Abstract
Arterial hypotension can be induced by sudden release of inflated thigh cuffs (THC), but its effects on the cerebral circulation have not been fully described. In nine healthy subjects [aged 59 (9) yr], bilateral cerebral blood flow velocity (CBFV) was recorded in the middle cerebral artery (MCA), noninvasive arterial blood pressure (BP) in the finger, and end-tidal CO2 (ETCO2) with nasal capnography. Three THC maneuvers were performed in each subject with cuff inflation 20 mmHg above systolic BP for 3 min before release. Beat-to-beat values were extracted for mean CBFV, BP, ETCO2, critical closing pressure (CrCP), resistance-area product (RAP), and heart rate (HR). Time-varying estimates of the autoregulation index [ARI(t)] were also obtained using an autoregressive-moving average model. Coherent averages synchronized by the instant of cuff release showed significant drops in mean BP, CBFV, and RAP with rapid return of CBFV to baseline. HR, ETCO2, and ARI(t) were transiently increased, but CrCP remained relatively constant. Mean values of ARI(t) for the 30 s following cuff release were not significantly different from the classical ARI [right MCA 5.9 (1.1) vs. 5.1 (1.6); left MCA 5.5 (1.4) vs. 4.9 (1.7)]. HR was strongly correlated with the ARI(t) peak after THC release (in 17/22 and 21/24 recordings), and ETCO2 was correlated with the subsequent drop in ARI(t) (19/22 and 20/24 recordings). These results suggest a complex cerebral autoregulatory response to the THC maneuver, dominated by myogenic mechanisms and influenced by concurrent changes in ETCO2 and possible involvement of the autonomic nervous system and baroreflex.
Keywords: cerebral autoregulation, cerebral blood flow, arterial hypotension, autonomic nervous system, critical closing pressure, cerebrovascular resistance
the thigh cuff (thc) maneuver involves the inflation of wide, above-knee cuffs, 20 mmHg or more above systolic blood pressure (BP) for 2 or 3 min, followed by their sudden deflation. The hyperemic response resulting from temporary arterial occlusion leads to a reduction in peripheral resistance and a sudden drop in mean BP (MBP) lasting ∼10–15 s. This approach to induce transient hypotension has been used as a stimulus to study different aspects of cardiovascular regulation at rest and during exercise (14, 16, 43).
In recent years, one of the main applications of the THC maneuver has been the study of cerebral blood flow (CBF) autoregulation. As shown by Aaslid et al. (2), the sudden drop in MBP induces a concomitant rapid drop in CBF, usually estimated from measurements of CBF velocity (CBFV) obtained with transcranial Doppler, which returns to its original value before the MBP signal, thus showing the dynamic nature of cerebral autoregulation (CA) (2, 39).
Different approaches have been adopted to quantify the transient relationship between CBFV and MBP following THC deflation, such as the rate of regulation (RoR) (2), the autoregulation index (ARI) (39), or other indexes (6). Although changes in key physiological variables such as heart rate (HR) and PaCO2 have been reported following the release of pressurized THC (8, 14, 16, 27), these potential confounders have been largely ignored in applications of the THC approach for assessment of dynamic CA. One exception though is the study of Ogoh et al. (27) where changes in HR were shown to be correlated to the RoR index following autonomic blockade.
More detailed analyses of the cerebrovascular effects of the THC maneuver and the influence of other physiological variables on dynamic CA indexes have the potential to advance our understanding of CBF regulation as well as improving current techniques for assessment of dynamic CA. One relevant recent development is the possibility of obtaining continuous estimates of ARI with temporal resolution of a few seconds. The feasibility of this approach has been demonstrated in recordings obtained at rest (31, 35), and during respiratory maneuvers such as breath holding or hyperventilation (10, 30), and during the Valsalva maneuver (5). However, time-varying changes in dynamic CA parameters have not been described during the THC maneuver. If dynamic CA is shown to vary during THC inflation, or following its sudden deflation, this will influence current conceptual models of CBF regulation and will also impact on the direction of future research on cerebral hemodynamics. Moreover, existing techniques for assessment of dynamic CA will also need to be revised with the prospect that a more refined approach to CA assessment might also lead to clinical benefits. To investigate this possibility, we tested the hypothesis that dynamic CA does not remain constant following the release of pressurized THC in healthy adult subjects and have also tested the potential contribution of HR and PaCO2 to explain these changes.
METHODS
Subjects and measurements.
Data for the current study were collected as part of a PhD project (for N. P. Saeed) and previously used as the control group for a clinical study (37). Reanalysis of the same set of data in the current study was performed to test different and original hypotheses. In brief, healthy subjects were recruited from departmental staff and their relatives. Subjects were free from any cardiovascular or neurological disease. The study was approved by the local ethics committee (09/H0403/25), and written informed consent was obtained from each subject.
Continuous noninvasive measurement of arterial BP was performed in the middle finger of the nondominant hand with the Finapres device (Ohmeda 2300). Casual BP was measured in the opposite upper arm using a validated sphygmomanometer (Omron 705CP-11). Bilateral measurements of CBFV were obtained in the middle cerebral arteries (MCA) with a 2-MHz probe supported by a headframe (Vyasis Companion III). A surface three-lead ECG was attached to the participants' arms and end-tidal carbon dioxide (ETCO2) was recorded using small nasal cannulae connected to an infrared capnograph (Capnocheck Plus). The servo adjust function of the Finapres was switched off during recordings.
After recordings were stable for at least 15 min, BP was measured with the automatic sphygmomanometer and a 5-min baseline recording was obtained with the subject breathing normally. This was followed by three separate THC maneuvers, each preceded by a BP measurement with the Omron while the servo-adjust function of the Finapres was turned on and then off again before the continuous measurements. Large bilateral THC (Hokanson model C22) were inflated 20 mmHg above systolic BP for 3 min. Rapid deflation of the cuffs was achieved with a special cuff deflator (Hokanson model RD2) to induce a sudden drop in BP, with recording of all signals continuing for another 90 s.
Continuous recordings of BP, CBFV, ETCO2, and ECG were sampled at 500 samples/s with 16-bit resolution and stored for further analysis.
Data analysis.
Data were carefully inspected and edited to remove narrow spikes (<100 ms) by linear interpolation.
The beginning and end of each cardiac cycle was automatically detected using a matched filter of the QRS complex of the ECG to obtain beat-to-beat values of HR. Mean, systolic, and diastolic values of BP and CBFV were extracted for each cardiac cycle. Resistance-area product (RAP) was calculated as RAP = P1/V1, where P1 and V1 are the first harmonics of the BP and CBFV waveforms for each cardiac cycle, respectively. RAP can also be calculated from the linear regression of the velocity-pressure scatterdiagram for each cardiac cycle, but it has been shown that the first harmonic method provides more robust estimates (34). With the use of this value of RAP, critical closing pressure (CrCP) can then be determined from CrCP = MBP − MCBFV.RAP, where MCBFV is the mean CBFV for each cardiac cycle. The end-tidal value of the continuous ETCO2 signal was automatically detected and resampled in synchrony with the cardiac cycle using linear interpolation. Beat-to-beat values of all extracted parameters were interpolated with a third order polynomial and resampled at 5 Hz to produce signals with a uniform time base. The resulting ensemble of time-varying parameters was visually inspected, and the position of the MBP drop was marked as a reference for further analyses. THC maneuvers were only accepted if the induced drop in MBP was >15 mmHg.
The efficiency of dynamic CA was assessed using the second-order differential equation model proposed by Tiecks et al. (39). The three main model coefficients were combined by Tiecks et al. (39) to provide an unidimensional scale characterized by a single parameter, the autoregulation index (ARI) that varies between 0 (absence of autoregulation) to 9 (best observed CA). For an idealized negative step change in MBP, each value of ARI leads to a CBFV step response that mimics the physiological response to the THC release. By comparing each one of these ideal responses to real data, usually based on least square curve fitting, it is possible to identify the value of ARI that best represents the efficiency of dynamic CA for each response to THC release. This approach will be referred to as “classical ARI” and was adopted to extract a single value of ARI for each THC maneuver.
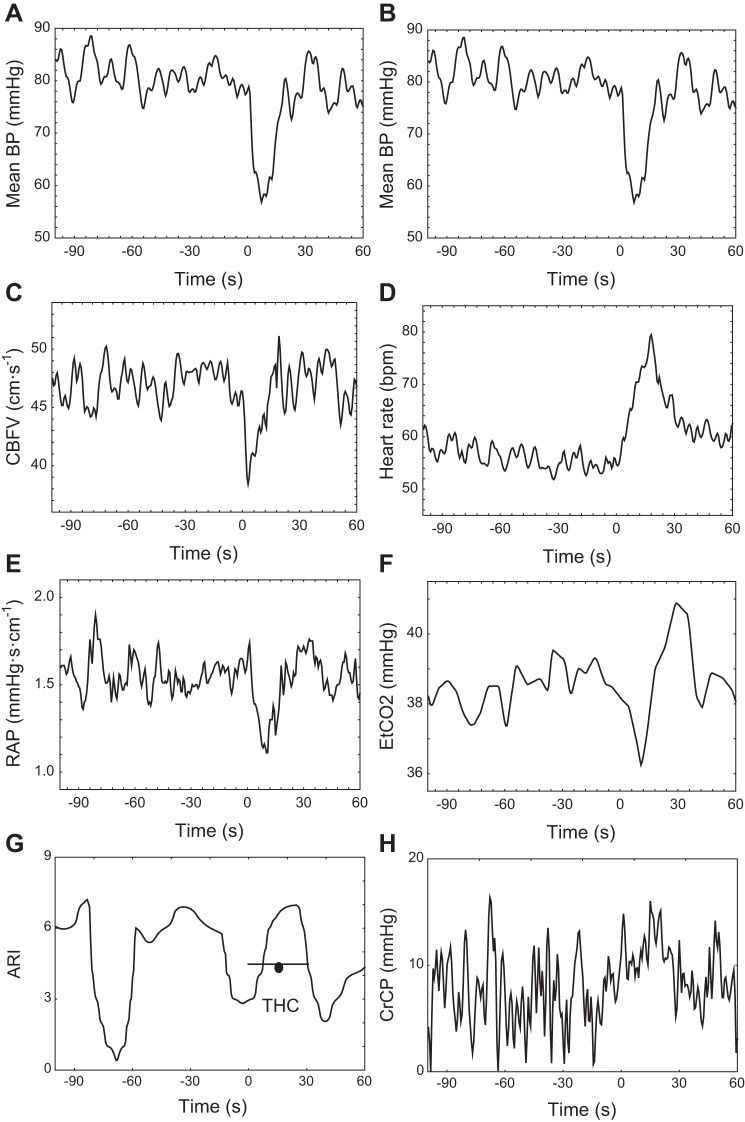
Continuous estimates of ARI, represented by ARI(t), can also be generated as described previously (30). For the entire recording, including the THC maneuver, the dynamic relationship between beat-to-beat values of MBP and CBFV was modeled in the time-domain, using an autoregressive-moving average model. Instead of the classical approach where coefficients are constants, in this case model coefficients were expanded as a Fourier series and their corresponding amplitudes also determined by least squares, using a maximum of 20 sinusoidal functions (45). With the use of the estimated time-varying model coefficients, it is then possible to obtain CBFV step responses for each time sample along the recording and, from each step response, one estimate of ARI(t), following the same procedure as for the classical ARI, that is by fitting one of the 10 template responses proposed by Tiecks et al. (30, 39). Time series of ARI(t) were obtained for each THC maneuver comprising 3 min before cuff release and 90 s postrelease after subsampling the data to time intervals of 0.6 s. An example of ARI(t) obtained with the sinusoidal expansion is given in Fig. 1.
Fig. 1.
Representative recordings from a 46 year old healthy male volunteer, with cerebral blood flow velocity (CBFV) recordings from the right middle cerebral arteries (MCA). Mean arterial blood pressure (BP; A and B), CBFV (C), heart rate (HR; D), resistance area-product (RAP; E), end-tidal CO2 (ETCO2; F), continuous autoregulation index (ARI; G), and critical closing pressure (CrCP; H). Time = 0 marks the instant of thigh cuff (THC) release. In G, the thick horizontal line with circle represents the classical ARI value obtained by direct fitting of Tiecks et al. (39) model for an interval of 30 s following THC release.
Statistical analysis.
For each subject, average curves of MBP, HR, ETCO2, mean CBFVR (right MCA), mean CBFVL (left MCA), RAPR, RAPL, CrCPR, CrCPL, ARIR(t), and ARIL(t) were obtained by coherent averaging using the time point of cuff release as the point of synchronism. From these, population mean and SD curves were obtained by averaging across subjects. Repeated-measures ANOVA was used to test for longitudinal changes in parameters at five time points corresponding to baseline (BSL), pre-THC release (pre-REL), and then 5, 20, and 40 s after release (REL5, REL20, and REL40, respectively). BSL corresponds to the average parameter value for 10 s at the beginning of cuff inflation. For pre-REL, REL5, REL20, and REL40, parameters were averaged for 5 s around the central point with pre-REL centered 5 s before release. Tukey's post hoc test was applied as appropriate. Paired t-tests were adopted to assess differences between BSL and pre-REL, using Bonferroni adjustment for multiple testing.
After THC release, the temporal pattern of ETCO2 and HR were cross correlated with ARI(t) using a 60-s window. The first maximum value of cross correlation was extracted for the HR-ARI(t) cross-correlation function and the first minimum value for the ETCO2-ARI(t) cross correlation. These values were compared with the 95% confidence limit of the cross-correlation function calculated as 1.96/√N, where N = 100 is the number of samples adopted for cross-correlation estimates, corresponding to 60 s. Differences were accepted as significant for P < 0.05.
RESULTS
Ten healthy volunteers were recruited to the study, but one subject could not complete her measurements due to intolerance to THC inflation. Complete measurements were obtained in nine subjects (seven males). Mean (SD) age was 59 (15) yr, and baseline characteristic values of main physiological parameters are given in Table 1. Of a total of 27 THC maneuvers, one was rejected due to a MBP drop <15 mmHg and in another ETCO2 could not be recorded due to technical problems. In one subject, CBFV measurements could not be obtained in the right MCA; in this case only parameters derived from the left MCA were considered for analysis. One recording for the left MCA could not be used for estimation of ARI(t) due to excessive noise. As a result, 22 THC maneuvers were available for analysis for the right MCA (8 subjects) and 24 for the left MCA (9 subjects), with a minimum of 2 THC maneuvers per subject.
Table 1.
Cerebral and peripheral hemodynamic parameters at baseline and 4 selected time points during the thigh cuff maneuver
| Parameter | BSL | Pre-REL | REL5 | REL20 | REL40 | P Value ANOVA |
|---|---|---|---|---|---|---|
| MAP, mmHg | 95.0 (23.2) | 97.0 (24.8) | 76.8 (27.0)a | 87.4 (28.6) | 91.0 (25.4) | 0.00001 |
| Heart rate, beats/min | 61.4 (7.3) | 60.9 (5.7) | 65.7 (4.6) | 66.7 (6.0)b | 60.5 (6.2) | 0.0085 |
| ETCO2, mmHg | 37.7 (3.9) | 37.8 (4.3) | 37.8 (4.1) | 39.0 (3.9)c | 38.4 (3.0) | N.S. |
| CBFV, cm/s | ||||||
| R | 49.5 (10.5) | 48.7 (8.4) | 43.4 (10.1)a | 48.2 (7.1)d | 48.0 (7.4)d | 0.0010 |
| L | 53.1 (10.7) | 52.9 (10.0)d | 47.2 (10.8)a | 52.9 (9.9) | 52.9 (10.2) | 0.0002 |
| RAP, mmHg·s·cm−1 | ||||||
| R | 1.65 (0.52)e | 1.75 (0.52) | 1.43 (0.39)a | 1.55 (0.47) | 1.62 (0.47) | 0.0013 |
| L | 1.58 (0.55) | 1.66 (0.60) | 1.39 (0.60)a | 1.52 (0.68) | 1.56 (0.68) | 0.0100 |
| CrCP, mmHg | ||||||
| R | 14.7 (10.3) | 12.5 (12.9) | 14.6 (8.6) | 13.2 (11.1) | 11.7 (12.8) | N.S. |
| L | 14.1 (11.5) | 12.5 (10.9) | 13.7 (8.6) | 10.4 (12.8) | 10.7 (11.8) | N.S. |
| ARI | ||||||
| R | 4.7 (2.0) | 2.9 (1.6) | 3.9 (1.9) | 6.7 (1.3)f | 4.6 (2.2) | 0.00058 |
| L | 4.4 (1.9) | 3.0 (1.6) | 2.8 (1.3) | 6.5 (1.7)f | 4.6 (1.9) | 0.00037 |
Values are means (SD). MAP, mean arterial blood pressure; ETCO2, end-tidal CO2; CBFV, cerebral blood flow velocity; RAP, resistance area product; CrCP, critical closing pressure; ARI, dynamic cerebral autoregulation index; R, right middle cerebral arteries (MCA); L, left MCA; BSL, baseline; pre-REL, 3 s before thigh cuff (THC) release; REL5, 5 s after THC release; REL20, 20 s after THC release; REL40, 40 s after THC release.
P < 0.01, post hoc Tukey's test compared with pre-REL.
P < 0.05, post hoc Tukey's test compared with REL40.
P < 0.05, paired t-test compared with REL5.
P < 0.05, post hoc Tukey's test compared with REL5.
P < 0.05, post hoc Tukey's test compared with REL5.
P < 0.01, post hoc Tuckey's test compared with pre-REL and REL5.
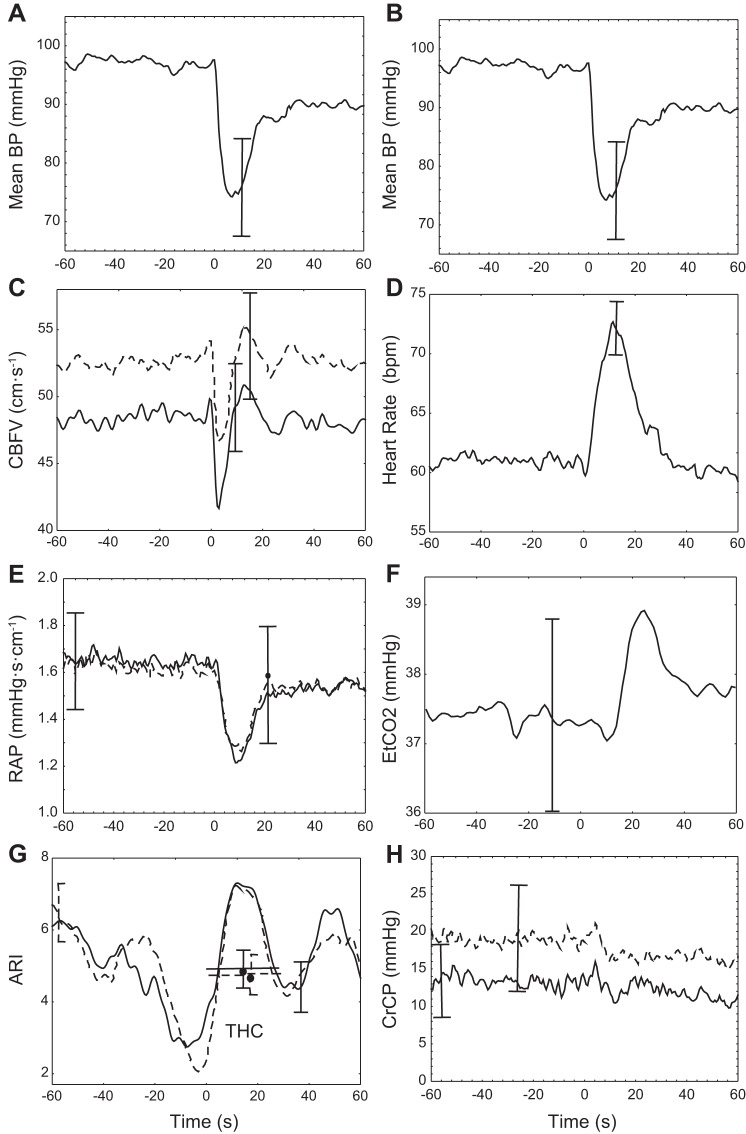
Representative temporal changes in physiological parameters are shown in Fig. 1 for a 46-yr-old male subject. Figure 2 gives corresponding population averages. In both figures, the MBP trace is repeated in both columns (Fig. 2, A and B) to facilitate comparison with other parameters. Following a population mean (SD) drop in MBP of 25.6 (9.7) mmHg, CBFV showed a rapid return to baseline values indicating a normal working dynamic CA in this group (Figs. 1C and 2C). Comparing Fig. 2, E and H, CBFV recovery seems to result mainly from a delayed reduction in RAP, instead of changes in CrCP. In both MCA, estimates of ARI(t) dropped before THC release and then rose to a peak ∼10–20 s after deflation. Following the drop in MBP, HR showed a pronounced peak (Figs. 1D and 2D) and there was also a delayed rise in ETCO2 (Figs. 1F and 2F).
Fig. 2.
Population coherent averages synchronized by the instant of THC release (t = 0). Mean arterial BP (A and B), CBFV (C), HR (D), RAP (E), ETCO2 (F), ARI (G), and CrCP (H). Averages from the right (continuous line) and left (dashed line) MCA are distinguished in C, E, G, and H. In G, the thick horizontal line with circle represents the population means ± SE classical ARI value obtained by direct fitting of Tiecks et al. (39) model, for an interval of 30 s following THC release. For the coherent averages, error bars represent the largest ± 1SE at the instant of occurrence.
Statistical analysis of parameter values at baseline and at four selected time points is summarized in Table 1. With the exception of ETCO2 and CrCP, all other parameters presented significant longitudinal differences on a repeated-measures ANOVA (P < 0.01). Paired t-tests did not show any significant differences between values at baseline (BSL) and 5 s before THC release (pre-REL; Table 1). Following THC release (REL5), the fall in MBP (P < 0.01), CBFV (P < 0.01) and RAP (P < 0.01) was all significant compared with prerelease values (Table 1). The rise in HR (P < 0.05) and ETCO2 (P < 0.05) ∼20 s after release (REL20) was also significant compared with preceding values. Finally, ARI(t) also presented highly significant changes, with the peak at REL20 showing marked differences compared with values at pre-REL (P < 0.01) and REL5 (P < 0.01, Table 1). Mean values of ARI(t) for the 30 s following cuff release were not significantly different from those obtained with the classical ARI [right MCA 5.9 (1.1) vs. 5.1 (1.6); left MCA 5.5 (1.4) vs. 4.9 (1.7)].
Representative cross-correlation functions are given in Fig. 3A for the same subject as in Fig. 1. Cross correlation of ETCO2 with ARI(t) in the 60 s after THC release showed a characteristic trough with time delays of 12.7 (11.7) s (ETCO2-ARIR) and 10.2 (11.0) s (ETCO2-ARIL). The positive time delays in this case indicate that changes in ETCO2 preceded corresponding changes in ARI(t). Figure 3A also illustrates the typical cross-correlation curve for the HR-ARI(t) relationship. For the population, peak values occurred for time delays of 0.46 (4.2) s (HR-ARIR) and 0.63 (4.6) s (HR-ARIL). As shown in Fig. 3, B and C, the large majority of recordings for the right and left MCA had significant minimum values for the ETCO2-ARI(t) cross-correlation and maximum values for the HR-ARI(t) cross correlation.
Fig. 3.
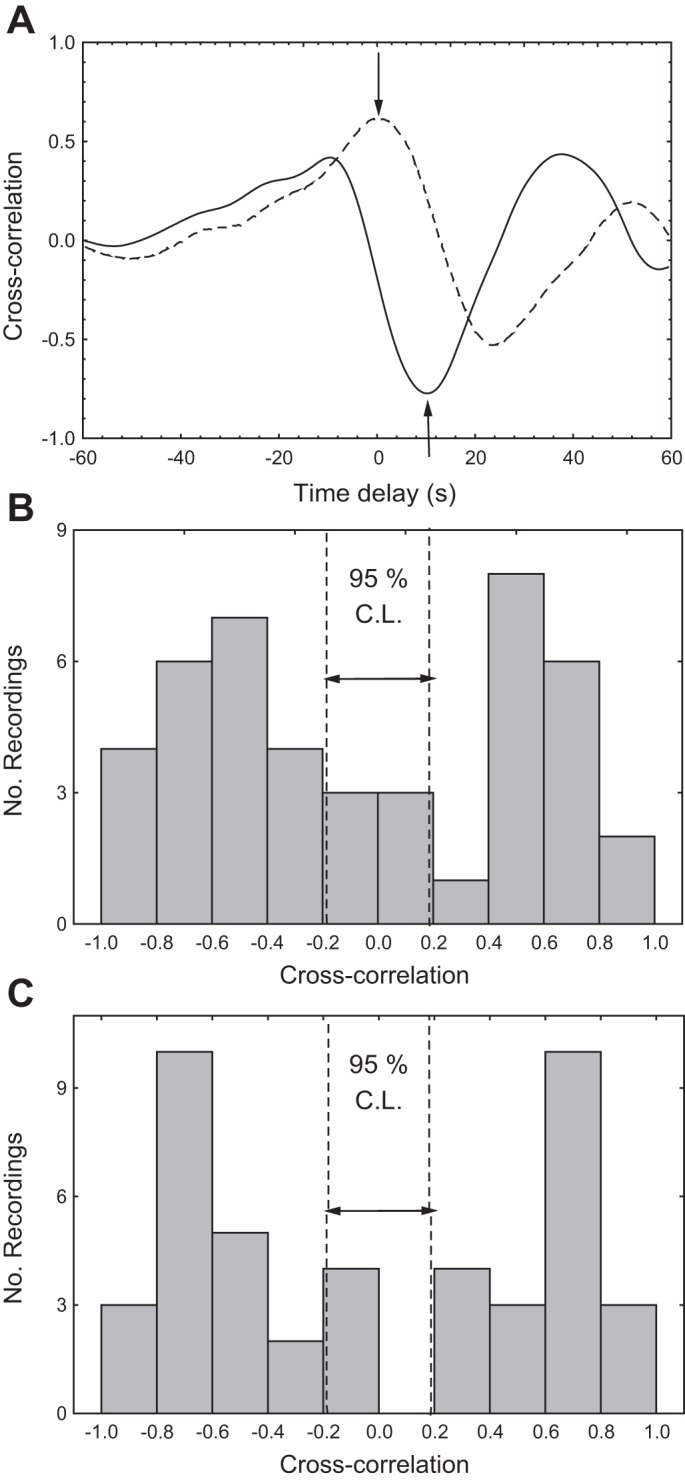
A: representative cross-correlation functions between continuous ARI and ETCO2 (continuous line) and HR (dashed line) for the same subject as in Fig. 1. The arrows indicate the position of maximum and minimum cross correlations detected in the respective curves. B: population distribution of minimum values of cross correlation between ETCO2 and ARI(t) (negative values) and maximum values of cross correlation between HR and ARI(t) (positive values) for the right MCA. C: similar representation for the left MCA. The vertical dashed lines represent the 95% confidence limit (C.L.) for cross-correlation amplitude.
DISCUSSION
Main findings.
Longitudinal changes in the performance of dynamic CA induced by the THC maneuver have not been described previously. The temporal changes observed in individual recordings (Fig. 1G) and in the population as a whole (Fig. 2G) were characterized by a prominent peak in ARI(t) ∼20 s after THC release. The temporal association of these changes with preceding changes in HR and ETCO2, and the results of the cross-correlation analysis, suggest that changes in ARI(t) could have been caused by one or both of these variables. The similarity of responses obtained from the right and left MCA, as shown in Figs. 2 and 3, adds confidence to the robustness of our findings.
Previous studies of dynamic CA based on the THC maneuver have estimated temporal changes in cardiovascular resistance by the ratio MBP/CBFV, mainly as a step towards calculation of the RoR index (2, 27, 42). However, cerebrovascular resistance does not provide a realistic representation of instantaneous pressure-flow curves of the cerebral circulation (7, 28). A considerable number of studies have shown that as MBP is reduced, CBF (or velocity) tends to reach zero for MBP values that are significantly above zero. This value of MBP has been called the CrCP of the cerebral circulation (1, 7, 9, 28). Conversely, the slope of instantaneous pressure-velocity relationships has been termed RAP to take into account the dimensional units of the ratio ΔMBP/ΔCBFV (11). To our knowledge, time-varying changes in RAP and CrCP during the THC maneuver have not been reported before. Following the drop in MBP, RAP is significantly reduced, with minimal changes in CrCP (Fig. 2, E and F). The relevance of this finding will be discussed below.
Finally, one important finding of our study was the lack of major physiological differences between baseline and pre-REL for most parameters studied (Table 1). Concerns have been raised in the literature that maintaining cuff compression for several minutes could lead to sympathetic nervous system activation, mainly due to the pain caused by THC inflation. In this case, the efficacy of dynamic CA could be affected, either directly (13, 26, 44) or due to changes in breathing patterns, that could then lead to alterations in PaCO2, a potent determinant of dynamic CA (2). Our results suggest that such concerns are unlikely to be founded given the lack of significant differences between the BSL and pre-REL stages in Table 1.
Hemodynamic changes during the THC maneuver.
The relatively narrow and transient CBFV response to the substantial MBP drop following THC release indicates that dynamic CA was active in this group of subjects. The related changes in HR and ETCO2 have been reported previously (8, 14, 16, 27), but their relevance to the cerebrovascular response has been largely ignored. The rise in HR following THC release has usually been interpreted as the baroreflex response to the rapid drop in MBP (8, 27, 40). Following parasympathetic blockade with atropine, the HR increase after THC release was not altered leading to the suggestion that the response was purely sympathetic in origin (40). On the other hand, Ogoh et al. (27) did not observe a significant change in the increase in HR following cuff release after β1-adrenergic blockade with metoprolol, but the response nearly disappeared when complete blockade was achieved by the addition of glycopyrrolate. The reasons for these diametrically opposed results are not clear, but could be due to methodological differences between studies. Ogoh et al. (27) measured the HR change only during the time interval 1–3.5 s after cuff release, while in our case and others, it is possible to see that the peak HR change only occurred ∼10 s after release. On the other hand, Toska et al. (40) produced very rapid inflation of THC that was maintained above 200 mmHg for less than a minute. Finally, persistence of the HR response after atropine, compared with blunting of the response with glycopyrrolate, could be due to different mechanisms of action of the two parasympathetic blockers (12).
The physiological origins of the rise in ETCO2, ∼10–30 s after THC release, are more debatable. One possible explanation would be the recirculation of blood trapped in the legs during arterial occlusion (14). Alternatively, the ETCO2 transient could reflect normalization of hyperventilation-induced hypocapnia preceding cuff release, but this was dismissed by Innes et al. (16) since the response was still present when subjects breathed 5% CO2 in air. Independent of its origins, PaCO2 has a well-known influence on the efficacy of both dynamic and static CA and this will be discussed in connection with the ARI(t) findings in the section that follows.
The different behavior of RAP and CrCP in response to a sudden drop in MBP (Fig. 2 and Table 1) is one of the most important findings of this study. Previous studies have shown that the increase in CBFV induced by hypercapnia was largely due to a reduction in CrCP, with limited contribution from RAP (28). Moreover, when CBFV was increased by cognitive or sensorimotor paradigms, this was achieved mainly through changes in CrCP, also with only limited contribution from RAP (20, 32, 33). These observations, and the close association of the RAP responses with preceding MBP changes (32), have led to the hypothesis that CrCP mainly reflects metabolic regulation of CBF while RAP is related to myogenic mechanisms controlling dynamic CA. Although much more work is needed to ascertain the validity and generalizability of these associations, the suggestion that the THC maneuver may involve an almost purely myogenic response could lead to more selective approaches to investigations of CBF regulation, compared with other methods, such as spontaneous fluctuations in MBP, changes in posture, synchronized breathing, or Valsalva maneuvers, that might (or might not) recruit a mixture of myogenic and metabolic regulatory mechanisms. For this reason, it would be important to perform similar investigations into relative changes in RAP and CrCP for a range of other methods that have been adopted for dynamic CA assessment. Differences in the extent of myogenic or metabolic involvement in dynamic CA responses to different stimuli that induce changes in MBP could help to explain the lack of intermethod agreement in studies of dynamic CA (41).
Nonstationarity of dynamic CA.
Time-varying estimates of dynamic CA indexes have been reported for spontaneous fluctuations in MBP (15, 18, 21, 31, 35), respiratory maneuvers (10), step changes in PaCO2 (17, 19), Valsalva maneuver (5), tilting (25), and the handgrip maneuver (24). Collectively, these studies demonstrate that parameters describing dynamic CA are not fixed quantities but, on the contrary, show considerable variability at rest or due to the influence of other physiological variables (4, 29). Results obtained during THC compression and release confirmed the nonstationarity of dynamic CA as expressed by ARI(t). For both MCA, ARI(t) showed a peak at ∼10–20 s after cuff release, significantly larger than preceding values. Comparison of the ARI(t) transient with peaks in HR and ETCO2 following THC release suggests possible associations. Although correlation can never be assumed to imply causality, in the case of cross correlations, the time delay corresponding to minimum or maximum values can add information to the likelihood of cause-effect mechanisms. The temporal association of the ARI(t) peak with the corresponding HR transient and time delays of less than a second suggest that ARI(t) is closely related to the baroreflex response triggered by the drop in MBP as proposed by Ogoh et al. (27). However, it is also possible that other physiological mechanism(s) could be behind these changes. In previous studies, temporary drops in ARI(t) were hypothesized to result from the alert reaction associated with cognitive or sensorimotor paradigms (10, 24, 32), which could also explain the reduction in dynamic CA efficiency due to visual stimulation as reported by Nakagawa et al. (22). The potential influence of an alert reaction, in anticipation to the release of THC, would provide a more cohesive interpretation of the changes in ARI(t) in Fig. 2G since there is a clear drop preceding cuff release, followed by the marked peak 10–20 s after THC release. If this is the case, why is the transient drop followed by the relatively large peak ∼10–20 s after THC release? Based on previous studies of the effects of autonomic blockade on dynamic CA (27, 44), one possibility would be sympathetic activation. Although it is generally accepted that increases in sympathetic activity tend to reduce CBF (38) and in the parasympathetic drive tend to induce vasodilation (12), the corresponding effects on dynamic CA are much more controversial (3). Assuming that sympathetic activation induces cerebral vasoconstriction, the expectation is that this would lead to improvements in dynamic CA, similar to the effects of hypocapnia. This interpretation is supported by the findings of Ogoh et al. (26) based on α1-adrenoreceptor blockade with prazosin during THC maneuvers and could also explain the proposed association of the baroreflex and dynamic CA (27).
If sympathetic activation, related to the baroreflex response, is driving ARI(t) upwards following release, why then is there a second trough occurring at ∼30–40 s following release? Our speculation is that in absence of the rise in ETCO2 (Fig. 2F), instead of the peak there would be a higher plateau with ARI(t) and then a gradual return to its baseline value. Instead, the ETCO2 transient is possibly inducing the second trough in ARI(t), as suggested by the highly significant negative cross-correlation values (Fig. 3) with time delays that are in excellent agreement with the time constants involved in the dynamic effects of PaCO2 on CBF and dynamic CA (17, 19, 36). Mean values of ARI(t) for 30 s following cuff release were not significantly different from the classical ARI (39) shown in Fig. 2G as horizontal bars. However, it is possible that selected temporal features of the ARI(t) transient response might be more sensitive and robust for clinical applications, something that warrants further investigation.
In summary, the temporal pattern of the ARI(t) response to the THC maneuver, which was highly consistent when compared between hemispheres (Fig. 2G), and clearly identifiable in most individual recordings (Fig. 1G), is likely to result from the complex interplay of autonomic nervous system influences and concurrent changes in PaCO2 induced by the maneuver. These results warrant further investigation, ideally coupling estimates of ARI(t), or other time-varying indexes of dynamic CA with selective autonomic blockade and manipulation of PaCO2 levels.
Limitations of the study.
Measurements of CBFV with transcranial Doppler can only be assumed to reflect changes in CBF if the cross-sectional area of the MCA remains constant. Strong evidence about the validity of this assumption for recordings performed during THC maneuvers comes from the validation study performed by Newell et al. (23). Noninvasive measurements of BP in the finger could also introduce spurious changes to the pressure-flow dynamic relationship, but comparisons with estimates of ARI(t) derived from intra-arterial BP recordings in the ascending aorta suggest that this is unlikely to contribute to any artifacts in ARI(t) (35).
Techniques for deriving estimates of ARI(t) are still at an early stage and considerable more work is needed to optimize their reliability. Compared with other techniques, such as the use of a 60-s moving window (10, 31), the alternative of expressing ARI(t) with an orthogonal decomposition, such as the Fourier series we adopted in this study, presents significant advantages as discussed previously (30). Nevertheless, either of these methods can display considerable intrasubject variability, as exemplified by the negative transient observed at t = −70 s in Fig. 1G. For this reason, caution should be exercised when analyzing individual recordings and the use of coherent averaging (Fig. 2) is recommended to obtain more robust results with these methods.
With the use of only 20 sinusoidal functions for the orthogonal decomposition of ARI(t) limits the frequency response of its temporal changes to ∼0.066 Hz for recordings with a 5-min duration. Increasing the number of functions would improve the temporal resolution of ARI(t) estimates but this would be penalized by greater computational complexity and also reduced reliability. Sensitivity analysis is one approach that could be adopted in the future to address this problem.
Our choice of performing repeated-measures ANOVA using five fixed time points could also be criticized due to the temporal variability of individual recordings, mainly regarding the REL20 and REL40 time points. One alternative would be to visually identify and extract individual values of peak ARI(t), peak HR, and peak ETCO2 following cuff release, for example. This procedure would undoubtedly accentuate differences in relation to other time points and lead to greater statistical significance. As observed in Fig. 2D, for example, on average peak HR occurs well before REL20 and the corresponding values in Table 1 are much less than the peak reached in the figure. Similar considerations would apply to characteristic features of ARI(t) and ETCO2 as well. Instead of this more selective approach though, we preferred the use of fixed time points as a more objective procedure, despite its reduced sensitivity. The temporal variability of characteristic features is likely to have influenced the lack of significance of the repeated-measures ANOVA for ETCO2 (Table 1), coupled with the large SD of population averages (Fig. 2F). For this reason, and given the presence of a clear peak around REL40 in most individual recordings, we applied a paired t-test to show that the peak amplitude was significantly different from preceding values at REL5.
Finally, we have only manipulated cerebral perfusion pressure, by inducing a sudden drop in MBP, while the strength of our conclusions would be reinforced by the additional manipulation of other variables, such as inducing concomitant changes in PaCO2 or HR, immediately before cuff release, which should be important targets for future research in this area.
Summary.
Analysis of multiple beat-to-beat cerebro-cardiovascular parameters, jointly with high-resolution estimates of ARI(t), an index of dynamic CA, has shown that in healthy subjects, the rapid recovery of CBFV following the sudden drop in MBP, resulting from the release of inflated THC, was mainly due to a drop in RAP without significant changes in CrCP. This finding suggests that the THC maneuver induces a purely myogenic response, without significant involvement of metabolic mechanisms. The temporal pattern of ARI(t) presented significant transient changes that were ascribed to multiple influences from the autonomic nervous system, possibly triggered by an alert reaction to THC deflation combined with the baroreflex response to the MBP drop, and fluctuations in PaCO2. No significant differences were observed in most cerebro-vascular parameters from the beginning of cuff inflation up to a few seconds before release. This is an important finding as it lessens concerns about any changes in normal physiological conditions that could be caused by the process of cuff inflation.
GRANTS
This study was funded by The Stroke Association (TSA2008/06), and N. P. Saeed received further funding from the Leicester Cardiovascular Biomedical Research Unit.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B.P. and T.G.R. conception and design of research; R.B.P. and N.P.S. analyzed data; R.B.P. and T.G.R. interpreted results of experiments; R.B.P. prepared figures; R.B.P. drafted manuscript; R.B.P., N.P.S., and T.G.R. edited and revised manuscript; R.B.P., N.P.S., and T.G.R. approved final version of manuscript; N.P.S. performed experiments.
REFERENCES
- 1.Aaslid R, Lash SR, Bardy GH, Gild WH, Newell DW. Dynamic pressure-flow velocity relationships in the human cerebral circulation. Stroke 34: 1645–1649, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Reports 6: 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodie FG, Atkins ER, Robinson TG, Panerai RB. Reliability of dynamic cerebral autoregulation measurements using spontaneous fluctuations in blood pressure. Clin Sci 116: 513–520, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Castro P, Santos R, Freitas J, Panerai RB, Azevedo E. Autonomic dysfunction affects dynamic cerebral autoregulation during Valsalva maneuver: comparison between healthy and autonomic dysfunction subjects. J Appl Physiol 117: 205–213, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Chacon M, Jara JL, Panerai RB. A new model-free index of dynamic cerebral blood flow autoregulation. PLoS One 9: 1–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czosnyka M, Smielewski P, Piechnik S, Al Rawi PG, Kirkpatrick PJ, Matta BF, Pickard JD. Critical closing pressure in cerebrovascular circulation. J Neurol Neurosurg Psychiatry 66: 606–611, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deegan BM, Devine ER, Geraghty MC, Jones E, Olaighin G, Serrador JM. The relationship between cardiac output and dynamic cerebral autoregulation in humans. J Appl Physiol 109: 1424–1431, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodunamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg 41: 597–606, 1974. [DOI] [PubMed] [Google Scholar]
- 10.Dineen NE, Brodie FG, Robinson TG, Panerai RB. Continuous estimates of dynamic cerebral autoregulation during transient hypocapnia and hypercapnia. J Appl Physiol 108: 604–613, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DH, Levene MI, Shortland DB, Archer LN. Resistance index, blood flow velocity, and resistance area product in the cerebral arteries of very low birth weight infants during the first week of life. Ultrasound Med Biol 14: 103–110, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Goadsby PJ. Autonomic nervous system control of the cerebral circulation. In: Handbook of Clinical Neurology. Autonomic Nervous System., edited by Buuijs RM, Swaab DF. Amsterdam, The Netherlands: Elsevier BV, 2013, p. 193–201. [DOI] [PubMed] [Google Scholar]
- 13.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke 41: 102–109, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildebrandt JR, Winn RK, Hildebrandt J. Cardiorespiratory responses to sudden release of circulatory occlusion during exercise. Respir Physiol 38: 83–92, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Hu K, Peng CK, Huang NE, Wu Z, Lipsitz LA, Cavallerano J, Novak V. Altered phase interactions between spontaneous blood pressure and flow fluctuations in type 2 diabetes mellitus: nonlinear assessment of cerebral autoregulation. Physica A 387: 2279–2292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Innes JA, Solarte I, Huszczuk A, Yeh E, Whipp BJ, Wasserman K. Respiration during recovery from exercise: effects of trapping and release of femoral blood flow. J Appl Physiol 67: 2608–2613, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Kostoglou K, Debert CT, Poulin MJ, Mitsis G. Nonstationary multivariate modeling of cerebral autoregulation during hypercapnia. Med Eng Phys 36: 592–600, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Latka M, Turalska M, Glaubic-Latka M, Kolodziej W, Latka D, West BJ. Phase dynamics in cerebral autoregulation. Am J Physiol Heart Circ Physiol 289: H2272–H2279, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Simpson DM, Kouchakpour H, Panerai RB, Chen J, Gao S, Zhang P, Wu X. Rapid pressure-to-flow dynamics of cerebral autoregulation induced by instantaneous changes in arterial CO2. Med Eng Phys 36: 1436–1443, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Maggio P, Salinet AS, Panerai RB, Robinson TG. Does hypercapnia-induced impairment of cerebral autoregulation affect neurovascular coupling? A functional TCD study. J Appl Physiol 115: 491–497, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsis G, Poulin MJ, Robbins PA, Marmarelis VZ. Nonlinear modeling of the dynamic effects of arterial pressure and CO2 variations on cerebral blood flow in healthy humans. IEEE Trans Biomed Eng 51: 1932–1943, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa K, Serrador JM, LaRose SL, Moslehi F, Lipsitz LA, Sorond FA. Autoregulation in the posterior circulation is altered by the metabolic state of the visual cortex. Stroke 40: 2062–2067, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 25: 793–797, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Nogueira RC, Bor-Seng-Shu E, Santos MR, Negrao CE, Teixeira MJ, Panerai RB. Dynamic cerebral autoregulation changes during sub-maximal handgrip maneuver. PLoS One 8: e70821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ocon AJ, Kulesa J, Clark D, Taneja I, Medow MS, Stewart JM. Increased phase synchronization and decreased cerebral autoregulation during fainting in the young. Am J Physiol Heart Circ Physiol 297: H2084–H2095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature. Acute hypotension. Stroke 39: 1979–1987, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Ogoh S, Tzeng YC, Lucas SJ, Galvin SD, Ainslie PN. Influence of baroreflex-mediated tachycardia on the regulation of dynamic cerebral perfusion during acute hypotension in humans. J Physiol 588.2: 365–371, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys 25: 621–632, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Panerai RB. Nonstationarity of dynamic cerebral autoregulation. Med Eng Phys 36: 576–584, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Panerai RB, Dineen NE, Brodie FG, Robinson TG. Spontaneous fluctuations in cerebral blood flow regulation: contribution of PaCO2. J Appl Physiol 109: 1860–1868, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Panerai RB, Eames PJ, Potter JF. Variability of time-domain indices of dynamic cerebral autoregulation. Physiol Meas 24: 367–381, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Panerai RB, Eyre M, Potter JF. Multivariate modeling of cognitive-motor stimulation on neurovascular coupling: transcranial Doppler used to characterize myogenic and metabolic influences. Am J Physiol Regul Integr Comp Physiol 303: R395–R407, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Panerai RB, Moody M, Eames PJ, Potter JF. Cerebral blood flow velocity during mental activation: interpretation with different models of the passive pressure-velocity relationship. J Appl Physiol 99: 2352–2362, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Panerai RB, Salinet AS, Brodie FG, Robinson TG. The influence of calculation method on estimates of cerebral critical closing pressure. Physiol Meas 32: 467–482, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Panerai RB, Sammons EL, Smith SM, Rathbone WE, Bentley S, Potter JF, Samani NJ. Continuous estimates of dynamic cerebral autoregulation: influence of non-invasive arterial blood pressure parameters. Physiol Meas 29: 497–513, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal Pco2 and Po2 in humans. J Appl Physiol 81: 1084–1095, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Saeed NP, Panerai RB, Horsfield MA, Robinson TG. Does stroke subtype and measurement technique influence estimation of cerebral autoregulation in acute ischaemic stroke? Cerebrovasc Dis 35: 257–261, 2013. [DOI] [PubMed] [Google Scholar]
- 38.ter Laan M, van Dijk JM, Elting JW, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 111: 361–367, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Toska K, Eriksen M, Walloe L. Short-term cardiovascular responses to a step decrease in peripheral conductance in humans. Am J Physiol Heart Circ Physiol 266: H199–H211, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, MacRae BA, Smirl JD, Horsman HM, Rickards CA. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol 303: H658–H671, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Tzeng YC, Lucas SJE, Atkinson G, Willie CK, Ainslie PN. Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol 117: 1037–1048, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Wray DW, Formes KJ, Weiss MS, Yurvati AH, Raven PB, Zhang R, Shi X. Vagal cardiac function and arterial blood pressure stability. Am J Physiol Heart Circ Physiol 281: H1870–H1880, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106: 1814–1820, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Zou R, Chon KH. Robust algorithm for estimation of time-varying transfer functions. IEEE Trans Biomed Eng 51: 219–228, 2004. [DOI] [PubMed] [Google Scholar]