Abstract
Type 2 diabetes mellitus patients (T2D) have elevated risk of stroke, suggesting that cerebrovascular function is impaired. Herein, we examined dynamic cerebral autoregulation (CA) at rest and during exercise in T2D patients and determined whether underlying systemic oxidative stress is associated with impairments in CA. Middle cerebral artery blood velocity and arterial blood pressure (BP) were measured at rest and during 2-min bouts of low- and high-intensity isometric handgrip performed at 20% and 40% maximum voluntary contraction, respectively, in seven normotensive and eight hypertensive T2D patients and eight healthy controls. Dynamic CA was estimated using the rate of regulation (RoR). Total reactive oxygen species (ROS) and superoxide levels were measured at rest. There were no differences in RoR at rest or during exercise between normotensive and hypertensive T2D patients. However, when compared with controls, T2D patients exhibited lower RoR at rest and during low-intensity handgrip indicating impaired dynamic CA. Moreover, the RoR was further reduced by 29 ± 4% during high-intensity handgrip in T2D patients (0.307 ± 0.012/s rest vs. 0.220 ± 0.014/s high intensity; P < 0.01), although well maintained in controls. T2D patients demonstrated greater baseline total ROS and superoxide compared with controls, both of which were negatively related to RoR during handgrip (e.g., total ROS: r = −0.71, P < 0.05; 40% maximum voluntary contraction). Collectively, these data demonstrate impaired dynamic CA at rest and during isometric handgrip in T2D patients, which may be, in part, related to greater underlying systemic oxidative stress. Additionally, dynamic CA is blunted further with high intensity isometric contractions potentially placing T2D patients at greater risk for cerebral events during such activities.
Keywords: static handgrip, cerebral blood flow, reactive oxygen species, oxidative stress
the incidence of stroke is elevated with type 2 diabetes mellitus (T2D), suggesting that cerebrovascular function is impaired (26, 49, 56). Dynamic cerebral autoregulation (CA) refers to the inherent ability of cerebral blood vessels to maintain blood flow in response to a rapid change in arterial blood pressure (BP) via rapid counter-regulatory changes in vascular resistance of the cerebral arterioles (1, 50). Although several studies have examined dynamic CA at rest in T2D (6, 16, 23, 30), it remains unclear as to how CA is impacted by T2D with equivocal results being reported. For example, Kim et al. (23) showed by using frequency domain analysis a reduced dynamic CA in T2D patients, suggesting less dampening of spontaneous beat-to-beat BP oscillations on cerebral blood vessels. In contrast, Huq et al. (16) reported no significant differences in dynamic CA at baseline or in response to respiratory maneuvers including breath holding and hyperventilation. The reason for these disparate findings is not clear, but additional work is needed to better understand the influence of T2D on dynamic CA, not only at rest but also under conditions when CA is challenged, such as isometric exercise. Short duration contractions involving an isometric component are part of many activities of daily living (e.g., carrying groceries) and the associated large elevations in BP (28) would potentially be deleterious to the cerebral vasculature if dynamic CA is impaired (23, 24).
Isometric exercise presents a challenge to CA, not only due to rapid and robust elevations in BP but also due to increases in sympathetic nerve activity (10) and cerebral metabolism (43, 52, 54). However, it is unknown how T2D influences the regulation of cerebral blood flow during exercise. This is important considering T2D-related impairments in peripheral vascular control have been reported at rest (32) and during exercise (46). Indeed, exaggerated sympathetic vasoconstrictor tone and blunted vasodilator responsiveness have been demonstrated in exercising skeletal muscle of T2D patients (15, 25, 46, 47). Whether these peripheral vascular abnormalities seen in T2D patients are manifest in the cerebral circulation and alter dynamic CA during exercise remains unknown.
Herein, we tested the hypothesis that patients with T2D would have an impaired dynamic CA during graded intensities of isometric handgrip exercise compared with healthy age, sex, and body-weight matched controls. Because hypertension is a common comorbidity in T2D patients (27, 45), we also studied a group of T2D patients with diagnosed hypertension. Furthermore, given recent evidence from rodent and human studies suggesting that an elevation in oxidative stress may impair cerebrovascular function (3, 9, 38), a second goal of the present study was to examine whether underlying oxidative stress is associated with impairments in dynamic CA at rest or during isometric exercise.
METHODS
Subjects.
A total of 23 subjects participated in the present study: seven normotensive patients with T2D (reported duration of disease: 4 ± 1 years), eight hypertensive patients with T2D (reported duration of disease: 6 ± 1 years), and eight normotensive controls with no family history of T2D and matched to T2D patients for age, sex, and body weight. Characteristics of the T2D patients and healthy control subjects are provided in Table 1. None of the T2D patients were being treated for or had symptoms of peripheral neuropathy. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board. Each subject received a verbal and written explanation of the study objectives, measurement techniques, and risks and benefits associated with the investigation. Before participation, each subject provided written informed consent and completed a medical health history questionnaire and a 12-h fasting blood chemistry screening including a lipid panel, metabolic panel, and HbA1c measurement.
Table 1.
Subject characteristics
| Controls | T2D Patients | T2D + HTN Patients | |
|---|---|---|---|
| Sex, male/female | 6/2 | 4/3 | 5/3 |
| Age, years | 55 ± 3 | 49 ± 3 | 55 ± 3 |
| Weight, kg | 91 ± 7.4 | 103 ± 8.1 | 90 ± 8.0 |
| Height, cm | 173 ± 2.8 | 169 ± 1.9 | 167 ± 3.4 |
| Body mass index, kg/m2 | 30 ± 2.3 | 36 ± 2.7 | 32 ± 1.9 |
| Glucose, mg/dL | 96 ± 5.3 | 164 ± 31* | 156 ± 25* |
| HbA1c, % | 5.5 ± 0.1 | 7.5 ± 0.1* | 7.5 ± 0.4* |
| Triglycerides, mg/dL | 154 ± 42 | 155 ± 26 | 177 ± 23 |
| LDL, mg/dL | 120 ± 9 | 110 ± 10 | 110 ± 11 |
| Hypoglycemic medications | |||
| n | |||
| Insulin | — | 1 | 1 |
| Biguanides | — | 6 | 7 |
| Sulfonylureas | — | 1 | 2 |
| Combination | — | 1 | 2 |
| Cardiovascular medications | |||
| n | |||
| ACE inhibitors | — | 1 | 5 |
| Angiotensin receptor blocker | — | 0 | 2 |
| Statin | — | 3 | 6 |
| Diuretic | — | 0 | 2 |
| β-Blocker | — | 1 | 1 |
Values are means ± SE.
T2D, type 2 diabetes mellitus; HTN, hypertensive.
Represents P < 0.05 vs. controls.
Cardiovascular measurements.
Heart rate (HR) was continuously monitored using a standard lead II surface electrocardiogram (ECG; Quinton Q710 Foremost Equipment, Rochester, NY). BP was measured on a beat-to-beat basis using servo-controlled finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) placed on the middle finger of the left hand and supported on a bedside table positioned at the level of the heart. The changes in arterial pressure measured by photoplethysmography have been shown to provide an accurate estimate of directly measured intra-arterial BP both at rest and during exercise (13, 44). In addition, an automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) recorded resting BP every minute by the auscultation of the brachial artery of the right arm to validate absolute BP measurements from the Finometer (7, 53). Respiratory movements were monitored using a strain-gauge pneumobelt placed in a stable position around the abdomen (Pneumotrace; UFI, Morro Bay, CA). Blood flow velocity in the middle cerebral artery (MCAV) was used to estimate changes in cerebral perfusion. The proximal segment of the MCAV was insonated through the temporal window using a 2-MHz pulsed-wave transcranial Doppler ultrasound (model 500V; Multigon Industries, Mt. Vernon, NY). The Doppler probe was placed over the temporal window and fixed with an adjustable headband. The MCAV was monitored on the same side as the exercising hand to avoid metabolic influence associated with this form of exercise (37, 43, 54). The cerebral vascular conductance index (CVCi) was calculated as MCAVmean divided by mean arterial pressure (MAP). End-tidal Pco2 (PetCO2) was obtained from a capnogram acquired by means of a nasal cannula connected to a rapid response infrared CO2 analyzer (Capnocheck Plus; Smith Medical PM, Waukesha, WI). Signal outputs were transmitted to an analog-to-digital converter (Chart v5.2; Powerlab, AD Instruments, Bella Vista, NSW, Australia), sampled at frequencies of 1,000 Hz, and stored on a personal computer for offline analysis.
Experimental protocols.
Before the actual experimental day, each subject was familiarized with the equipment and the study protocol including inflation of bilateral thigh cuffs for dynamic CA measures (1, 48), as described in detail below. On the experimental day, T2D patients were instructed to refrain from medication use. Although medications being withheld on the morning of the study would not completely eliminate the impact medications may be having on experimental measures, because of the high risk in this patient group it would neither be safe nor ethical to completely discontinue medications for an extended period of time. Moreover, we would suggest that the clinical applicability of our results is enhanced by the patients being on their existing medications as this addresses the true clinical picture of T2D patients. All subjects arrived at the laboratory a minimum of 2 h after a light meal. Subjects were also asked to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol for at least 24 h before experimental sessions. All experiments were performed at an ambient room temperature of 22°–24°C with external stimuli minimized. The procedures listed below were performed.
Total reactive oxygen species and superoxide.
After resting quietly for a minimum of 15 min, blood samples were obtained from the antecubital vein for the determination of total reactive oxygen species (ROS) and superoxide using electron paramagnetic resonance (EPR) spectroscopy (8). These measures were only obtained at rest because isometric handgrip does not increase systemic oxidative stress even when performed to fatigue at high intensities (2). Samples contained 3.5 mM of deferoxamine methanesulfonate salt (DF; Noxygen Science Transfer & Diagnostics GmbH, Elzach, Germany) and 9.08 mM of diethyldithiocarbamic acid sodium (DETC). Initially, samples assigned for the measurements of total ROS and superoxide were incubated with Krebs-HEPES buffer solution at 37°C for 15 min. Subsequently, both samples were incubated with methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) spin probe at 37°C for 15 min. After complete mixing, 50 μl of each sample were loaded into a 1-cc syringe and flash frozen between buffer solutions to form a continuous frozen plug using liquid nitrogen. Samples were then stored at −80°C and shipped to the University of Nebraska Medical Center for analyses. Total ROS was measured directly using the CMH probe, while superoxide was calculated indirectly by subtracting superoxide dismutase-treated samples from total ROS.
Dynamic cerebral autoregulation at rest.
Subjects were seated in a semi-recumbent position on a medical examination table and instrumented for measures of HR, BP, respiration, MCAV, and PetCO2. After instrumentation, cuffs (SC12; 13 × 85 cm; Hokanson) were placed around both thighs for the assessment of dynamic CA (1, 48). Subjects then rested quietly for 10 min followed by a 3-min baseline data collection period, and bilateral inflation of the thigh cuffs to suprasystolic pressure (220 mmHg) for 3 min, after which the thigh cuffs were deflated and continuous data collection occurred for an additional 3 min. Cuffs were always deflated at end expiration, observed from the respiratory band, to eliminate potential respiratory influences. This protocol was performed twice with the trials separated by a minimum of 20 min, and the average of the trials was used to estimate resting dynamic CA for each subject (37).
Dynamic cerebral autoregulation during exercise.
Subjects had both arms supported on adjustable bedside tables with a handgrip dynamometer held in the dominant hand (model 78010; Lafayette Instrument, Lafayette, IN) and beat-to-beat Finometer BP measures made on the opposite hand. Maximum voluntary contraction (MVC) was taken as the highest force produced during 3 to 5 maximal efforts, each separated by at least 1 min. To estimate dynamic CA during exercise, 1.5 min after thigh cuffs were inflated, subjects initiated isometric handgrip exercise at either low (20% MVC) or high (40% MVC) intensity for 2 min and cuffs were then deflated at 1.5 min of exercise at end expiration, as previously described (37). Exercise bouts were performed in random order with two bouts performed at each intensity. The exercise bouts were separated by a minimum of 20 min, and the average of the trials for each intensity was used to estimate dynamic CA during exercise for each subject.
Data analyses.
Resting baseline values for HR, MAP, MCAVmean, and PetCO2 were calculated as mean values over a 1-min steady-state period at rest before bilateral cuff inflation and exercising values were calculated as mean values over the last 30 s of handgrip bouts before bilateral cuff release. For dynamic CA measures, control values of MAP, MCAVmean, and calculated CVCi (MCAVmean/MAP) were taken as 4-s averages immediately before thigh-cuff release. Changes in MAP, MCAVmean, and CVCi during cuff release were then determined relative to these control values and calculated from nadir values at the time of 1–3.5 s from cuff release. The rate of regulation (RoR) was then calculated as an index of dynamic CA (1, 34, 48): RoR = (Δrelative CVCi/ΔT)/Δrelative MAP, where (Δ relative CVCi/ΔT) is the slope of the linear regression between relative CVCi and time (T), and Δrelative MAP, the magnitude of the transient fall in MAP, which was calculated by subtracting control MAP from averaged MAP during the interval from 1.0 to 3.5 s (1). The rate of change in CVCi between 1.0 and 3.5 s from cuff release has been shown to be directly related to dynamic CA (1, 34, 48).
Statistical analysis.
All data are reported as means ± SE. Comparisons of baseline subject characteristics, total ROS, and superoxide between groups were made using a simple ANOVA followed by a multiple comparison test when appropriate. Statistical comparisons of physiological variables were made using repeated-measures two-way ANOVA with the Green-house-Geisser correction, in which condition (i.e., baseline, 20% handgrip and 40% handgrip) and group [controls, T2D patients, T2D + hypertensive (HTN) patients] were the main factors. A Bonferroni test was used post hoc to investigate significant main effects and interactions when present. Statistical significance was set at P < 0.05. The relationships of baseline and exercise RoR with total ROS, superoxide, fasting glucose, and HbA1C were examined with Pearson correlations. Because there were no differences in RoR between normotensive and hypertensive T2D patients, these groups were combined for all correlation analyses. Relationships were also evaluated by using a partial correlation approach to account for the potential confounding influences of lipids, body mass index, age, duration of the disease, fasting glucose, and HbA1c. All statistical analyses were performed using SPSS version 20 (SPSS, Chicago, IL).
RESULTS
Resting HR, MAP, MCAVmean, CVCi, and PetCO2 were not significantly different between any of the subject groups (P > 0.05; Table 2). Likewise, PetCO2, cerebral and cardiovascular responses to both low- and high-intensity handgrip were similar between groups.
Table 2.
Physiological measurements at baseline and during low and high intensity isometric handgrip exercise
| Heart Rate, beats/min | Mean Arterial Pressure, mmHg | Middle Cerebral Artery Mean Blood Velocity, cm/s | Cerebrovascular Conductance Index, cm·s−1·mmHg−1 | PETCO2*,mmHg | |
|---|---|---|---|---|---|
| Baseline | |||||
| Controls | 69 ± 3 | 91 ± 3 | 52 ± 3 | 0.58 ± 0.03 | 42 ± 1 |
| T2D patients | 68 ± 5 | 94 ± 2 | 50 ± 4 | 0.53 ± 0.04 | 42 ± 1 |
| T2D + HTN patients | 77 ± 4 | 96 ± 4 | 45 ± 3 | 0.47 ± 0.04 | 41 ± 1 |
| 20% Handgrip | |||||
| Controls | 71 ± 3 | 104 ± 3 | 53 ± 3 | 0.51 ± 0.04 | 42 ± 1 |
| T2D patients | 74 ± 3 | 111 ± 1 | 48 ± 4 | 0.44 ± 0.04 | 41 ± 1 |
| T2D HTN patients | 81 ± 5 | 111 ± 4 | 46 ± 3 | 0.42 ± 0.03 | 41 ± 1 |
| 40% Handgrip | |||||
| Controls | 79 ± 4 | 121 ± 5 | 56 ± 4 | 0.46 ± 0.04 | 41 ± 1 |
| T2D patients | 84 ± 3 | 127 ± 2 | 52 ± 4 | 0.41 ± 0.03 | 42 ± 2 |
| T2D + HTN patients | 87 ± 5 | 127 ± 8 | 48 ± 3 | 0.39 ± 0.03 | 41 ± 2 |
| Condition | <0.001 | <0.001 | 0.005 | <0.001 | 0.263 |
| Group | 0.203 | 0.621 | 0.360 | 0.308 | 0.472 |
| Interaction | 0.527 | 0.767 | 0.803 | 0.394 | 0.636 |
Values are means ± SE.
The RoR was attenuated in both T2D and T2D + HTN patients compared with healthy controls at baseline, indicating an impaired dynamic CA (P < 0.05; Fig. 1). During low-intensity handgrip, the RoR was not significantly different from baseline in controls or T2D patients and thus remained lower in the T2D and T2D + HTN patients. Although RoR was well maintained in controls during high-intensity handgrip, it was reduced by 28 ± 5% from baseline in normotensive T2D patients and by 30 ± 4% in T2D + HTN patients (Fig. 1).
Fig. 1.
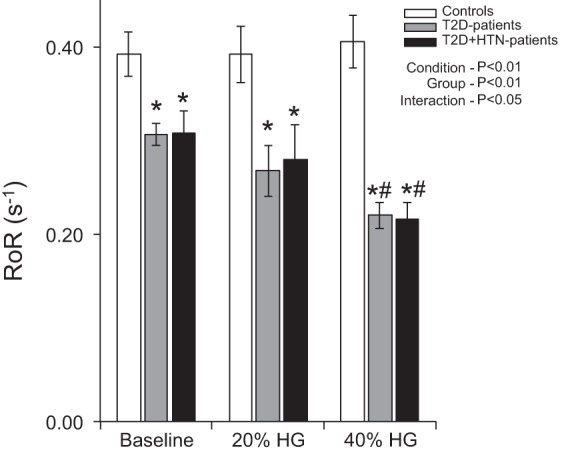
Summary data showing group means for rate of regulation (RoR) at baseline and during low [20% maximum voluntary contraction (MVC)]- and high-intensity (40% MVC) handgrip (HG) in healthy controls, normotensive type 2 diabetes mellitus (T2D) patients, and T2D + hypertensive (HTN) patients. *P < 0.05 vs. controls; #P < 0.05 vs. baseline.
Peak decreases in MAP and MCAVmean to release of the thigh cuffs were similar between controls and T2D patients at rest and during handgrip exercise (Fig. 2). Likewise, the reflex tachycardia to the acute hypotension induced by cuff release was not statistically different between controls (+9 ± 2, +10 ± 2, +9 ± 3 beats/min during baseline, 20% and 40% MVC handgrip, respectively), T2D patients (+8 ± 2, +7 ± 2, +7 ± 2 beats/min during baseline, 20% and 40% MVC handgrip, respectively), and T2D + HTN patients (+7 ± 3, +8 ± 2, +8 ± 3 beats/min during baseline, 20% and 40% MVC handgrip, respectively).
Fig. 2.
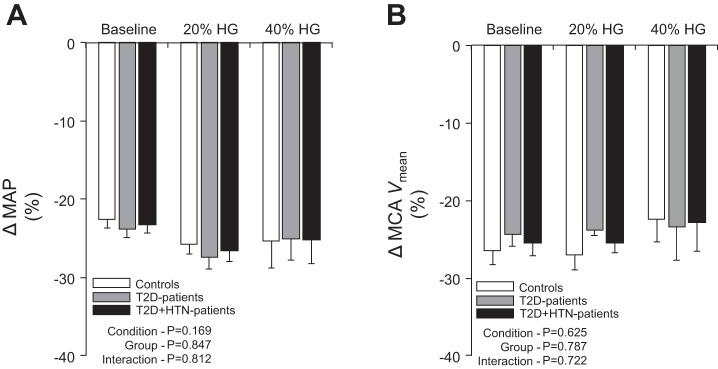
Summary data showing relative changes in mean arterial pressure (MAP; A) and middle cerebral artery blood velocity (MCAVmean; B) to thigh cuff release at baseline and during low (20% HG) and high-intensity (40% HG) handgrip in healthy controls, normotensive T2D patients, and T2D + HTN patients.
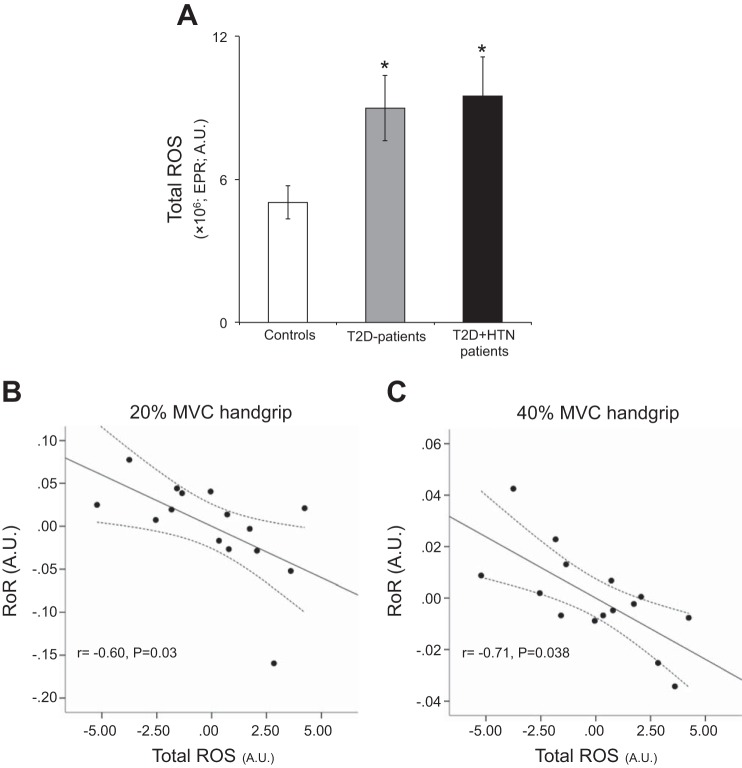
Normotensive T2D patients and T2D + HTN patients demonstrated greater resting total ROS (P < 0.05; Fig. 3A) and superoxide (2.9 ± 0.7 × 106 normotensive T2D patients and 1.7 ± 0.5 × 106 T2D + HTN patients; EPR; arbitrary units) compared with controls (0.3 ± 0.1 × 106; EPR; arbitrary units; P < 0.05). Baseline total ROS and superoxide did not demonstrate any significant relationships with resting dynamic CA measures in healthy controls or T2D patients (Table 3). However, for T2D patients, baseline total ROS (Fig. 3, B and C) and superoxide (Table 3) demonstrated significant inverse relationships with dynamic CA obtained during low- and high-intensity handgrip after adjusting for other potential contributing variables including fasting glucose and HbA1C with partial correlation analysis. For healthy controls, an inverse relationship between dynamic CA and total ROS and superoxide was found only for high-intensity handgrip (Table 3). Notably, no relationships were found between fasting glucose or HbA1C and dynamic CA obtained at rest or during low- and high-intensity handgrip in T2D patients (e.g., 40% MVC; RoR vs. glucose: r = 0.09, P > 0.05; RoR vs. HbA1C: r = −0.05, P > 0.05).
Fig. 3.
Summary data showing total reactive oxygen species (ROS) in healthy controls, normotensive T2D patients, and T2D + HTN patients (A). B and C: partial correlation plots in T2D patients with and without hypertension demonstrating an inverse relationship between total ROS and RoR during 20% and 40% MVC handgrip, respectively. Partial correlation was used to account for the potential confounding influence of lipids, body mass index, age, duration of the disease, fasting glucose, and HbA1c. *P < 0.05 vs. controls. EPR, electron paramagnetic resonance; AU, arbitrary units.
Table 3.
Partial correlation of baseline and exercise rate of regulation with total reactive oxygen species and superoxide
| Rate of Regulation, s−1 |
|||
|---|---|---|---|
| Baseline | 20% Handgrip | 40% Handgrip | |
| Total reactive oxygen species | |||
| Controls | −0.65 | 0.21 | −0.94* |
| T2D and T2D + HTN patients | −0.02 | −0.60* | −0.71* |
| Superoxide | |||
| Controls | 0.25 | −0.48 | −0.81* |
| T2D and T2D + HTN patients | 0.14 | −0.88* | −0.81* |
Represents P < 0.05 for r values.
DISCUSSION
Herein, we provide novel information regarding the dynamics of the cerebrovascular autoregulatory capacity in T2D patients at rest and during isometric handgrip exercise. The major findings are twofold. First, patients with T2D exhibited lower dynamic CA at rest and during low-intensity handgrip compared with age, sex, and body weight-matched healthy controls. Moreover, this reduction in dynamic CA in T2D patients was further exaggerated during high-intensity exercise. Such impairments in dynamic CA manifest with T2D were not different between normotensive and hypertensive T2D patients. Second, RoR obtained during handgrip was inversely related to baseline total ROS and superoxide in T2D patients, suggesting that greater underlying systemic oxidative stress may contribute to the impairment in dynamic CA in T2D. Collectively, we demonstrate impaired dynamic CA at rest and during isometric handgrip in T2D patients that may be, in part, related to greater underlying systemic oxidative stress. Additionally, dynamic CA was blunted further with high-intensity isometric contractions potentially placing T2D patients at greater risk for cerebral events during such activities. These findings are of particular importance given the number of common daily activities that require an isometric muscle contraction (e.g., carrying groceries, lifting a child).
Although various reports have indicated that an impaired CA is an initial event in the development of stroke (12, 17, 21, 40, 41), the underlying mechanisms impairing CA remain unclear. In this regard, T2D is a major risk factor for the development of stroke and has been one of leading causes of mortality in the world. However, human studies investigating the effects of T2D on the regulation of cerebral blood flow are limited and have reported inconsistent findings. Indeed, recent findings suggested normal (16) or impaired (23) dynamic CA in T2D patients. One possible explanation for these disparate findings might be related to the method used to estimate dynamic CA (i.e., frequency domain analysis). Frequency domain analysis estimates dynamic CA efficacy in response to normal oscillations in BP (51), whereas in the present study we used the RoR index derived from transient hypotension induced with thigh cuff deflation (1, 34, 48). The RoR index was consistently lower in patients with T2D than in controls, indicating impaired dynamic CA.
Besides our findings on dynamic CA at rest, the main purpose of the present study was to assess the dynamic CA during graded intensities of isometric handgrip exercise in patients with T2D. Previous studies indicated that dynamic CA is well preserved during low- to moderate-intensity exercise in healthy young and older subjects (11, 37). In contrast, intense exercise has the potential to induce impairments in dynamic CA even in healthy humans (3, 35, 43). The findings of the present study indicated that dynamic CA is well maintained in controls during high-intensity handgrip, whereas the RoR was reduced by ∼29% (impaired dynamic CA) from baseline in T2D patients. This suggests that in patients with T2D arterial pressure surges are buffered less efficiently, with more passive transmission of BP to the cerebral vasculature (5). Importantly, even during low-intensity handgrip dynamic CA was lower in T2D owing to the impairment in dynamic CA manifest at rest. Interestingly, despite the additional cardiovascular burden of hypertension, we did not find a difference in dynamic CA at rest or during handgrip exercise between the hypertensive and normotensive T2D patients. This potentially could be a consequence of their BP being well controlled.
Several mechanisms likely contribute to the impaired dynamic CA observed at rest and during isometric exercise in patients with T2D. The presence of vascular abnormalities in diabetes is well established and is characterized by increased vascular permeability (22), endothelial dysfunction (33), and capillary basement membrane thickening (19). In addition, autonomic dysfunction has also been reported in T2D patients and may contribute (15, 42). Nevertheless, a secondary aim of the present study was to examine whether underlying oxidative stress would be associated with impairments in dynamic CA at rest or during isometric exercise. This premise was based on recent studies suggesting that an elevation in oxidative stress may impair cerebrovascular function (3, 9, 38). Investigations have indicated that ROS reduces endothelial function by scavenging nitric oxide and generating highly reactive peroxynitrite (4, 9). In addition, chronic increases in oxidative stress promote vascular smooth muscle hypertrophy and remodeling that in turn alters the structural properties of the vessel wall to respond to rapid hemodynamic changes (18). However, in the present study, systemic ROS and superoxide were only inversely associated with the RoR obtained during low- and high-intensity handgrip. These data suggest that the deleterious impact of elevated ROS and superoxide on CA may not become manifest until the system is stressed. In other words, it is plausible that the challenges to dynamic CA mediated by handgrip exercise (e.g., BP elevation) cannot be adequately regulated due to the elevations in oxidative stress that are present in T2D patients. Additional studies are warranted. Also, the lack of a relationship between baseline RoR and oxidative stress measures does not definitively rule out underlying oxidative stress in contributing to the impairments in dynamic CA at rest. Overall, further investigations are needed to establish any causality between elevated oxidative stress and RoR at rest and during exercise in T2D patients.
Other mechanisms aside from oxidative stress need to be considered in mediating the greater impairment seen during high intensity handgrip in T2D patients since further increases in oxidative stress would not be expected during or following a 2-min isometric exercise bout even when performed at high intensity to fatigue (2). One possibility is the augmented sympatho-excitation evoked by high intensity handgrip (10). Indeed, although the increase in sympathetic nerve activity would be minimal during 2 min of 20% MVC handgrip, a robust increase would be expected during 40% MVC handgrip (29, 36, 55). Although somewhat controversial, several studies have demonstrated a role for the sympathetic nervous system in the control of the cerebral vasculature (20, 34, 55a). Moreover, patients with T2D have been reported to have an increased α-adrenergic responsiveness (14). Thus the robust sympatho-excitation during high intensity handgrip may lead to a greater vasoconstrictor tone in T2D patients, thereby contributing to a reduced ability to rapidly restore cerebral blood flow in face of changes in BP. In support of this idea, Jordan et al. (20) demonstrated that sympathetic overactivity contributes to impairments in cerebrovascular vasodilation in patients with idiopathic orthostatic intolerance. In addition, this impairment was partially restored by blocking the vascular α-adrenergic receptors (20).
Perspectives.
We demonstrate that dynamic CA is impaired at rest and during low-intensity handgrip in T2D patients with further impairments observed during high intensity handgrip. Such impairments in dynamic CA manifest with T2D were not different between normotensive and hypertensive T2D patients. Collectively, our results suggest that the cerebral vasculature of T2D patients is at a greater risk to fluctuations in BP, particularly during activities encompassing isometric contractions. These findings are of particular importance given the number of common daily activities that require an isometric muscle contraction (e.g., carrying groceries, lifting a child). Moreover, combined aerobic and resistance training has emerged as ideal to account for both vascular and nonvascular complications in T2D (31). Similar to aerobic exercise, resistance training has been shown to improve whole-body glucose utilization (31) but it also involves repeated straining like maneuvers with rapid and robust swings in BP (28, 37, 54). Hence, the integrity of the autoregulatory mechanisms protecting the brain becomes quite important under these conditions. The findings of the present study, however, indicate that transmission of BP surges to the cerebral circulation is dampened less effectively in patients with T2D, in particular during high-intensity handgrip. In this sense, lower resistance exercise intensities may be more appropriate for exercise prescription in T2D patients. Interestingly, in the T2D patient cohort studied, we did not find any relationships between fasting glucose or HbA1C and impaired dynamic CA obtained at rest or during low- and high-intensity handgrip. However, systemic ROS was inversely associated with dynamic CA during isometric handgrip, suggesting that greater underlying oxidative stress may contribute to exercise-induced impairments in dynamic CA in T2D. Given this, elevated oxidative stress may represent a potential therapeutic target for improving cerebral vascular responses during stressors in T2D patients (3, 9, 38).
In summary, our findings demonstrate that T2D impairs dynamic CA at rest and during isometric handgrip, which may be, in part, related to greater underlying systemic oxidative stress.
GRANTS
This work was partially supported by an American Physiological Society Arthur C. Guyton Awards for Excellence in Integrative Physiology (to P. J. Fadel). The EPR spectroscopy studies were performed in the EPR Core Facility at the University of Nebraska Medical Center, which is supported by National Institute of General Medical Sciences Grant 1P30GM103335 awarded to the University of Nebraska's Redox Biology Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.C.V. and P.J.F. conception and design of research; L.C.V., S.H.D., A.K.J., and S.W.H. performed experiments; L.C.V., S.W.H., and M.C.Z. analyzed data; L.C.V., S.H.D., and P.J.F. interpreted results of experiments; L.C.V. prepared figures; L.C.V. drafted manuscript; L.C.V., S.H.D., A.K.J., S.W.H., M.C.Z., and P.J.F. approved final version of manuscript; S.H.D., S.W.H., M.C.Z., and P.J.F. edited and revised manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all the volunteer subjects is greatly appreciated.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc 32: 1576–1581, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DM, Evans KA, McEneny J, Young IS, Hullin DA, James PE, Ogoh S, Ainslie PN, Lucchesi C, Rockenbauer A, Culcasi M, Pietri S. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp Physiol 96: 1196–1207, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bor-Seng-Shu E, Kita WS, Figueiredo EG, Paiva WS, Fonoff ET, Teixeira MJ, Panerai RB. Cerebral hemodynamics: concepts of clinical importance. Arq Neuropsiquiatr 70: 352–356, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Brown CM, Marthol H, Zikeli U, Ziegler D, Hilz MJ. A simple deep breathing test reveals altered cerebral autoregulation in type 2 diabetic patients. Diabetologia 51: 756–761, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol 306: H1417–H1425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deo SH, Fisher JP, Vianna LC, Kim A, Chockalingam A, Zimmerman MC, Zucker IH, Fadel PJ. Statin therapy lowers muscle sympathetic nerve activity and oxidative stress in patients with heart failure. Am J Physiol Heart Circ Physiol 303: H377–H385, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes 53: 1352–1359, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Fadel PJ, Wang Z, Tuncel M, Watanabe H, Abbas A, Arbique D, Vongpatanasin W, Haley RW, Victor RG, Thomas GD. Reflex sympathetic activation during static exercise is severely impaired in patients with myophosphorylase deficiency. J Physiol 548: 983–993, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JP, Ogoh S, Young CN, Raven PB, Fadel PJ. Regulation of middle cerebral artery blood velocity during dynamic exercise in humans: influence of aging. J Appl Physiol (1985) 105: 266–273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo ZN, Liu J, Xing Y, Yan S, Lv C, Jin H, Yang Y. Dynamic cerebral autoregulation is heterogeneous in different subtypes of acute ischemic stroke. PLoS One 9: e93213, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999. [PubMed] [Google Scholar]
- 14.Hogikyan RV, Galecki AT, Halter JB, Supiano MA. Heightened norepinephrine-mediated vasoconstriction in type 2 diabetes. Metabolism 48: 1536–1541, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 108: 3097–3101, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Huq R, Philbey CE, Mistri AK, Panerai RB, Robinson TG. Dynamic cerebral autoregulation assessed by respiratory manoeuvres in non-insulin-treated Type 2 diabetes mellitus. Diabet Med 29: 609–613, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Immink RV, van Montfrans GA, Stam J, Karemaker JM, Diamant M, van Lieshout JJ. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke 36: 2595–2600, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 87: 179–183, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PC, Brendel K, Meezan E. Thickened cerebral cortical capillary basement membranes in diabetics. Arch Pathol Lab Med 106: 214–217, 1982. [PubMed] [Google Scholar]
- 20.Jordan J, Shannon JR, Black BK, Paranjape SY, Barwise J, Robertson D. Raised cerebrovascular resistance in idiopathic orthostatic intolerance: evidence for sympathetic vasoconstriction. Hypertension 32: 699–704, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Jordan JD, Powers WJ. Cerebral autoregulation and acute ischemic stroke. Am J Hypertens 25: 946–950, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Joyner WL, Mayhan WG, Johnson RL, Phares CK. Microvascular alterations develop in Syrian hamsters after the induction of diabetes mellitus by streptozotocin. Diabetes 30: 93–100, 1981. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Immink RV, Stok WJ, Karemaker JM, Secher NH, van Lieshout JJ. Dynamic cerebral autoregulatory capacity is affected early in Type 2 diabetes. Clin Sci (Lond) 115: 255–262, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Secher NH, van Lieshout JJ. Resistance exercise and control of cerebral blood flow in type 2 diabetes. Diabetologia 51: 1755–1756, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 28: 355–359, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J Clin Hypertens (Greenwich) 13: 244–251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 58: 785–790, 1985. [DOI] [PubMed] [Google Scholar]
- 29.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. [DOI] [PubMed] [Google Scholar]
- 30.Marthol H, Zikeli U, Brown CM, Tutaj M, Hilz MJ. Cardiovascular and cerebrovascular responses to lower body negative pressure in type 2 diabetic patients. J Neurol Sci 252: 99–105, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, Blumenthal RS, Philippides G, Rocchini A. Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119: 3244–3262, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, Uptergrove GM, Deo SH, Kim A, Kanaley JA, Fadel PJ, Thyfault JP. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. J Appl Physiol (1985) 111: 657–664, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nazir FS, Alem M, Small M, Connell JM, Lees KR, Walters MR, Cleland SJ. Blunted response to systemic nitric oxide synthase inhibition in the cerebral circulation of patients with Type 2 diabetes. Diabet Med 23: 398–402, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke 39: 1979–1987, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol 288: H1461–H1467, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416–H1424, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogoh S, Sato K, Akimoto T, Oue A, Hirasawa A, Sadamoto T. Dynamic cerebral autoregulation during and after handgrip exercise in humans. J Appl Physiol (1985) 108: 1701–1705, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Pena Silva RA, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, Heistad DD. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 43: 3358–3363, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinhard M, Roth M, Guschlbauer B, Harloff A, Timmer J, Czosnyka M, Hetzel A. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke 36: 1684–1689, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Reinhard M, Wihler C, Roth M, Harloff A, Niesen WD, Timmer J, Weiller C, Hetzel A. Cerebral autoregulation dynamics in acute ischemic stroke after rtPA thrombolysis. Cerebrovasc Dis 26: 147–155, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Sanya EO, Brown CM, Dutsch M, Zikeli U, Neundorfer B, Hilz MJ. Impaired cardiovagal and vasomotor responses to baroreceptor stimulation in type II diabetes mellitus. Eur J Clin Invest 33: 582–588, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol (1985) 104: 306–314, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Shi X, Gallagher KM, SASM, Bryant KH, Raven PB. Diminished forearm vasomotor response to central hypervolemic loading in aerobically fit individuals. Med Sci Sports Exerc 28: 1388–1395, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension 26: 869–879, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Thaning P, Bune LT, Hellsten Y, Pilegaard H, Saltin B, Rosenmeier JB. Attenuated purinergic receptor function in patients with type 2 diabetes. Diabetes 59: 182–189, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thaning P, Bune LT, Zaar M, Saltin B, Rosenmeier JB. Functional sympatholysis during exercise in patients with type 2 diabetes with intact response to acetylcholine. Diabetes Care 34: 1186–1191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 370: 233–244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzeng YC, Ainslie PN. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol 114: 545–559, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzeng YC, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 56: 268–273, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Vianna LC, Araujo CG, Fisher JP. Influence of central command and muscle afferent activation on anterior cerebral artery blood velocity responses to calf exercise in humans. J Appl Physiol (1985) 107: 1113–1120, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vianna LC, Sales AR, da Nobrega AC. Cerebrovascular responses to cold pressor test during static exercise in humans. Clin Physiol Funct Imaging 32: 59–64, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest 79: 508–516, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106: 1814–1820, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. Sex differences in the risk of stroke and HbA among diabetic patients. Diabetologia 57: 918–926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]