 μm thick) were cut using a cryostat (model CM3050S; Leica Microsystems, Wetzlar, Germany). Sections were air-dried at least 30 min, then fixed with ice-cold acetone for 10 min, followed by washing with room temperature (RT) PBS for 10 min. Sections were blocked with 1% BSA and 2% goat serum in PBS for 1 h, and then incubated with primary anti-BKα antibody (1:200; Cat. No. APC-151; Alomone Labs, Jerusalem, Israel) either at RT for 1 h or at 4°C overnight (∼16 h). Samples were then washed three times at RT in PBS for 10 min each. Then samples were incubated with goat anti-rabbit secondary antibody (1:300) conjugated with Alexa 488 (green; Cat. No. A11008, Life Technologies, Carlsbad, CA) at RT for 40 min in the dark. Sections were either counterstained for 15 min with wheat germ agglutinin (WGA) conjugated to Alexa 594 (AF-594 conjugated WGA; 1:300), a general membrane marker, or with recombinant cholera toxin subunit B (ChTx) conjugated to Alexa 594 (5 μg/ml), a GM1 marker of lipid rafts (18, 40) (red; Cat. No. W11262 and V34777, respectively; Life Technologies) and then with Hoechst dye 33342 (0.01 μg/ml; blue; Cat. No. H1399; Life Technologies) for 10 min to label cell nuclei. Coverslips (No. 1.5, VWR; 161.3 ± 1.25 μm thickness; n = 8) were then applied to samples.
Prepared slides were viewed and imaged with a LSM710 NLO Confocal system (Carl Zeiss) equipped with 63× (n.a. 1.40) oil-immersion objective. Images were acquired with Zen software (Zeiss) at 1,024 × 1,024 pixels, where each pixel was 0.09 × 0.09 μm. To reduce background noise the pixel dwell time was 0.50 μs, and four lines were averaged. To maximize imaging of intact myocytes, care was taken to image from the middle of cut sections. For cluster analysis, we used the particle analysis function of ImageJ software (51; http://rsb.info.nih.gov/ij) with procedures similar to Kirby et al. (26). To measure BKα and ChTx clusters, we examined fluorescence at several incremental intensities above mean levels (57). Particle intensities were examined by converting from gray scale to binary images based on circularity and size criteria. Circularity criterion was set at >0.1, where circularity (4π area/perimeter2) can range from 0 (infinitely elongated polygon) to 1 (perfect circle). Size criterion was set at >0.2 μm. Intensity data meeting criteria were collected, saved, and analyzed. Data were collected from an area of 20 × 40 μm per section. BKα and ChTx clusters were based on positive staining for BKα-like green and ChTx-like red fluorescence, respectively. For most purposes we used intensities 3.5-fold above mean intensities as the threshold to define cluster because it was the lowest threshold yielding significant differences between fetal and adult intensities. Statistical analysis used GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Two-way ANOVA with post hoc test comparison and unpaired t-test of data sets were performed for each experiment.
Two independent, negative controls were performed to determine specificity of staining for the BKα antibody in immunohistochemistry and colocalization studies. On each experimental day, adjacent tissue slices were incubated with fluorophore-conjugated secondary antibody, but not primary antibody. Images were made at the same excitation and emission settings for experimental and control samples of each day. Fluorescent signals were then measured for three ∼35 × 75 μm perimeter regions of interest in each sample, and statistical comparisons were made by a Mann Whitney U test. All experimental conditions had ∼7–10 times greater fluorescence relative to control (P < 0.0001). Separately, the primary antibody was incubated with an epitope-blocking peptide. The fluorescence signal for regions of interest in fetal arterial samples was reduced nearly 10-fold by pre-absorbing antibody with blocking peptide (P < 0.0001, unpaired t-test).
For analysis of colocalization, threshold values were set using automated criteria within Coloc_2 software (http://fiji.sc/Coloc_2/), where pixels below threshold had null or anti-correlated intensities. This method gives a Pearson's correlation coefficient (r) of zero for the pixels below the threshold. The correlation coefficients for areas of overlapping expression of BK subunits with ChTx-positive fluorescence were then measured. For ChTx clusters, a threshold of threefold above mean was used, whereas for BKα clusters 3.5-fold was used.
Reagents and solutions.
Papain was obtained from Worthington Biochemical (Lakewood, NJ). Calcium standards and Fura-2 were obtained from Molecular Probes (Eugene, OR). Free calcium concentrations of patch-clamp solutions were first estimated with Max Chelator Sliders software (C. Patton; Stanford University, Stanford, CA) (47) and adjusted using fluorometric measurements with Fura-2 and Fura-6 and Ca2+ standard kits 2 and 3 (Molecular Probes) for calibration. All other chemicals were obtained from Sigma (St. Louis, MO). For cell isolation, the cell isolation solution contained (in mM) 55 NaCl, 80 Na+-glutamate, 5.6 KCl, 2 MgCl2, 10 glucose, and 10 HEPES adjusted to pH 7.3 with NaOH. For perforated-patch recording, the bathing solution contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 10 glucose, 2 CaCl2, and 10 HEPES adjusted to pH 7.4 with NaOH. The pipette solution for perforated-patch recordings contained (in mM) 110 K+-aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA adjusted to pH 7.2 with KOH, containing 200 μg/ml amphotericin B. For conventional whole-cell recording, the bathing solution contained (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 1.5 CaCl2, and 10 HEPES adjusted to pH 7.4 with NaOH. The pipette solution for whole-cell recordings contained (in mM) 130 K+-gluconate, 30 KCl, 1 MgCl2, 0.1 CaCl2, 0.5 EGTA, and 5 HEPES adjusted to pH 7.2 with KOH. The single-channel bathing solution contained (in mM) 140 KCl, 1 Mg2+, 10 HEPES, and 5 EGTA adjusted to pH 7.2 with KOH with different free Ca2+ concentrations (∼0.3, 1, 3, and 10 μM) measured fluorometrically using Fura-2. The single-channel pipette solution had the same composition as the bathing solution with ∼3 μM free Ca2+.
Data analysis and statistics.
All values were calculated and displayed as means ± SE. In all cases, n refers to the number of replicate cells. All statistical comparisons were performed at the 95% confidence level using two-sample, unpaired t-tests. A P value of <0.05 was considered to be statistically significant. We verified all sample populations to be normally distributed. For comparisons of values that were not significantly different, power analyses were performed to confirm that statistical power was 0.7 and the probability of Type II errors was acceptably small. Curve fitting was performed with GraphPad Prism 5 (GraphPad Software).
RESULTS
Comparison of LTH adult and fetal whole-cell currents.
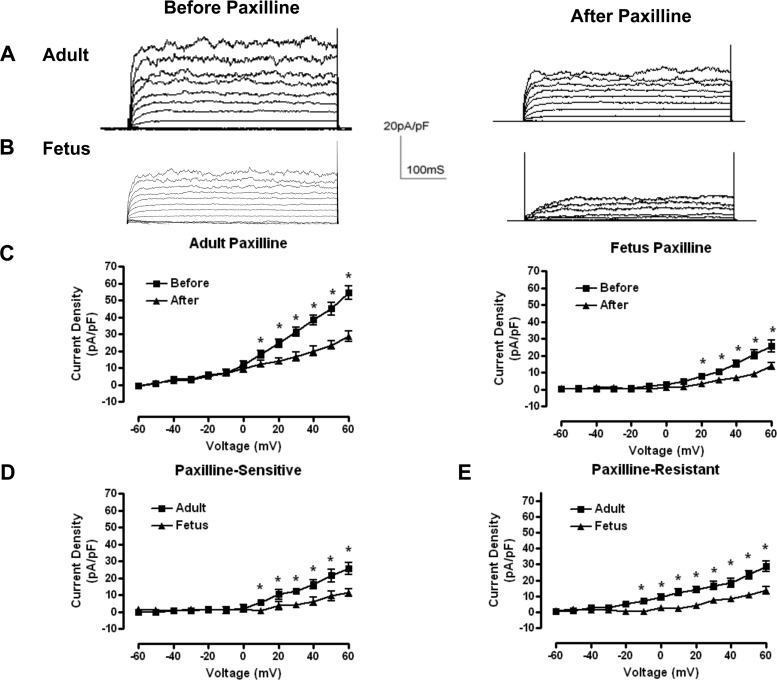
In conventional whole-cell preparations, we recorded outward currents from LTH adult and fetal basilar arterial myocytes. Cell capacitances from LTH adult and fetal myocytes were 15.2 ± 0.9 pF (n = 6) and 8.3 ± 0.4 pF (n = 7), respectively (P < 0.05; Table 1). We recorded total outward currents from cells held at −60 mV followed by a series of depolarizing steps over the range of −60 to +60 mV. Because isolated adult myocytes present about 80% more plasma membrane surface area to the bathing medium than those of the fetus, we normalized raw whole-cell outward currents to membrane capacitance and present current measurements as current densities (Fig. 1, A and B). The steady-state outward current density at +60 mV in LTH adult (54.2 ± 4.1 pA/pF; n = 7) was about twice that of fetal myocytes (24.8 ± 3.0 pA/pF; n = 8; P < 0.01; Fig. 1C and Table 1).
Table 1.
Summary of smooth myocyte conventional and perforated-patch whole cell, and single-channel recordings
| LTH |
NXa |
|||
|---|---|---|---|---|
| Adult | Fetus | Adult | Fetus | |
| Capacitance, pF | ||||
| Conventional whole-cell | 15.2 ± 0.9 (6) | 8.3 ± 0.4 (7)* | # | # |
| Perforated-patch | 16.1 ± 1.3 (7) | 9.4 ± 1.7 (6)* | 15.7 ± 0.6 | 9.1 ± 0.6** |
| Current density, pA/pF | ||||
| Conventional whole-cellb | ||||
| Outward current | 54.2 ± 4.1 (7) | 24.8 ± 3.0 (8)** | # | # |
| BK current | 25.7 ± 3.4 | 11.2 ± 2.2* | # | # |
| Non-BK current | 28.8 ± 3.2 | 13.6 ± 2.3* | ∼29a | ∼26a |
| Perforated-patchb | ||||
| Outward current | 75.1 ± 5.9 (5) | 71.6 ± 13 (6) | 37.9 ± 1.8 | 57.9 ± 6.7* |
| BK current | 46.3c | 58.0c | 10a | 29a |
| Resting membrane potential, mV | −28.7 ± 5.5 (8) | −39.4 ± 7.0 (8)* | −30.1 ± 4.0(13) | −23.8 ± 2.6 (17) |
| BK single-channel parameters | ||||
| Slope, mV/log [Ca2+]i | 65.9 ± 3.3 | 66.8 ± 3.8 | 67.1 ± 2.5 | 67.6 ± 2.7 |
| Ca2+ set point, Cao, μM | 3.6 | 3.0 | 8.8 | 4.7 |
| Hill coefficient, rH | 3.3 ± 0.2 | 3.0 ± 0.3 | 2.9 ± 0.1 | 2.9 ± 0.2 |
| Unitary conductance, pS | 215 ± 12 | 228 ± 7 | 221 ± 8 | 229 ± 5 |
Values are means ± SE, based on a sample size of (n). Conventional (Fig. 1) and perforated-patch (Fig. 9) whole-cell recording yield membrane capacitance and current density. Single-channel recording and analysis (Fig. 2) reveal large-conductance Ca2+-activated K+ (BK) channel parameters and properties. LTH, long-term hypoxia; NX, normoxic.
From Lin et al. (33);
measured at +60 mV;
estimated by subtracting Non-BK current from perforated-patch outward current;
not measured. When compared against adult of same treatment:
P < 0.05;
P < 0.01.
Fig. 1.
Whole-cell currents from long-term hypoxia (LTH) adult and fetal smooth muscle cells. A and B: representative whole-cell outward membrane current density traces elicited by a series of 10-mV depolarizing steps (−60 to +60 mV) from a holding potential of −60 mV. Traces before (left) and after (right) paxilline application are shown in typical isolated LTH adult (A) and fetal (B) basilar artery myocytes. Whole-cell current density is obtained from normalized whole-cell currents to membrane capacitance to account for size differences between adult and fetal myocytes. C: averaged steady-state current-voltage plot of outward current density in myocytes obtained from LTH adult (n = 6) and fetal (n = 7) basilar arteries before and after treatment with 5 × 10−7 M paxilline. D and E: averaged steady-state paxilline-sensitive large-conductance Ca2+-activated K+ channel (BK) currents (left) and residual, paxilline-insensitive currents (right) obtained from digital subtraction of the individual traces such as in A and B. *Significant difference with P < 0.05.
To determine the contribution of BK current to total whole-cell current density, we applied paxilline (5 × 10−7 M) to inhibit BK current (58). Paxilline significantly reduced whole-cell current density. The paxilline-sensitive (i.e., BK) current density at +60 mV constituted about half (adult: 47.1%, P < 0.001; and fetus: 45.2%, P < 0.001) of the total outward current densities (Fig. 1C). Both paxilline-sensitive (Fig. 1D) and paxilline-resistant (Fig. 1E) current densities were twofold greater in LTH adult than in fetal myocytes (Fig. 1C). At physiological membrane potentials, BK channels normally activate in response to transient micromolar elevations of intracellular Ca2+. Because intracellular Ca2+ levels were buffered at resting levels of 100 nM, BK channel activation was right shifted to nonphysiological potentials.
Several factors could account for the difference in BK activities between LTH adult and fetal basilar arterial myocytes. These include differences in channel affinity for Ca2+, differential phosphorylation, and differential expression of BK subunits (e.g., β or α). Previously, in normoxic control animals, we showed that higher BK current density in the normoxic fetus was attributable to a higher intracellular affinity for Ca2+, as compared with that of the adult (31). Consequently, we hypothesized that higher BK current density in LTH adult was due to a higher affinity for intracellular Ca2+.
Effects of LTH on BK channel Ca2+ affinity.
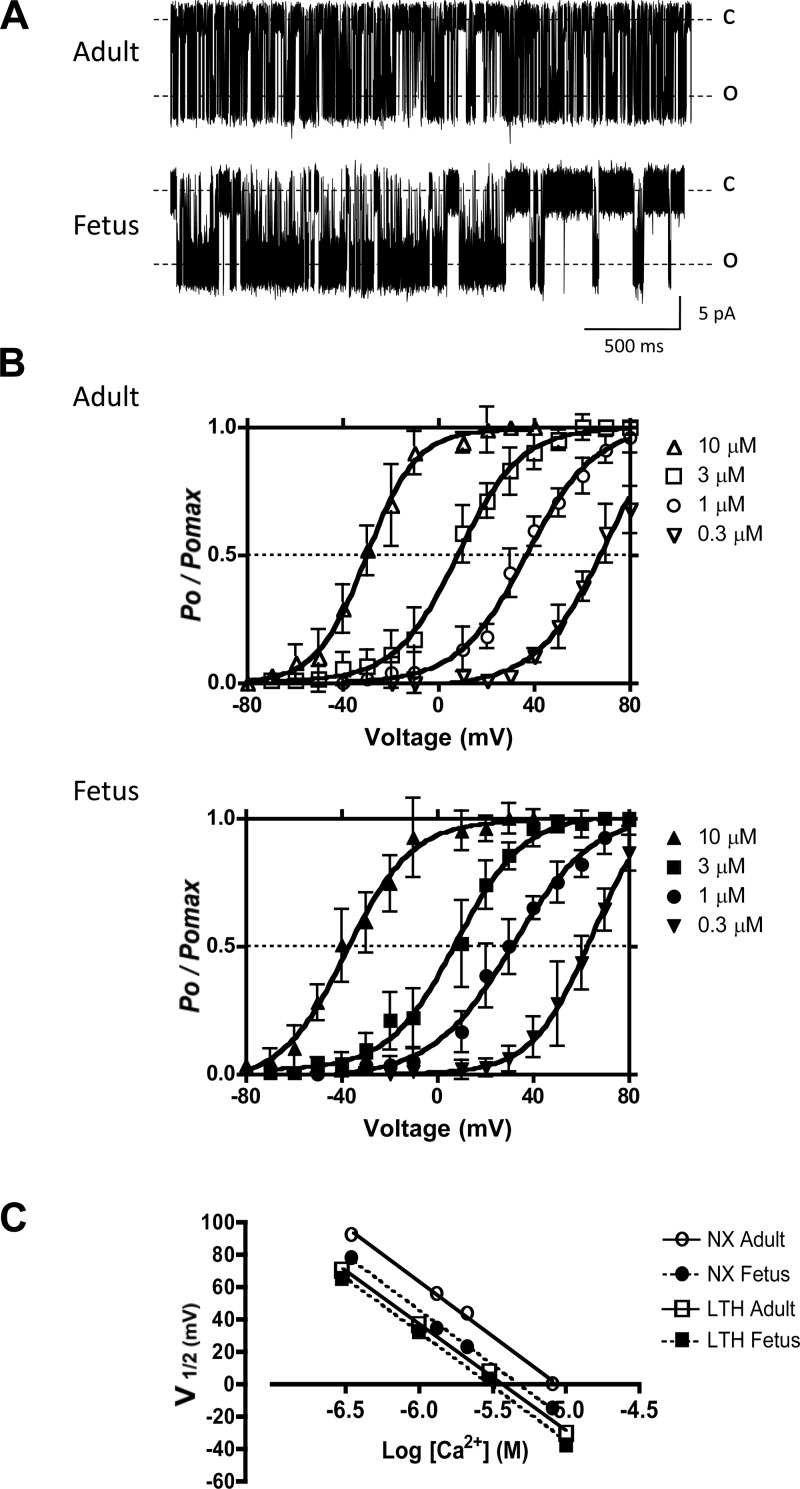
To compare the Ca2+ affinity of adult and fetal BK channels, we determined Ca2+ set points (Ca0), where Ca0 is the Ca2+ concentration that half-activates BK channels at 0 mV. The Ca0 equals the Kd for Ca2+ at 0 mV (33). We recorded BK channel activity in inside out, excised patch preparations from LTH adult and fetal basilar arterial myocyte membranes (Fig. 2A) and plotted BK channel open probabilities at different voltages and Ca2+ concentrations (Fig. 2B). Data were fitted to the Boltzmann equation and the membrane potential required for 50% activation of channels (V1/2), and we plotted the V1/2 values against log [Ca2+] (Fig. 2C). From the equation for the line fitted to these data, we estimated the calcium sensitivities from the change in V1/2 for a 10-fold change in Ca2+ concentration (ΔV1/2) (Table 1). The calcium sensitivities of these two age groups did not differ significantly, nor did they differ significantly from their normoxic controls (Table 1). For LTH adult and fetal BK channels the Ca0 values estimated by linear regression were found to be 3.6 μM and 3.0 μM Ca2+, respectively. However, both were lower than their corresponding normoxic controls (Table 1). Of note, similar Ca0 values, Hill coefficients, and unitary conductances (Table 1) for LTH adult and fetal BK channels could not account for the twofold difference in their whole-cell BK channel current densities.
Fig. 2.
BK channel open probabilities and calcium set points. A: representative inside-out patch recordings of BK channel from LTH adult and fetal smooth muscle cells in symmetrical 140 mM KCl. In both cases, command potentials were −30 mV making BK channels experience +30 mV depolarizations. The bath [Ca2+] was 1.0 μM. Dotted lines represent the channels in closed (C) and open (O) states. B: voltage activation curves at different membrane potentials in 10-mV increments for various [Ca2+]i. Data are channel open probability (Po) expressed relative to maximum channel open probability (Pomax). Solid lines indicate best-fit curves to the Boltzmann equation: Po/Pomax = 1{1 + exp[(V1/2 − Vm)/K]}, where V1/2 is the membrane potential (Vm) required for half-maximal activation of the channel and K is the logarithmic voltage sensitivity (change in voltage required for an e-fold increase in activity). The voltage sensitivities estimated from the fitted curves were similar for all concentrations of Ca2+ tested and indicated that channel activity increased e-fold (∼2.72 times) for 23.5 ± 1.8 mV (n = 4, adult) and 25.0 ± 2.1 mV (n = 4, fetus) depolarizations. C: estimation of changes in V1/2 for a 10-fold change in [Ca2+]i (ΔV1/2) and estimation of the Ca2+ axis intercept (calcium set point, Ca0) for both adult and fetal BK channels. V1/2 values were obtained from B. The lines represent the best linear regression fits. LTH adult and fetal Ca0 values were calculated to be 3.6 μM and 3.0 μM, respectively. NX, normoxic.
Effects of phosphorylation on BK channel activity.
Under appropriately controlled conditions (i.e., identical BKα isoforms and BKβ expression levels), we have shown that the Ca0 is a surrogate measure of the extent of channel phosphorylation, with lower Ca0 values correlating with greater extents of phosphorylation by either PKA or PKG (33). Because BK channels from LTH animals show similar Ca0 values that are lower than those from normoxic controls (Table 1) (31), we hypothesized that LTH adult and fetal BK channels are both phosphorylated similarly and to a greater extent than the normoxic channels. To test these hypotheses, we compared BK channel voltage-activation from different phosphorylation states by applying exogenous alkaline phosphatase and protein kinases using inside-out patches. We plotted single-channel open probability, Po/Po max, against membrane potential and fitted the Boltzmann equation to data (Fig. 3).
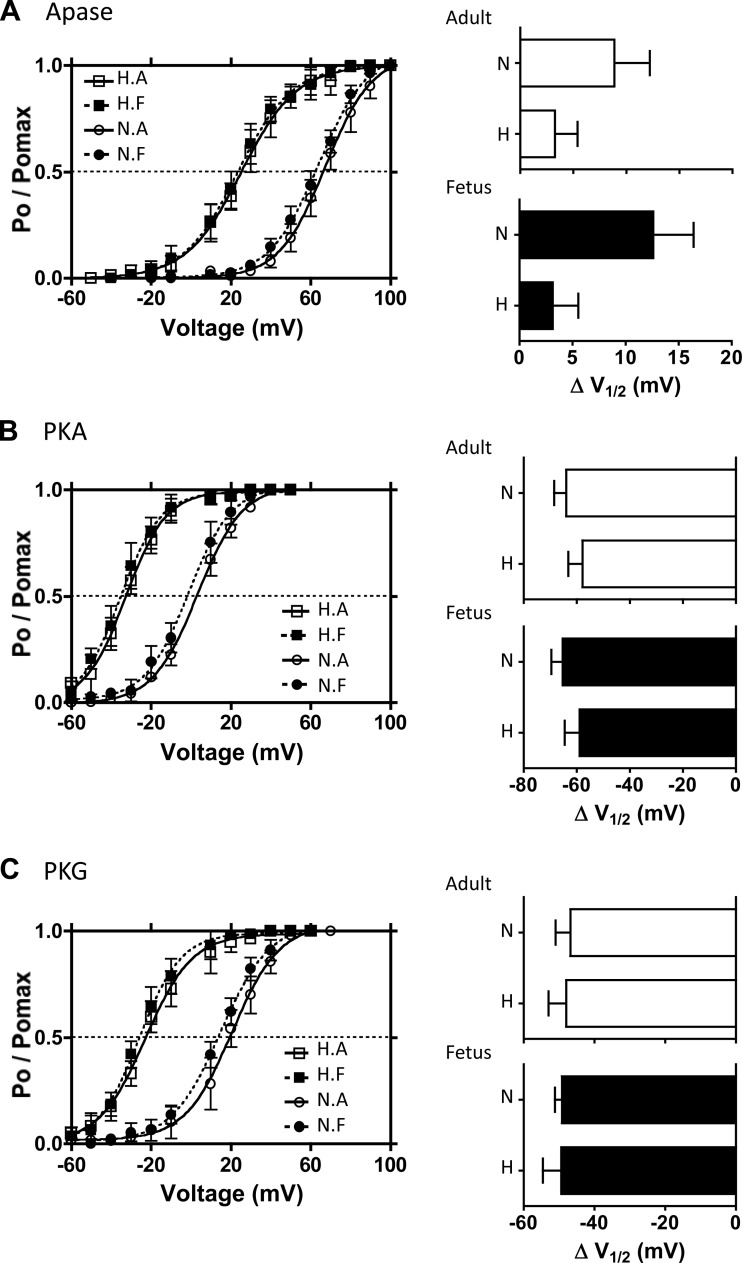
Fig. 3.
Effects of exogenous phosphorylation and dephosphorylation on BK channel activities. Single BK channel recordings of BK channels from inside-out micro patches were obtained in 3 μM free [Ca2+] from the isolated myocytes of the 4 experimental animal groups: LTH (H) and normoxic (N) and adult (A) and fetus (F). A: voltage-activation curves of BK channels with alkaline phosphatase (Apase, 350 U/ml) in the bathing medium. Bar graphs summarize the extent to which Apase treatment right-shifts the activation curves in terms of change in V1/2 values for adult (top) and fetal (bottom) groups. B: voltage activation curves of BK channels in the presence of exogenous PKA. After phosphatase pretreatment, purified PKA catalytic subunit (cPKA, 30 U/ml) was added in the presence of KT5823, OA, and ATP. The extent to which PKA left-shifts V1/2 values is summarized on the bar graphs. C: voltage activation curves of BK channels in the presence of exogenous PKG. Following phosphatase pretreatment, purified PKG (2,000 U/ml) was added in the presence of KT5720, OA, and ATP. Effect of PKG left-shift V1/2 values is summarized on the right bar graphs. Solid lines show the best-fit curves to the Boltzmann equation from which V1/2 values were calculated.
To compare the voltage-activation of BK channels in the fully dephosphorylated state from each group, we added alkaline phosphatase (Apase, 350 U/ml) to the bath on the cytoplasmic side of the plasma membrane. Apase right-shifted normoxic adult and fetal BK voltage-activation curves to the same extent (Fig. 3A). The voltage-activation curves of LTH adult and fetal myocytes were also right shifted to the same extent by Apase. However, the shift in their V1/2 values was substantially less and about −40 mV to the left (i.e., more negative) relative to normoxic V1/2 values (Fig. 3A and Table 2). Despite the difference, BK channel voltage sensitivities did not differ among the four groups of myocytes. Bar graphs in Fig. 3A represent Apase-induced changes of V1/2 values (ΔV1/2) from their previous endogenous (native) state. Consistent with our first hypothesis, native LTH adult and fetal BK channels were similarly phosphorylated. Unexpectedly, dephosphorylation with Apase right-shifted both V1/2 values only by ∼3 mV, indicating that LTH BK channels were less phosphorylated relative to normoxic controls. The ΔV1/2 values from native state to dephosphorylated state for both LTH adult and fetus were about one-third and one-fourth of normoxic controls, respectively. Thus, when compared with normoxic groups, the lower Ca2+ set point values of LTH groups (Table 1) are unlikely due to BK channels being more highly phosphorylated.
Table 2.
Summary of BK V1/2 and differences in V1/2 values in different phosphorylation states
| LTH |
NX |
NX-LTH V1/2 Diff |
||||
|---|---|---|---|---|---|---|
| Phosphorylation State | Adult | Fetus | Adult | Fetus | Adult | Fetus |
| Native | 22.0 ± 6.5 (8) | 20.5 ± 4.9 (6) | 58.4 ± 5.4* (11) | 51.5 ± 3.9* (16) | 36.4 | 31.0 |
| Dephosphorylated | 25.3 ± 5.2 (8) | 23.6 ± 6.1 (7) | 67.3 ± 6.6* (12) | 64.1 ± 4.9* (14) | 42.0 | 40.5 |
| PKA | −32.6 ± 6.2 (8) | −35.3 ± 5.6 (6) | 3.3 ± 6.5* (14) | −1.2 ± 5.0* (15) | 35.9 | 34.1 |
| PKG | −22.8 ± 5.5 (9) | −25.9 ± 7.0 (6) | 20.6 ± 4.5* (11) | 14.9 ± 4.9* (11) | 43.4 | 40.8 |
Values are means ± SE, based on a sample size of (n). V1/2 values were obtained from BK channel voltage-activation curves at each phosphorylation state. Values for native controls, dephosphorylation, PKA, and PKG phosphorylation states were obtained from Fig. 3. NX-LTH V1/2 diff values show BK V1/2 differences between LTH and NX animal groups. When compared against LTH counterparts:
P < 0.05.
Nevertheless, to examine the effects of phosphorylation on BK channel voltage-activation, we first dephosphorylated the channels with 350 U/ml Apase, followed by removing Apase from the bath and exposing BK channels to purified catalytic subunit of protein kinase A (cPKA; 30 U/ml) in the presence of KT-5823 (PKG inhibitor, 1 μM), okadaic acid (OA, 1 μM), and ATP (0.5 mM). KT-5823 and OA were used to inhibit the endogenous, channel-associated PKG and phosphatase activities, respectively (32). cPKA left-shifted the voltage-activation curves of both LTH and normoxic BK channels by ∼60 mV (Fig. 3B), but the V1/2 values for LTH channels were about −35 mV to the left of those for normoxic channels (Table 2).
Similarly, we studied the effect of PKG phosphorylation on BK channel activity. Following Apase pre-treatment and subsequent washout, addition of exogenous PKG (2,000 U/ml), KT-5720 (PKA inhibitor; 0.3 μM), OA, and ATP left-shifted the voltage-activation curves of both LTH and normoxic BK channels by ∼50 mV (Fig. 3C), but the V1/2 values for LTH adult and fetal myocytes were about −40 mV to the left of that for normoxic myocytes (Table 2). The bar graphs show that PKA (Fig. 3B) and PKG (Fig. 3C) phosphorylation shifted the V1/2 values of BK channels from all four groups to a similar extent toward more negative potentials.
Taken together, inducing changes in phosphorylation status consistently segregated voltage-activation curves for both LTH age groups from their comparable normoxic controls. In each of the three defined phosphorylation states, V1/2 values for BK channels from LTH myocytes were consistently −35 to −40 mV more negative relative to those from normoxic myocytes (Table 2), demonstrating that intrinsic functional differences exist between LTH and normoxic BK channels.
Gating kinetics.
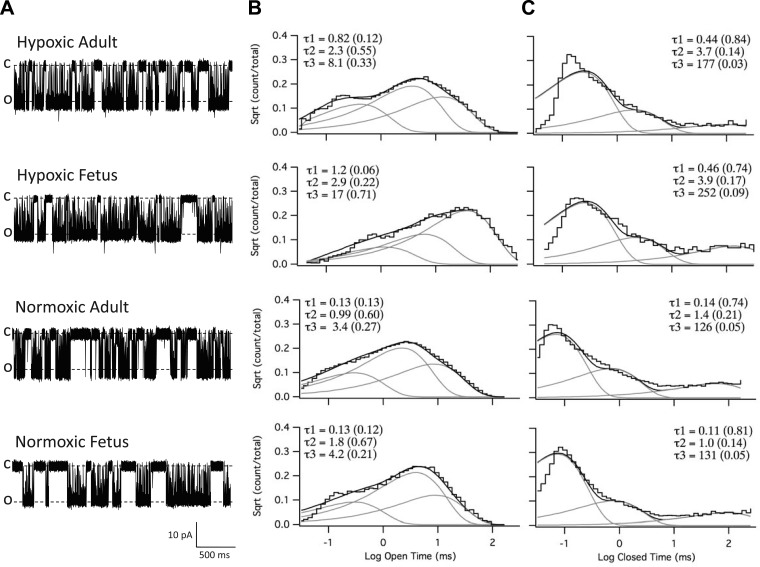
Despite BK channels from LTH adult and fetal myocytes being from developmentally different populations, they exhibited similar Ca2+ affinities (Cao; Fig. 2C) and voltage-activation (V1/2; Fig. 3). However, single-channel BK channel recordings (Fig. 2A) suggest different gating kinetics. Therefore, we compared gating kinetics by measuring open and closed dwell times of LTH and normoxic adult and fetal BK channels from single-channel, inside-out preparations. Figure 4A shows representative traces of single BK channel recordings from the four groups in their native state (i.e., endogenous controls). BK channel dwell times were plotted as the square root of event fraction versus the logarithmic open or closed dwell times. The histograms to the right were set to 50 bins per decade, and the plots were best fitted to 3-component exponential functions to display open (Fig. 4B) or closed (Fig. 4C) components. By summing the products of the component mean dwell times (τ1, τ2, τ3) and their respective weight factors (w1, w2, w3; Fig. 4, B and C, shown in parentheses), we calculated the weighted mean open (τo) and closed (τc) times, which are represented as τ = (w1τ1 + w2τ2 + w3τ3)/(w1 + w2 + w3), where (w1 + w2 + w3) = 1. The τo and τc of BK channels in the native state from LTH fetus were more than three times longer than that of the other three groups (Table 3).
Fig. 4.
BK channel dwell time analysis. A: representative inside-out patch recordings of BK channels from hypoxic (LTH) adult and fetal, and normoxic adult and fetal myocytes in symmetrical 140 mM KCl solutions with 3 μM free Ca2+. Recordings were done at +60 mV depolarizing potential. C, closed state; O, open state. B and C: plots of open and closed dwell times. Channel open and closed dwell times were plotted on a logarithmic time abscissa as a function of the square root (Sqrt) of the number of events per bin on the ordinate axis. The bin density is 50 bins per decade. Both the open (B) and closed (C) plots were best fitted to exponential functions with 3 components using QuB software (see methods). The lines for the sum and each component exponential fit are shown. The time constants (τ) and their relative weight contributions (in parentheses) of each component to the composite fit are listed.
Table 3.
Summary of weighted mean open and closed dwell-times
|
τO |
τC |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTH |
NX |
LTH |
NX |
||||||
| Phosphorylation State | Adult | Fetus | Adult | Fetus | Adult | Fetus | Adult | Fetus | |
| Native | 3.84 (8) | 13.81 (7) | 1.84 (5) | 2.09 (6) | 8.32 | 27.03 | 6.64 | 6.69 | |
| Dephosphorylated | 2.77 (6) | 9.65 (6) | 1.66 (5) | 1.63 (6) | 8.59 | 21.07 | 26.37 | 19.88 | |
| PKA | 1.31 (5) | 1.83 (7) | 1.73 (6) | 1.48 (7) | 14.61 | 4.98 | 8.71 | 5.48 | |
| PKG | 1.33 (6) | 0.92 (5) | 1.45 (5) | 1.85 (5) | 6.19 | 13.89 | 2.06 | 28.83 | |
Dwell-times were obtained from square-root versus logarithmic time plots (Fig. 4, B and C) best fitted to an exponential function with 3 components. Mean time constants (τ) were multiplied by corresponding weighing factor (see Supplemental Table S1, including P values), and the 3 products were summed to yield weighted mean open (τo) and closed (τc) times for different phosphorylation states: native control; dephosphorylated (alkaline phosphatase, Apase); and phosphorylation by added PKA and PKG following pretreatment with Apase. Sample sizes (n) are shown in parentheses.
To determine the extent to which the longer dwell times of LTH fetal BK channels may be attributable to differential phosphorylation, we examined the effects of BK channel dephosphorylation and phosphorylation on dwell times. We treated inside-out patches in Apase to dephosphorylate or in PKA or in PKG to phosphorylate BK channels (identical to the procedures for Fig. 3). Table 3 summarizes the compiled weighted mean open (τo) and closed dwell times (τc) for BK channels from each of four animal groups in three defined phosphorylation states: dephosphorylated and PKA- and PKG-phosphorylated. Changes in phosphorylation state did not influence normoxic BK weighted mean open dwell times significantly, while both protein kinases A and G decreased open dwell times in LTH groups. Moreover, dephosphorylation with Apase had little effect on LTH BK open or closed dwell times. Consistent with findings in Fig. 3, these results indicated that BK channels from LTH adult and fetus in the native state were essentially dephosphorylated compared with those native state normoxic controls.
Expression of cell surface BK β1.
Because increases in BK β1 subunit expression have been associated with increases in channel gating kinetics (7, 42), increases in channel Ca2+ affinity (i.e., lower Ca0; 50), and left-shifted voltage activation (44), we tested the hypothesis that myocyte cell surface BK β1 subunit expression was upregulated in LTH fetal myocytes. To test this proposal, we used flow cytometry with a primary antibody directed against a conserved, extracellular BK β1 subunit epitope (Fig. 5). An epitope-blocking peptide was used as a negative control (Fig. 5, E and F). The specificity of the antibody was tested in Western immunoblots, which showed BK β1 expression in ovine fetal and adult pulmonary arteries, as previously reported by Resnik et al. (52), but not in ovine adult brain, which predominantly expresses the BK β4 isoform (data not shown) (4, 6). To eliminate effects due to variation in cell size and surface area, we normalized cell surface BK β1 expression (fluorescence units, FL) to relative surface area based on measured cell capacitances (i.e., pF) (Table 1). We thereby converted flow cytometric data (FL) for surface BK β1 into units of relative surface density (i.e., FL/pF) (Table 4). Our data indicate that BK β1 surface density on LTH fetal myocytes was three times greater than that of LTH adult cells and two times greater than that of either normoxic group. Based upon this analysis, long-term hypoxia enhances BK β1 surface expression on fetal myocytes relative to that of LTH adult myocytes and both normoxic control myocytes.
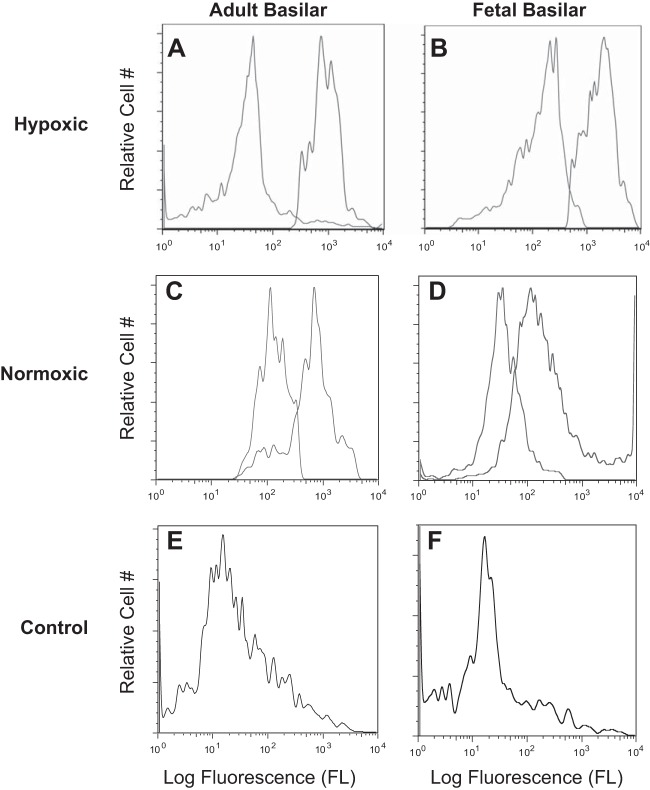
Fig. 5.
Representative flow cytometric distributions of cell surface BK channel βl subunit. A–D: isolated, intact basilar artery smooth myocytes were treated with either primary anti-BK βl (black trace) plus secondary antibody or with secondary antibody alone (gray trace). E and F: primary anti-BK βl antibody was pre-incubated with 70-fold molar excess βl epitopic peptide overnight on ice. Isolated, intact basilar artery smooth myocytes then were treated with the primary antibody and peptide mixture followed by secondary antibody to serve as antibody specificity controls (gray trace). A: LTH adult (n = 8); B: LTH near-term fetus (n = 9); C: normoxic adult (n = 13); D: normoxic near-term fetus (n = 13); E: normoxic adult (n = 13); and F: normoxic fetus (n = 13).
Table 4.
Summary of BKβ and BKα surface densities and BKα clustering
| LTH |
NX |
|||
|---|---|---|---|---|
| Adult | Fetus | Adult | Fetus | |
| Flow cytometry | ||||
| Geometric mean, FL | 299 ± 17* | 546 ± 68 | 452 ± 27 | 206 ± 13* |
| Mean cell count/sample | 1.2 × 104 | 3 × 104 | 3 × 104 | 6.5 × 105 |
| Relative surface area, pFa | 16.1 ± 1.3 | 9.4 ± 1.7 | 15.7 ± 0.6 | 9.1 ± 0.6 |
| BKβ1 surface density, FL/pF | 18.6 | 58.1 | 28.8 | 22.6 |
| Channels per micropatch | ||||
| Mean BK channel per patch | 2.1 ± 0.2 | 2.6 ± 0.3 | 2.2 ± 0.2 | 2.6 ± 0.2 |
| Mean tip resistance, MΩ | 15.2 ± 0.1 | 15.9 ± 0.1 | 15.3 ± 0.1 | 15.5 ± 0.1 |
| BK per patch, minus empties | 2.3 ± 0.2* | 3.7 ± 0.3 | 2.5 ± 0.2 | 2.6 ± 0.2* |
| %Patches with no BK channels | 8.3 | 31.7 | 7.0 | 1.6 |
| %Patches with 1 BK channel | 27.8 | 7.1 | 27.9 | 29.0 |
| %BK channels clusteredb | 33.3 | 50.0 | 37.2 | 53.2 |
| BK clustering | ||||
| Total BKα, (103)c | 9.2 ± 1.1 | 6.0 ± 1.1 | 9.8 ± 1.3 | 5.8 ± 1.1 |
| BKα surface density, (102)d | 8.0 ± 1.2 | 6.6 ± 1.0 | 7.6 ± 1.0 | 6.8 ± 0.9 |
| BKα clusters/total BKα, (10−3)e | 1.4 ± 0.2* | 3.8 ± 0.4 | 1.4 ± 0.2* | 3.4 ± 0.2 |
| Cluster colocalization | ||||
| Number of BKα clusters (103)f | 11.6 ± 2.8 | 20.0 ± 0.4 | 12.5 ± 4.3 | 15.6 ± 4.8 |
| Number of cholera toxin clusters (102)g | 7.5 ± 0.9 | 9.2 ± 1.9 | 5.8 ± 1.7 | 10.0 ± 2.1 |
| Colocalized clusters (103)h | 2.6 ± 1.2* | 7.8 ± 1.6 | 2.8 ± 0.8* | 9.0 ± 1.6 |
| %BKα clusters colocalized | 31.9 | 39.0 | 32.0 | 53.8 |
Values are means ± SE, as appropriate, with n values indicated in accompanying text or figures. Flow cytometry was used to measure BK β1 surface expression (Fig. 5) and calculate relative β1 surface density. Channels per micropatch are estimated BK channels on excised micro-patches.
Measured in perforated-patch mode (Table 1);
% of channels associated with 3 or more other channels in patch. BK clustering is the extent of BK channel clustering.
From Fig. 8A;
from Fig. 8B;
from Fig. 8F. Cluster colocalization is colocalization of BK clusters with clusters of cholera toxin.
From Fig. 9A;
from Fig. 9B;
from Fig. 9F.
Compared with LTH fetus (P < 0.05).
Channel surface density.
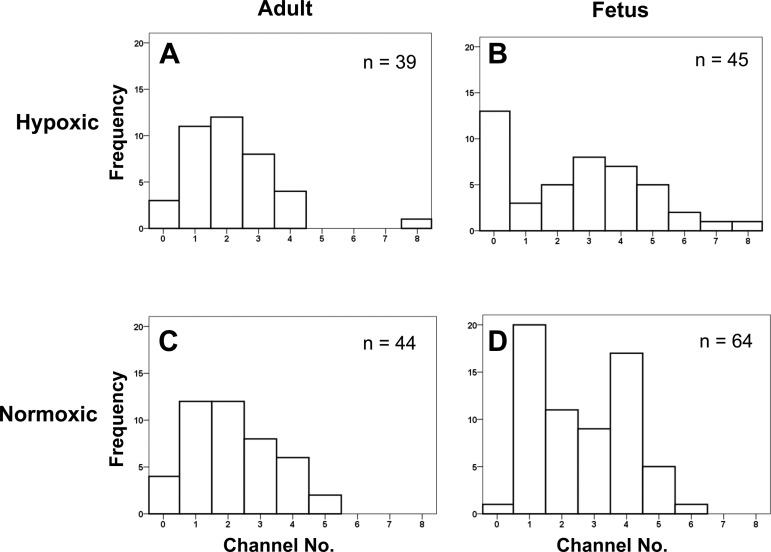
Because myocyte BK β1 surface density was significantly greater in the LTH fetus than in the other three groups, we measured the corresponding BK channel surface density. For this purpose, we counted BK channels in excised membrane patches from micropipettes of similar tip diameter and resistance (15.5 ± 0.10 MΩ; n = 192) (3, 56). The data show that channel surface densities did not differ between treatment groups (Table 4). However, frequency histograms of number of channels per excised patch (Fig. 6) suggest different patterns of BK surface distribution between groups. In the LTH fetus, many patches did not have channels (31.7%) and few patches contained one channel (7.1%), whereas the largest percentage of patches had three or more BK channels (50.0%). The two adult groups had fewer patches containing three or more BK channels (33.3% and 37.2%), whereas the normoxic fetal group had an intermediate percentage (53.2%) (Table 4). These findings suggest that myocyte BK channels of the LTH fetus and the normoxic fetus are more clustered than those of the adult.
Fig. 6.
Number (No.) of BK channels in excised micro patches. The number of BK channels in inside/out patches was determined at +60 mV in symmetrical KCl solutions with 3 mM Ca2+ in the bath solution to ensure maximal channel activation. Patch electrode tip resistances averaged 15.5 ± 0.1 MΩ (n = 192). Frequency histograms of the number of BK channels per patch preparation with distribution curve overlays were displayed. A: LTH adult (n = 39); B: LTH near-term fetus (n = 45); C: normoxic adult (n = 44); and D: normoxic near-term fetus (n = 64).
BKα expression and clustering.
To test further the hypothesis that BK channels are more clustered in the fetal groups, we used confocal microscopy to measure BKα channel expression and extent of BKα clustering. The representative micrographs (Fig. 7) show that myocytes of the four treatment groups exhibited BKα in both dispersed and clustered forms. Such variation in expression is consistent with our electrophysiological recordings of excised patches (Fig. 6).
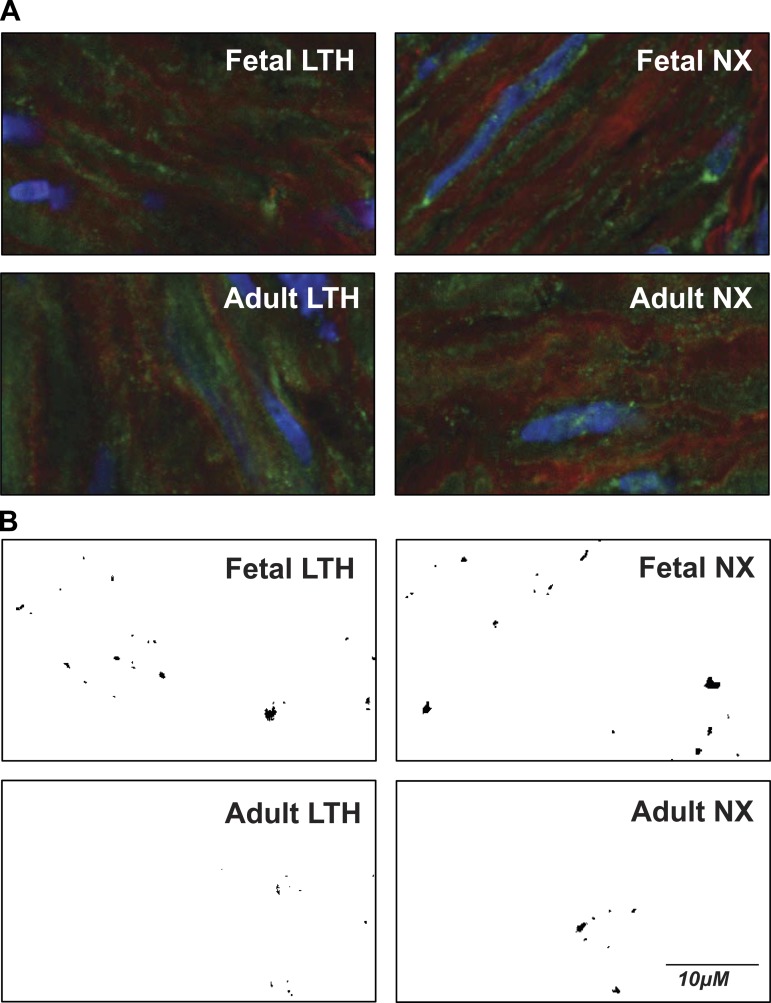
Fig. 7.
Representative confocal microscopic images of arterial myocytes reveal presence of dispersed and clustered BK channels. A: representative color images from adult LTH, fetal LTH, adult NX (normoxic), and fetal NX. Viewed areas measure 20 × 40 μm. Green color indicates presence of BK channels. B: green channel (BK fluorescence) intensities converted to binary image from same areas as above (A) after masking out all values below threshold (3.5× mean intensity). BK clusters show as black areas of different size and shape. Controls with secondary antibody alone or with primary antibody pre-absorbed with antigenic peptide revealed little to no detectable BKα fluorescence (data not shown).
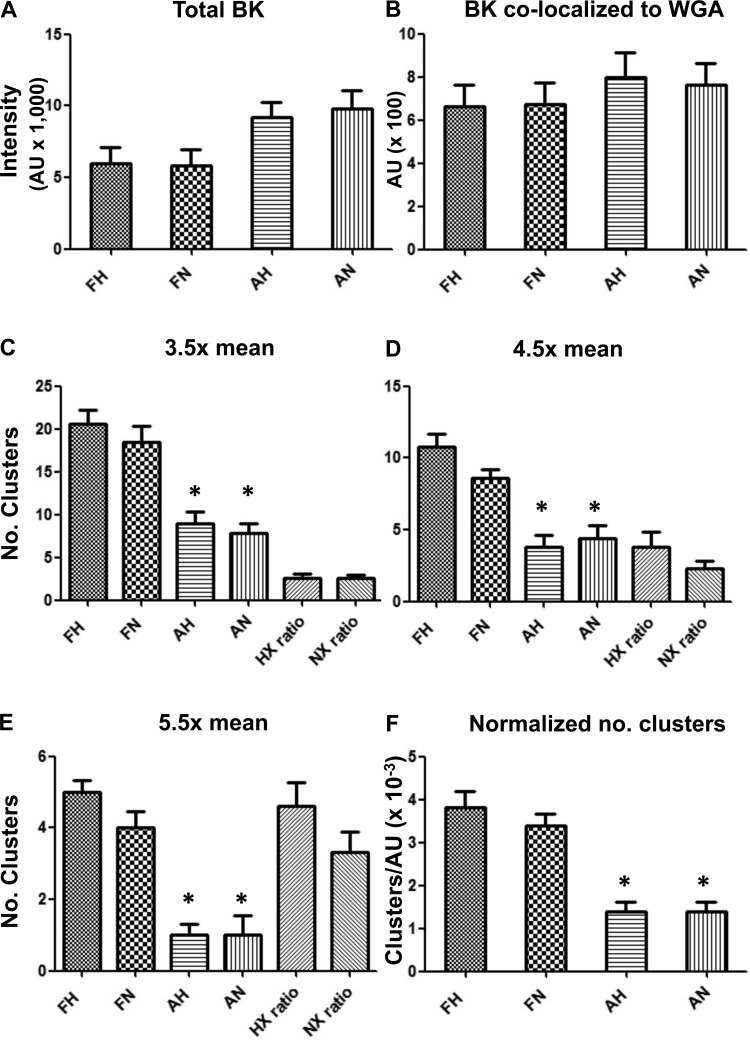
Myocytes from normoxic and LTH adult groups expressed 35% (P < 0.05) and 31% (P < 0.05) more total BKα per cross-sectional area than their fetal counterparts, respectively (Fig. 8A and Table 4). However, when BKα fluorescence was colocalized to the cell surface marker, AF-594 conjugated WGA, cell surface expression of BKα did not differ among the groups (Fig. 8B and Table 4). Again, this is consistent with our findings from counting channels in micropatches (Table 4). Adjacent sample sections treated with secondary antibody alone showed only dark backgrounds with diffuse, faint, nonlocalized fluorescence. BK-transfected HEK293 cells treated with primary antibody showed intense intracellular and cell surface fluorescence, whereas primary antibody pre-absorbed with BKα epitopic peptide revealed little cellular fluorescence (not shown).
Fig. 8.
Total BK channel density, BK surface density, and BK clustering measured in confocal images of intact basilar artery myocytes. A: total BK fluorescence intensity in arbitrary units (AU; means ± SE; n = 5), where FH is fetal hypoxic (LTH), FN is fetal normoxic, AH is adult hypoxic, and AN is adult normoxic. B: BK colocalized with the surrogate surface membrane marker, wheat germ agglutinin (WGA; n = 6). C: number (No.) of BK clusters measured at 3.5 times above mean intensity (n = 7). D: number of BK clusters measured at 4.5 times above mean intensity (n = 6). E: number of BK clusters measured at 5.5 times above mean intensity (n = 6). F: number of BK clusters at 3.5 times mean intensity per total BK intensity (n = 6). Imaged areas measured 20 × 40 μm. Number of animals in each group was either 3 or 4. *Significant difference with P < 0.001 relative to either fetal group. HX ratio and NX ratio in C, D, and E refer to FH:AH and FN:AN, respectively.
In contrast with total expression and surface expression, the fetal groups exhibited significantly more BKα clusters than their corresponding adult groups across a range of intensity thresholds above mean BKα fluorescence (e.g., Fig. 8, C–E). What is more, at higher intensity thresholds the ratio of fetal cluster numbers to adult clusters increased (Fig. 8E), suggesting that fetal groups have larger clusters than the adult counterparts. LTH and normoxic fetal groups expressed 2.7 (P < 0.01) and 2.4 (P < 0.01) times more BK clusters, respectively, than their corresponding adult groups (Fig. 8F and Table 4) after normalizing the number of BK clusters (e.g., Fig. 8C) to total BKα fluorescence (Fig. 8A). These results confirm our hypothesis that BK channels on fetal myocytes are more clustered (Fig. 8F).
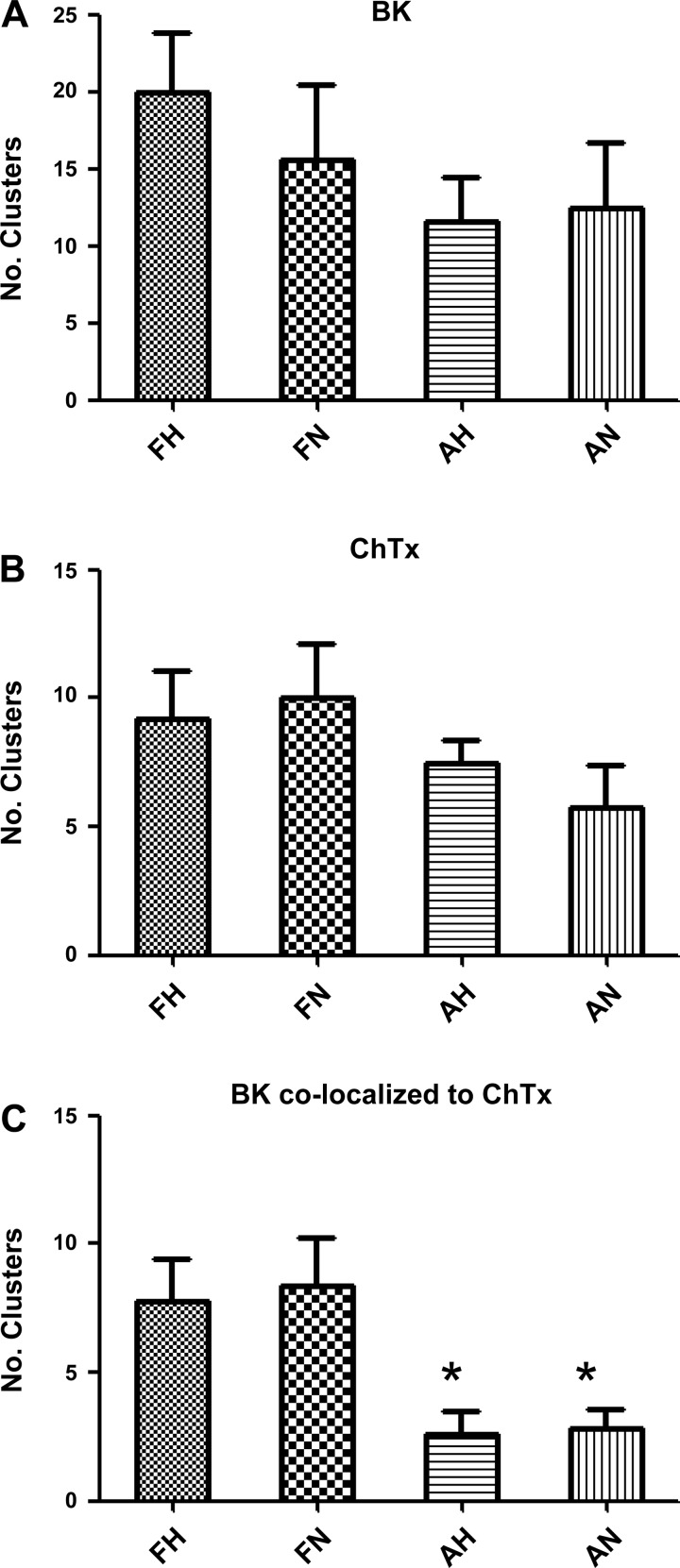
BK channels in vascular myocytes are known to localize on lipid rafts (2, 39). Therefore, we hypothesized that BK channel clusters colocalize with lipid rafts and that these fetuses would have greater lipid raft associated clusters as compared with adults, independent of altitude. To address this hypothesis, we measured BK channel clustering by examining cholera toxin B subunit-Alex 594 conjugate (ChTx) as a marker of GM1-containing lipid rafts (40, 46), such as caveolae (18). Operationally, we defined lipid rafts as sites of ChTx clusters and correlated this with BKα fluorescence using the methodology described for Fig. 8. Although slightly more ChTx clusters occur in the fetal groups than in the adults (Fig. 9B), the number of BK clusters that colocalize to ChTx clusters is two times higher in the fetal groups than their corresponding adult groups (P < 0.05) (Fig. 9C and Table 4).
Fig. 9.
BK channel clusters colocalized to cholera toxin clusters. A: number of BK clusters measured at 3.5 times above mean intensity (means ± SE; n = 5), where FH is fetal hypoxic (LTH), FN is fetal normoxic, AH is adult hypoxic, and AN is adult normoxic. B: number of cholera toxin (ChTx) clusters measured at 3.0 times above mean intensity (n = 5). C: number BK clusters colocalized with ChTx clusters. Imaged areas measured 20 × 40 μm. Number of animals in each group was 3. *Significant difference with P < 0.05 relative to either fetal group.
Perforated-patch whole-cell currents.
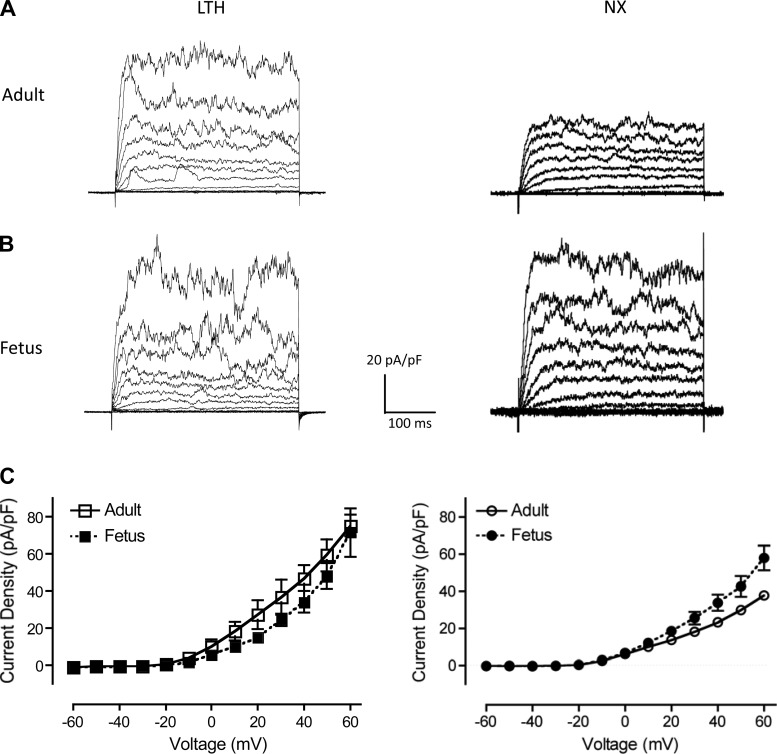
Because BK clusters colocalize to lipid rafts more in both fetal groups than in adults, we hypothesized that outward currents recorded from the two fetal groups would increase more relative to the adult groups while recording under conditions that permit spark activity (49). To test our prediction, we recorded whole-cell outward currents in perforated-patch mode (Fig. 10), which permits Ca2+ spark activity, and compared currents with conventional whole-cell mode (Fig. 1), which suppresses sparks. Membrane capacitances were similar to those from conventional whole-cell mode (Table 1). As predicted, outward current densities were higher in perforated-patch mode (Fig. 10) with LTH adult currents increasing by 39%, whereas LTH fetal currents increased by 189% (Table 1). In addition, normoxic fetal outward currents were higher than normoxic adults (Fig. 10 and Table 1). These results suggest that normoxic and LTH fetal BK channels may be more sensitive to endogenous Ca2+ sparks than adults (49, 59). Consistent with these results, resting membrane potentials from the LTH fetus were more negatively polarized than those of the other three groups (Table 1). In future work, we plan to examine sparks and spontaneous transient outward currents between adult and fetal groups in this ovine model.
Fig. 10.
Perforated-patch, whole-cell outward current density recordings. A and B: representative whole-cell outward membrane current density traces are shown from isolated LTH and normoxic (NX) adult (A) and fetal (B) basilar artery myocytes. Currents were elicited by a series of 10-mV depolarizing steps (−60 to +60 mV) from a holding potential of −60 mV. Whole-cell current density was used to normalize whole cell currents for size differences between adult and fetal myocytes. C: averaged steady-state current-voltage plot of outward current density in myocytes obtained from LTH (left) adult (n = 5) and fetal (n = 6) and normoxic (right; taken from Ref. 3) adult (n = 4) and fetal (n = 5) basilar arteries.
DISCUSSION
Despite the recognized physiological importance of BK channels in regulating vascular tone and maintaining adequate cerebral blood flow (28), the present study is the first to directly examine the effects of LTH on BK channels of the cerebral vasculature. Our present findings show that BK channels of basilar artery smooth muscle in LTH acclimatized adult and near-term fetus are significantly more active than their normoxic counterparts. Such LTH acclimatization involves lowering the Ca2+ set point, left shifting voltage activation independently of channel phosphorylation, and upregulating accessory BK β-l subunit expression.
LTH increased BK activity independent of age.
Several features distinguished LTH BK channels from normoxic controls, regardless of age group. The LTH BK channels exhibited 1) increased Ca2+ affinity (i.e., lower Ca2+ set points) (Fig. 2 and Table 1); 2) left-shifted V1/2 values (i.e., more negative) in each of three defined phosphorylation states (Fig. 3 and Table 2); 3) longer weighted mean open dwell times (Fig. 4 and Table 3); and 4) an apparent lower extent of phosphorylation in the endogenous native state (Fig. 3A). Together these features suggest that LTH acclimatization increases BK channel activity, which, in turn, may help provide adequate brain oxygen in the face of lowered arterial oxygen levels by maintaining CBF (29, 37).
The physiological challenge of high altitude is accentuated in the LTH fetus by additional demands of cerebral growth and development and by being in utero at lower arterial PO2 values (34). In apparent response to these additional demands, the LTH fetus upregulates cell surface expression of BK β1 (Fig. 5 and Table 4). It is known that decreased expression of BK β1 uncouples BK channels from Ca2+ sparks, increases vascular tone (4, 35, 49, 59), and produces hypertension in mice (3). Thus, an increased expression of BK β1 may enhance coupling of BK channels to Ca2+ sparks and decrease vascular tone in the LTH fetus. The increased BK channel activity in LTH fetal myocytes (Table 1) is further supported by their significantly more negative resting membrane potentials than the other three groups (Table 1). In keeping with these findings, we observed an estimated fivefold increase in BK current density in the LTH fetus in perforated-patch mode over conventional whole-cell mode, but less than a twofold increase in the LTH adult (Table 1).
Previously, our functional studies showed that LTH reduces NS1619-induced BK channel activation-mediated vasorelaxation of middle cerebral artery segments in the near-term fetus (36). This was attributed to either decreased BK channel expression or decreased sensitivity to Ca2+ (14). In contrast, our direct measurements presented in this study showed that in LTH fetal basilar arteries neither BK channel expression (Figs. 6 and 8B) nor affinity to Ca2+ decreased (Fig. 2 and Table 1). These present findings are consistent with our in vivo studies showing that CBF is near normal in the ovine LTH fetus (25, 48, 63). A possible explanation for conflicting ex vivo functional studies (14, 36) could stem from reported nonselective, off-site effects of NS1619 that may have offset the relaxation effects of BK channel activation when used on intact tissues (62). Such nonselective effects include inhibition of L-type Ca2+ channels (10, 19; 45), stimulation of Ca2+ mobilization from ryanodine-sensitive Ca2+ stores (27, 67), and stimulation of Ca2+-gated, Cl− currents (55).
One confounding result in the same ex vivo study (36) was that the LTH fetus showed greater sensitivity to iberiotoxin (IBTX; lower pD2 values) and greater extent of contraction at high doses of IBTX than the normoxic fetus despite significantly lower [Ca2+]i in the LTH fetal vessels (41). Taking into account that LTH fetal basilar arterial myocytes were also more hyperpolarized (Table 1), a consistent picture emerges with the BK channel activity of the LTH fetus being higher than that of its normoxic control (Table 1). Given the greater selectivity of IBTX for BK channels than NS1619 in tissue preparations, we suggest that our previous ex vivo studies (36) using the same model reflect the current findings of enhanced BK channel Ca2+ affinity and activity in the LTH fetus.
In a rat model of chronic hypoxia, the hind-limb vascular endothelial BK channel activity increased to reduce myogenic responsiveness and vasoconstriction (21). This hypoxia-induced increase in BK activity was accompanied by increased channel sensitivity to Ca2+ and channel colocalization to caveolin-1 (54), similar to what we found for the LTH fetus in the present study (Figs. 2 and 9, respectively). In addition, although vascular smooth muscle hyperpolarization occurred in both the LTH rat and ovine fetal models, the effect required an intact endothelium in the rat model (9), but in the ovine LTH fetal model was recorded in the absence of endothelium.
BK activity increases in both fetal groups.
Normoxic and LTH fetuses exhibit increased BK channel activity compared with their adult counterparts. To ensure adequate blood flow to the developing brain, the fetus appears to increase vascular BK channel activity without changing the level of BKα expression (Table 4 and Figs. 6 and 8). Both fetal groups increased BK channel affinity to Ca2+ (Table 1) (37), clustering (Fig. 8), and colocalization to lipid rafts (Fig. 9), however, via different mechanisms to increase the affinity to Ca2+. On the one hand, the normoxic fetus increases channel Ca2+ affinity (Table 1) by increasing channel phosphorylation (32, 33). On the other hand, the LTH fetus upregulates BK β1 surface expression (Fig. 5 and Table 4), increases open and closed dwell times (Fig. 4 and Table 3), and left-shifts voltage activation (Fig. 3 and Table 2). Furthermore, the LTH fetal channels appear relatively dephosphorylated (Fig. 3A), which potentially provides the LTH fetal channels with a capacity of up to a 10-fold increase in Ca2+ affinity (33), depending upon extent of PKA or PKG signaling pathway stimulation (5, 32).
Perspective.
It has been suggested that BK channels may respond directly or indirectly to acute hypoxia. The present study underscores an important role of cerebral artery BK channels as a long-term hypoxia mediator in regulating vascular tone and CBF. Long-term hypoxia-induced increases cerebrovascular BK channel activity may be a partial physiological basis by which sheep and other herbivores (Fam. Bovidae) can successfully acclimatize to long-term high altitude as compared with many mammals that cannot (53).
GRANTS
This work was supported in part by National Institutes of Health Grants HD-003807-41 and HD031226-20 (to L. D. Longo) and HD06946 (to S. Wilson). A portion of this study was performed in the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core that is supported by the National Science Foundation under Major Research Instrumentation, Division of Biological Infrastructure Grant No. 0923559 (to S. Wilson) and the Loma Linda University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.T., M.T.L., and G.U.T. performed experiments; X.T., M.T.L., G.U.T., S.M.W., and D.A.H. analyzed data; X.T., M.T.L., and G.U.T. prepared figures; X.T., M.T.L., L.D.L., and D.A.H. drafted manuscript; X.T., M.T.L., G.U.T., S.M.W., L.D.L., and D.A.H. edited and revised manuscript; X.T., M.T.L., G.U.T., S.M.W., L.D.L., and D.A.H. approved final version of manuscript; S.M.W. and D.A.H. interpreted results of experiments; L.D.L. and D.A.H. conceived of and designed the research.
ACKNOWLEDGMENTS
We are indebted to Monica Romero for assistance with confocal microscopy. We appreciate the help of Dr. Pooja Mujumdar with aspects of data presentation and statistical analysis.
REFERENCES
- 1.Ainslie PN, Subudhi AW. Cerebral blood flow at high altitude. High Alt Med Biol 15: 133–140, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo 1 caveolin-binding motif, a mechanism of caveolin-Slo 1 interaction regulating Slo 1 surface expression. J Biol Chem 283: 4809–4817, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest 112: 717–724, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Brugniaux JV, Hodges ANH, Hanly PJ, Poulin MJ. Cerebrovascular responses to altitude. Resp Physiol Neurobiol 158: 212–223, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Earley S, Naik JS, Walker BR. 48-h Hypoxic exposure results in endothelium-dependent systemic vascular smooth muscle cell hyperpolarization. Am J Physiol Regul Integr Comp Physiol 283: R79–R85, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol 113: 1538–1547, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferriero DM. Neonatal brain injury. N Engl J Med 351: 1985–1995, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology 21: 29–37, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Gebremedhin D, Bonnet P, Greene AS, England SK, Rusch NJ, Lombard JH, Harder DR. Hypoxia increases the activity of Ca2+-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflügers Arch 428: 621–630, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert RD, Pearce WH, Longo LD. Fetal cardiac and cerebrovascular acclimatization responses to high altitude, long-term hypoxia. High Alt Med Biol 4: 203–213, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Goddard-Finegold J, Mizrahi EM. Understanding and preventing perinatal, intracerebral, peri- and intraventricular hemorrhage. J Child Neurol 2: 170–185, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Hackett PH, Roach RC. High altitude cerebral edema. High Alt Med Biol 5: 136–146, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- 18.Pang H, Le PU, Nabi IR. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J Cell Sci 117: 1421–1430, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol 117: 119–129, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92: 145–159, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol 299: H1439–H1450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter CJ, Blood AB, White CR, Pearce WJ, Power GG. Role of nitric oxide in hypoxic capillary flow response to hypoxia. J Physiol (Lond) 549: 625–633, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen GFA, Kagenaar DA, Basnyat B, Odoom JA. Basilar artery blood flow velocity and the ventilatory response to acute hypoxia in mountaineers. Resp Physiol Neurobiol 133: 65–74, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Jensen JB, Wright AD, Lassen NA, Harvey TC, Winterborn MH, Bradwell AR. Cerebral blood flow in acute mountain sickness. J Appl Physiol 69: 430–433, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Kamitomo M, Alonso JG, Okai T, Longo LD, Gilbert RD. Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169: 701–707, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Kirby BS, Bruhl A, Sullivan MN, Francis M, Dinenno FA, Earley S. Robust internal elastic lamina fenestration in skeletal muscle arteries. PLoS One 8: e54849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korper S, Nolte F, Rojewski MT, Thiel E, Schrezenmeier H. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34 cell line KG-1a. Exp Hematol 31: 815–823, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–79, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Hatran DP, Tomimatsu T, Peña JP, McAuley G, Longo LD. Fetal cerebral blood flow, electrocorticographic activity, and oxygenation: responses to acute hypoxia. J Physiol 587: 2033–2047, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin MT, Adelman JP, Maylie J. Modulation of endothelial SK3 channel activity by Ca2+ dependent caveolar trafficking. Am J Physiol Cell Physiol 303: C318–C327, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin MT, Hessinger DA, Pearce WJ, Longo LD. Developmental differences in Ca2+-activated K+ channel activity in ovine basilar artery. Am J Physiol Heart Circ Physiol 285: H701–H709, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Lin MT, Longo LD, Pearce WJ, Hessinger DA. Ca2+-activated K+ channel-associated phosphatase and kinase activities during development. Am J Physiol Heart Circ Physiol 289: H414–H425, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Lin MT, Hessinger DA, Pearce WJ, Longo LD. Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol 291: H732–H740, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Llanos AJ, Riquelme RA, Sanheuza EM, Hanson MA, Blanco CE, Parer JT, Herrera EA, Pulgar VM, Reyes RV, Cabello G, Giussani DA. The fetal llama versus the fetal sheep: different strategies to withstand hypoxia. High Alt Med Biol 4: 193–202, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Lohn M, Lauterbach B, Haller H, Pongs O, Luft FC, Gollasch M. β1-Subunit of BK channels regulates arterial wall [Ca2+] and diameter in mouse cerebral arteries. J Appl Physiol 91: 1350–1354, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Long W, Zhang L, Longo LD. Fetal and adult cerebral artery KATP and KCa channel responses to long-term hypoxia. J Appl Physiol 92: 1692–1701, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Longo LD, Pearce WJ. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp Biochem Physiol A 119: 683–694, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol 288: R16–R24, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Lu T, Zhang DM, Wang XL, He T, Wang RX, Chai Q, Katusic ZS, Lee HC. Regulation of coronary arterial BK channels by caveolae-mediated angiotensin II signaling in diabetes mellitus. Circ Res 106: 1164–1173, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massol RH, Larsen JE, Fujinaga Y, Lencer WI, Kirchhausen T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol Biol Cell 15: 3631–3641, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauli SM, Williams JM, Gerthoffer WT, Pearce WJ. Chronic hypoxia modulates relations among calcium, myosin light chain phosphorylation, and force differently in fetal and adult ovine basilar arteries. J Appl Physiol 99: 120–127, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Navarro-Antolín J, Levitsky KL, Calderón E, Ordóñez A, López-Barneo J. Decreased expression of maxi-K channel beta 1-subunit and altered vasoregulation in hypoxia. Circulation 112: 1309–1315, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Nelson KB. Can we prevent cerebral palsy? N Engl J Med 349: 1765–1769, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Orio P, Latorre R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. J Gen Physiol 125: 395–411, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park WS, Kang SH, Son YK, Kim N, Ko JH, Kim HK, Ko EA, Kim CD, Han J. The mitochondrial Ca2+-activated K+ channel activator, NS 1619 inhibits L-type Ca2+ channels in rat ventricular myocytes. Biochem Biophys Res Commun 362: 31–36, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveoli. J Histochem Cytochem 42: 155–166, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium 35: 427–431, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Pereyra Peña JL, Tomimatsu T, Hatran DP, McGill LL, Longo LD. Cerebral blood flow and oxygenation in the ovine fetus: responses to superimposed hypoxia at both low and high altitude. J Physiol (Lond) 578: 359–370, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 87: 53–60, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Qian X, Magleby KL. β1 subunits facilitate gating of BK channels by acting through the Ca2+, but not the Mg2+, activating mechanisms. Proc Natl Acad Sci 100: 10061–10066, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasband WS. ImageJ. U. S. National Institutes of Health, Bethesda, MD; http://imagej.nih.gov/ij/, 1997–2014[1 Aug 2014]. [Google Scholar]
- 52.Resnik E, Herron J, Fu R, Ivy DD, Cornfield DN. Oxygen tension modulates the expression of pulmonary vascular BKCa channel α and β subunits. Am J Physiol Lung Cell Mol Physiol 290: L761–L768, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Rhodes J. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol 98: 1092–1100, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Riddle MA, Hughes JM, Walker BR. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am J Physiol Cell Physiol 301: C1404–C1414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleh SN, Angermann JE, Sones WR, Leblanc N, Greeenwood IA. Stimulation of Ca++-gated Cl− currents by the calcium-dependent K+ channel modulators NS1619 (1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-H-benzimidazol-2-one) and isopimaric acid. J Pharmacol Exp Ther 321: 1075–1084, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Sakmann B, Neher E. Single-Channel Recording. New York: Plenum, 1995, p. 627–650. [Google Scholar]
- 57.Samaranayake H, Saunders JC, Greene MI, Navaratnam DS. Ca2+ and K+ (BK) channels in chick hair cells are clustered and colocalized with apical-basal and tonotopic gradients. J Physiol 560: 13–20, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacol 35: 963–968, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel β1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol 29: 802–810, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Severinghaus JW, Chiodi H, Eger EIII, Branstater B, Hornbein TF. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res 19: 274–282, 1966. [DOI] [PubMed] [Google Scholar]
- 61.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szewczyk A, Kajma A, Malinska D, Wrzosek A, Bednarczyk P, Zabocka B, Doowy K. Pharmacology of mitochondrial potassium channels: dark side of the field. FEBS Letters 584: 2063–2069, 2010. [DOI] [PubMed] [Google Scholar]
- 63.Tomimatsu T, Pereyra-Peña JL, Hatran DP, Longo LD. Maternal oxygen administration and fetal cerebral oxygenation: studies on near-term fetal lambs at both low and high altitude. Am J Obstet Gynecol 195: 535–541, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Vargas M, Ororio J, Moraga D, Sepulveda M, Del Solar J, Hudson C, Cortes G, Leon A. Acute mountain sickness at 3500 and 4250 m. A study of symptom incidence and severity. Rev Med Chile 127: 166–172, 2001. [PubMed] [Google Scholar]
- 65.Volpe A. Brain injury in the premature infant: neuropathology, clinical aspects, pathogenesis and prevention. Clin Perinatol 24: 567–587, 1997. [PubMed] [Google Scholar]
- 66.Wilson MH, Edsell MEG, Davagnanam I, Hirani SP, Martin DS, Levett DZH, Thornton JS, Golay X, Strycharczuk L, Newman SP, Montgomery HE, Grocott MPW, Imray CHE. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia—an ultrasound and MRI study. J Cerebral Blood Flow Metab 31: 2019–2029, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamura H, Ohi Y, Muraki K, Watanabe M, Imaizumi Y. BK channel activation by NS-1619 is partially mediated by intracellular Ca2+ release in smooth muscle cells of porcine coronary artery. Brit J Pharmacol 132: 828–834, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]